Abstract
Aim
To determine the risk of adverse outcomes across the spectrum of glycated haemoglobin (HbA1c) levels among hospitalized COVID‐19 patients with and without diabetes.
Materials and methods
Danish nationwide registries were used to study the association between HbA1c levels and 30‐day risk of all‐cause mortality and the composite of severe COVID‐19 infection, intensive care unit (ICU) admission and all‐cause mortality. The study population comprised patients hospitalized with COVID‐19 (3 March 2020 to 31 December 2020) with a positive polymerase chain reaction (PCR) test and an available HbA1c ≤ 6 months before the first positive PCR test. All patients had at least 30 days of follow‐up. Among patients with diabetes, HbA1c was categorized as <48 mmol/mol, 48 to 53 mmol/mol, 54 to 58 mmol/mol, 59 to 64 mmol/mol (reference) and >64 mmol/mol. Among patients without diabetes, HbA1c was stratified into <31 mmol/mol, 31 to 36 mmol/mol (reference), 37 to 41 mmol/mol and 42 to 47 mmol/mol. Thirty‐day standardized absolute risks and standardized absolute risk differences are reported.
Results
We identified 3295 hospitalized COVID‐19 patients with an available HbA1c (56.2% male, median age 73.9 years), of whom 35.8% had diabetes. The median HbA1c was 54 and 37 mmol/mol among patients with and without diabetes, respectively. Among patients with diabetes, the standardized absolute risk difference of the composite outcome was higher with HbA1c < 48 mmol/mol (12.0% [95% confidence interval {CI} 3.3% to 20.8%]) and HbA1c > 64 mmol/mol (15.1% [95% CI 6.2% to 24.0%]), compared with HbA1c 59 to 64 mmol/mol (reference). Among patients without diabetes, the standardized absolute risk difference of the composite outcome was greater with HbA1c < 31 mmol/mol (8.5% [95% CI 0.5% to 16.5%]) and HbA1c 42 to 47 mmol/mol (6.7% [95% CI 1.3% to 12.1%]), compared with HbA1c 31 to 36 mmol/mol (reference).
Conclusions
Patients with COVID‐19 and HbA1c < 48 mmol/mol or HbA1c > 64 mmol/mol had a higher associated risk of the composite outcome. Similarly, among patients without diabetes, varying HbA1c levels were associated with higher risk of the composite outcome.
Keywords: antidiabetic drug, cardiovascular disease, database research, glycaemic control, hypoglycaemia, population study
1. INTRODUCTION
Coronavirus disease‐2019 (COVID‐19) infection, caused by severe acute respiratory syndrome coronavirus 2(SARS‐CoV‐2), was declared a global pandemic by the World Health Organization on 11 March 2020. 1 , 2
In previous studies, in a non‐COVID‐19 setting, glycated haemoglobin (HbA1c) has been shown to be a clinically useful marker for predicting all‐cause mortality among patients both with and without diabetes. 3 , 4 , 5 , 6 In a COVID‐19 setting, many studies have demonstrated that diabetes mellitus is a substantial risk factor for increased severity of COVID‐19 infection, admission to an intensive care unit (ICU), and COVID‐19 related mortality. 7 , 8 , 9 , 10 Patients with diabetes and higher levels of HbA1c or hyperglycaemia also have higher levels of inflammatory markers, which could indicate a more severe inflammatory response than those without diabetes or with normoglycaemia. 11 , 12 , 13 , 14 , 15 , 16 However, to date, few studies have examined the relationship between varying HbA1c levels and the associated risk of mortality and adverse outcomes among COVID‐19 patients, and these studies have conflicting results. 17 , 18 , 19 The studies focused primarily on the association between HbA1c and mortality among COVID‐19 patients with diabetes. Previously, Holman et al 18 identified an independent association between high HbA1c levels and COVID‐19‐related mortality among over 3 million people with type 1 diabetes and type 2 diabetes. However, the absolute risk of mortality according to HbA1c level after COVID‐19 infection was not reported, only patients with diabetes were included, and important outcomes such as severe COVID‐19 infection or ICU admission were also not examined.
In a COVID‐19 setting, more information is needed on the association between varying HbA1c testing levels prior to a positive SARS‐CoV‐2 polymerase chain reaction (PCR) test and risk of mortality and, particularly, adverse outcomes among patients with and without diabetes. Thus, the purpose of the present Danish registry‐based cohort study was to determine the risks of severe COVID‐19 infection, ICU admission, or all‐cause mortality among COVID‐19 patients, with and without diabetes, associated with varying levels of HbA1c.
2. MATERIALS AND METHODS
2.1. Data sources
All Danish residents are assigned a unique and personal civil registration number, which allows individual linkage of nationwide administrative registries. The data used in the present study were obtained from the Danish National Patient Registry, the Danish Microbiology Database, the Clinical Laboratory Information System, the Danish National Prescription Registry, and the Danish Population Registry. The Danish National Patient Registry provides data on all hospital admissions and outpatient contacts since 1977 according to the International Classification of Diseases (8th Revision [ICD‐8] until 1993 and 10th Revision [ICD‐10] thereafter) as well as surgical procedures, registered based on the Nordic Medico‐Statistical Committee (NOMESCO) Classification of Surgical Procedures (NCSP). 20 SARS‐CoV‐2 PCR data were obtained from the Danish Microbiology Database, which provides nationwide, complete reports from all Danish departments of clinical microbiology. 21 Information on HbA1c measurements was gathered from the Clinical Laboratory Information System, which holds information on all blood samples obtained from hospitals and medical practices in two Danish regions, representing one‐third of the Danish population. In Denmark, the HbA1c measurement has been reported in mmol/mol according to the International Federation of Clinical Chemistry and Laboratory Medicine Reference Measurement Procedure since 2010. The blood samples are coded according to the international Nomenclature, Properties and Units coding system (see Appendix S1, Table S1). 22 The Danish National Prescription Registry provides detailed information on all dispensed drug prescriptions from Danish pharmacies according to the Anatomic Therapeutic Chemical (ATC) Classification System since 1995. 23 The Danish Civil Registration System holds information on birth date, sex and vital status (ie, whether a person is alive and a citizen in Denmark, emigrated, or dead, along with the date of these events). 24 The Danish registries are of high quality and complete, and the definitions and codes obtained from the registries have been previously validated. 20 , 25 , 26
2.2. Study population
The study population included all Danish citizens with a positive SARS‐CoV‐2 PCR test and a COVID‐19‐related hospitalization between 26 February 2020 and 31 December 2020. The index date was defined as the first positive PCR test result. A COVID‐19‐related hospitalization was defined as a hospital admission within 14 days after the first positive PCR test result with a length of stay of at least 12 hours. Information on the most recent HbA1c for each patient was identified from blood samples ≤ 6 months before index date. Only patients with information on HbA1c were eligible for inclusion.
2.3. Patient characteristics, comorbidities and pharmacotherapy
Patients with diabetes were identified according to diabetes‐related hospital admissions or outpatient contacts (ICD‐8 code: 250 and ICD‐10 codes: E10‐E14) any time before index date or by dispensed prescriptions for an antidiabetic drug (ATC code: A10) or HbA1c ≥ 48 mmol/mol (6.5%) ≤ 6 months before index date.
Patient comorbidity was determined from the Danish National Patient Registry using hospital admissions or outpatient contacts (ie, primary or secondary diagnosis codes) any time prior to index date (Table S2). Concomitant pharmacotherapy was identified from the Danish Prescription Registry as a dispensed prescription within 6 months prior to index date (Table S3). Patients with hypertension were identified from combination treatment with at least two dispensed antihypertensive drug prescriptions, as described previously. 27
2.4. Follow‐up and outcomes
The primary study outcome was a composite of COVID‐19 severe acute respiratory syndrome (ie, severe COVID‐19 infection [ICD‐10 code: B972A]), ICU admission (NCSP codes: NABE, NABB, BGDA), or all‐cause mortality. The secondary study outcome was all‐cause mortality. All patients included had at least 30 days of potential follow‐up from the first positive PCR test result. Patients were followed from the day of the first positive PCR test until the occurrence of a study outcome, death, or end of study (31 January 2021), whichever came first.
2.5. Statistical methods
Continuous variables are presented as medians with interquartile ranges (IQRs), and differences were assessed by the Kruskal‐Wallis test. Categorical variables are presented as numbers with proportions, and differences were assessed using the chi‐squared test.
To test the association of varying HbA1c levels among patients with and without diabetes separately, we performed stratified analyses according to diabetes status. Patients with diabetes were grouped according to the HbA1c thresholds used by the International Diabetes Federation and the American Diabetes Association 28 , 29 , 30 , 31 : <48 mmol/mol (6.5%), 48 to 53 mmol/mol (6.5% to 7.0%), 54 to 58 mmol/mol (7.1% to 7.5%), 59 to 64 mmol/mol (7.5% to 8.0%), and >64 mmol/mol (8.0%). For this analysis, HbA1c 59 to 64 mmol/mol (7.5% to 8.0%) was used as the comparative reference as the International Diabetes Federation recommended this HbA1c target among patients using multiple medications, including glucose‐lowering drugs, who have a reduced life expectancy (eg,<10 years) and multiple comorbidities. 31 Among patients without diabetes, HbA1c was stratified into <31 mmol/mol (5.0%), 31 to 36 mmol/mol (5.0% to 5.4%; reference), 37 to 41 mmol/mol (5.5% to 5.9%), and 42 to 47 mmol/mol (6.0% to 6.5%), as done previously. 4 , 32
Using working Cox regression models, we standardized the 30‐day risks of all‐cause mortality and the composite outcome in the aforementioned HbA1c groups among patients with and without diabetes, according to the distribution of sex, age as a continuous variable, history of ischaemic heart disease, heart failure, atrial fibrillation, stroke, peripheral artery disease, hypertension, chronic obstructive pulmonary disease, cancer, chronic renal disease and use of cholesterol‐lowering drugs, beta‐blockers, calcium channel blockers, renin‐angiotensin system inhibitors, aspirin, and anticoagulants in the study population. We report 30‐day standardized absolute outcome risks and risk differences with 95% confidence intervals (CIs) of outcomes. 33
To test the robustness of our results, we performed several sensitivity analyses. First, the model assumptions of the working Cox regression model (proportional hazards, linearity of effects and absence of interactions) were tested, estimating standardized 30‐day outcome risks based on a random survival forest model among patients with and without diabetes, respectively. 34 Second, we report hazard ratios (HRs) with 95% CIs during complete follow‐up among patients with and without diabetes, respectively. Third, to visualize the association between continuous HbA1c and the rates of the study outcomes, we fitted a Cox regression model, which used restricted cubic splines with three knots to illustrate the association between HbA1c and the outcome rates adjusted for all other variables of the main analysis. Fourth, to assess if HbA1c per se has a significant biological effect, we compared patients with and without diabetes but with the same range of HbA1c levels. The combined population was stratified into the following groups: HbA1c < 39 mmol/mol (5.7%) without diabetes, HbA1c < 39 mmol/mol with diabetes, HbA1c 39 to 47 mmol/mol (5.7% to 6.5%) without diabetes, HbA1c 39 to 47 mmol/mol with diabetes, 48 to 53 mmol/mol, 54 to 58 mmol/mol, 59 to 64 mmol/mol, and >64 mmol/mol (Table S8). Fifth, a sensitivity analysis was conducted to assess if random blood glucose at the time of admission plays a critical role in the prognosis of COVID‐19 patients in terms of all‐cause mortality or the composite outcome. Sixth, to determine possible generalizability of the present study findings, we compared patient characteristics for the included study patients with COVID‐19 patients with no information on HbA1c levels according to diabetes status. Seventh, to test if time of diagnosis influenced our findings, the populations with and without diabetes were stratified in two periods before and after 1 August 2020, and the main analyses were repeated. Eighth, to test whether time of HbA1c measurement prior to index date would influence our results, sensitivity analyses using HbA1c obtained 3 months before index date instead of 6 months were performed for both study outcomes among patients with and without diabetes as the main analyses.
The main statistical coding was conducted using the SAS (version 9.4; SAS Institute Inc., Cary, North Carolina), and all statistical analyses were obtained using R (version 4.0.3). The level of statistical significance was set at 5%.
2.6. Ethics
In Denmark, registry‐based studies using de‐identifiable data do not require ethical approval. The present study was approved by the data responsible institute (the Capital Region of Denmark‐Approval number:P‐2019‐191) in accordance with the General Data Protection Regulation. Danish law prohibits reporting of low group numbers below 3, which were replaced with “≤3” throughout the paper. The exact numbers are known to the investigators.
3. RESULTS
Of the 4 198 450 individuals in Denmark who underwent a PCR test, 163 908 Danish citizens had a positive test for SARS‐CoV‐2 from 26 February 2020 to 31 December 2020, of whom 7865 (4.8%) had a COVID‐19‐related hospitalization. Among these, 3295 (41.9%) individuals had an HbA1c value ≤6 months prior to index date and comprised the study population in the present study. A total of 1178 (35.8%) patients had diabetes. The first patient was included on 3 March 2020, and the last patient was included on 31 December 2020. Among patients with diabetes, the median (IQR) follow‐up time to all‐cause mortality and to the composite outcome was 50.5 (32‐118) days and 36 (6‐89) days, respectively. Among patients without diabetes, the median (IQR) follow‐up time to all‐cause mortality and to the composite outcome was 54 (35‐187) days and 43 (9‐107) days, respectively.
Patient characteristics according to diabetes status are listed in Table 1. Compared with patients without diabetes, patients with diabetes were more likely to be male (62.7%) and to have comorbidities. The patient characteristics according to HbA1c group among patients with and without diabetes are listed in Tables 2 and 3, respectively.
TABLE 1.
Baseline characteristics according to diabetes status
| Total (N = 3295) | Patients without diabetes (N = 2117) | Patients with diabetes (N = 1178) | P‐value | ||
|---|---|---|---|---|---|
| Sex: male, n (%) | 1853 (56.2) | 1114 (52.6) | 739 (62.7) | <0.001 | |
| Age, median (IQR), years | 73.9 (62.0, 81.8) | 73.6 (59.9, 82.7) | 74.3 (64.9, 80.8) | 0.26 | |
| HbA1c, median (IQR), mmol/mol | 40 (36, 48) | 37 (34, 40) | 54 (47, 65) | <0.001 | |
| Blood glucose, median (IQR), mmol/L | 6.9 (5.9,8.8) | 6.3 (5.7, 7.4) | 9.3 (7.0, 12.1) | <0.001 | |
| Missing | 999 | 620 | 379 | ||
| C‐reactive protein, median (IQR), mg/L | 25 (4.3,72.0) | 21 (4, 66) | 30 (6.7, 86.0) | <0.001 | |
| Missing | 468 | 256 | 212 | ||
| Leukocytes, median (IQR), x 109/L | 6.8 (5.2,9.0) | 6.7 (5.0, 8.7) | 7.2 (5.5, 9.2) | <0.001 | |
| Missing | 340 | 159 | 181 | ||
| Comorbidities, n (%) | |||||
| Ischaemic heart disease | 759 (23.0) | 404 (19.1) | 355 (30.1) | <0.001 | |
| Heart failure | 338 (10.3) | 183 (8.6) | 155 (13.2) | <0.001 | |
| Previous myocardial infarction | 300 (9.1) | 172 (8.1) | 128 (10.9) | 0.01 | |
| Atrial fibrillation | 562 (17.1) | 346 (16.3) | 216 (18.3) | 0.16 | |
| Stroke | 316 (9.6) | 190 (9.0) | 126 (10.7) | 0.12 | |
| Peripheral artery disease | 136 (4.1) | 72 (3.4) | 64 (5.4) | 0.007 | |
| Hypertension | 1444 (43.8) | 770 (36.4) | 674 (57.2) | <0.001 | |
| Chronic obstructive pulmonary disease | 344 (10.4) | 207 (9.8) | 137 (11.6) | 0.11 | |
| Cancer | 646 (19.6) | 427 (20.2) | 219 (18.6) | 0.29 | |
| Liver disease | 128 (3.9) | 72 (3.4) | 56 (4.8) | 0.067 | |
| Rheumatic disease | 258 (7.8) | 163 (7.7) | 95 (8.1) | 0.76 | |
| Chronic renal disease | 385 (11.7) | 150 (7.1) | 235 (19.9) | <0.001 | |
| Lipidaemia | 470 (14.3) | 290 (13.7) | 180 (15.3) | 0.23 | |
| Concomitant pharmacotherapy, n (%) | |||||
| Antidiabetics | 951 (28.9) | 0 (0.0) | 951 (80.7) | <0.001 | |
| Insulin | 395 (12.0) | 0 (0.0) | 395 (33.5) | <0.001 | |
| Oral glucose‐lowering drugs | 801 (24.3) | 0 (0.0) | 801 (68.0) | <0.001 | |
| Cholesterol‐lowering drugs | 1344 (40.8) | 628 (29.7) | 716 (60.8) | <0.001 | |
| Beta‐blockers | 927 (28.1) | 494 (23.3) | 433 (36.8) | <0.001 | |
| Calcium channel blockers | 786 (23.9) | 440 (20.8) | 346 (29.4) | <0.001 | |
| Renin angiotensin system inhibitors | 1375 (41.7) | 738 (34.9) | 637 (54.1) | <0.001 | |
| Thiazides | 319 (9.7) | 204 (9.6) | 115 (9.8) | 0.96 | |
| Loop diuretics | 776 (23.6) | 393 (18.6) | 383 (32.5) | <0.001 | |
| Spironolactone | 181 (5.5) | 94 (4.4) | 87 (7.4) | <0.001 | |
| Digoxin | 144 (4.4) | 83 (3.9) | 61 (5.2) | 0.11 | |
| Aspirin | 542 (16.4) | 280 (13.2) | 262 (22.2) | <0.001 | |
| Adenosine diphosphate receptor inhibitors | 407 (12.4) | 245 (11.6) | 162 (13.8) | 0.077 | |
| Anticoagulants | 683 (20.7) | 407 (19.2) | 276 (23.4) | 0.005 |
Abbreviations: HbA1c, glycated haemoglobin; IQR, interquartile range.
TABLE 2.
Baseline characteristics according to HbA1c groups among hospitalized COVID‐19 patients with diabetes
| Total (N = 1178) | HbA1c < 48 mmol/mol (N = 317) | HbA1c 48‐53 mmol/mol (N = 272) | HbA1c 54‐58 mmol/mol (N = 141) | HbA1c 59‐64 mmol/mol (N = 136) | HbA1c > 64 mmol/mol (N = 312) | P‐value | ||
|---|---|---|---|---|---|---|---|---|
| HbA1c < 6.5% | HbA1c 6.5%‐7.0% | HbA1c 7.1%‐7.5% | HbA1c 7.5%‐8.0% | HbA1c > 8.0% | ||||
| Sex: male, n (%) | 739 (62.7) | 191 (60.3) | 185 (68.0) | 79 (56.0) | 86 (63.2) | 198 (63.5) | 0.14 | |
| Age, median (IQR), years | 74.3 (64.9, 80.8) | 75.5 (68.0, 81.4) | 75.3 (65.3, 80.3) | 73.4 (64.6, 80.7) | 74.1 (67.2, 81.2) | 72.8 (60.6, 79.9) | 0.01 | |
| HbA1c, median (IQR), mmol/mol | 54 (47, 65) | 43 (39, 45) | 50 (49, 52) | 55 (55, 57) | 61 (60, 63) | 75 (69, 84) | <0.001 | |
| Blood glucose, median (IQR), mmol/L | 9.3 (7.0, 12.1) | 7.6 (6.4, 9.5) | 8.7 (7.1, 11.3) | 9 (7.2, 11.8) | 9.9 (8.3, 11.9) | 11.6 (8.8, 15.4) | <0.001 | |
| Missing | 379 | 108 | 102 | 44 | 42 | 83 | ||
| C‐reactive protein, median (IQR), mg/L | 30 (6.7, 86.0) | 25 (6, 80) | 39 (7, 95) | 24.5 (6.5, 63.0) | 24.5 (6.2, 77.2) | 30.5 (7.8, 89.0) | 0.50 | |
| Missing | 212 | 48 | 53 | 35 | 24 | 52 | ||
| Leukocytes, median (IQR), x 109/L | 7.2 (5.5, 9.2) | 7.3 (5.3, 9.7) | 7 (5.4, 9.2) | 7.2 (5.6, 9.1) | 6.7 (5.5, 9.3) | 7.2 (5.8, 9.1) | 0.81 | |
| Missing | 181 | 35 | 54 | 29 | 19 | 44 | ||
| Comorbidity, n (%) | ||||||||
| Ischaemic heart disease | 355 (30.1) | 106 (33.4) | 75 (27.6) | 39 (27.7) | 47 (34.6) | 88 (28.2) | 0.32 | |
| Heart failure | 155 (13.2) | 43 (13.6) | 29 (10.7) | 17 (12.1) | 19 (14.0) | 47 (15.1) | 0.60 | |
| Previous myocardial infarction | 128 (10.9) | 34 (10.7) | 31 (11.4) | 13 (9.2) | 15 (11.0) | 35 (11.2) | 0.97 | |
| Atrial fibrillation | 216 (18.3) | 72 (22.7) | 44 (16.2) | 20 (14.2) | 23 (16.9) | 57 (18.3) | 0.15 | |
| Stroke | 126 (10.7) | 37 (11.7) | 37 (13.6) | 11 (7.8) | 14 (10.3) | 27 (8.7) | 0.25 | |
| Peripheral artery disease | ‐ | 15 (4.7) | 15 (5.5) | ≤3 | 14 (10.3) | 17 (5.4) | ‐ | |
| Hypertension | 674 (57.2) | 180 (56.8) | 155 (57.0) | 80 (56.7) | 82 (60.3) | 177 (56.7) | 0.96 | |
| Chronic obstructive pulmonary disease | 137 (11.6) | 45 (14.2) | 31 (11.4) | 10 (7.1) | 20 (14.7) | 31 (9.9) | 0.14 | |
| Cancer | 219 (18.6) | 71 (22.4) | 50 (18.4) | 25 (17.7) | 26 (19.1) | 47 (15.1) | 0.22 | |
| Liver disease | ‐ | 18 (5.7) | 11 (4.0) | 7 (5.0) | ≤3 | 17 (5.4) | ‐ | |
| Rheumatic disease | 95 (8.1) | 35 (11.0) | 16 (5.9) | 14 (9.9) | 7 (5.1) | 23 (7.4) | 0.093 | |
| Chronic renal disease | 235 (19.9) | 67 (21.1) | 32 (11.8) | 22 (15.6) | 31 (22.8) | 83 (26.6) | <0.001 | |
| Lipidaemia | 180 (15.3) | 55 (17.4) | 36 (13.2) | 23 (16.3) | 28 (20.6) | 38 (12.2) | 0.12 | |
| Concomitant pharmacotherapy, n (%) | ||||||||
| Antidiabetics | 951 (80.7) | 226 (71.3) | 185 (68.0) | 129 (91.5) | 122 (89.7) | 289 (92.6) | <0.001 | |
| Insulin | 395 (33.5) | 48 (15.1) | 49 (18.0) | 52 (36.9) | 66 (48.5) | 180 (57.7) | <0.001 | |
| Oral glucose‐lowering drugs | 801 (68.0) | 206 (65.0) | 161 (59.2) | 109 (77.3) | 101 (74.3) | 224 (71.8) | <0.001 | |
| Cholesterol‐lowering drugs | 716 (60.8) | 186 (58.7) | 162 (59.6) | 89 (63.1) | 92 (67.6) | 187 (59.9) | 0.42 | |
| Beta‐blockers | 433 (36.8) | 128 (40.4) | 105 (38.6) | 47 (33.3) | 53 (39.0) | 100 (32.1) | 0.19 | |
| Calcium channel blockers | 346 (29.4) | 92 (29.0) | 76 (27.9) | 39 (27.7) | 48 (35.3) | 91 (29.2) | 0.59 | |
| Renin angiotensin system inhibitors | 637 (54.1) | 159 (50.2) | 149 (54.8) | 75 (53.2) | 85 (62.5) | 169 (54.2) | 0.20 | |
| Thiazides | 115 (9.8) | 26 (8.2) | 33 (12.1) | 18 (12.8) | 14 (10.3) | 24 (7.7) | 0.23 | |
| Loop diuretics | 383 (32.5) | 105 (33.1) | 83 (30.5) | 45 (31.9) | 45 (33.1) | 105 (33.7) | 0.94 | |
| Spironolactone | 87 (7.4) | 33 (10.4) | 15 (5.5) | 10 (7.1) | 8 (5.9) | 21 (6.7) | 0.18 | |
| Digoxin | ‐ | 20 (6.3) | 13 (4.8) | 10 (7.1) | ≤3 | 16 (5.1) | ‐ | |
| Aspirin | 262 (22.2) | 65 (20.5) | 57 (21.0) | 31 (22.0) | 38 (27.9) | 71 (22.8) | 0.49 | |
| Adenosine diphosphate receptor inhibitors | 162 (13.8) | 42 (13.2) | 42 (15.4) | 16 (11.3) | 20 (14.7) | 42 (13.5) | 0.82 | |
| Anticoagulants | 276 (23.4) | 90 (28.4) | 60 (22.1) | 31 (22.0) | 32 (23.5) | 63 (20.2) | 0.16 |
Abbreviations: HbA1c, glycated haemoglobin; IQR, interquartile range.
TABLE 3.
Baseline characteristics according to HbA1c groups among hospitalized COVID‐19 patients without diabetes
| Total (N = 2117) | HbA1c < 31 mmol/mol (N = 142) | HbA1c 31‐36 mmol/mol (N = 773) | HbA1c 37‐41 mmol/mol (N = 831) | HbA1c 42‐47 mmol/mol (N = 371) | P‐value | ||
|---|---|---|---|---|---|---|---|
| HbA1c < 5.0% | HbA1c 5.0%‐5.4% | HbA1c 5.5%‐5.9% | HbA1c 6.0%‐6.5% | ||||
| Sex: male, n (%) | 1114 (52.6) | 71 (50.0) | 380 (49.2) | 462 (55.6) | 201 (54.2) | 0.060 | |
| Age, median (IQR), years | 73.6 (59.9, 82.7) | 69 (52.2, 82.9) | 71 (55.1, 81.3) | 74.5 (62.5, 83.0) | 75 (67.2, 83.2) | <0.001 | |
| HbA1c, median (IQR), mmol/mol | 37 (34, 40) | 29 (27, 30) | 34 (33, 35) | 39 (38, 40) | 44 (42, 45) | <0.001 | |
| Blood glucose, median (IQR), mmol/L | 6.3 (5.7, 7.4) | 6 (5.2, 6.8) | 6.1 (5.5, 7.0) | 6.5 (5.7, 7.5) | 6.9 (6, 8) | <0.001 | |
| Missing | 620 | 48 | 225 | 238 | 109 | ||
| C‐reactive protein, median (IQR), mg/L | 21 (4, 66) | 13 (4, 44) | 14 (4.0, 60.5) | 23 (4, 69) | 34 (6.0, 80.8) | <0.001 | |
| Missing | 256 | 13 | 93 | 117 | 33 | ||
| Leukocytes, median (IQR), x 109/L | 6.7 (5.0, 8.7) | 6.7 (4.7, 8.1) | 6.4 (4.9, 8.4) | 6.8 (5.1, 8.9) | 7.1 (5.4, 9.0) | 0.03 | |
| Missing | 159 | 9 | 51 | 81 | 18 | ||
| Comorbidities, n (%) | |||||||
| Ischaemic heart disease | 404 (19.1) | 18 (12.7) | 107 (13.8) | 183 (22.0) | 96 (25.9) | <0.001 | |
| Heart failure | 183 (8.6) | 11 (7.7) | 54 (7.0) | 72 (8.7) | 46 (12.4) | 0.02 | |
| Previous myocardial infarction | 172 (8.1) | 10 (7.0) | 42 (5.4) | 73 (8.8) | 47 (12.7) | <0.001 | |
| Atrial fibrillation | 346 (16.3) | 26 (18.3) | 108 (14.0) | 152 (18.3) | 60 (16.2) | 0.12 | |
| Stroke | 190 (9.0) | 13 (9.2) | 63 (8.2) | 86 (10.3) | 28 (7.5) | 0.32 | |
| Peripheral artery disease | 72 (3.4) | 10 (7.0) | 24 (3.1) | 22 (2.6) | 16 (4.3) | 0.04 | |
| Hypertension | 770 (36.4) | 41 (28.9) | 230 (29.8) | 315 (37.9) | 184 (49.6) | <0.001 | |
| Chronic obstructive pulmonary disease | 207 (9.8) | 16 (11.3) | 56 (7.2) | 85 (10.2) | 50 (13.5) | 0.008 | |
| Cancer | 427 (20.2) | 36 (25.4) | 139 (18.0) | 170 (20.5) | 82 (22.1) | 0.15 | |
| Liver disease | 72 (3.4) | 12 (8.5) | 30 (3.9) | 16 (1.9) | 14 (3.8) | <0.001 | |
| Rheumatic disease | 163 (7.7) | 9 (6.3) | 62 (8.0) | 57 (6.9) | 35 (9.4) | 0.41 | |
| Chronic renal disease | 150 (7.1) | 10 (7.0) | 53 (6.9) | 63 (7.6) | 24 (6.5) | 0.90 | |
| Lipidaemia | 290 (13.7) | 12 (8.5) | 77 (10.0) | 135 (16.2) | 66 (17.8) | <0.001 | |
| Concomitant pharmacotherapy, n (%) | |||||||
| Cholesterol‐lowering drugs | 628 (29.7) | 24 (16.9) | 170 (22.0) | 284 (34.2) | 150 (40.4) | <0.001 | |
| Beta‐blockers | 494 (23.3) | 34 (23.9) | 143 (18.5) | 203 (24.4) | 114 (30.7) | <0.001 | |
| Calcium channel blockers | 440 (20.8) | 19 (13.4) | 142 (18.4) | 195 (23.5) | 84 (22.6) | 0.008 | |
| Renin angiotensin system inhibitors | 738 (34.9) | 38 (26.8) | 227 (29.4) | 297 (35.7) | 176 (47.4) | <0.001 | |
| Thiazides | 204 (9.6) | 6 (4.2) | 66 (8.5) | 89 (10.7) | 43 (11.6) | 0.04 | |
| Loop diuretics | 393 (18.6) | 36 (25.4) | 104 (13.5) | 149 (17.9) | 104 (28.0) | <0.001 | |
| Spironolactone | 94 (4.4) | 7 (4.9) | 28 (3.6) | 36 (4.3) | 23 (6.2) | 0.26 | |
| Digoxin | 83 (3.9) | 5 (3.5) | 26 (3.4) | 34 (4.1) | 18 (4.9) | 0.66 | |
| Aspirin | 280 (13.2) | 17 (12.0) | 82 (10.6) | 109 (13.1) | 72 (19.4) | <0.001 | |
| Adenosine diphosphate receptor inhibitors | 245 (11.6) | 20 (14.1) | 78 (10.1) | 96 (11.6) | 51 (13.7) | 0.24 | |
| Anticoagulants | 407 (19.2) | 27 (19.0) | 127 (16.4) | 171 (20.6) | 82 (22.1) | 0.077 | |
Abbreviations: HbA1c, glycated haemoglobin; IQR, interquartile range.
3.1. Risk of mortality
Among patients with diabetes, 272 (23.1%) died within 30 days of follow‐up, of whom 94 (29.7%) died with HbA1c < 48 mmol/mol, 55 (20.2%) with HbA1c 48 to 53 mmol/mol, 29 (20.6%) with HbA1c 54 to 58 mmol/mol, 23 (16.9%) with HbA1c 59 to 64 mmol/mol, and 71 (22.8%) with HbA1c > 64 mmol/mol. Among patients with diabetes, HbA1c < 48 mmol/mol, 54 to 58 mmol/mol, and >64 mmol/mol were associated with significantly higher 30‐day standardized absolute outcome risk differences of all‐cause mortality, compared with HbA1c 59 to 64 mmol/mol (9.8% [95% CI 3.5% to 16.0%], 9.7% [95% CI 1.7% to 17.8%] and 8.8% [95% CI 2.3% to 15.3%], respectively [Figure 1]). Furthermore, Figure S1 shows the adjusted HRs from the Cox regression analysis for all‐cause mortality among patients with diabetes. Illustrated in Figure S2 is a restricted cubic spline reporting the continuous association between HbA1c and the mortality rate among patients with diabetes.
FIGURE 1.
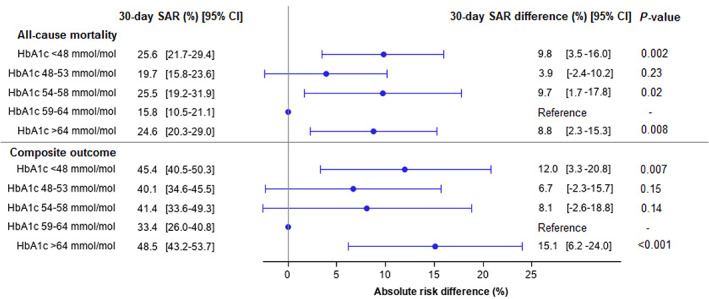
Standardized 30‐day absolute risks and standardized 30‐day absolute risk differences for all‐cause mortality and a composite of severe COVID‐19 infection, admission to intensive care unit, or all‐cause mortality according to glycated haemoglobin (HbA1c) level among hospitalized COVID‐19 patients with diabetes. Standardized to age, sex, history of ischaemic heart disease, heart failure, atrial fibrillation, stroke, peripheral artery disease, hypertension, chronic obstructive pulmonary disease, cancer, chronic renal disease, and use of cholesterol‐lowering drugs, beta‐blockers, calcium channel blockers, renin‐angiotensin system inhibitors, aspirin, and anticoagulants. SAR, standardized absolute risk
In total, 401 (18.9%) patients without diabetes died during 30 days of follow‐up, of whom 40 (28.2%) died with HbA1c < 31 mmol/mol, 122 (15.8%) with HbA1c 31 to 36 mmol/mol, 160 (19.3%) with HbA1c 37 to 41 mmol/mol, and 79 (21.3%) with HbA1c 42 to 47 mmol/mol. Among patients without diabetes, HbA1c < 31 mmol/mol was associated with higher 30‐day standardized absolute outcome risk difference of all‐cause mortality (11.2% [95% CI 5.0% to 17.5%]), compared with HbA1c 31 to 36 mmol/mol (Figure 2). Adjusted HRs from the Cox regression analysis and a restricted cubic spline for mortality among patients without diabetes are depicted in Figures S3 and S4, respectively.
FIGURE 2.
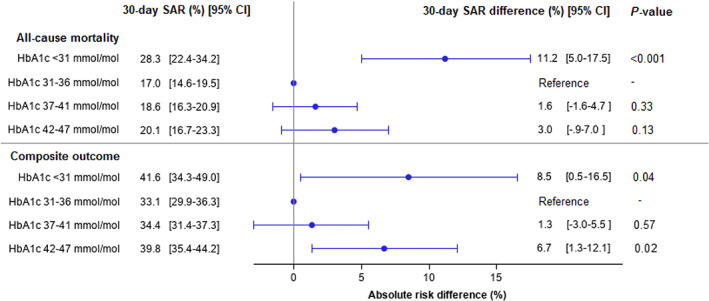
Standardized 30‐day absolute risks and standardized 30‐day absolute risk differences for all‐cause mortality and a composite of severe COVID‐19 infection, admission to intensive care unit, or all‐cause mortality according to glycated haemoglobin (HbA1c) level among hospitalized COVID‐19 patients without diabetes. Standardized to age, sex, history of ischaemic heart disease, heart failure, atrial fibrillation, stroke, peripheral artery disease, hypertension, chronic obstructive pulmonary disease, cancer, chronic renal disease, and use of cholesterol‐lowering drugs, beta‐blockers, calcium channel blockers, renin‐angiotensin system inhibitors, aspirin, and anticoagulants. SAR, standardized absolute risk
3.2. Risk of severe COVID‐19 infection, ICU admission or all‐cause mortality
Among patients with diabetes, the composite outcome of severe COVID‐19 infection, ICU admission, or all‐cause mortality occurred in 512 (43.5%) within 30 days of follow‐up; it occurred in 148 patients (46.7%) with HbA1c < 48 mmol/mol, 109 (40.1%) with HbA1c 48 to 53 mmol/mol, 55 (39.0%) with HbA1c 54 to 58 mmol/mol, 48 (35.3%) with HbA1c 59 to 64 mmol/mol, and 152 (48.7%) with HbA1c > 64 mmol/mol. Among patients with diabetes, HbA1c < 48 mmol/mol and HbA1c > 64 mmol/mol were associated with significantly increased 30‐day standardized absolute outcome risk differences of the composite outcome, compared with HbA1c 59 to 64 mmol/mol, (12.0% [95% CI 3.3% to 20.8%]) and (15.1% [95% CI 6.2% to 24.0%]), respectively (Figure 1). The HRs from the Cox regression analysis for the composite outcome among patients with diabetes are depicted in Figure S5. A restricted cubic spline reporting the continuous association between HbA1c and the rate of the composite outcome among patients with diabetes is illustrated in Figure S6.
The composite outcome occurred in 757 (35.8%) patients without diabetes during 30 days of follow‐up; 56 (39.4%) with HbA1c < 31 mmol/mol, 246 (31.8%) with HbA1c 31 to 36 mmol/mol, 298 (35.9%) with HbA1c 37 to 41 mmol/mol, and 157 (42.3%) with HbA1c 42 to 47 mmol/mol. Compared with HbA1c 31 to 36 mmol/mol, HbA1c < 31 mmol/mol and 42 to 47 mmol/mol were associated with higher 30‐day standardized absolute outcome risk differences of the composite outcome, (8.5% [95% CI 0.5% to 16.5%]) and (6.7% [95% CI 1.3% to 12.1%]), respectively (Figure 2). Finally, Figures S7 and S8 depict HRs from the Cox regression analysis and a restricted cubic spline for the composite outcome among patients without diabetes, respectively.
3.3. Sensitivity analyses
The model assumptions of the Cox regression analysis, based on a random survival forest model, were fulfilled for both study outcomes among COVID‐19 patients with and without diabetes (Tables S4 and S5, respectively).
The comparison between patients with and without diabetes with the same range of HbA1c levels in a pooled population showed that having diabetes and an HbA1c level 39 to 47 mmol/mol or an HbA1c level > 64 mmol/mol was associated with a higher risk of the composite outcome (Figure S9). However, an increased risk of the composite outcome was not observed in patients without diabetes.
According to baseline characteristics, a dose‐response relationship was identified between increasing levels of HbA1c groups and median levels of blood glucose among patients with and without diabetes (Tables 2 and 3). Among patients with diabetes, blood glucose levels 11.1 to 13.0 mmol/L and blood glucose levels > 13.0 mmol/L were associated with a higher risk of the composite outcome (Figure S10). A dose‐response relationship was identified between increasing blood glucose levels and the composite outcome among patients without diabetes (Figure S11).
Overall, few differences were observed in baseline characteristics in hospitalized COVID‐19 patients with diabetes according to available HbA1c value (Table S6). Baseline differences among hospitalized COVID‐19 patients but without diabetes are listed in Table S7.
To test whether time affected our findings, we performed sensitivity analyses for the periods from 3 March 2020 to 1 August 2020 and 1 August 2020 to 31 December 2020 according to diabetes status for both study outcomes, which all yielded similar results to the main analyses (data not shown). To test whether time of HbA1c collection affected our findings, we performed sensitivity analyses using HbA1c measurements collected at <3 months prior to index date according to diabetes status. These analyses yielded the same results as the main analyses for both study outcomes (data not shown).
4. DISCUSSION
In this Danish registry‐based cohort study, we examined the association between varying HbA1c levels and the risk of severe COVID‐19 infection, ICU admission or all‐cause mortality among COVID‐19 patients with and without diabetes. This study yielded two principal findings. First, among COVID‐19 patients with diabetes, both high HbA1c levels >64 mmol/mol and low HbA1c levels <48 mmol/mol were associated with increased risk of all‐cause mortality and the composite of severe COVID‐19 infection, ICU admission or all‐cause mortality, compared with HbA1c 59 to 64 mmol/mol. Second, among COVID‐19 patients without diabetes, HbA1c < 31 mmol/mol and HbA1c 42 to 47 mmol/mol were associated with elevated risk of the composite outcome, compared with HbA1c 31 to 36 mmol/mol.
A meta‐analysis by Mantovani et al 10 showed that COVID‐19 patients with pre‐existing diabetes were associated with a twofold increased risk of critical COVID‐19 illness requiring admission to an ICU (n = 22 studies) and a threefold increased risk of in‐hospital mortality (n = 15 studies), compared with patients without diabetes. In accordance with the findings of Mantovani et al, the present study highlights that patients with dysregulated diabetes (ie, HbA1c >64 mmol/mol) are also more likely to experience adverse outcomes in a COVID‐19 setting, compared with patients with well‐regulated diabetes or without diabetes. Consistent with the present study findings, hyperglycaemia was also independently associated with COVID‐19‐related mortality in a population‐based cohort study by Holman et al 18 that examined risk factors for COVID‐19‐related mortality among people with diabetes. Among 264 390 people with type 1 diabetes, only HbA1c ≥ 86 mmol/mol (10.0%) was associated with an increased mortality rate, compared with HbA1c 48 to 53 mmol/mol. Notably, among 2 874 020 people with type 2 diabetes, a graded relationship was identified between increasing HbA1c ≥ 59 mmol/mol and mortality. 18 Collectively, both the present study and the study by Holman et al 18 showed that hyperglycaemia measured by HbA1c is a strong predictor of mortality, which may partly be due to the fact that people with poor glycaemic control are at greater risk of many serious infections. 35 , 36 Moreover, in line with previous COVID‐19 studies, patients with diabetes and poorly regulated HbA1c or hyperglycaemia have higher levels of inflammatory markers, compared with patients without diabetes or with normoglycaemia. 11 , 12 , 13 , 14 , 15 , 16 Similarly, we also found higher, although not statistically significant, C‐reactive protein values to be correlated with higher HbA1c, which could suggest a more severe course of infection among patients with diabetes and high HbA1c levels.
In the present study, HbA1c levels >64 mmol/mol were associated with both increased risk of all‐cause mortality and the composite of severe COVID‐19 infection, ICU admission, or all‐cause mortality among COVID‐19 patients with diabetes. Conversely, a nationwide observational study by Cariou et al 17 (CORONADO study) of 1317 hospitalized COVID‐19 patients with diabetes demonstrated no association between HbA1c and mortality or the composite of tracheal intubation for mechanical ventilation and mortality within 7 days of admission, even with the highest values of HbA1c > 75 mmol/mol (9.0%). 17 Correspondingly, a study by Myers et al 37 of 4413 COVID‐19 patients with type 2 diabetes reported no difference in mortality between those with HbA1c ≥ 75 mmol/mol and HbA1c < 75 mmol/mol. The findings of the CORONADO study conflict with the present study and could be attributed to missing HbA1c measurements among one‐third of patients as well as a very short 7‐day observation period. The findings of the present study suggest that poorly regulated HbA1c among patients with diabetes may affect the severity of COVID‐19, leading to ICU admission and mortality. In support of the latter notion, a small study by Smith et al 38 demonstrated a higher prevalence of intubation among hospitalized COVID‐19 patients with HbA1c ≥ 58 mmol/mol, compared with HbA1c < 58 mmol/mol. Furthermore, a recent meta‐analysis by Prattichizzo et al 39 demonstrated that HbA1c was linearly associated with a higher COVID‐19‐related‐mortality or worsening. However, the small number and the large heterogenicity of the studies performed to date should be taken into consideration. The meta‐analysis calls for more studies as no definitive conclusions can be drawn concerning the role of glycaemic control in providing COVID‐19 prognosis or mortality among patients with diabetes.
In the present study, COVID‐19 patients with diabetes and HbA1c < 48 mmol/mol had an increased risk of all‐cause mortality and the composite outcome. Similarly, a study by Holman et al 18 reported significantly higher COVID‐19‐related mortality among patients with type 2 diabetes and HbA1c < 48 mmol/mol, compared with HbA1c 48 to 53 mmol/mol but, in contrast to the present study, outcomes other than mortality were not examined in their study. A retrospective study by Yuan et al 19 concluded that both lower HbA1c levels 10 to 30 mmol/mol (3.0% to 4.9%) and higher HbA1c levels ≥42 (≥6.0%) mmol/mol were associated with increased all‐cause mortality, compared with HbA1c levels 31 to 41 mmol/mol (5.0% to 5.9%), in a pooled population of 922 COVID‐19 patients with and without diabetes. Notably, both the present study and the study by Yuan et al demonstrated that, although lower HbA1c levels were associated with greater mortality, lower blood glucose levels were associated with decreased mortality. However, patients with HbA1c 10 to 30 mmol/mol had higher fasting blood glucose in the study by Yuan et al, which may be attributable to a statistically underpowered HbA1c group with only 14 included patients. In the present study, blood glucose increased with increasing HbA1c level, indicating that HbA1c is a sensitive and plausible marker of hyperglycaemia. Importantly, the study by Yuan et al did not investigate varying HbA1c levels in a stratified analysis according to diabetes status, and outcomes such as severe COVID‐19 infection and ICU admission were not examined. In the present study, a low HbA1c level as a risk factor for severe outcomes among COVID‐19 patients with diabetes adds to the growing literature suggesting that tight glycaemic control or hypoglycaemia may lead to more severe outcomes. As investigated in previous studies in non‐COVID‐19 settings, 40 , 41 a randomized clinical trial by Gerstein et al 40 reported greater mortality among patients with type 2 diabetes randomized to receive intensive therapy targeting HbA1c < 42 mmol/mol, compared with patients with type 2 diabetes receiving standard therapy (58‐63 mmol/mol [7.5% to 7.9%]) for 3.5 years. Notably, the intensive therapy group had significantly elevated rates of hypoglycaemia requiring assistance, compared with the standard therapy group. Taking these findings together, HbA1c may play an important role in predicting the prognosis of COVID‐19 patients with and without diabetes.
Among patients without diabetes, we demonstrated that HbA1c levels < 31 mmol/mol, compared with HbA1c levels 31 to 36 mmol/mol, were associated with increased risk of both all‐cause mortality and the composite outcome. However, HbA1c level 42 to 47 mmol/mol was only associated with the composite outcome. In conformity with the HbA1c groups of the present study, a non‐COVID‐19 study by Selvin et al 4 reported a greater risk of all‐cause mortality among patients with HbA1c < 31 mmol/mol, HbA1c 37 to 41 mmol/mol, HbA1c 42 to 47 mmol/mol, and HbA1c > 48 mmol/mol, compared with HbA1c 31 to 36 mmol/mol. Overall, HbA1c levels 39 to 47 mmol/mol (5.7% to 6.5%) could be indicative of a prediabetic state according to American Diabetes Association diagnostic criteria, 28 who are susceptible to more adverse outcomes, or normoglycaemia among patients without diabetes. In the present study, patients with HbA1c levels 42 to 47 mmol/mol tended to have more comorbidities and had a higher median age, which could also imply more severe clinical outcomes. A study by Vargas‐Vázquez et al 42 of 317 hospitalized COVID‐19 patients concluded that prediabetes (39‐47 mmol/mol) was associated with higher odds of severe COVID‐19 infection. Notably, other previous studies, in non‐COVID‐19 settings among patients without diabetes, have demonstrated that HbA1c levels in the range of 39 to 47 mmol/mol or 42 to 47 mmol/mol were associated with increased risk of all‐cause mortality. 4 , 6 , 32 , 43 , 44 , 45 Collectively, both low and high HbA1c levels may help identify hospitalized COVID‐19 patients who are at greater risk of mortality and adverse outcomes. Accordingly, we recommend assessing HbA1c levels in all patients who are admitted to a hospital with a positive SARS‐CoV‐2 PCR test in order to optimize treatment and improve patient outcomes. More studies are needed to elucidate the potential effect of varying HbA1c levels among COVID‐19 patients with and without diabetes and the risk of severe COVID‐19 infection, ICU admission or all‐cause mortality.
To the best of our knowledge, the present study is the first to provide a large amount of data on the association between varying HbA1c levels and adverse outcomes among hospitalized COVID‐19 patients with and without diabetes. Because of the observational study design, no causal inference should be made from the findings in the present study, but the results should instead be interpreted as associations. Thus, we acknowledge the risk of residual confounding, although we tried to eliminate the effect of these. The risk of confounding by indication cannot be excluded as HbA1c is an independent risk factor for mortality regardless of COVID‐19 infection. Moreover, we are also unable to determine the reasons for which physicians decided to examine HbA1c levels in patients, which may have influenced the study findings.
The Danish National Health Service system ensures equal access for all Danish citizens irrespective of socioeconomic status, funded by taxes. Moreover, the study is based on large Danish nationwide registries of high quality and completeness. However, risk of selection bias cannot be excluded based on available HbA1c measurements gathered from the Clinical Laboratory Information System. Thus, caution needs to be taken when extrapolating the present findings to other populations with COVID‐19. The median age of admission for the present study population was 73.9 years, which is slightly older than for the remainder of the Danish population (median age: 71.2 years) hospitalized with COVID‐19 during the same time period. Thus, overall, according to the Danish Health Authority, patients aged 70 to 79 years comprised the largest age group of hospitalized patients with confirmed COVID‐19. 46
The HbA1c measurements were obtained up to 6 months prior to the index date, which may have influenced our findings, although sensitivity analyses using HbA1c levels gathered up to 3 months before the index date yielded the same results as for the main analyses. In addition, we were not able to confidently distinguish type 1 and type 2 diabetes, which may have influenced our study findings.
In conclusion, in the present registry‐based observational study, we demonstrated that tightly regulated diabetes (HbA1c < 48 mmol/mol) and severely dysregulated diabetes (HbA1c > 64 mmol/mol) were associated with both increased risk of all‐cause mortality and the composite outcome of severe COVID‐19 infection, ICU admission, or all‐cause mortality, compared with HbA1c levels 59 to 64 mmol/mol among hospitalized patients with COVID‐19. Among hospitalized COVID‐19 patients without diabetes, HbA1c levels < 31 mmol/mol and 42 to 47 mmol/mol were associated with a greater risk of the composite outcome. Collectively, in a COVID‐19 setting, HbA1c is an important marker to identify patients at risk of all‐cause mortality and adverse outcomes among patients with and without diabetes.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
Amna Alhakak: performed the main statistical coding, analysed the data, interpreted the results, and wrote the manuscript. Peter E. Weeke, Lars Køber and Christian Torp‐Pedersen: contributed to the primary study conception, design and interpretation of the results. Jawad H. Butt and Thomas A. Gerds: contributed to the statistical analyses and interpretation of results. The manuscript was reviewed and revised critically for important intellectual content by all authors. The final version of the manuscript was approved by all authors. Amna Alhakak is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14604.
Supporting information
Appendix S1. Supporting Information.
ACKNOWLEDGMENTS
This work was supported by the Independent Research Fund Denmark [9149‐00019B].
Alhakak A, Butt JH, Gerds TA, et al. Glycated haemoglobin levels among 3295 hospitalized COVID‐19 patients, with and without diabetes, and risk of severe infection, admission to an intensive care unit and all‐cause mortality. Diabetes Obes Metab. 2022;24(3):499‐510. doi: 10.1111/dom.14604
Funding information Independent Research Fund Denmark, Grant/Award Number: 9149‐00019B
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from Statistics Denmark but are not publicly available due to Danish legislation on data protection.
REFERENCES
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization 2020. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020. Accessed November 2, 2021
- 3. Currie CJ, Peters JR, Tynan A, et al. Survival as a function of HbA(1c) in people with type 2 diabetes: a retrospective cohort study. Lancet. 2010;375(9713):481‐489. [DOI] [PubMed] [Google Scholar]
- 4. Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010;362(9):800‐811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Palta P, Huang ES, Kalyani RR, Golden SH, Yeh HC. Hemoglobin A(1c) and mortality in older adults with and without diabetes: results from the National Health and Nutrition Examination Surveys (1988‐2011). Diabetes Care. 2017;40(4):453‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carson AP, Fox CS, McGuire DK, et al. Low hemoglobin A1c and risk of all‐cause mortality among US adults without diabetes. Circ Cardiovasc Qual Outcomes. 2010;3(6):661‐667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID‐19‐related death using OpenSAFELY. Nature. 2020;584(7821):430‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tan E, Song J, Deane AM, Plummer MP. Global impact of coronavirus disease 2019 infection requiring admission to the ICU: a systematic review and meta‐analysis. Chest. 2021;159(2):524‐536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dorjee K, Kim H, Bonomo E, Dolma R. Prevalence and predictors of death and severe disease in patients hospitalized due to COVID‐19: a comprehensive systematic review and meta‐analysis of 77 studies and 38,000 patients. PLoS One. 2020;15(12):e0243191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mantovani A, Byrne CD, Zheng MH, Targher G. Diabetes as a risk factor for greater COVID‐19 severity and in‐hospital death: a meta‐analysis of observational studies. Nutr Metab Cardiovasc Dis. 2020;30(8):1236‐1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yan Y, Yang Y, Wang F, et al. Clinical characteristics and outcomes of patients with severe covid‐19 with diabetes. BMJ Open Diabetes Res Care. 2020;8(1):e001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang Y, Li H, Zhang J, et al. The clinical characteristics and outcomes of diabetes mellitus and secondary Hyperglycaemia patients with coronavirus disease 2019: a single‐center, retrospective, observational study in Wuhan. Diabetes Obes Metab. 2020;22(8):1443‐1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhu L, She ZG, Cheng X, et al. Association of blood glucose control and outcomes in patients with COVID‐19 and pre‐existing type 2 diabetes. Cell Metab. 2020;31(6):1068‐77.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang J, Kong W, Xia P, et al. Impaired fasting glucose and diabetes are related to higher risks of complications and mortality among patients with coronavirus disease 2019. Front Endocrinol. 2020;11:525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen Y, Yang D, Cheng B, et al. Clinical characteristics and outcomes of patients with diabetes and COVID‐19 in association with glucose‐lowering medication. Diabetes Care. 2020;43(7):1399‐1407. [DOI] [PubMed] [Google Scholar]
- 16. Ling P, Luo S, Zheng X, Cai G, Weng J. Elevated fasting blood glucose within the first week of hospitalization was associated with progression to severe illness of COVID‐19 in patients with preexisting diabetes: a multicenter observational study. J Diabetes. 2021;13(1):89‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cariou B, Hadjadj S, Wargny M, et al. Phenotypic characteristics and prognosis of inpatients with COVID‐19 and diabetes: the CORONADO study. Diabetologia. 2020;1‐16:1500‐1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Holman N, Knighton P, Kar P, et al. Risk factors for COVID‐19‐related mortality in people with type 1 and type 2 diabetes in England: a population‐based cohort study. Lancet Diabetes Endocrinol. 2020;8(10):823‐833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yuan S, Li H, Chen C, Wang F, Wang DW. Association of glycosylated haemoglobin HbA1c levels with outcome in patients with COVID‐19: a retrospective study. J Cell Mol Med. 2021;25:3484‐3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Voldstedlund M, Haarh M, Mølbak K. The Danish microbiology database (MiBa) 2010 to 2013. Euro Surveill. 2014;19(1):20667. [DOI] [PubMed] [Google Scholar]
- 22. Grann AF, Erichsen R, Nielsen AG, Frøslev T, Thomsen RW. Existing data sources for clinical epidemiology: the clinical laboratory information system (LABKA) research database at Aarhus University, Denmark. Clin Epidemiol. 2011;3:133‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kildemoes HW, Sorensen HT, Hallas J. The Danish National Prescription Registry. Scand J Public Health. 2011;39(7 Suppl):38‐41. [DOI] [PubMed] [Google Scholar]
- 24. Schmidt M, Pedersen L, Sorensen HT. The Danish civil registration system as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541‐549. [DOI] [PubMed] [Google Scholar]
- 25. Schmidt M, Schmidt SAJ, Adelborg K, et al. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol. 2019;11:563‐591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sundbøll J, Adelborg K, Munch T, et al. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open. 2016;6(11):e012832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Olesen JB, Lip GYH, Kamper A‐L, et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med. 2012;367(7):625‐635. [DOI] [PubMed] [Google Scholar]
- 28. American Diabetes Association . 2. Classification and diagnosis of diabetes: standards of medical care in Diabetes—2020. Diabetes Care. 2020;43(suppl 1):S14‐S31. [DOI] [PubMed] [Google Scholar]
- 29. American Diabetes Association . 12. Older adults: standards of medical care in diabetes—2021. Diabetes Care. 2021;44(suppl 1):S168‐S179. [DOI] [PubMed] [Google Scholar]
- 30. American Diabetes Association . 13. Children and adolescents: standards of medical care in diabetes—2021. Diabetes Care. 2021;44(suppl 1):S180‐S199. [DOI] [PubMed] [Google Scholar]
- 31.IDF clinical practice recommendations for managing type 2 diabetes in primary care: International Diabetes Federation, 2017. https://www.idf.org/e‐library/guidelines/128‐idf‐clinical‐practice‐recommendations‐for‐managing‐type‐2‐diabetes‐in‐primary‐care.html#.YGIp0kJn_VU.link. Accessed November 2, 2021. [DOI] [PubMed]
- 32. Sakurai M, Saitoh S, Miura K, et al. HbA1c and the risks for all‐cause and cardiovascular mortality in the general Japanese population: NIPPON DATA90. Diabetes Care. 2013;36(11):3759‐3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ozenne BMH, Scheike TH, Staerk L, Gerds TA. On the estimation of average treatment effects with right‐censored time to event outcome and competing risks. Biom J. 2020;62(3):751‐763. [DOI] [PubMed] [Google Scholar]
- 34. Ishwaran H, Kogalur UB, Blackstone EH, Lauer MS. Random survival forests. Ann Appl Stat. 2008;2(3):841‐860. [Google Scholar]
- 35. Carey IM, Critchley JA, DeWilde S, Harris T, Hosking FJ, Cook DG. Risk of infection in type 1 and type 2 diabetes compared with the general population: a matched cohort study. Diabetes Care. 2018;41(3):513‐521. [DOI] [PubMed] [Google Scholar]
- 36. Critchley JA, Carey IM, Harris T, DeWilde S, Hosking FJ, Cook DG. Glycemic control and risk of infections among people with type 1 or type 2 diabetes in a large primary care cohort study. Diabetes Care. 2018;41(10):2127‐2135. [DOI] [PubMed] [Google Scholar]
- 37. Myers AK, Kim TS, Zhu X, Liu Y, Qiu M, Pekmezaris R. Predictors of mortality in a multiracial urban cohort of persons with type 2 diabetes and novel coronavirus 19. J Diabetes. 2021;13:430‐438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smith SM, Boppana A, Traupman JA, et al. Impaired glucose metabolism in patients with diabetes, prediabetes and obesity is associated with severe Covid‐19. J Med Virol. 2020;93(1):409‐415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Prattichizzo F, de Candia P, Nicolucci A, Ceriello A. Elevated HbA1c levels in pre‐Covid‐19 infection increases the risk of mortality: a sistematic review and meta‐analysis. Diabetes Metab Res Rev. 2021;e3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545‐2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lind M, Svensson AM, Kosiborod M, et al. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med. 2014;371(21):1972‐1982. [DOI] [PubMed] [Google Scholar]
- 42. Vargas‐Vázquez A, Bello‐Chavolla OY, Ortiz‐Brizuela E, et al. Impact of undiagnosed type 2 diabetes and pre‐diabetes on severity and mortality for SARS‐CoV‐2 infection. BMJ Open Diabetes Res Care. 2021;9(1):e002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Aggarwal V, Schneider AL, Selvin E. Low hemoglobin A(1c) in nondiabetic adults: an elevated risk state? Diabetes Care. 2012;35(10):2055‐2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Warren B, Pankow JS, Matsushita K, et al. Comparative prognostic performance of definitions of prediabetes: a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol. 2017;5(1):34‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pfister R, Sharp SJ, Luben R, Khaw KT, Wareham NJ. No evidence of an increased mortality risk associated with low levels of glycated haemoglobin in a non‐diabetic UK population. Diabetologia. 2011;54(8):2025‐2032. [DOI] [PubMed] [Google Scholar]
- 46. COVID‐19 surveillance: Danish Health Authority ; 2021. https://www.sst.dk/da/corona/covid-19-og-ny-coronavirus/coronatal. Accessed November 2, 2021
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.
Data Availability Statement
The data that support the findings of this study are available from Statistics Denmark but are not publicly available due to Danish legislation on data protection.


