Summary
Bleeding and thrombosis are major complications in patients supported with extracorporeal membrane oxygenation (ECMO). In this multicentre observational study of 152 consecutive patients (≥18 years) with severe COVID‐19 supported by veno‐venous (VV) ECMO in four UK commissioned centres during the first wave of the COVID‐19 pandemic (1 March to 31 May 2020), we assessed the incidence of major bleeding and thrombosis and their association with 180‐day mortality. Median age (range) was 47 years (23–65) and 75% were male. Overall, the 180‐day survival was 70·4% (107/152). The rate of major bleeding was 30·9% (47/152), of which intracranial bleeding (ICH) was 34% (16/47). There were 96 thrombotic events (63·1%) consisting of venous 44·7% [68/152 of which 66·2% were pulmonary embolism (PE)], arterial 18·6% (13/152) and ECMO circuit thrombosis 9·9% (15/152). In multivariate analysis, only raised lactate dehydrogenase (LDH) at the initiation of VV ECMO was associated with an increased risk of thrombosis [hazard ratio (HR) 1·92, 95% CI 1·21‐3·03]. Major bleeding and ICH were associated with 3·87‐fold (95% CI 2·10–7·23) and 5·97‐fold [95% confidence interval (CI) 2·36–15·04] increased risk of mortality and PE with a 2·00‐fold (95% CI1·09–3·56) risk of mortality. This highlights the difficult balancing act often encountered when managing coagulopathy in COVID‐19 patients supported with ECMO.
Keywords: COVID‐19, extracorporeal membrane oxygenation, bleeding, thrombosis, mortality
Introduction
The WHO, 1 Surviving Sepsis Campaign, 2 Extracorporeal Life Support Organization (ELSO) 3 and the National Institute of Clinical Excellence (NICE) in the UK 4 , 5 recommend the consideration of extracorporeal membrane oxygenation (ECMO) for patients with coronavirus [Severe Acute Respiratory Syndrome Coronavirus‐2 (SARS‐CoV‐2)]‐induced severe respiratory failure not responding to optimal conventional management, including mechanical ventilation and proning. Clinical evidence supporting the use of veno‐venous (VV) ECMO in the management of viral pneumonia comes mainly from experience during the H1N1 pandemic of 2009. 6
In the context of coronavirus disease 2019 (COVID‐19), the potential benefits of VV ECMO are supported by an international cohort study of the ELSO registry which reported an estimated cumulative in‐hospital mortality 90 days after the initiation of ECMO of 37·4% (95% CI 34·6–41·5). 7 This mortality rate is similar to that in patients with severe adult respiratory distress syndrome (ARDS) due to other causes supported by ECMO. 8
Bleeding and thrombosis are major complications in patients supported with ECMO irrespective of the cause of underlying respiratory failure 9 and thrombosis is itself a prominent feature of severe COVID‐19. 10 No large studies have yet assessed bleeding and thrombosis and their association with clinical outcome in patients who have been supported with VV ECMO. The continuing high rates of infection mean that the success or failure of ECMO remains of major interest. We aimed to analyse factors associated with bleeding and thrombosis at the initiation of VV ECMO and the effect of major bleeding and thrombosis on 180‐day mortality in severe COVID‐19 patients supported with VV ECMO.
Materials and methods
Study design, population, and data collection
This is a multicentre observational study within four nationally commissioned ECMO centres in the UK. Criteria for the referral of patients to ECMO centres are listed in Table S1. All consecutive adult patients (≥18 years) supported with VV ECMO for at least 48 h during the first wave of the COVID‐19 pandemic in the UK (1 March 2020–31 May 2020) were included in the study. All patients had SARS‐CoV‐2 infection confirmed by real‐time polymerase chain reaction (RT‐PCR) on nasopharyngeal swabs or lower respiratory tract aspirates.
The study was approved by the human research authority (HRA) and health and care Research Wales and the local Caldicott Guardian at Scotland (reference number: 20/HRA/1785). All patients lacked capacity, and the need for individual informed consent was waived because of the observational nature of the study.
Data collection
Data were collected both retrospectively and prospectively using a pre‐designed standardised case record form (CRF) by clinicians directly involved in patient care with no breach of privacy or anonymity to a central electronic database [coagulopathy associated with COVID‐19 (CA‐COVID‐19)] (REDcap v10·0.10; Vanderbitt University, Nashville, TN, USA) hosted by Imperial College London (https://clinicaltrials.gov/ct2/show/NCT04405232). CA‐COVID‐19 is a multicentre study across the UK to assess the natural history of patients admitted to hospital and up to 180 days. However, this paper includes only the patients supported with VV ECMO since patients supported with VV ECMO behave differently to patients not supported with VV ECMO and may have more bleeding and thrombotic complications. Outcome of the patients who did not receive ECMO support were analysed separately. Patient demographics, comorbidities, laboratory results from the day of ECMO initiation and then weekly until the date of death, discharge, or transfer to another hospital after decannulation from ECMO, additional treatment interventions such as use of steroids, plasmapheresis and intravenous (IV) immunoglobulins were recorded. At the time of writing this paper, ECMO support had ended for all patients and follow‐up to 180‐day post VV ECMO initiation or death had been completed.
Anti‐coagulation protocol
Intravenous unfractionated heparin (UFH) was used as the first line anti‐coagulation and argatroban was used in the context of confirmed heparin‐induced thrombocytopenia (HIT) or those who failed to achieve adequate anti‐coagulation with UFH despite increasing the dose of UFH as per local protcols. 11 Anti‐coagulant protocols were not specified but in general, all patients received UFH with heparin anti‐Xa of 0·2–0·3 IU/ml or equivalent (local) activated partial thromboplastin time (APTT) unless they had major bleeding. For patients with thrombosis at the initiation or during ECMO the targets were increased up to anti‐Xa of 0·5–0·7 IU/ml or equivalent APTT at local clinical discretion.
Heparin‐induced thrombocytopenia
Heparin‐induced thrombocytopenia was suspected and a screening test was performed in patients who developed thrombocytopenia with a pattern suggestive of HIT, with or without objectively proven thrombosis. Patients with suspected HIT had a pre‐test probability score (PTPS) of ≥4 [calculated by two clinicians (requesting clinician and on‐call haematologist)]. Patients with low PTPS (< 4) were not tested except for a selected group with a score of 3 but in whom alternative causes for thrombocytopenia were discounted by the clinicians. Patients with positive screen results were tested using an enzyme‐linked immunosorbent assay (ELISA; HYPHEN BioMed, Neuville‐sur‐Oise, France) in all centres and with further confirmatory tests such Hemosil AcuStar HIT‐IgG (PF4‐H; Werfen, Warrington, Cheshire, UK) which is an automated chemiluminescent immunoassay or platelet aggregation assay. Patients with HIT were anti‐coagulated with argatroban.
Use of blood products and haemostatic support
The optimal transfusion threshold for patients supported with VV ECMO remains controversial and centres used thresholds of haemoglobin 70–100 g/l, assessing for additional risk factors such as underlying cardiac disease. 12 Platelet transfusion was generally given to maintain a platelet count of ≥50 × 109/l if no bleeding or ≥100 × 109/l in the presence of bleeding. Fresh frozen plasma (FFP) or cryoprecipitate was given in the presence of major bleeding or clinically relevant non‐major bleeding (CRNMB) 13 associated with prolonged prothrombin time (PT) or APTT after cessation of anti‐coagulation. Fibrinogen concentrate or cryoprecipitate was given to maintain a fibrinogen level of 1·5–2·0 g/l in the presence of bleeding. In addition to blood product support, further haemostatic support with intravenous stat dose tranexamic acid 1 g or three times daily doses were given in the presence of major or clinically relevant minor bleeding especially in the mucosal surfaces, but was stopped immediately once the bleeding settled. Intravenous vitamin K (10 mg IV one to three days) was given in patients with PT prolongation with or without evidence of liver disease only if there was evidence of major or clinically relevant minor bleeding.
Objectives and outcomes
To determine the incidence of thrombosis and bleeding.
To determine the clinical and laboratory characteristics that are associated with thrombosis and major bleeding
To assess the effect of major bleeding and thrombosis on 180‐day mortality in severe COVID‐19 patients supported with VV ECMO.
Definitions of clinical outcomes
Bleeding and thrombotic complications. Major bleeding and minor bleeding were defined as per International Society on Thrombosis and Haemostasis (ISTH) criteria for major or clinically relevant non‐major bleeding (CRNMB) in non‐surgical patients 13 (Table S2).
Routine computed tomography (CT) imaging was performed within 24 h of admission for VV ECMO. Additional imaging for bleeding or thrombosis was performed as clinically indicated. Intracranial haemorrhage (ICH) was diagnosed using non‐contrast CT. During ECMO, brain CT was performed for clinical suspicion of acute neurological injury.
Thrombotic events were defined as image‐confirmed pulmonary embolism (PE), deep vein thrombosis (DVT) or arterial thrombosis or thrombosis of the ECMO circuit.
Statistical analysis
Standard descriptive parameters were calculated for categorical and quantitative variables. Survival probabilities were calculated using the Kaplan–Meier method, and groups compared using the log‐rank test. Variables identified from univariate analyses with P values < 0·2 were entered into a backward stepping Cox regression analysis to find independent prognostic factors significant at P < 0·05. To assess the influence of complications following initiation of ECMO on the risk of mortality, each complication was independently entered as a time‐dependent variable. Identification of significant independent prognostic factors for the thrombosis and major bleeding required the use of the cumulative incidence procedure with Gray’s test to compare groups, and the Fine and Gray model for the multivariate setting. Death in the absence of thrombosis or major bleeding was considered the competing event. The effects of secondary outcomes on mortality were assessed by Cox proportional hazards models incorporating the secondary outcomes as time‐dependent covariates. Multiple imputation was used to account for missing laboratory values but not for comorbidities or clinical outcomes as there were no missing values. The multiple imputation by chained equation (MICE) technique with its regression imputation model was used for this imputation with ten iterative cycles. Once imputation was done, results were reviewed for each imputed feature to make sure that the imputation has generated plausible data. (Scatterplots for the imputed features were used for the review.) We used variables which are predictive of missing values by considering a MAR (Missing At Random) assumption, which means that the probability that a value is missing depends only on observed values and not on unobserved values. All tests were two‐sided, and P values <0·05 were deemed statistically significant. All analyses were performed using either SPSS version 27 (SPSS v27; IBM, Armonk, NY, USA), R (v4.0.3, Open‐source software) or Stata (v17, StataCorp LLC, College Station, TX, USA) and open‐source software programming languages and libraries (Python [v3.7, Open‐source software], panda [v1.3.3, Open‐source software], numpy [v1.21.2, Open‐source software], scikit learn [v0.22.1, Open‐source software]).
Results
A total of 152 patients were included in the analysis. Median age (range) was 47 (23–65) years and 75% (114/152) were male. Median duration on ECMO was 17·5 days [interquartile range (IQR) 11–30 days]. Overall, 180‐day survival was 70·4% (107/152). Demographics, clinical and laboratory characteristics at the initiation of ECMO are summarised in Table I. Comorbidities were present in 61·2% (93/152) of the patients prior to the diagnosis of COVID‐19 and 53·8% (50/93) of these had only one comorbidity. Supportive care and therapies delivered during support with VV ECMO are summarised in Table II.
Table I.
Demographics, comorbidities, and laboratory parameters at the initiation of VV ECMO.
| Demographics | n (%) | |
|---|---|---|
| Patient age (years) | ||
| <40 | 34 (22·4) | |
| 40–50 | 66 (43·4) | |
| >50 | 52 (34·2) | |
| Gender | ||
| Male | 114 (75·0) | |
| Female | 38 (25·0) | |
| Ethnicity | ||
| Caucasian | 57 (37·5) | |
| Asian | 50 (32·9) | |
| Black or African American | 17 (11·2) | |
| Mixed | 4 (2·6) | |
| Other | 24 (15·8) | |
| Body mass index (kg/m2) | ||
| 18–24·99 | 32 (21·1) | |
| 25–29·99 | 45 (29·6) | |
| 30–39·99 | 53 (34·9) | |
| ≥40 | 22 (14·5) | |
| Comorbidities | ||
| Liver disease | 2 (1·3) | |
| Lung disease | 24 (15·8) | |
| Diabetes | 37 (24·3) | |
| Hypercholesterolemia | 15 (9·9) | |
| Hypertension | 44 (28·9) | |
| Auto‐immune disease | 5 (3·3) | |
| Combined co‐morbidities | ||
| 0 | 58 (38·4) | |
| 1 | 50 (32·9) | |
| >1 | 43 (28·3) | |
| Smoking history | ||
| None | 83 (54·6) | |
| Current | 7 (4·6) | |
| Ex‐smoker | 19 (12·5) | |
| Unknown | 43 (28·3) | |
| Duration mechanical ventilation pre‐ECMO: | ||
| 1‐6 days | 108 (71·1) | |
| ≥7 days | 44 (28·9) | |
| PaO2/FiO2 ratio (kPa)* | 9·4 (8·3–10·7) | |
| PaCO2 (kPa)* | 7·8 (6·4–9·2) |
| Laboratory parameters | Median (IQR) | Reference range |
|---|---|---|
| Haemoglobin g/l | 100 (91, 110·5) | (130–160) |
| Platelets 109/l | 264 (193·8, 333) | (150–400) |
| WBC 109/l | 11·4 (8·592, 14·425) | (4·1–11·1) |
| Neutrophils 109/l | 9·655 (7·1, 12·915) | (2·1–6·7) |
| Lymphocytes 109/l | 0·7 (0·5, 1·115) | (1·3–3·7) |
| Prothrombin time (seconds) | 14·4 (13·3, 15·7) | (10·2–13·2) |
| APTT (seconds) | 41·7 (32·75, 66·33) | (26·0–36·0) |
| Fibrinogen g/l | 6·1 (4·7, 7·5) | (1·5–4·5) |
| D‐dimer ng/ml | 3 206 (1 400, 6 391) | (0–500) |
| Anti‐thrombin IU/dl; | (70–140) | |
| Lactate mmol/l | 1·5 (1·1, 1·82) | (0·5–2·0) |
| Troponin I ng/l | 33·4 (12·6, 107·17) | (<19·8) |
| Ferritin μg/l | 1 301 (691·5, 2 136·9) | (20–186) |
| LDH IU/l | 773·6 (583·9, 1 030·2) | (266–500) |
| CRP mg/l | 239·5 (146·8, 294) | (0–10) |
| ALT IU/l | 49 (33·5, 78·75) | (8–40) |
| Bilirubin μmol/l | 13 (9, 23) | (1–17) |
| Creatinine μmol/l | 77·5 (53·75, 150·5) | (60–120) |
| Heparin anti‐Xa (IU/ml at the initiation of ECMO) | ||
| 0 – 0·29* | 73 (48·02%) | Normal (0) |
| 0·30 – 0·70 | 55 (36·18%) | prophylaxis <0·3 |
| > 0. 70 | 24 (15·78%) | Treatment levels (0·30–0·70) |
| Blood group | ||
| O | 53 (34·86%) | |
| A | 61 (40·13%) | |
| B | 27 (17·76%) | |
| AB | 11 (7·24%) |
ALT, alanine aminotransferase; APTT, activated partial thromboplastin time; CRP, C‐reactive protein; LDH, lactate dehydrogenase; VV ECMO, veno‐venous extracorporeal membrane oxygenation; WBC, white blood cells.
All laboratory parameters, PaO2/FiO2 ratio and PaCO2 values are at the initiation of ECMO (prior to cannulation).
Median and interquartile range.
Table II.
Supportive care and therapies delivered during support with VV ECMO.
| Intervention/treatment | Total number = 152 |
|---|---|
| IV Immunoglobulins | 5 (3·3%) |
| Tocilizumab | 8 (5·3%) |
| Plasmapheresis | 14 (9·2%) |
| Steroids | 91 (59·9%) |
| Haemostatic with tranexamic acid | 14 (9·2%) |
| Blood product support | |
| Red cell units (median and range) | 7 (0–57) |
| Platelet units (median and range) | 0 (0–10) |
| FFP units (median and range) | 0 (0–48) |
| Cryoprecipitate units (median and range) | 0 (0–12) |
| Fibrinogen (g; median and range) | 0 (0–12) |
VV ECMO, veno‐venous extracorporeal membrane oxygenation; FFP, fresh frozen plasma; IV, intravenous.
Supportive care and therapies were involving based on clinical studies and experience during the pandemic and not all available at the start of the study.
Major bleeding
Major bleeding was diagnosed in 30·9% (47/152) of patients occurring at a median of nine days (range 0–31) from initiation of VV ECMO. The cumulative incidence of major bleeding is presented in Figure 1A. The most frequent major bleeding was intracranial (ICH) accounting for 34% (16/47), followed by pulmonary haemorrhage (26%, 12/47), gastrointestinal haemorrhage (11%, 5/47) and bleeding at other sites with a fall of haemoglobin of >20 g/l (6%, 3/47). Eleven patients developed major bleeding at more than one site (23%, 11/47; Table III). Of the 47 patients who developed major bleeding events, 19·1% (9/47) occurred within 24 h of initiation of ECMO [comprising 12·8% (6/47) ICH, 4·3% (2/47) pulmonary haemorrhage and one gastrointestinal bleeding dropping haemoglobin by >20 g/l]. No bleeding events were diagnosed after decannulation of VV ECMO.
Fig 1.
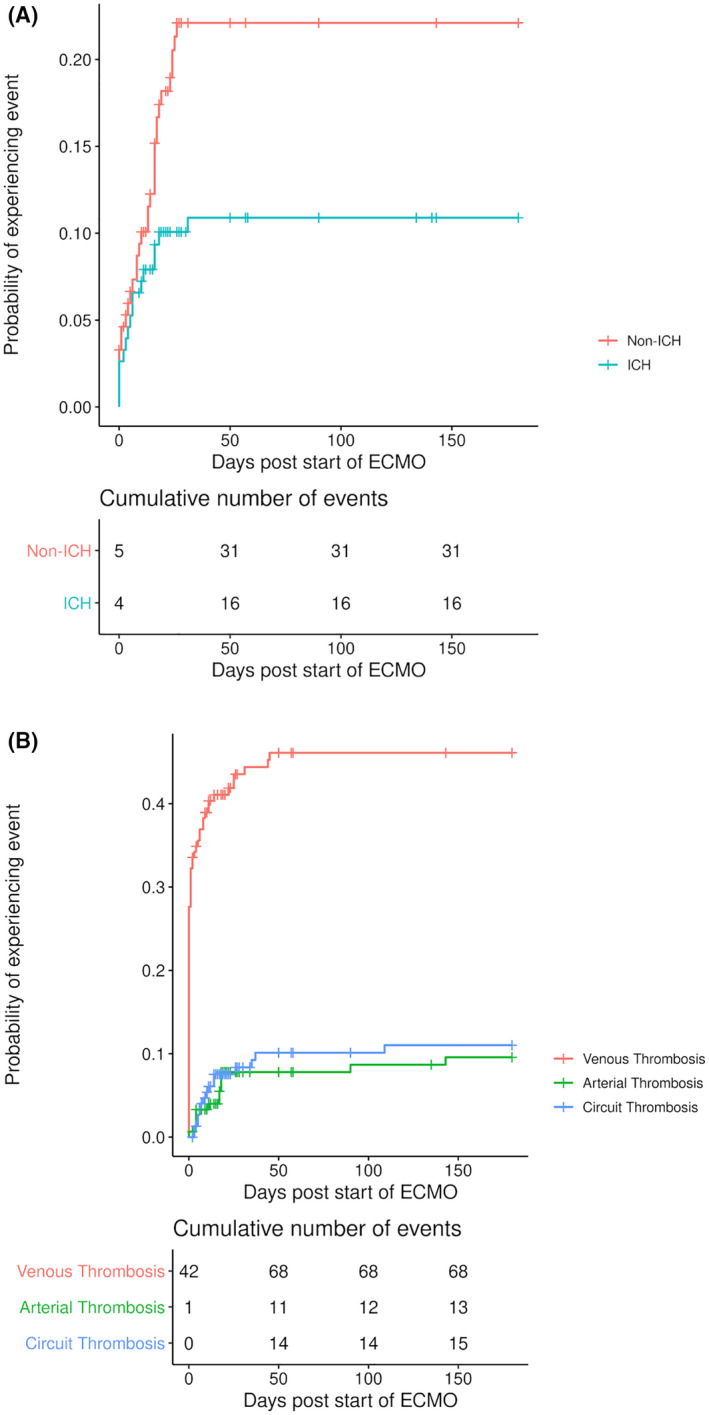
(A) Cumulative incidence of major bleeding (intracerebral and bleeding at other sites) since initiation of veno‐venous extracorporeal membrane oxygenation (VV ECMO) in patients with COVID‐19. (B) Cumulative incidence of thrombosis (venous, arterial or ECMO circuit) since initiation of VV ECMO in patients with COVID‐19. ICH, Intracranial bleeding
Table III .
Impact of major bleeding and thrombosis on mortality following initiation of VV ECMO.
| Complication | n = (152) | Alive (n = 107) | Dead (n = 45) | Crude time‐adjusted HR (95% CI) | Time‐adjusted HR* (95% CI) |
|---|---|---|---|---|---|
| Major bleeding | 47 (30·9%) | 23 (48·9%) | 24 (51·1%) | 3·01 (1·63–5·51) | 3·87 (2·10–7·23) |
| ICH | 16 (34%) | 6 (37·5%) | 10 (62·5%) | 3·30 (1·36–7·76) | 5·97 (2·36–15·04) |
| GI | 5 (11%) | 2 (40%) | 3 (60%) | ||
| Other | 3 (6%) | 2 (66%) | 1 (33%) | ||
| Pulmonary haemorrhage | 12 (26%) | 5 (41·7%) | 7 (58·3%) | ||
| >1 Bleeding site | 11 (23%) | 8 (72·7%) | 3 (27·3%) | ||
| Venous thrombosis | 68 (44·7%) | 48 (70·6%) | 20 (29·4%) | 1·14 (0·61–2·14) | 1·63 (0·94–3·04) |
| PE | 45 (66·2%) | 28 (26·2%) | 17 (37·8%) | 2·12 (1·19–3·76) | 2·00 (1·09–3·56) |
| DVT | 13 (19·1%) | 11 (84·6%) | 2 (15·4%) | ||
| PE and DVT | 10 (14·7%) | 9 (90%) | 1 (10%) | ||
| Arterial thrombosis | 13 (8·6%) | 6 (46·2%) | 7 (53·8%) | 1·74 (1·24–6·14) | 1·70 (0·71–3·92) |
| ECMO circuit thrombosis | 15 (9·9%) | 12 (80·0%) | 3 (20·0%) | 0·92 (0·26–3·04) | 0·79 (0·34–2·78) |
CI, confidence interval; DVT, deep vein thrombosis; GI, gastrointestinal; HR, hazard ratio; ICH, intracranial haemorrhage; PE, pulmonary embolism; VV ECMO, veno‐venous extracorporeal membrane oxygenation.
Numbers in bold type indicate complications that are associated with increased risk of mortality.
Adjusted for patient age and duration of mechanical ventilation. All HRs are shown relative to not having the condition of interest (HR 1·00).
Following univariate analysis of the factors described in Table I, nine variables [age, blood group, white blood cell count, platelets, alanine transferase, C‐reactive protein, bilirubin, ferritin, and mechanical ventilation (MV) prior to initiation of ECMO] with P values <0·2 were included in a multivariate analysis. Presence of white cell count larger than the normal range showed a trend towards reduction in risk of major bleeding (HR 0·57, 95% CI 0·32–1·02) whilst duration of MV >6 days) prior to initiation of VV ECMO showed a trend towards an increased risk of major bleeding (HR 1·72, 95% CI 0·98–3·04), though both these factors were not statistically significant (Table IV). Although thrombocytopenia (<150 × 109/l) and blood group (group A) had increased risks of major bleeding in the univariate analysis (Table IV), these were not significant in the multivariate analysis.
Table IV.
Univariate and multivariate factors associated with the probabilities of a major bleed following initiation of VV ECMO.
| Subgroup | n | Probability of major bleed % (95% CI) | P | HR (95% CI) | P | |
|---|---|---|---|---|---|---|
| Overall | 152 | 30·9 (24–39) | ||||
| Patient age (years) | <50 | 100 | 27·0 (20–37) | 0·15 | ||
| >50 | 52 | 38·5 (27–55) | ||||
| Blood group | A | 55 | 45·5 (34–61) | 0·036 | ||
| B | 25 | 32·0 (17–58) | ||||
| AB | 50 | 20·0 (11–35) | ||||
| O | 10 | 30·0 (11–82) | ||||
| Duration of mechanical ventilation (days) | <7 | 108 | 23·7 (16–36) | 0·049 | 1·00 | 0·057 |
| >6 | 44 | 38·2 (29–51) | 1·72 (0·98–3·04) | |||
| Ferritin (μg/l) | <1301 | 76 | 36·8 (27–50) | 0·083 | ||
| >1 300 | 76 | 25·0 (17–37) | ||||
| Platelets (109/l) | Below normal (<150) | 18 | 55·6 (36–86) | 0·041 | ||
| Normal (150–400) | 116 | 26·7 (20–36) | ||||
| Above normal (>400) | 18 | 33·3 (17–66) | ||||
| WBC (109/l) | Normal (4·1–11·1) | 72 | 38·9 (29–52) | 0·044 | 1·00 | 0·060 |
| Above normal (>11·1) | 80 | 23·7 (16–35) | 0·57 (0·32–1·02) | |||
| ALT (IU/l) | Normal (8–40) | 57 | 40·4 (29–56) | 0·054 | ||
| Above normal (>40) | 95 | 25·3 (18–36) | ||||
| Bilirubin (µmol/l) | Normal (0–20) | 108 | 35·2 (27–46) | 0·082 | ||
| Above normal (>20) | 44 | 20·5 (11–37) | ||||
| CRP (mg/l) | <240 | 76 | 23·7 (16–36) | 0·051 | ||
| >239·9 | 76 | 38·2 (29–51) |
ALT, alanine aminotransferase; CRP, C reactive protein; VV ECMO, veno‐venous extracorporeal membrane oxygenation;WBC, white blood cells.
Values in bold are significant.
Thrombosis
There was a total of 96 thrombotic events comprising venous, arterial or ECMO circuit thrombosis rates of 44·7% (68/152), 18·6% (13/152) and 9·9% (15/152) respectively (Table IV). Of patients who developed venous thrombosis (VTE), isolated PE was the most common (66·2%, 45/68) followed by DVT (19·1%, 13/68) and combined PE and DVT (14·7%, 10/68) (Table IV). Of the 81 venous or arterial thrombotic events, 51·8% (42/81) were diagnosed within 24 h of initiation of ECMO. Of the 42, 16·7% 7 events were arterial (six ischaemic strokes and one with thrombus in the right atrium) and 83·3% (35) were venous (17 isolated PE, 18 PE and DVT). Median duration from initiation of ECMO to thrombosis was six days (IQR 2–17).
Cumulative incidence of thrombosis is presented Figure 1B. Of the total 68 VTE, 13 were diagnosed after ECMO decannulation of which 11 were central line‐related thromboses and two were PE. Of the total 13 patients who developed arterial thrombosis, 46·2% (6/13) had ischaemic stroke.
In univariate analysis, only three variables were significant at P < 0·2, fibrinogen (P = 0·14), presence of diabetes (P = 0·094) and lactate dehydrogenase (LDH; P = 0·005). Only raised LDH at the initiation of ECMO was significantly associated with thrombosis in multivariate analysis [HR 1·92 (95% CI 1·21–3·03), P = 0·005].
Impact of bleeding and thrombosis on overall survival
In crude time‐adjusted analysis, the development of major bleeding following initiation of ECMO conferred a 3·01‐fold increased risk of mortality (95% CI 1·63–5·51). This remained significant after adjusting for patient age and duration of MV prior to ECMO which were significantly associated with increased risk of death in multivariate analysis of baseline clinical and laboratory characteristics at the initiation of ECMO [HR 3·87 (95% CI 2·10–7·23)]. The HR for mortality was higher in patients who developed ICH [3·30 (95%1·36–7·76) and was 5·97 (95% CI 2·36–15·04)] after adjusting for the patient age and duration of MV. Although association with overall venous thrombosis and circuit thrombosis did not reach statistical significance, presence of PE was associated with increased risk of mortality by 2·12‐fold (95% CI 1·19–3·76) which remained significant after adjusting for patient age and the duration of MV prior to ECMO (HR 2·00, 95% CI 1·09–3·56). Patients who developed arterial thrombosis had higher mortality (HR 1·74, 95% CI 1·24–6·14) in crude time‐adjusted analysis but this was lost after adjustment for age and duration of MV prior to ECMO (HR 1·70, 95% CI 0·71–3·92; Table IV).
Clinically relevant non‐major bleeding events
A total of 58 patients had Clinically relevant non‐major bleeding events (CRNMB) following initiation of ECMO. The median duration from initiation of ECMO to CRNMB was six days (IQR 4–14). Of those bleeding events, four patients had gastrointestinal bleeding only, 12 patients had mucosal bleeding only, 24 had bleeding from line or cannula site, six had bleeding into the lungs and 12 patients had bleeding into more than one site. Five episodes of CRNMB occured post decannulation of ECMO and all were related to tracheostomy sites.
Fluctuations of haematological parameters over time on ECMO
Fluctuations of haematological parameters over time on ECMO are presented in graphical form comparing patients with major bleeding vs no major bleeding (Figs S1 and S2) or thrombosis vs no thrombosis (Figs S3 and S4) up to 56 days on ECMO since 142/152 (93·42%) of patients had ECMO <56 days.
Use of blood products over time on ECMO in patients with bleeding vs no bleeding
Blood product transfusion over time in patients with major bleeding vs no major bleeding is presented in Figures S5–S8. A total of 140/152 (92·10%) received one or more red cell unit whilst 39/152 (25·65%), 24/152 (22·36%) and 40/152 (26·31%) received one or more unit of platelets, fresh frozen plasma, or cryoprecipitate, respectively, in the whole cohort.
Heparin‐induced thrombocytopenia
HIT was diagnosed in 16/152 (10·52%) of the patients (Table V) after a median of 12·5 days (range 3–25 days) on ECMO. Ten patients had thromboses (62·5%) associated with HIT, which are included in the total thrombotic events reported.
Table V.
Characteristics and clinical outcomes of the patients developed heparin induced thrombocytopenia during VV ECMO.
| Patient | Age (years) | Sex | Duration on ECMO prior to diagnosis of HIT in days | Type of thrombotic events | Clinical outcome |
|---|---|---|---|---|---|
| 1 | 49 | Male | 18 | Deep vein thrombosis, renal circuit thrombosis, ECMO circuit thrombosis | Discharged home |
| 2 | 40 | Male | 15 | None | Discharged home |
| 3 | 47 | Male | 14 | None | Discharged home |
| 4 | 45 | Male | 17 | None | In‐hospital mortality whilst on ECMO |
| 5 | 47 | Male | 10 | Only renal circuit thrombosis, | Discharged home |
| 6 | 43 | Male | 3 | Pulmonary embolism, renal circuit thrombosis | Discharged home |
| 7 | 63 | Male | 10 | Pulmonary embolism | Discharged home |
| 8 | 49 | Male | 13 | Pulmonary embolism, renal circuit thrombosis | Discharged home |
| 9 | 39 | Male | 16 | Pulmonary embolism | Discharged home |
| 10 | 39 | Female | 19 | None | In‐hospital mortality whilst on ECMO |
| 11 | 38 | Female | 11 | Deep vein thrombosis, pulmonary embolism | In‐hospital mortality whilst on ECMO |
| 12 | 49 | Male | 10 | Pulmonary embolism, renal circuit thrombosis | Discharged home |
| 13 | 36 | Male | 25 | Pulmonary embolism | Discharged home |
| 14 | 51 | Male | 9 | Deep vein thrombosis, pulmonary embolism | Discharged home |
| 15 | 51 | Male | 6 | Pulmonary embolism | Discharged home |
| 16 | 49 | Male | 12 | None | Discharged home |
HIT, heparin‐induced thrombocytopenia; VV ECMO, veno‐venous extracorporeal membrane oxygenation.
Discussion
In this multicentre observational study, comprising a uniform population of patients with COVID‐19 supported with VV ECMO, thrombosis and major bleeding events were frequent. Major bleeding (in particular ICH) and PE, were associated with increased risk of mortality after adjusting for age and duration of MV prior to ECMO which were independently associated with increased risk of mortality.
The major bleeding rate of 30·9% in our study was lower than in another cohort study (42%). 14 Overall our thrombosis rates were much higher (63·1%) compared to previous reports in patients supported with ECMO 14 and from systematic review and meta‐analysis data from patients with severe COVID‐19 treated in intensive‐care units. 15 The differences in bleeding and thrombosis may in part be due to different rates of imaging, variation in the anti‐coagulation practice, difference in the study population, ECMO technique and heterogeneity of the follow‐up.
We performed routine CT imaging within 24 h of initiation of VV ECMO. Additional imaging for bleeding or thrombosis was performed as clinically indicated. Thrombosis and bleeding rates reported in our study include all events throughout the ECMO run. Most thrombotic events and all major bleeding events occurred in the first 50 days from initiation of ECMO with most of venous thrombosis and 9/47 major bleeding events detected within 24 h of initiation of ECMO. It is uncertain whether these events occurred prior to ECMO or soon after ECMO or were precipitated by ECMO initiation and associated coagulation derangements. As all patients have been sedated and ventilated prior to initiation of ECMO without performing imaging per and post initiation ECMO, this differentiation is not possible. A separate study, conducted by one of the UK ECMO centres, compared the rate of ICH in patients with COVID‐19 vs patients with influenza supported with VV ECMO. 16 The rates of ICH at the time of cannulation were high in both patient cohorts (16% with COVID‐19 vs 14% with influenza; P = 0·8). 16
During the early stage of the COVID‐19 pandemic, anti‐coagulation was intensified in some centres due to increased rates of thrombosis observed with COVID‐19. However, we did not alter the existing anti‐coagulant protocols of patients supported with VV ECMO for COVID −19, whereas Schmidt et al. increased the target APTT to 60–75 s or anti‐Xa level 0·3–0·5 IU/ml. 14 A recently published multiplatform trial showed that empirical therapeutic‐dose anti‐coagulation was associated with poorer outcomes in the critically ill patients [requiring ICU‐level respiratory or cardiovascular support or extracorporeal life support (ELS) at enrolment]. 17 However, it is not clear how many of the 1 098 patients in this trial were supported with ELS. 17 Patients supported with ECMO already have high rates of bleeding and thrombosis at the initiation of ECMO 9 in addition to risk of thrombosis and bleeding during ECMO. Hence generalisation of the trial findings to patients not supported with ECMO is not appropriate. Additionally, tighter anti‐coagulant control (as shown in Fig S2, heparin anti‐Xa level remained within therapeutic range during ECMO) and exclusion of veno‐arterial (VA)‐ECMO may have contributed to the lower bleeding rate in our cohort. However, despite the lower overall major bleeding rate, ICH was the most common major bleeding event and constituted 34% of the major bleeding events which had a direct impact on mortality.
Occurrence of ICH precludes anti‐coagulation for at least 7–10 days according to severity of the bleeding. Although heparin increases the risk of bleeding, it has anti‐inflammatory, anti‐viral and anti‐complement effects and the loss of these in patients with major bleeding may have contributed to worse outcomes. 18 , 19 Previous studies in patients with COVID‐19 not requiring ECMO support demonstrated an increased risk of mortality in patients with PE. 20 This emphasises the challenge of balancing bleeding vs thrombosis in patients supported with ECMO.
The incidence of HIT in the study group was higher than other studies of ECMO‐supported patients with 14 or without COVID‐19· 21 This may reflect the intensity of the immune response in patients with COVID‐19, or increased diagnosis frequency based on previous experience. 21
Many studies have shown that raised D‐dimer is a predictor of increased mortality in patients with COVID‐19 22 , 23 but we did not see any difference between survivors and non‐survivors or those with or without thrombosis. As all patients had equally severe disease at the initiation of ECMO, the predictive power of D‐dimer appears to be lost in these patients.
Thrombosis and bleeding complications during ECMO are likely multifactorial in origin. Anti‐coagulation, acquired von Willebrand syndrome (AVWS), thrombocytopenia and platelet dysfunction 9 , 24 are all identified bleeding risks on ECMO. Interestingly, a white cell count above the normal range was associated with a 53% lower risk of major bleeding and inflammation is known induce a prothrombotic response with raised coagulation factors and endothelial upregulation. Duration of MV (>6 days) prior to initiation of VV ECMO was associated with a two‐fold increased risk of major bleeding. After adjusting for competing risks for mortality these factors were no longer significant. In multivariate analysis, only raised LDH at the initiation of ECMO was associated with increased risk of thrombosis. Raised LDH is a marker of acute or chronic tissue damage including acute and severe lung damage and is considered an inflammatory marker which can contribute to increase risk of thrombosis. 25 , 26 However, due to the complex interactions of the ECMO circuit, patient factors and anti‐coagulation, it is not possible to distinguish specifically the contributors to thrombosis and haemorrhagic risk in these patients. The higher prevalence of ICH in these patients could be due to greater thrombotic burden necessitating therapeutic anti‐coagulation in most of these patients and the presence of ischaemic stroke rendered them more vulnerable to haemorrhagic transformation. At the same time, contact activation on the ECMO circuit and inflammation increases thrombotic risk.
The main limitation of this study is that some of the data were collected retrospectively, but all relevant information and clinical outcomes were recorded directly using a predefined well‐structured electronic CRF. Although the number of patients included into study is relatively small, this study included all consecutive patients supported with VV ECMO from four nationally commissioned ECMO centres in UK. Additionally, we used the ISTH criteria to assess the CRNMB, although this has not been validated for patients supported with ECMO. Absence of imaging at decannulation in some asymptomatic patients may have contributed to underreporting of thrombosis.
As this study included the patients supported with VV ECMO from the first wave of the COVID‐19 pandemic, not all patients received steroids (i.e dexamethasone), and it is possible that patients who progressed to have severe disease requiring ECMO support are having more refractory disease since steroids are given to patients early in the ICU admission. However, 91 /152 (59·9%) of the patients in our study received steroids and the main focus of this paper is bleeding and thrombosis and their impact on clinical outcome; we would not expect these outcomes to change significantly during the latter part of the pandemic, especially given the fact that we did not alter our anti‐coagulant strategy. There are no published data comparing the bleeding, thrombosis, and their impact on mortality in patients supported with VV ECMO from the first and second wave of the COVID‐19 pandemic. The decision to treat with steroids and other supportive interventions during the ECMO run was taken by the multidisciplinary team. Decisions were based on clinical, radiological and biological parameters and guided by previous experience on patients with severe ARDS. Additionally, small proportion of patients received steroids through the RECOVERY trial.
The study’s strengths include the inclusion of consecutive patients supported with VV ECMO (unform population) in four participating centres working collaboratively, and the inclusion of all relevant laboratory parameters on admission in assessing their relevance to clinical outcomes. This is the first multicentre study to assess bleeding and thrombosis and their association with clinical outcome in patients supported with VV ECMO.
Conclusions
In patients with COVID 19 pneumonitis requiring VV ECMO support, thrombosis and major bleeding were frequent. Major bleeding, especially ICH and PE, were independently associated with increased mortality. This emphasises the difficult balancing act between bleeding and thrombosis in COVID 19 patients supported with ECMO.
Funding
Bayer plc supported the study by providing investigator‐initiated funding (P87339) to set up the multicentre database for the study. The funder had no access to the data and played no part in analysis or writing. The corresponding author is responsible for the study design, had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Author Contributions
DJA conceived the study, acquired the funding, and was involved in data collection, data verification, data analysis, figures, data interpretation, writing the original draft, reviewing and editing the manuscript. IR, ZO, WB, and RS were involved in data verification, data analysis, figures, data interpretation and reviewing and editing the manuscript. IS, MG, AV, GI, LC, JW, LS, ML, SL, RJ, AV and HY were involved in data collection, data interpretation, reviewing and editing the manuscript. All authors interpreted the results, reviewed, and approved the final work, and are accountable for the accuracy and integrity of the work.
Conflicts of interest
DJA received funding from Bayer plc to setup the multicentre database of the study as an investigator‐initiated funding. Other authors have no conflict of interest to declare.
Supporting information
Table SI. Criteria for the referral of patients to extracorporeal membrane oxygenation (ECMO) centres NHS England and Scotland
Table SII. International Society on Thrombosis and Haemostasis criteria for major and clinically relevant non‐major bleeding.
Fig S1. Fluctuations in haemoglobin, platelet count, prothrombin time and activated partial thromboplastin time in patients developing major bleeding vs no major bleeding during extracorporeal membrane oxygenation (ECMO).
Fig S2. Fluctuations in heparin anti‐Xa level, fibrinogen, anti‐thrombin and D‐dimer levels, in patients developing major bleeding vs no major bleeding during extracorporeal membrane oxygenation (ECMO).
Fig S3. Fluctuations in haemoglobin, platelet count, prothrombin time and activated partial thromboplastin time in patients developing thrombosis vs no thrombosis during extracorporeal membrane oxygenation (ECMO).
Fig S4. Fluctuations in heparin anti‐Xa level, fibrinogen, anti‐thrombin and D‐dimer levels in patients developing thrombosis vs no thrombosis during extracorporeal membrane oxygenation (ECMO).
Fig S5. Red cell transfusion in patients with major bleeding vs no major bleeding during extracorporeal membrane oxygenation (ECMO).
Fig S6. Platelet transfusion in patients with major bleeding vs no major bleeding during extracorporeal membrane oxygenation (ECMO).
Fig S7. Fresh frozen plasma transfusion in patients with major bleeding vs no major bleeding during extracorporeal membrane oxygenation (ECMO).
Acknowledgements
The authors would like to thank Mr Feargus Hosking‐Jervis for developing the study database, all clinical and laboratory staff who were involved in care of the patients at participating ECMO centres. Collaborators are listed in the appendix.
References
- 1. https://www.who.int/publications/i/item/clinical‐management‐of‐covid‐19. 2020
- 2. Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, et al. Surviving sepsis campaign: Guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID‐19). Crit Care Med. 2020;48(6):e440–e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Badulak J, Antonini MV, Stead CM, Shekerdemian L, Raman L, Paden ML, et al. ECMO for COVID‐19: updated 2021 guidelines from the extracorporeal life support organization (ELSO). Asaio J. 2021;67:485–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.COVID‐19 rapid guideline: critical care in adults. 2020. https://www.nice.org.uk/guidance/ng159 [PubMed]
- 5. Camporota L, Meadows C, Ledot S, Scott I, Harvey C, Garcia M, et al. Consensus on the referral and admission of patients with severe respiratory failure to the NHS ECMO service. Lancet Respir Med. 2021;9(2):e16–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Noah MA, Peek GJ, Finney SJ, Griffiths MJ, Harrison DA, Grieve R, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA. 2011;306(15):1659–68. [DOI] [PubMed] [Google Scholar]
- 7. Barbaro RP, MacLaren G, Boonstra PS, Iwashyna TJ, Slutsky AS, Fan E, et al. Extracorporeal membrane oxygenation support in COVID‐19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet. 2020;396(10257):1071–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thiagarajan RR, Barbaro RP, Rycus PT, McMullan DM, Conrad SA, Fortenberry JD, et al. Extracorporeal life support organization registry International Report 2016. Asaio J. 2017;63(1):60–7. [DOI] [PubMed] [Google Scholar]
- 9. Arachchillage DRJ, Passariello M, Laffan M, Aw TC, Owen L, Banya W, et al. Intracranial hemorrhage and early mortality in patients receiving extracorporeal membrane oxygenation for severe respiratory failure. Semin Thromb Hemost. 2018;44(3):276–86. [DOI] [PubMed] [Google Scholar]
- 10. Patel BV, Arachchillage DJ, Ridge CA, Bianchi P, Doyle JF, Garfield B, et al. Pulmonary angiopathy in severe COVID‐19: physiologic, imaging, and hematologic observations. Am J Respir Crit Care Med. 2020;202(5):690–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arachchillage DJ, Remmington C, Rosenberg A, Xu T, Passariello M, Hall D, et al. Anticoagulation with argatroban in patients with acute antithrombin deficiency in severe COVID‐19. Br J Haematol. 2020;190(5):e286–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arachchillage DRJ, Owen S, Anievas M, Gaspar M, Laffan M. Red cell alloimmunisation in patients receiving veno‐venous extracorporeal membrane oxygenation (VV‐ECMO). Intensive Care Med. 2020;46(10):1932–3. [DOI] [PubMed] [Google Scholar]
- 13. Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost. 2005;3(4):692–4. [DOI] [PubMed] [Google Scholar]
- 14. Schmidt M, Hajage D, Lebreton G, Monsel A, Voiriot G, Levy D, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID‐19: a retrospective cohort study. Lancet Respir Med. 2020;8(11):1121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nopp S, Moik F, Jilma B, Pabinger I, Ay C. Risk of venous thromboembolism in patients with COVID‐19: A systematic review and meta‐analysis. Res Pract Thromb Haemost. 2020;4(7):1178–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Doyle AJ, Hunt BJ, Sanderson B, Zhang J, Mak SM, Benedetti G, et al. A Comparison of thrombosis and hemorrhage rates in patients with severe respiratory failure due to coronavirus disease 2019 and influenza requiring extracorporeal membrane oxygenation. Crit Care Med. 2021;49:e663–e672. [DOI] [PubMed] [Google Scholar]
- 17. Goligher EC, Bradbury CA, McVerry BJ, Lawler PR, Berger JS, Gong MN, et al. Therapeutic anticoagulation with heparin in critically Ill patients with Covid‐19. N Engl J Med. 2021;385:777–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gozzo L, Viale P, Longo L, Vitale DC, Drago F. The potential role of heparin in patients with COVID‐19: beyond the anticoagulant effect. A Review. Front Pharmacol. 2020;11:1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alijotas‐Reig J, Esteve‐Valverde E, Belizna C, Selva‐O'Callaghan A, Pardos‐Gea J, Quintana A, et al. Immunomodulatory therapy for the management of severe COVID‐19. Beyond the anti‐viral therapy: A comprehensive review. Autoimmun Rev. 2020;19(7):102569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liao SC, Shao SC, Chen YT, Chen YC, Hung MJ. Incidence and mortality of pulmonary embolism in COVID‐19: a systematic review and meta‐analysis. Crit Care. 2020;24(1):464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arachchillage DRJ, Laffan M, Khanna S, Vandenbriele C, Kamani F, Passariello M, et al. Frequency of thrombocytopenia and heparin‐induced thrombocytopenia in patients receiving extracorporeal membrane oxygenation compared with cardiopulmonary bypass and the limited sensitivity of pretest probability score. Crit Care Med. 2020;48(5):e371–e9. [DOI] [PubMed] [Google Scholar]
- 22. Yao Y, Cao J, Wang Q, Shi Q, Liu K, Luo Z, et al. D‐dimer as a biomarker for disease severity and mortality in COVID‐19 patients: a case control study. J Intensive Care. 2020;8:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sakka M, Connors JM, Hékimian G, Martin‐Toutain I, Crichi B, Colmegna I, et al. Association between D‐Dimer levels and mortality in patients with coronavirus disease 2019 (COVID‐19): a systematic review and pooled analysis. J Med Vasc. 2020;45(5):268–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lukito P, Wong A, Jing J, Arthur JF, Marasco SF, Murphy DA, et al. Mechanical circulatory support is associated with loss of platelet receptors glycoprotein Ibα and glycoprotein VI. J Thromb Haemost. 2016;14(11):2253–60. [DOI] [PubMed] [Google Scholar]
- 25. Xiong X, Chi J, Gao Q. Prevalence and risk factors of thrombotic events on patients with COVID‐19: a systematic review and meta‐analysis. Thromb J. 2021;19(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Figueira Gonçalves JM, Hernández Pérez JM, Acosta Sorensen M, Wangüemert Pérez AL, Ruiz M, de la Rosa E, et al. Biomarkers of acute respiratory distress syndrome in adults hospitalised for severe SARS‐CoV‐2 infection in Tenerife Island, Spain. BMC Res Notes. 2020;13(1):555. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table SI. Criteria for the referral of patients to extracorporeal membrane oxygenation (ECMO) centres NHS England and Scotland
Table SII. International Society on Thrombosis and Haemostasis criteria for major and clinically relevant non‐major bleeding.
Fig S1. Fluctuations in haemoglobin, platelet count, prothrombin time and activated partial thromboplastin time in patients developing major bleeding vs no major bleeding during extracorporeal membrane oxygenation (ECMO).
Fig S2. Fluctuations in heparin anti‐Xa level, fibrinogen, anti‐thrombin and D‐dimer levels, in patients developing major bleeding vs no major bleeding during extracorporeal membrane oxygenation (ECMO).
Fig S3. Fluctuations in haemoglobin, platelet count, prothrombin time and activated partial thromboplastin time in patients developing thrombosis vs no thrombosis during extracorporeal membrane oxygenation (ECMO).
Fig S4. Fluctuations in heparin anti‐Xa level, fibrinogen, anti‐thrombin and D‐dimer levels in patients developing thrombosis vs no thrombosis during extracorporeal membrane oxygenation (ECMO).
Fig S5. Red cell transfusion in patients with major bleeding vs no major bleeding during extracorporeal membrane oxygenation (ECMO).
Fig S6. Platelet transfusion in patients with major bleeding vs no major bleeding during extracorporeal membrane oxygenation (ECMO).
Fig S7. Fresh frozen plasma transfusion in patients with major bleeding vs no major bleeding during extracorporeal membrane oxygenation (ECMO).


