Abstract
Objectives
This study examines the histological findings of tracheal tissue samples obtained from COVID‐19 positive mechanically ventilated patients, to assess the degree of tracheal inflammation/ulceration present.
Design and participants
Retrospective single‐centre observational cohort study. All patients admitted to Adult Intensive Care Unit (AICU) with COVID‐19 infection, requiring mechanical ventilation and surgical tracheostomy between 1 April and 1 May 2020, were included (Group 1). Tracheal windows excised at tracheostomy underwent histological analysis. Comparison was made with: tracheal windows from COVID‐19 positive AICU ventilated patients admitted between 1 January and 1 March 2021 (Group 2); tracheal windows from COVID‐19 negative AICU ventilated patients (Group 3); and, tracheal autopsy samples from COVID‐19 positive patients that died without undergoing prolonged mechanical ventilation (Group 4).
Results
G roup 1 demonstrated mild/moderate inflammation (tracheitis) in nearly all samples (15/16, 93.8%), with infrequent micro‐ulceration (2/16, 12.5%). G roup 2 demonstrated similar mild/moderate inflammation in all samples (17/17, 100%), with no ulceration. Histological findings of Groups 1 and 2 COVID‐19 positive patients were similar to Group 3 COVID‐19 negative patients, which demonstrated mild/moderate inflammation (5/5, 100%), with uncommon superficial erosion (1/5, 20%). Group 4 demonstrated mild chronic inflammation or no significant inflammation, with uncommon micro‐ulceration (1/4, 25%).
Conclusions
Severe tracheal inflammation was not demonstrated in mechanically ventilated COVID‐19 positive patients at the level of the second/third tracheal rings, at the stage of disease patients underwent tracheostomy. Histological findings were similar between mechanically ventilated COVID‐19 positive and negative patients. Tracheal ulceration may be a feature of early or severe COVID‐19 disease.
Keywords: COVID‐19, histology, inflammation, mechanically ventilated, tracheal, ulceration
Key Points.
Severe inflammation/ulceration was not demonstrated in the tracheal window samples of COVID‐19 positive mechanically ventilated patients;
The degree of inflammation and ulceration in tracheal window samples were similar in COVID‐19 positive and negative mechanically ventilated patients;
The degree of inflammation and ulceration in tracheal window samples was similar in COVID‐19 positive patients from first and second COVID‐19 pandemic surges;
Tracheal micro‐ulceration may be a feature of early or severe disease having been identified in one tracheal autopsy sample and two samples from COVID‐19 positive mechanically ventilated patients;
This is the first study to examine histology in live COVID‐19 positive mechanically ventilated patients. The minor degree of tracheal inflammation/ulceration found is not consistent with more severe changes reported in previous bronchoscopic and radiological studies.
1. OBJECTIVES
The degree of tracheal inflammation/ulceration present in critically ill mechanically ventilated (MV) patients with coronavirus disease 2019 (COVID‐19) is unknown. Routine tracheal inspection using a bronchoscope is relatively contraindicated due to the perceived risk of viral aerosolisation. However, clinically observed severe laryngo‐tracheitis has been described in this patient population,1, 2 with one study reporting 47% full‐thickness tracheal erosions or tracheo‐oesophageal fistulae, based upon radiological and/or bronchoscopic evaluation. 3 Tracheal inflammation is a recognised complication of prolonged MV 4 ; however, there is a rising number of case reports indicating increased incidence of pneumothorax, pneumomediastinum and subcutaneous emphysema (‘barotrauma’) in COVID‐19 positive critically ill patients. 5 Notably, these potentially life‐threatening complications appear to be more common in patients in whom severe tracheal inflammation/ulceration is present. 3 The aim of this study was to investigate the frequency and degree of tracheal inflammation/ulceration, by histological analysis of tracheal tissue samples obtained at surgical tracheostomy (ST) and at autopsy in critically ill patients with COVID‐19. To our knowledge, there is currently no existing published data on tracheal histology in live COVID‐19 patients.
2. DESIGN, SETTINGS, PARTICIPANTS, MAIN OUTCOME MEASURES
This retrospective observational cross‐sectional study was conducted at a single site, Chelsea and Westminster Hospital, London, UK. All patients admitted to Adult Intensive Care Unit (AICU), diagnosed with severe COVID‐19 infection, requiring MV and ST between 1 April and 1 May 2020 were included (Group 1, COVID‐19 pandemic, UK first wave). A second group of AICU patients, with severe COVID‐19 infection, requiring MV and ST between 1 January and 1 March 2021 were included for comparison (Group 2, COVID‐19 pandemic, UK second wave). A third group of patients, comprising COVID‐19 negative AICU patients, requiring MV and ST between 1 April 2020 and 1 March 2021, were included as a control group (Group 3). All ST were performed as part of patients’ routine AICU treatment, and followed a standardised open surgical approach, between the second and third tracheal rings, with excision of a tracheal window (TW). Routine analysis of TWs is rarely undertaken, with available tissue usually discarded. TWs were placed immediately in 10% neutral buffered formalin, processed using standard techniques (formalin‐fixed, paraffin‐embedded blocks, with six haematoxylin and eosin‐stained sections cut for histological analysis) and reported by a Consultant Histopathologist, specialising in head and neck pathology.
Tracheal tissue samples from a fourth group of patients, that died between 1 March 2020 and 1 May 2020 (COVID‐19 pandemic, UK first wave) due to severe COVID‐19 disease without undergoing prolonged MV were also analysed (Group 4). Sections of the trachea, at the same level as a typical TW, were taken at autopsy, analysed and reported (as above).
COVID‐19 diagnoses (positive or negative) were based upon detection (or absence) of SARS‐CoV‐2 antigen on respiratory swab specimens.
The diagnosis of barotrauma (pneumothoraces, pneumomediastinum, and/or subcutaneous emphysema) was based upon clinical findings and confirmed on chest computed tomography (CT). All COVID‐19 positive AICU patients underwent CT thorax (+/‐ pulmonary angiogram) on AICU admission, and repeated if clinically indicated.
This study was registered with Chelsea and Westminster Hospital Governance Department. The data were extracted and anonymised in accordance with internal information governance review, NHS Trust information governance approval, and Caldicott Guardian procedures outlined under the Strategic Research Agreement. Research Ethics Committee approval was obtained for analysis of SARS‐COV‐2 virus and patho‐mechanisms in tissues derived from diagnostic samples in live patients and from post‐mortem examination (R20012). Tissue samples were provided by Imperial College Healthcare NHS Trust Tissue Bank, funded by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Imperial College NHS Trust and Imperial College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or Department of Health. The authors have no conflicts of interest to declare.
This study followed the STROBE reporting guideline. Data were retrospectively collected from hospital electronic patient records. Descriptive statistics were determined for all variables, using SPSS® version 22 IBM®, Chicago, IL, USA.
3. RESULTS
A similar degree of mild/moderate tracheal inflammation was observed in the COVID‐19 positive MV groups (Groups 1 and 2), and the COVID‐19 negative MV group (Group 3). The autopsy (Group 4) samples demonstrated only mild chronic inflammation or no inflammation.
3.1. Group 1
Sixteen patients underwent MV and ST [median (IQR) age 54 (9.5); male gender 12/16 (75%)]. All TWs were made available for histological analysis (Table 1). In fifteen of the sixteen samples (15/16, 93.8%), diffuse mild/moderate inflammation (tracheitis) was observed, with neutrophils within the respiratory epithelium and active chronic inflammatory infiltrate in the submucosa (Figure 1). In two of the samples, punctate micro‐ulceration was also observed (2/16, 12.5%). The remaining sample (1/16, 6.25%) showed no significant acute inflammation, with only mild chronic inflammatory infiltrate, with scattered eosinophils but virtually no interstitial neutrophils. None of the sixteen samples demonstrated severe tracheitis, significant ulceration, cartilage damage, perichondritis, or necrosis. Only one patient had previously undergone attempted (failed) tracheal extubation prior to ST. The sample from this patient demonstrated very mild acute tracheitis only. All other patients underwent ST to assist with respiratory weaning.
TABLE 1.
Summary of comparison of severity of inflammation and ulceration in tracheal histology samples of COVID‐19 positive and negative patients
| Group 1 COVID−19 positive tracheostomy (pandemic wave 1) (n = 16) | Group 2 COVID−19 positive tracheostomy (pandemic wave 2) (n = 17) | Group 3 COVID−19 negative tracheostomy (n = 5) | Group 4 COVID−19 positive tracheal autopsy (pandemic wave 1) (n = 4) | |
|---|---|---|---|---|
| Age, years, median (IQR) | 54 (9.5) | 64 (17.0) | 57 (8.0) | 70 (31.8) |
| Gender, n (%) | ||||
| Male | 12 (75.0) | 8 (47.1) | 3 (60.0) | 3 (75.0) |
| Female | 4 (25.0) | 9 (52.9) | 2 (40.0) | 1 (25.0) |
| Duration of invasive mechanical ventilation prior to tracheal sampling, days, median (IQR) | 17 (6.0) | 22 (9.0) | 26 (19.0) | 0 (0.0) |
| Barotrauma, n (%) (pneumothorax/pneumomediastinum/s/c emphysema) | 3 (18.8) | 4 (23.5) | 0 (0.0) | 0 (0.0) |
| Tracheal histology, n (%) | ||||
| No significant inflammation | 1(6.25) | 0 (0.0) | 0 (0.0) | 1 (25.0) |
| Mild or moderate inflammation | 15 (93.4) | 17 (100.0) | 5 (100.0) | 3 (75.0) |
| Severe inflammation | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Micro‐ulceration/superficial erosion | 2 (12.5) | 0 (0.0) | 1 (20.0) | 1 (25.0) |
| Severe full‐thickness ulceration | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Tracheo‐oesophageal fistulae | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
FIGURE 1.
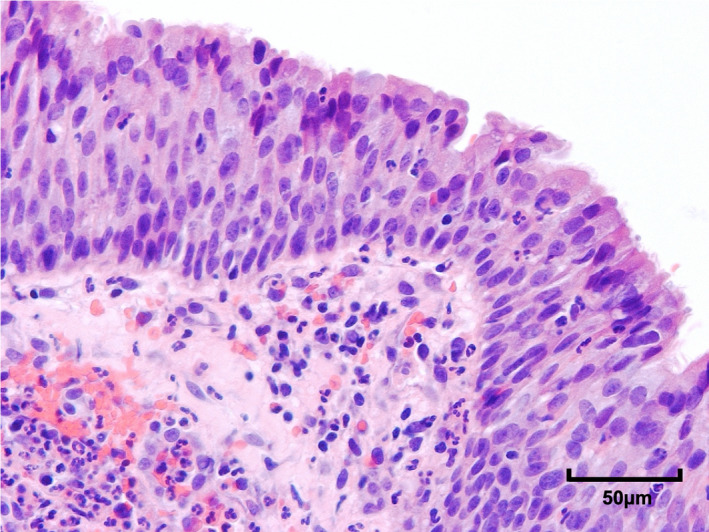
Photomicrograph of tracheal respiratory epithelium 400× from an excised tracheostomy window taken from a COVID‐19 positive mechanically ventilated patient. Neutrophils are present in the epithelium and in the underlying submucosa, indicative of acute tracheitis
Barotrauma was identified in three patients (3/16, 18.8%). No tracheo‐oesophageal fistulae were demonstrated. Mild/moderate tracheitis was found in all three corresponding samples; focal punctate micro‐ulceration was observed in one sample. This patient underwent the longest duration of MV prior to ST, of Group 1 patients (26 days).
3.2. Group 2
Eighteen patients underwent MV and ST; however, the TW from one patient was inadvertently discarded and therefore not available for analysis. This patient was omitted from the study, yielding seventeen samples for analysis [median (IQR) age 64 (17); male gender 8/17 (47.1%)]. All seventeen patients underwent ST to assist with respiratory weaning (tracheal extubation was not attempted in any patients). Mild/moderate tracheal inflammation was observed in all samples (17/17, 100%), with no ulceration or erosions. No samples demonstrated severe tracheitis, significant ulceration, cartilage damage, perichondritis or necrosis (Table 1).
Barotrauma was identified in four patients (4/17, 23.5%). No tracheo‐oesophageal fistulae were demonstrated. Mild chronic inflammation was seen in all four corresponding samples. The duration of MV prior to ST in these patients was 15, 16, 23 and 49 days respectively.
3.3. Group 3
Five COVID‐19 negative patients underwent MV and ST [median (IQR) age 57 (8); male gender 3/5 (60.0%)], with all TWs analysed (Table 1). All five patients underwent ST to assist with respiratory weaning (tracheal extubation was not attempted in any patients). Mild/moderate tracheitis was observed in all five samples (5/5,100%), with no severe inflammation or full‐thickness ulceration. Focal superficial erosion* was observed in one sample (1/5, 20%). There was no radiological evidence of barotrauma or tracheo‐oesophageal fistulae. These patients underwent the longest duration of MV (median 26 days) of all patient groups.
*Erosion is characterised by partial loss of epithelium, with the basement membrane left intact; Ulceration refers to segmental or more extensive loss of epithelium, including the basement membrane.
3.4. Group 4
Autopsy tracheal samples from four selected patients [median (IQR) age 70 (31.8); male gender 3/4 (75.0%)] were obtained and analysed for comparison (Table 1). Only one patient underwent tracheal intubation and MV (for less than 24 hours) before death. One sample revealed punctate micro‐ulceration (1/4, 25%) with background mild acute inflammation only (Figure 2), similar to the minor ulceration observed in two COVID‐19 positive TWs (Group 1). The other three autopsy samples demonstrated mild chronic inflammation or no significant inflammatory change. No evidence of barotrauma or tracheo‐oesophageal fistulae was found on autopsy in these four patients.
FIGURE 2.
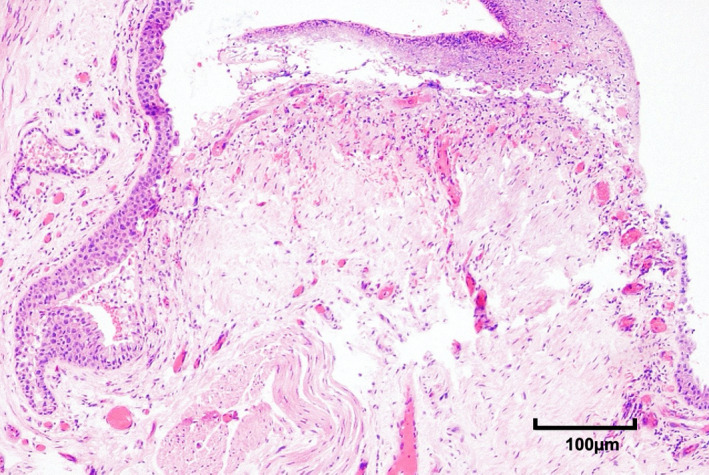
Photomicrograph of tracheal ulceration 200× in tracheal tissue samples obtained at autopsy from a COVID‐19 positive deceased patient that had not undergone tracheal intubation and invasive mechanical ventilation
4. DISCUSSION
A similar degree of mild/moderate tracheal inflammation was observed in the COVID‐19 positive MV groups (Groups 1 and 2), and the COVID‐19 negative MV group (Group 3). The autopsy (Group 4) samples demonstrated only mild chronic inflammation or no inflammation. Therefore, the relatively minor degree of tissue inflammation demonstrated in Groups 1, 2 and 3 is most likely due to mechanical irritation and tracheal cuff pressure effects, and does not appear to be COVID‐19 disease‐specific. These histological appearances are largely consistent with those described in historical studies of mechanically ventilated COVID‐19 negative patients.6, 7
Minor ulceration was an infrequent finding. Only two TWs (from Group 1) demonstrated punctate microscopic ulceration. Neither of these two patients had undergone difficult tracheal intubation or failed extubation prior to ST. This finding may be attributed to prolonged MV prior to ST, as this was slightly longer than the median value of 17 days in both patients (18 and 26 days respectively). However, similar punctate micro‐ulceration was also seen in one autopsy sample – obtained from a patient that had not undergone tracheal intubation or MV. Furthermore, no ulceration was demonstrated in any Group 3 samples, which included a patient that had undergone MV for 49 days. It is therefore possible that ulceration is a feature of the COVID‐19 disease process alone, and not necessarily potentiated by MV.
Previous studies2, 3 have demonstrated bronchoscopic and/or radiological evidence of severe laryngo‐tracheitis, full‐thickness ulceration and tracheo‐oesophageal fistulae in COVID‐19 positive patients undergoing MV; however, the severity of histological tracheal inflammation/ulceration found in our study was significantly less than that reported in these studies.
Barotrauma was only demonstrated in COVID‐19 positive mechanically ventilated patients (Groups 1 and 2). These findings are consistent with international reports of increased barotrauma in COVID‐19 patients. However, the frequency of barotrauma was relatively low (18.8% and 23.5% respectively) compared with the 47% reported by Fiacchini, 3 and there was no evidence of tracheo‐oesophageal fistulae. Barotrauma did not appear to correlate with severity of inflammation/ulceration on histological samples, with mild/moderate inflammation demonstrated in all samples from patients with barotrauma, with micro‐ulceration observed in only one of these.
It has been postulated that the degree of airway inflammation/oedema in patients with severe COVID‐19 may exceed levels normally associated with intubation, prolonged MV, and extubation of the critically ill patient. High rates of failed first tracheal intubation attempt, intubation complications, and failed extubation have been reported. 8 Virally mediated inflammation, the use of subglottic drainage tracheal tubes (larger external diameters), repeated prone positioning manoeuvres, excessive tracheal cuff pressures (to prevent aerosolisation), mucosal damage from repeated hypoxemic and thrombotic events, and high‐dose steroid treatment have been suggested as potential contributory factors.1, 3
Larger multi‐centre investigation is needed to fully explore the implications of MV, localised tracheal tube/cuff effects and AICU treatments (e.g. high‐dose steroids, antithrombotic medications, prone positioning) on tracheal inflammation in COVID‐19 patients. In the meantime, it is crucial that AICU multidisciplinary teams continue to employ protective strategies to minimise potential iatrogenic contributions, including judicious tracheal tube selection (diameter), frequent cuff manometry checks, meticulous head and neck positioning during prone positioning manoeuvres, and avoidance of unnecessary breathing circuits disconnections (reduced hypoxic events).
This study has several limitations. The study was undertaken at a single centre, was retrospective and of limited sample size. Samples were analysed by a single Consultant Histopathologist, which ensured reporting consistency (and accuracy due to their subspecialty experience); however, consensus of a team of histopathologists would improve reliability.
The study periods for first and second pandemic waves were selected to match respective peaks in AICU admissions and, therefore, ST. In order to yield a similar number of patients in each group (for better comparison), the study period for the second wave was set at 2 months rather than one. This reflects a change in tracheostomy service delivery, rather than a true reduction in the peak of COVID‐19 patients requiring ST in the second wave (i.e. a similar number of patients were spread over a longer period due to a change in our service model – reflected in the increased number of days between intubation and ST in second wave patients). Tracheostomy UK guidance, 9 prompted this change from a 7‐days‐a‐week service to twice‐weekly scheduled operating lists, staffed by a select number of familiar staff members on a regular basis, to improve teamworking and increase patient safety.
Groups 3 and 4 patient numbers were limited. Despite all COVID‐19 negative patients undergoing ST being included, the low number reflects the predominance of COVID‐19‐related clinical activity at our institution during this period. Group 4 patient numbers were restricted by the limited number of autopsies being undertaken at the time.
None of the COVID‐19 positive MV AICU patients underwent early ST (all patients >10 days), and it is therefore possible that significant inflammation/ulceration is a feature of early severe COVID‐19 disease, that may not be detected within tracheal samples taken later in the disease process (and following supportive AICU treatment). This hypothesis would seem supported by the observation that patients with barotrauma appeared to incur these complications at an early stage of disease, coinciding with maximal inflammation/tissue friability (maximal viral replication within tissues), and by the time they underwent ST, there was both clinical and radiological improvement (usually following chest drain insertion for pneumothoraces, and conservative management of pneumomediastinum). Bradley et al. 10 demonstrated viral particles in tracheal epithelium in autopsy patients, and a similar technique of RNA analysis (nanostring) and electron microscopy (EM) could be used in live patient TW samples to demonstrate whether viral particles were still present at the latter stage of disease when these patients underwent ST (EM not available at our institution).
Not all patients underwent bronchoscopic airway luminal assessment, which may have identified some patients where more significant tracheal inflammation/ulceration was present, but distal to the second/third tracheal ring. However, it also seems logical that tracheal inflammation/ulceration is likely to be maximal where the tube/cuff reside, and that these changes would therefore be identified within TW samples. Indeed, Oliver et al. 2 describe a well‐demarcated zone of severe ulceration involving the proximal tracheal rings (with distal sparing), which would have been captured by TW sampling. It also seems likely that any significant inflammation/ulceration will be exacerbated by prolonged MV, localised tube/cuff effects and repeated prone positioning manoeuvres, and is unlikely to have resolved completely by the time patients underwent ST, despite this being >2 weeks since the onset of disease.
Whilst our COVID‐19 cohort appears relatively generalisable (age and gender), different COVID‐19 viral strains may cause varying disease severity/pathology, and account for the reduced tracheal inflammation/ulceration we identified compared with the more severe bronchoscopic/radiological findings reported by other international institutions. Of course, the differing modes of assessment/detection also invite variability in reporting, therefore further multi‐centre investigation is warranted, where all three methods of analysis (radiological, bronchoscopic and histological) are synchronously undertaken.
5. CONCLUSIONS
The degree of tracheitis seen on histological analysis of TW samples from MV COVID‐19 positive patients does not appear to be more severe than that found in COVID‐19 negative MV patients at our institution. We believe that the minor acute inflammation common to nearly all samples is likely caused by tracheal intubation, local tube and cuff pressure effects, and MV. COVID‐19 infection (and associated AICU treatments) does not appear to predispose patients to increased inflammation within TW samples. However, micro‐ulceration may be a feature of COVID‐19 infection, having been identified in two samples from COVID‐19 tracheostomy patients and from one COVID‐19 autopsy sample. These preliminary conclusions are based upon a small patient sample, and analysis of a larger population is warranted, which together with adjuvant techniques such as immunohistochemistry, would help inform our understanding of the pathophysiology of this disease.
AUTHOR CONTRIBUTIONS
P.A. Ward ‐ concept, design, principal anaesthetist for surgical tracheostomy procedures, data collection, data analysis, manuscript preparation, submission. J. Collier ‐ concept, co‐surgical lead for surgical tracheostomy procedures, data analysis, manuscript preparation. J. Weir ‐ concept, lead pathologist for study, analysis of tracheostomy window samples, data input, manuscript preparation. M. Osborn ‐ supporting pathologist for study, analysis of tracheostomy samples, data input, manuscript preparation. B. Hanley ‐ supporting pathologist for study, analysis of tracheostomy samples, data input, manuscript preparation. W.J.B. Smellie – concept, co‐surgical lead for surgical tracheostomy procedures, data analysis, manuscript preparation.
ETHICAL STATEMENT
This study was registered with Chelsea and Westminster Hospital Governance Department. The data were extracted and anonymised in accordance with internal information governance review, NHS Trust information governance approval, and Caldicott Guardian procedures outlined under the Strategic Research Agreement. Research Ethics Committee approval was obtained for analysis of SARS‐COV‐2 virus and patho‐mechanisms in tissues derived from diagnostic samples in live patients and from post‐mortem examination (R20012). Tissue samples were provided by Imperial College Healthcare NHS Trust Tissue Bank, funded by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Imperial College NHS Trust and Imperial College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or Department of Health. The authors have no conflicts of interest to declare.
Supporting information
File S1
File S2
ACKNOWLEDGEMENTS
Chelwest COVID‐19 Tracheostomy Group – see Supplementary File S2 for list of contributors.
Ward PA, Collier JM, Weir J, et al; Chelwest COVID‐19 Tracheostomy Group . Histological findings of tracheal samples from COVID‐19 positive critically ill mechanically ventilated patients. Clin Otolaryngol. 2022;47:131–137. 10.1111/coa.13872
Funding information
No funding was received for this study.
Contributor Information
Patrick Alexander Ward, Email: patrickward81@hotmail.com.
Chelwest COVID‐19 Tracheostomy Group:
Peter Brooks, Mark Cox, Juliet Dunn, Jacqueline Durbridge, Gabriela Frunza, Seth Galton, Kevin Haire, Seleena Haque, Ami Kotecha, Manisha Kulkarni, Corina Lee, Alex Li, Ganga Liyanage, Alexia Paolineli, Kate Richardson, Atika Sabharwal, John Thornton, Annette Volger, Patrick Ward, Linsey Christie, Roger Davies, Michelle Hayes, Rick Keays, Christopher Lockie, Suveer Singh, Alice Sisson, Marcela P Vizcaychipi, Brian Hanley, Michael Osborn, Justin Weir, Christopher Abela, Jonathan Collier, Declan Collins, Lisa Greaney, Isabel Jones, James Smellie, Jorge Leon Villapalos, and Andrew Williams
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the [Link], [Link] of this article.
REFERENCES
- 1. McGrath BA, Wallace S, Goswamy J. Laryngeal oedema associated with COVID‐19 complicating airway management. Anaesthesia. 2020;75(7):972. 10.1111/anae.15092. Epub 2020 Apr 26. PMID: 32302417. [DOI] [PubMed] [Google Scholar]
- 2. Oliver CM, Campbell M, Dulan O, Hamilton N, Birchall M. Appearance and management of COVID‐19 laryngo‐tracheitis: two case reports [version 2; peer review: 2 approved]. F1000Research. 2020;9:310. 10.12688/f1000research.23204.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fiacchini G, Tricò D, Ribechini A, et al. Evaluation of the incidence and potential mechanisms of tracheal complications in patients with COVID‐19. JAMA Otolaryngol Head Neck Surg. 2020;147(1):70‐76. 10.1001/jamaoto.2020.4148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brodsky MB, Levy MJ, Jedlanek E, et al. Laryngeal injury and upper airway symptoms after oral endotracheal intubation with mechanical ventilation during critical care: a systematic review. Crit Care Med. 2018;46(12):2010‐2017. 10.1097/CCM.0000000000003368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wali A, Rizzo V, Bille A, Routledge T, Chambers AJ. Pneumomediastinum following intubation in COVID‐19 patients: a case series. Anaesthesia. 2020;75(8):1076‐1081. 10.1111/anae.15113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Badr El Din MH, Ahmed MR, Hinnis AR, Abd El Baky MS. Serial histopathological tracheal changes from prolonged intubations. Egypt J Otolaryngol. 2014;30(2):142‐146. [Google Scholar]
- 7. Cooper JD, Grillo HC. The evolution of tracheal injury due to ventilatory assistance through cuffed tubes: a pathologic study. Ann Surg. 1969;169(3):334‐348. 10.1097/00000658-196903000-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yao W, Wang T, Jiang B, et al. Emergency tracheal intubation in 202 patients with COVID‐19 in Wuhan, China: lessons learnt and expert recommendations. BrJ Anaesth. 2020;125(1), e28‐e37. Epub 10 April. 10.1016/j.bja.2020.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McGrath BA, Ashby N, Birchall M, et al. Multidisciplinary guidance for safe tracheostomy care during the COVID‐19 pandemic: the NHS National Patient Safety Improvement Programme (NatPatSIP). Anaesthesia. 2020;75:1659‐1670. 10.1111/anae.15120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bradley BT, Maioli H, Johnston R, et al. Histopathology and ultrastructural findings of fatal COVID‐19 infections in Washington State: a case series. Lancet. 2020;396(10247):320‐332. 10.1101/2020.04.17.20058545 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1
File S2
Data Availability Statement
The data that supports the findings of this study are available in the [Link], [Link] of this article.


