CONFLICT OF INTEREST
Nothing to report.
To the editor,
The vaccines against the severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) were granted a fast‐track authorization due to the catastrophic consequences of the coronavirus disease 2019 pandemic. Although these vaccines have proven their efficacy, safety has been a concern. Up till now, two cases of autoimmune hepatitis (AIH) after anti‐SARS‐CoV‐2 vaccination have been reported.[ 1 , 2 ]
We report a case of AIH‐like syndrome in a 40‐year‐old Caucasian woman after Pfizer‐BioNTech‐mRNA vaccination. The patient had a medical history of sarcoidosis for which she had never received treatment. On routine blood testing 1 month after completing her vaccination, abnormal liver function tests were found, with serum transaminases being 4× upper limit of normal (ULN). The patient was referred to our hepatology department for evaluation.
On arrival, the patient’s physical examination and upper abdominal ultrasound were normal. Testing for hepatitis B, C, and E; Epstein‐Barr virus; cytomegalovirus; and HIV infections was negative. Antinuclear‐antibodies testing was positive with a titer of 1/640; testing for antimitochondrial, antismooth muscle and liver‐kidney‐microsome type‐1 antibodies was also negative. Total IgG serum levels were markedly raised at 2.4 g/dl (normal values 0.7–1.2 g/dl).
Due to the possibility of vaccine‐induced transaminasemia, monthly follow‐up was decided. During the next 5 months, serum transaminases fluctuated around 3–4 × ULN, so a liver biopsy (LB) was performed.
LB revealed active hepatitis with significant interface necroinflammation and severe lobular inflammatory infiltration composed predominantly of lymphocytes with an admixture of plasma cells. Portal/periportal fibrosis was evident as well as fibrous septa with occasional bridging. Granulomas were not encountered (Figure 1A,B)
FIGURE 1.
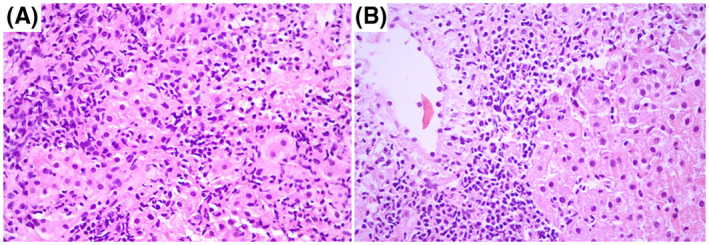
Active hepatitis. (A) Interface activity. (B) Centrilobular lymphoplasmacytic inflammation. (A, B) ×400
Based on elevated levels of serum transaminases and IgG and LB findings, the patient was started on 40 mg prednisolone. One‐week after treatment initiation, serum transaminases declined to normal levels.
Because anti‐SARs‐Cov‐2 vaccination might rarely cause transaminasemia,[ 3 , 4 ] drug‐induced liver injury was a possible diagnosis in our patient, so LB was performed 6 months after vaccination due to persisting transaminasemia. This 6‐month period coupled with the high serum IgG levels and the absence of recent use of hepatotoxic drugs and granulomas in LB make vaccine‐induced AIH a probable diagnosis. However, because serum transaminases improved within a week post‐treatment, AIH‐like syndrome due to molecular mimicry to the vaccine is more likely. What makes our patient unique is the fact that she was neither postpartum nor was under any medical treatment as previously reported patients.[ 1 , 2 ]
Overall, we believe that AIH‐like syndrome may be a rare complication of SARS‐CoV‐2 vaccination and should be considered in cases of postvaccination persistent transaminasemia. However, under no circumstances should this rare side effect restrain patients from anti‐SARS‐CoV‐2 vaccination because coronavirus disease poses a major threat to patients with liver diseases.[ 5 ]
REFERENCES
- 1. Bril F, Al Diffalha S, Dean M, Fettig DM. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID‐19) vaccine: causality or casualty? J Hepatol. 2021;75(1):222–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tan CK, Wong YJ, Wang LM, Ang TL, Kumar R. Autoimmune hepatitis following COVID‐19 vaccination: true causality or mere association? J Hepatol. 2021;75(5):1250–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mann R, Sekhon S, Sekhon S. Drug‐induced liver injury after COVID‐19 vaccine. Cureus. 2021;13(7):e16491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. COVID‐19 mRNA Pfizer‐BioNTech vaccine analysis print. Accessed September 3, 2021. https://www.gov.uk/government/publications/coronavirus‐covid‐19‐vaccine‐adverse‐reactions/coronavirus‐vaccine‐summary‐of‐yellow‐card‐reporting [Google Scholar]
- 5. Jothimani D, Venugopal R, Abedin MF, Kaliamoorthy I, Rela M. COVID‐19 and the liver. J Hepatol. 2020;73(5):1231–40. [DOI] [PMC free article] [PubMed] [Google Scholar]


