Abstract
As the COVID‐19 pandemic continues to rise, the development of effective vaccines is of crucial importance to prevent further morbidity and mortality. In parallel, some rare adverse events related to COVID‐19 vaccines, have been reported, most of them mild. Here we report the case of a previously healthy 19‐year‐old woman who developed optic neuritis 1 week after single dose of Ad26.COV2.S vaccine with marked improvement after management with steroids. Although causality cannot be confirmed due to lack of a biological marker, this case may help to guide further research for potential pathogenic mechanism.
Keywords: adverse effects, optic neuritis, SARS‐Cov2, vaccination, viral vector vaccine
Optic neuritis after COVID‐19 vaccination.
1. INTRODUCTION
Coronavirus disease 19 (COVID) caused by SARS‐Cov‐2 represents a priority health issue since its first reports in November 2019, manifesting with a wide variety of primarily respiratory symptoms ranging from asymptomatic to life‐threatening conditions such as severe acute respiratory syndrome with over 4 million deaths worldwide. 1 As the COVID‐19 pandemic continues to rise, the development of effective vaccines is of crucial importance to prevent further morbidity and mortality. At the time of writing this manuscript, six COVID vaccines have been granted emergency use by regulatory authorities (AZD1222 (ChAdOx1‐S), Sputnik V (Gam‐COVID‐Vac), Ad26.COV2.S, BBV152, BNT162b2 and mRNA‐1273) resulting in nearly 6 billion people vaccinated up to date. (Johns Hopkins University Coronavirus Resource Center, https://coronavirus.jhu.edu/map.html, accessed on September 20, 2021). In parallel, some rare adverse events related to COVID‐19 vaccines, have been reported, most of them mild. Here we report the case of a 19‐year‐old woman who developed optic neuritis 1 week after single dose of Ad26.COV2.S vaccine.
2. CASE PRESENTATION
A 19‐year‐old woman with no relevant medical history attended to emergency department due to ocular pain and bilateral vision loss. She received single‐dose of the Ad26.COV2.S vaccine against COVID‐19 3 weeks prior to her first assessment. One week after vaccine application, the patient complained of left ocular pain, mainly with horizontal movement. During the next 5 days she noticed visual loss of the left eye. She denied the presence of fever, respiratory or other types of symptoms. At clinical evaluation, the patient was found with left amaurosis, afferent pupillary defect and fundoscopic examination revealed papillitis on the left eye (Figure 1). The right eye showed no abnormalities. No other neurological disturbance was observed. Clinical diagnosis of inflammatory optic neuritis was established.
FIGURE 1.
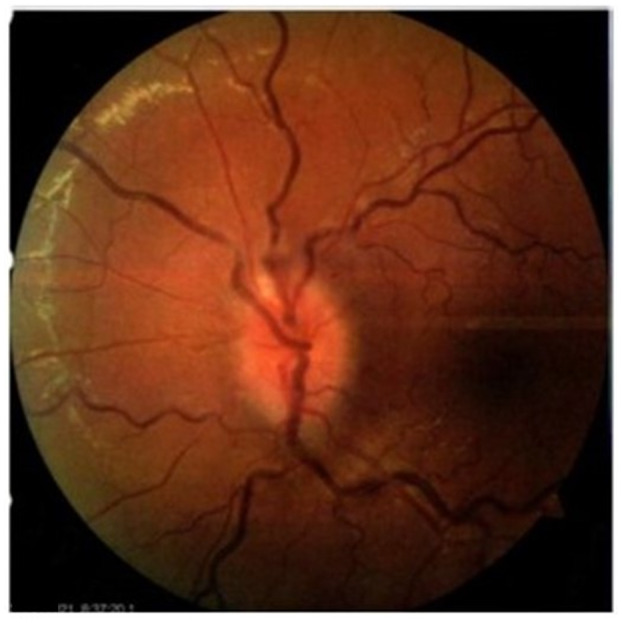
Left funduscopic examination showing papillitis and tortuous retinal vessels
Initial work‐up including complete blood count, serum glucose, kidney and liver function test were normal. CRP was 1.18 mg/dL and ESR was 21 mm/h. Brain MRI showed swelling of the left optic nerve, enhancing after gadolinium administration (Figure 2). No parenchymal lesions were observed. Immunologic panel including ANA, anti‐DNA, ANCA, anti‐Ro, anti‐La and antiphospholipid antibodies were negative. CSF reported only four leucocytes and normal glucose and protein levels. Positive CFS‐restrictive oligoclonal bands (OCB) were reported. CSF encephalitis/meningitis panel was negative for pathogens. Serum anti‐aquaporin‐4 (AQP4) antibodies were also negative. Serum anti‐myelin‐oligodendrocyte‐glycoprotein (MOG) antibodies were not commercially available. PCR for SARS‐Cov2 in nasopharyngeal swab was negative.
FIGURE 2.
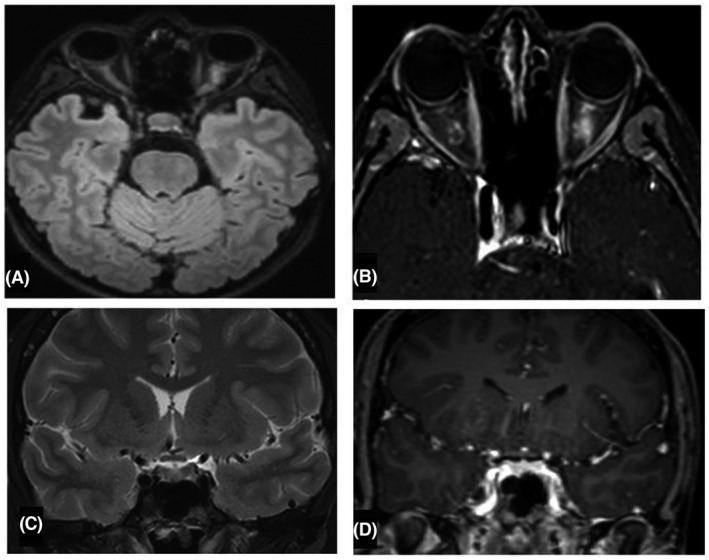
(A,B) Axial FLAIR and T1‐Gd weighted MRI evidencing swelling of the left optic nerve, enhancing after gadolinium administration. (C,D) Sagittal T2 and T1‐Gd weighted MRI, no enhancing of the optic chiasma is observed.
The patient received high dose IV steroids (methylprednisolone 1 g daily for 5 days) with improvement in visual acuity from day 2, with nearly complete resolution at day 5 followed by oral prednisolone (1 mg/kg) on tapering schedule over 4 weeks. On last checkup visual acuity of the left eye was 20/20 and papillitis was resolved.
3. DISCUSSION
Here we present the case of a 19‐year‐old woman with optic neuritis after COVID‐19 vaccination. As COVID‐19 vaccination spreads worldwide it is expected that side effects of these vaccines emerge. Ad26.COV2.S vaccine (Janssen Pharmaceutical Companies) contains a replicant deficient human adenovirus type 26 vector and is safe and effective against severe disease. 2 On February 26, 2021, the vaccine received FDA emergency use authorization as single dose to prevent COVID‐19 in individuals of 18 years and older. In the following months, although rare, some relevant neurological side effects were reported, such as vaccine‐induced immune thrombotic thrombocytopenia 3 , 4 manifesting with disseminated thrombosis in atypical sites, mainly in the cerebral venous system associated with thrombocytopenia and the production of autoantibody against platelet‐factor 4 (PF4). 5
Regarding demyelinating diseases, three cases of transverse myelitis were reported in the interim analysis of the other viral vector vaccine (ChAdOx1 nCoV‐19, AstraZeneca) 6 ; only one of them was considered as possibly related to vaccination. Other important neurological side effects such as Guillain‐Barre syndrome (GBS), stroke, facial palsy, and acute disseminated encephalomyelitis (ADEM) have been reported. 7
Optic neuritis (ON) can result from inflammatory, autoimmune, or infectious disorders. ON is the most common demyelinating condition associated with vaccination 8 including various types of infectious agents such as hepatitis A and B, influenza, measles, pneumococcal vaccine, human papilloma virus, rabies, and BCG. 9 To date, no cases of optic neuritis associated with SARS‐Cov‐2 vaccination have been reported. The mechanisms underlying these autoimmune/inflammatory events are mostly unknown, but some points are remarkable. First, some vaccine adjuvants induce activation of the NLRP3 inflammasome, inciting inflammation an immunity. 10 Molecular mimicry is another proposed mechanism in COVID‐19 related immune phenomena, where viral proteins expressed after mRNA translation have elicited immune cross‐reactivity with human tissue. 11 It has been proposed that the active component itself of the Ad26.COV2.S vaccine (adenovirus type 26 vector containing the DNA of spike) and the specific B cell response targeting the vector‐based vaccine could trigger a dysregulated immunological process, causing the aforementioned adverse reactions in certain individuals. This cross reactivity relates to several environmental and genetic factors, such as aberrant major histocompatibility complex (MHC) class II antigen presentation to autoreactive T cells. 12
We recognize that the good clinical response to steroids highly suggest the possibility of MOG‐associated disease (MOGAD). Unfortunately, anti‐MOG antibodies were not commercially available. Nevertheless, we cannot ignore the strong temporal relation of the vaccine and the potentially immune mechanisms triggered by its components.
Our rationale is that the viral vector vaccine may access the cerebral neuronal pathways to replicate and trigger a pro‐inflammatory response resulting in optic neuritis. Despite these hypotheses, we are aware of the difficulty to establish causality between vaccination and optic neuritis, nevertheless, the close temporal association since vaccine application to symptoms initiation strongly suggest common mechanisms.
4. CONCLUSION
We present the case of a previously healthy 19‐year‐old woman who developed optic neuritis with close temporal association to SARS‐Cov‐2 vaccination. Although causality cannot be confirmed due to the lack of a biological marker, this case may help to guide further research into a potential pathogenic mechanism.
CONFLICT OF INTEREST
None declared.
ETHICAL STATEMENT
Approval of the research protocol: N/A
Informed Consent: informed consent was obtained from the subject
Registry and the Registration No. of the study/trial: N/A
Animal Studies: N/A
García‐Estrada C, Gómez‐Figueroa E, Alban L, Arias‐Cárdenas A. Optic neuritis after COVID‐19 vaccine application. Clin Exp Neuroimmunol. 2022;13:72–74. 10.1111/cen3.12682
REFERENCES
- 1. COVID‐19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). https://coronavirus.jhu.edu/map.html
- 2. Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, et al.; ENSEMBLE Study Group . Safety and efficacy of single‐dose Ad26.COV2.S vaccine against Covid‐19. N Engl J Med. 2021;384(23):2187–201. 10.1056/NEJMoa2101544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Muir KL, Kallam A, Koepsell SA, Gundabolu K. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination. N Engl J Med. 2021;384(20):1964–5. 10.1056/NEJMc2105869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. See I, Su JR, Lale A, Woo EJ, Guh AY, Shimabukuro TT, et al. US Case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325(24):2448–56. 10.1001/jama.2021.7517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dotan A, Shoenfeld Y. Perspectives on vaccine induced thrombotic thrombocytopenia. J Autoimmun. 2021;121:102663. 10.1016/j.jaut.2021.102663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy ofthe ChAdOx1 nCoV‐19 vaccine (AZD1222) against SARS‐CoV‐2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. 10.1016/s0140-6736(20)32661-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goss AL, Samudralwar RD, Das RR, Nath A. ANA investigates: neurological complications of COVID‐19 vaccines. Ann Neurol. 2021;856–7. 10.1002/ana.26065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheng JY, Margo CE. Ocular adverse events following vaccination: overview and update. Surv Ophthalmol. 2021;S0039–6257(21):00099. 10.1016/j.survophthal.2021.04.001 [DOI] [PubMed] [Google Scholar]
- 9. Karussis D, Petrou P. The spectrum of post‐vaccination inflammatory CNS demyelinating syndromes. Autoimmun Rev. 2014;13(3):215–24. 10.1016/j.autrev.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 10. Teijaro JR, Farber DL. COVID‐19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol. 2021;21(4):195–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kanduc D, Shoenfeld Y. On the molecular determinants of the SARS‐CoV‐2 attack. Clin Immun. 2020;215:108426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Segal Y, Shoenfeld Y. Vaccine‐induced autoimmunity: the role of molecular mimicry and immune crossreaction. Cell Mol Immunol. 2018;15(6):586–94. [DOI] [PMC free article] [PubMed] [Google Scholar]


