Abstract
Objective
A wide variety of mouth rinses are available to combat micro‐organisms in the oral cavity. At the present global pandemic, the need of the hour is to control the viral infection due to the novel corona virus SARS‐COV‐2, as its port of entry is through the receptors located in the oral and pharyngeal mucosa. This systematic literature review focuses on the in vivo studies [randomized control trials (RCTs)] done on the efficacy of existing mouth rinses which have been used in reducing the viral loads.
Methods
The electronic database which includes PubMed‐MEDLINE, Google scholar, Scopus, Web of Science, EMBASE, ProQuest and CINAHL was searched from December 2019 to June 2021 with appropriate Medical Subject Headings (MeSH) terms and Boolean operators. Two reviewers independently reviewed the abstracts.
Results
Of the 2438 retrieved titles, 905 remained after removing duplicates. Twelve articles were eligible to be included in this review of which seven were randomized with adequate sample size.
Conclusions
Mouth washes containing povidone iodine and chlorhexidine decrease the viral load transiently. Large amount of in vivo studies are of paramount importance, especially RCTs, to prove the efficacy of these mouth rinses.
Keywords: cetylpyridinium chloride, chlorhexidine chloride, citrox, covid‐19, essential oil, hydrogen peroxide, oral rinse, pandemic, povidone iodine, SARS‐Cov‐2, virus
1. INTRODUCTION
The advent of severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) pandemic has resulted in more than 3,233,845 deaths around the World as on May 6, 2021, and has created an unprecedented healthcare, social and economic disaster. 1 , 2 Recently, the National Institute of Virology (NIV) of India detected a strain of coronavirus with a double mutation named B.1.617 in samples collected from major Indian states having mutations from two separate virus variants, namely E484Q and L452R. 3
Virus spread can be halted/minimized by protective measures like use of N95 masks, hand sanitizers/washing and by social distancing. But, sometimes despite all the precautions, some people are at a higher risk of contracting this infection including dental professionals due to their close proximity to patients while working and generation of aerosols in most of the dental procedures, and asymptomatic patients pose more risk. Various studies have emphasized the importance of oral health and how the oral cavity is an entry point for numerous viral diseases, including the corona virus. 4 , 5 , 6 Although COVID‐19 is considered a disease of respiratory system primarily affecting the lungs, studies have shown that SARS‐CoV‐2 virus can also invade the oral mucosa and salivary gland epithelium due to high expression of angiotensin converting enzyme 2 (ACE2) receptors at these sites, thereby leading to an increase in viral load in the oral cavity. 5 , 7 , 8 Keeping in view this concept, it seems logical to curb this virus at the entry point itself. This review mainly focuses on chief antimicrobial ingredients of various nasal and oral rinses that could reduce the viral load in the oral cavity, oro‐ and nasopharynx. We aim to summarize the information related to antiviral agents acting against SARS‐CoV‐2 till date and potential ingredients present in oral rinses which could be effective in patients.
SARS‐CoV‐2 virus belongs to the family of enveloped RNA viruses, coronaviridae. The presence of ‘spike protein’ (S protein) on its membrane envelope plays a significant role in the pathogenesis of this disease (Figure 1). This S protein interacts mainly with the ACE2 receptors whose distribution in different parts of oral cavity indicates several virus entry points like non‐keratinized mucosa of the mouth, epithelial cells of tongue and salivary glands. 5 Priming of the virus S protein is carried out with the help of the cellular transmembrane serine protease 2 (TMPRSS2). 5 Interestingly, individuals with healthy or poor oral hygiene status have reported viral load of SARS‐CoV‐2 in their saliva. 9 , 10 This indicates that the oral tissues are a probable reservoir from which the SARS‐CoV‐2 virus can be transmissible during breathing, coughing, sneezing and talking. Although patients infected with this virus do not require hospitalization very often, the ones with comorbidities are prone to complications such as pneumonia, respiratory failure and multiple organ collapse. 11 Little is known about how transmission occurs from the oral cavity to the rest of the body causing systemic inflammatory response syndrome (SIRS) and multiple organ dysfunction syndrome (MODS) in severe and fatal cases. 5 One of the theories can be that it invades through a periodontal pocket, then into the tissues and, finally into the blood stream as it is said that periodontitis seems to worsen the symptoms of COVID‐19. 9 Another theory could be the settling of the virus on the tonsillar crypts and the oropharynx further going into the gastrointestinal tract and cause symptoms like diarrhoea.
FIGURE 1.
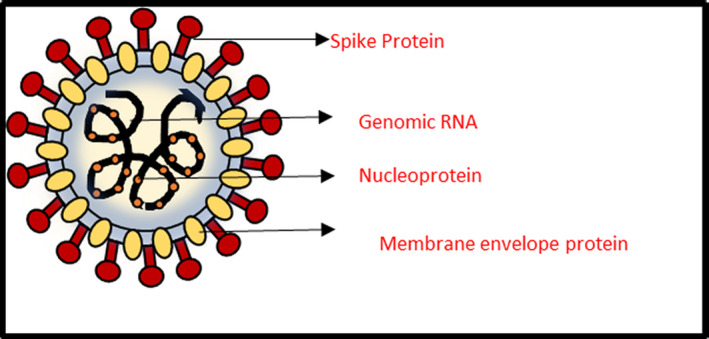
Structure of SARS‐CoV‐2 virus
Cerebral involvement, considered a deadly form of this disease, is believed to be caused by the cellular receptor neuropilin‐1 which binds with furin cleaved substrates forming a path of progression through central nervous system. 12 , 13 Hence, a good understanding of its pathogenesis, site of entry, identifying and treating the disease at its earliest course can be useful for saving the humankind from this pandemic (Figures 2 and 3).
FIGURE 2.
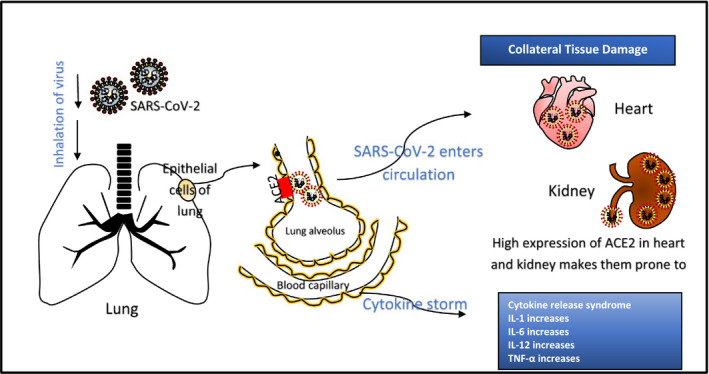
Pathogenesis of COVID‐19 disease
FIGURE 3.
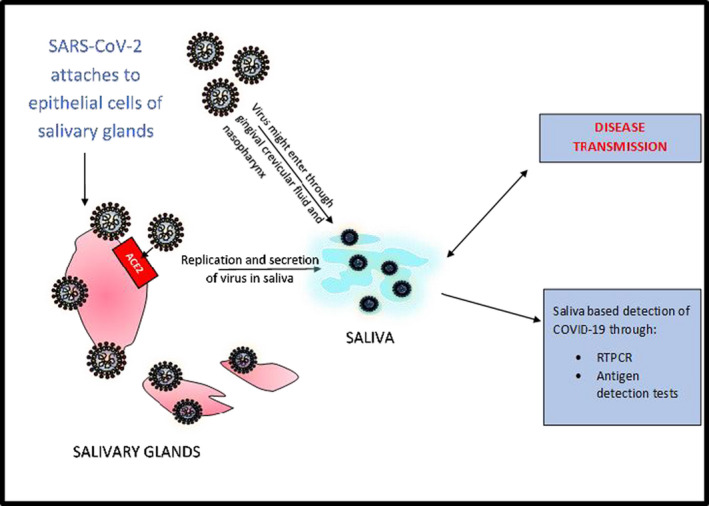
Pathogenesis of SARS‐CoV‐2 in oral cavity
One of the striking symptoms which most of the people suffer when infected with COVID‐19 is loss of smell seen even in severe allergic rhinitis or after an attack of cold. 12 , 14 This could occur as the virus invades and binds to ACE2 receptors which support the nerve cells that detect smell and thereby their inflammation can cause anosmia. Although it is not known as to why there is a loss in taste, it is said that this is the consequence of loss of smell. 14 Another reason that could be the cause of ageusia is the alterations in protein and substance composition of the saliva. There are many substances in saliva that have affinity to bind with different foods of different taste and it is said that when COVID‐19 invades the cells of salivary glands, it can cause alteration either in the composition or in the amount of saliva produced. 15 , 16
Oral rinses or mouthwashes are frequently used for rinsing the teeth, gums, mouth and halitosis, before and after oral surgery/dental procedures because they contain antiseptic agents which help in killing the harmful oral microbes and reduce the microbial load in the aerosols generated during dental procedures. 17 , 18 , 19 Although there is a lack of evidence if mouthwashes act as effective antiviral agents that will reduce the SARS‐CoV‐2 transmission, the American Dental Association (ADA) has recommended the usage of preprocedural mouth wash povidone iodine (PVP‐I; 0.2%) before any oral procedures for the safety of healthcare professionals and patients. 17 Due to the surge in COVID‐19 cases and its high transmissible rates, researchers have carried in vitro studies to test the virucidal activity of common over‐the‐counter oral and nasal rinses as well as tailored formulae and have yielded promising results and this has paved the way for in vivo studies. There is a growing need for more clinical trials to test the safety and effectiveness of oral and nasal rinses and a study of long‐term adverse effects too, if any.
Although mouthwashes contain many ingredients such as antimicrobials, antivirals, flavouring agents, excipients, anti‐halitosis agents, etc., we aim to focus on antiviral properties, since most of these mouthwashes are known to have antimicrobial properties. The World Health Organization (WHO) has not specified on the use of mouth rinses as a preventive or viral eradication measure in controlling COVID‐19 but they have been used in controlling similar type of viral diseases.
2. MATERIALS AND METHODS
The present review followed the preferred reporting items for systematic reviews and meta‐analysis (PRISMA) statement guidelines. 20
2.1. Search strategy
The objective of the review was to find the efficacy of oral rinses on virucidal property against n‐coronavirus (in vivo studies). Search engines used for this study included Medline (via PubMed), Scopus, Web of Science, EMBASE, CINAHL, Google scholar, Clinical Trial Registry and ProQuest and were searched electronically to retrieve all data using MeSH terms and Boolean operators: ‘mouthwash’ OR ‘oral rinse’ OR ‘mouth rinse’ OR ‘povidone iodine’ OR ‘chlorhexidine chloride’ OR ‘hydrogen peroxide’ OR ‘cetylpyridinium chloride’ OR ‘essential oil’ OR ‘phthalocyanine derivatives’ OR ‘ethanol’ OR ‘citrox’ AND ‘COVID‐19’ OR ‘SARS‐CoV‐2’ OR ‘SARS’. Search strings were developed as appropriate to the different databases.
The search was complemented by hand searching of the reference list of included relevant articles. Articles published from December 2019 to June 2021 were included. Two reviewers (GG & LT) independently searched the databases and screened abstract and title. Third reviewer (SC) resolved the discrepancies between two reviewers. Two reviewers (NU & SC) performed full‐text screening and data extraction. Any disagreement between the researchers was resolved by consensus and researcher (considered unbiased) who moderated the research activity.
2.2. Inclusion criteria
Clinical or in vivo studies that used mouthwashes, a form of intervention as a hypothesis for decreasing the viral load in saliva, were included.
2.3. Exclusion criteria
Studies that evaluated mouthwashes for viruses other than SARS‐CoV‐2 infected individuals/saliva
Studies where the evaluation was carried with ingredients (antiseptic ingredients present in mouthwashes) other than saliva
Descriptive studies such as reviews, conference abstracts, expert opinions, chapter in books and case reports.
2.4. Risk of bias
The risk of bias (RoB) of individual studies was independently assessed by two authors (NU & SC) using the check list presented in Appendix S1. Quality criteria were designated with a positive sign (+) if an informative description was present, a negative sign (‐) and if the informative description did not meet the criteria and a question mark (?) if the information was missing or insufficient. A study was classified to be ‘low risk of bias’ with all positive scores, assigned to criteria of random allocation, defined inclusion/exclusion criteria, blinding to product and examiner, identical treatment between groups and reporting of follow‐up. When two or more of these criteria were missing, the study was considered to have a high potential risk of bias. 21 The data were extracted using a data extraction sheet which included name of the first author, title, year of publication, country, study design, age and gender, sample size, type of intervention and control, time of testing the viral load, method of testing, bio‐efficacy rate, statistical test used and limitation.
3. RESULTS
The total number of articles from various databases were as follows: PubMed: 278, Scopus: 178, Web of Science: 363, EMBASE: 608, Google scholar: 705, ProQuest: 193, CINAHL: 60, Clinical Trial Registry: 34 and hand searching (of included studies): 19. From 2438 articles, 905 remained after removing the duplicates. After a thorough screening process, 12 articles were eligible to be included in this review (Figure 4). Five studies identified for inclusion in the review were non‐randomized in vivo studies with small sample size (Table 1, Clinical heterogeneity). Furthermore, it was observed in all RCTs (Table 2, online Appendix S1 and S2), although PVP‐I was found to be efficient, the percentage and the time interval taken to test the efficacy against this virus was not consistent (methodological heterogeneity). 22 , 23 , 24 Chlorhexidine (CHX) as a combination of mouth rinse and oropharyngeal spray was found to be more effective than mouth rinse alone; cetylpyridinium chloride (CPC) did show promising results (Table 2). The summary on the potential RoB estimated on various studies, as presented in the online Appendix S1, estimates high RoB in all study. 23 , 24 , 25 , 26 , 27 , 28 A study related to oral rinse PVP‐I reported thyroid dysfunction in 42% of their patients, the symptoms resolved following treatment discontinuation. 26
FIGURE 4.
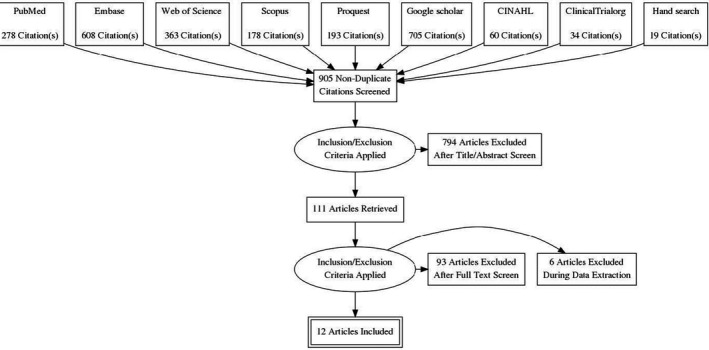
PRISMA flow diagram showing the process of surveying, screening and selecting the articles for systematic review (December 2019 to June 2021)
TABLE 1.
Evidence‐based non‐randomized in vivo studies on efficacy of oral rinses against SARS‐CoV‐2
| Intervention | Biological efficacy | Limitations | References |
|---|---|---|---|
| Hydrogen peroxide (1%) | No significant reduction in viral load | Small sample size, different concentrations and contact times were not measured, no control group | Gottsauner et al. 2020 22 |
| Povidone iodine (1%) | Viral load decreases transiently for 3 h | Small sample size, no control group | Martínez et al. 2020 33 |
| Chlorhexidine (0.12%) | Viral load decreases transiently for 2 h after mouthwash, but increased again at 2–4 h post‐mouthwash | Small sample size, absence of negative control, pts were on intraoral viral therapy, no control group | Yoon et al. 2020 6 |
| Phthalocyanine derivate (5 ml) | Reduction in clinical symptoms | Small sample size, no control group | da Fonseca et al. 2021 53 |
| Chlorhexidine (0.2%) and Chlorine dioxide (0.1%) | Reduction in clinical symptoms | No control group | Avhad et al. 2020 44 |
TABLE 2.
Evidence‐based randomized control trials on efficacy of oral rinses against SARS‐CoV‐2
| Sample size and Time of testing | Intervention | Biological efficacy | References |
|---|---|---|---|
|
No. of pts: 61 Saliva samples for RTPCR taken before and after 5 min of applying the intervention |
PVP‐I (1%) and CHX (0.2%) mouth rinsed for 30 s | Significant difference was noted between the Ct value of distilled water and each of the two solutions. Both are effective in preventing SARS‐CoV‐2 infection | Elzein et al. 36 |
|
No. of pts: 24 Day 1: RTPCR pre‐rinse (baseline) followed by 3 h after application of PVP‐I (nasal & oral) Day 7: Repeat RTPCR after 3 h application of PVP‐I |
25 ml of 1% aqueous PVP‐I solution each, followed by 2.5 ml nasal pulverization of the same solution into each nostril using an intranasal mucosal atomization device and a dab of 10% PVP‐I ointment over nasal mucosa |
Mean relative difference in viral titres between baseline and day 1 was 75% in the intervention group and 32% in the control group. No change in reduction of viral load over 7 days |
Guenezan et al. 38 |
|
No. of pts: 294 RTPCR test was done 4 days post‐rinse |
CHX (0.12%) used as oral rinse for 30 s twice daily, oropharyngeal spray (1.5 ml) three times per day | Combination was found to be more effective when compared to CHX oral rinse alone | Huang et al. 45 |
|
No. of pts: 40 Subjects advised to vigorously rinse with a total of 15 ml (7.5 ml each) at intervals of 30 s each for 60 s. Saliva samples were collected at 15 and 45 min post‐rinsing for RTPCR |
15 ml of normal saline, 1% hydrogen peroxide, 0.12% CHX or 0.5% PVP‐I |
All four mouth rinses decreased viral load by 61–89% at 15 min, and by 70–97% at 45 min |
Chaudhary PP et al. 47 |
|
No. of pts: 60 Unstimulated saliva collected at baseline (T0), immediately after rinsing (T1), 30 min after rinsing (T2) and 60 min after rinsing (T3) and subjected to RTPCR analysis |
Placebo (oral rinsing with distilled water), CPC (0.075%)+Zn (0.28%) group: rinse with 20 ml for 30 s; hydrogen peroxide (1.5%) group: rinse with 10 ml for 1 min; CHX group: rinse with 15 ml for 30 s; hydrogen peroxide+CHX group: rinse with 10 ml of hydrogen peroxide for 1 min, followed by rinsing with 15 ml of CHX for 30 s | CPC+Zinc mouthwash and CHX mouthwash provided a significant reduction in the SARS‐CoV‐2 viral load in saliva up to 60 min after rinsing, while HP provided a significant reduction of up to 30 min after rinsing | Eduardo et al. 46 |
|
No. of pts: 36 Saliva samples collected at baseline (pre‐rinse) & post‐rinse (5 min, 3 & 6 h) & subjected to RTPCR analysis |
PVP‐I 0.5% (10 ml betadine gargle and mouthwash) CHX 0.2% (pearly white Chlor‐Rinse) CPC 0.075% (Colgate Plax mouthwash) & sterile water |
No significant difference in salivary Ct values within each group at the described intervals. Compared with the water group a significant decrease in the viral load, while a significant decrease in the CPC group at 5 min and 6 h and in the PVP‐I group only at 6 h | Seneviratne et al. 27 |
|
No. of pts: 176 Participants were instructed to use mouthwashes three times per day, followed by saliva testing for RTPCR at intervals of T1 (at 09.00 h: before the first mouthwash) and then at T2 (13.00 h) and T3 (18.00 h). Only one sample was taken at 15.00 h on day 6 |
Placebo or β‐cyclodextrin (0.1%) and citrox (0.01%) rinse, 30 ml of mouthwash | Combination of CDCM had a significant beneficial effect on reducing SARS‐CoV‐2 salivary viral load 4 h after the initial dose. For long‐term effect, the benefit to recommend CDMC appears limited | Carrouel F et al. 62 |
Abbreviations: CDCM: β‐cyclodextrin & citrox; CHX: chlorhexidine; CPC: cetylpyridinium chloride; Ct: cycle threshold; PVP‐I: povidone iodine.
4. DISCUSSION
Hydrogen peroxide (H2O2): is a widely used antimicrobial agent and studies have been conducted to demonstrate its effect on several viruses including SARS‐CoV‐2 and influenza. The oral microbiota produce H2O2 physiologically and maintain a balance of oral microenvironment and it act on the epithelial cells that contain an enzyme, superoxide dismutase which catalyses the reaction converting H2O2 into ion superoxide. This oxidative stress activates NF‐ĸβ, leading to a local innate response that plays a major role in regulating host immune system and acting against viral infections. 8 So, it was proposed that washing nose, mouth and throat with H2O2 may improve local innate responses to SARS‐CoV‐2 virus and increase protection against COVID‐19 disease.
In vitro studies have been done to test the action of H2O2 against SARS‐CoV‐2 virus or just its S protein. 22 , 23 , 24 , 25 , 26 , 29 A corona surrogate, transmissible gastroenteritis virus (TGEV), was dried on stainless steel to H2O2 vapour (20 µl) for 2–3 h and was seen that about 5log10 reduction in viral load. This study proved H2O2 to have good surface decontamination ability, but concerns were on relative susceptibility of viruses in vivo and safety dosage when used as oral/nasal rinse. 30 On the contrary, an in vivo study on 12 COVID‐19 patients instructed to gargle mouth and throat with 20 ml of 1% H2O2 for 30 s with a repeat RTPCR test after 30 min concluded no significant reduction in oral viral load and its use was questionable. 22 , 23 More in vivo studies would give a clear scientific background of the use of this rinse.
Povidone iodine (PVP‐I): is composed of water‐soluble polymer and polyvinyl pyrrolidone that acts as an antimicrobial agent which dissociates to release iodine, disrupting the membrane protein of microbes and is used as an antiseptic for skin disinfection before and after surgery. 8 Usually used topically, with the outbreak of COVID‐19, its usage both nasally and orally was suggested by many frontline workers and researchers. 31 , 32
Pelletier et al. 31 reported the first anti‐SARS‐CoV‐2 estimation of a nasal antiseptic and an oral rinse antiseptic containing PVP‐I, which have been developed specifically for routine intranasal or oral use. Many authors have recommended the use of 0.5%–1% of PVP‐I as mouth rinse for 30–60 s. 33 , 34 , 35 , 36 , 37 , 38 It has a good virucidal activity as confirmed by real‐time reverse PCR (rRTPCR). 37 The viral load of SARS‐CoV‐2 is as high in asymptomatic patients as those with symptoms and inactivation of this virus has been documented to a usage of 0.5% PVP‐I for 15 s. 26 , 39 PVP‐I is said to be safe with a concentration of up to 2.5% intraorally up to 5 months as it also maintains the oral ecosystem. 40 , 41 ADA has advised mouth rinse of PVP‐I 0.2%, while the amount of PVP‐I ranged from 0.3 ml (nasal) to 9 ml (mouth rinse and gargling). However, its use is contraindicated in patients with allergy to iodine, thyroid disease and pregnancy. 42 Recently, it has been proposed that a minimum of 0.23% concentration of PVP‐I for at least 15 s before any procedure is effective in decreasing viral load, and hence has been indicated in COVID‐19‐positive patients. 43
Chlorhexidine (CHX): is a broad‐spectrum antiseptic, acts primarily against lipid‐enveloped viruses and so there is a high possibility that it may act against enveloped coronaviruses. 8 A review by Bernstein et al. 42 stated that CHX usage reduces viral transmission through aerosol generation but its action still remains debatable. 6 CHX (0.12%) of 15 ml decreased the viral load 2 h post‐rinse; however, viral load increased again: the challenge faced in the study was a small sample size and changes were also observed with reduction in clinical signs and symptoms. 6 , 44 A RCT by Huang also proves the efficacy of CHX (0.12%) for 30 s twice daily. 45 Similar findings at same concentration for 60 s were proved by few researchers. 46 , 47 Hence, more studies are required to understand the efficacy of this mouthwash.
Cetylpyridinium chloride (CPC): is a quaternary ammonium compound having a broad antimicrobial activity, primarily on gram‐positive bacteria. It has a lysosomotropic action and is able to destroy viral capsids. 48 It also shows a fungicidal effect on yeasts. As CPC is an antimicrobial, antiviral and antifungal agent, it was thought that it might have an action against enveloped viruses too, such as coronavirus. 8 This compound has been regarded as safe by Food Drug Administration and has the ability to act against SARS‐CoV‐2 virus. 49 A RCT concluded that salivary viral load decreased significantly with PVP‐I and CPC mouth rinses at 6 h and a similar type of study has proved its efficacy against the virus. 27 , 50 Even a long‐term use of CPC (around 6 weeks) does not disrupt the equilibrium of oral microbiota. 35 , 50 A combination of CPC (0.075%) and zinc lactate (0.28%) proves its efficacy in saliva up to 60 min after rinsing. 46 Since just a handful of studies are available with respect to (w.r.t) CPC and its action against coronavirus, this is an area of research that has a lot of potential to be explored. Long‐term use of these mouth washes needs to be monitored.
4.1. Excipients in oral rinses
Ethanol is a common excipient used in several oral rinses and has been observed as an effective agent against SARS‐CoV‐2 virus. It acts by attacking the lipid membrane of the micro‐organisms, denatures proteins and lipid structure. It primarily inactivates enveloped virus at a higher concentration than what is deemed safe for oral use and is added in lower concentrations in many mouthwashes (14–27% weight/volume). 27 , 51 , 52 Bidra et al. 26 reported that use of 70% of ethanol for 30 s inactivated SARS‐CoV‐2 virus. Studies are required to standardize the use of non‐toxic concentrations of ethanol which will inactivate SARS‐CoV‐2 virus. Therefore, in vitro and in vivo studies should be carried out in order to define its role against SARS‐CoV‐2 prior to its use in oral and nasal rinses.
Combinations of antimicrobial and antiviral agents: are also being tested so as to have a more effective bactericidal and virucidal activity owing to a cumulative effect of such combinations. Most of the mouthwashes are alcohol based owing to increased antibacterial and antiviral activity. Precautions need to be taken with usage of alcohol‐based rinses in vulnerable groups (children, pregnant women and people with previous alcohol addiction). CHX 0.06%, sodium fluoride (NaF, 0.025%) and CPC 0.03% proved to be effective as non‐alcohol‐based oral rinses. 52 We believe that such combinations should be used and their activity should even be tested against SARS‐CoV‐2 virus too.
4.2. Phthalocyanine derivate
A study by de Fonseca et al. 53 indicated that a phthalocyanine derivate‐based mouthwash shows promising action in reducing SARS‐CoV‐2 viral load. Five millilitre of phthalocyanine derivate mouthwash for 1 min, five times daily for 2 weeks, reduced the clinical symptoms of the disease. The limitations of this study is a small sample size, lack of control group and the fact that lack of RTPCR test on patients’ salivary samples before and after use of the mouth rinse so as to evaluate its effectiveness in decreasing the viral load with specification of concentration of phthalocyanine derivate.
Essential oils (EOs):are natural, volatile and fragrant substances obtained from medicinal plants and herbs. They show antimicrobial, anticancer and anti‐inflammatory actions. Studies on commercially available EOs containing mouthwashes are said to decrease the viral load in vivo and in vitro. Listerine mouth washes have shown a log reduction value (LRV) of viricidal effect, viz Listerine Total Care (eucalyptol, thymol, menthol, NaF and zinc fluoride); after 1 min, the LRV was ≥4.1 (3.8–4.4). 54 Also another commercial product, Listerine cool mint reduced the viral load by ≥3.11 in one of the virus strains. 55 Several in silico studies projected antiviral effects of EOs against SARS‐CoV‐2: 17 compounds of garlic oil were predicted to interact with viral main protease of SARS‐CoV‐2. 56 , 57 , 58 EOs have been shown to have incredible antiviral potential. A mixture of oleoresins and EOs extracted from aromatic herbs showed antiviral effect against infectious bronchitis virus (IBV) in both in vitro and in vivo studies. 58 There was a reduction in severity of clinical symptoms like sore throat, fever, cough and shortness of breath when patients treated with 5 ml of thyme EO, 3 times daily for a week. It was also said that it stimulates the immune system by increasing the lymphocyte count, decreases inflammation by decreasing neutrophils and promotes healing. 59 Among the EOs, geranium and lemon oil is said to have a significant antiviral effect, it was observed that citronellol, geraniol and neryl acetate of geranium oil and limonene, which represented major compounds of lemon oil, showed down‐regulated ACE2 expression in epithelial cells, suggesting that these antiviral agents could be used as promising oral rinses requiring further validation. 60
4.3. Mouth rinses containing Citrox and β‐cyclodextrins
Citrox, a derivative of citrus fruits, a soluble bioflavonoid and inhibitor of the 3‐chymotrypsin–like protease of the SARS‐CoV‐2 is a protein vital to virus replication or inhibits binding to ACE2 receptors. 61 It has the property of oxidizing the virus, and thus reduces its salivary load. Similarly, β‐cyclodextrin (β‐CD), a modified sugar molecule, has the property of targeting its lipid bilayer by sequestering cholesterol and attracts viruses before inactivating it irreversibly. 61 , 62 Thus, a combination of these agents (Cyclodextrins combined with Citrox) were used as mouth rinses and/or nasal rinse in a RCT that found a remarkable decrease in the salivary viral load on day 1, 4 h after the initial dose and a modest decrease in the viral load was observed as a long‐term effect (7 days). 62
4.4. Home remedies to decrease viral load
Salt water gargle, as well as nasal irrigation, is said to decrease the viral load. 28
5. CONCLUSION
As the COVID‐19 second‐wave reaches its peak, it has gotten all the more very importance that we ramp up our clinical testing, in vitro and in vivo studies, as we seem to have quite a few positive outcomes in various studies mentioned in this review. Some agents such as EOs and phthalocyanine derivates seem to aid in reducing the clinical severity of the symptoms, and others like PVP‐I, CHX and CPC actually result in reduction in salivary viral load of SARS‐CoV‐2. As soon as more evidence‐based studies and clinical trials prove the safety and efficacy of these agents, they should be put into use as a part of daily routine as well as in healthcare practices to fight this pandemic and future viral infections too. Home remedies have proven to act as a preventive measure and in reducing the severity of clinical symptoms in novel coronavirus‐19 disease. Over‐the‐counter oral rinses, instead of being provided in expensive bottles, should be made available in small sachets for a quick and cost‐effective use by the common man. In this difficult time of pandemic, there is a need to make protective measures like hand sanitizers, masks and oral rinses cost‐effective, easy to use and readily available at small local stores. It is time that we made a translational step towards a safer clinical practice and community‐based prophylactic measures that may help in estimating and controlling the viral load in individuals as well as their transmission within populations.
6. CLINICAL RELEVANCE
The RCTs of oral rinse/mouthwashes on SARCoV‐2 give an evidence‐based approach on the use of the antiseptic (PVP‐I of 0.5%–1%) having virucidal properties, as a preprocedural rinse such that it will decrease the viral load in both asymptomatic and symptomatic patients, and also in preventing cross‐infections between patients and treating dental or medical personnel. As per the occupational safety and health administration guidelines, this mouth rinse is safe and cost‐effective, and could also be used as a prophylactic measure in venerable population.
CONFLICT OF INTEREST
There are no conflicts of interest.
AUTHOR CONTRIBUTIONS
Gargi Gandhi, first author, contributed to the acquisition, analysis and interpretation of data, and drafted the manuscript. Latha Thimmappa contributed to the design of study, the acquisition, analysis and interpretation of data, and drafted the manuscript. Nagaraja Upadhya contributed to the design of study and critically revised the manuscript. Sunitha Carnelio contributed to the conception and design of the study, supported the analysis and interpretation of the data and critically revised the manuscript. All authors gave final approval and agreed to be accountable for all aspects of this work, ensuring its integrity and accuracy.
ETHICAL STATEMENT
Ethical committee clearance is not required as this is a systematic review based on the published articles.
Supporting information
Appendix S1‐S2
Supplementary Material
ACKNOWLEDGMENT
None.
Gandhi G, Thimmappa L, Upadhya N, Carnelio S. Could mouth rinses be an adjuvant in the treatment of SARS‐CoV‐2 patients? An appraisal with a systematic review. Int J Dent Hygiene.2022;20:136–144. 10.1111/idh.12555
Funding information
Not obtained.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in [repository name] at [DOI].
REFERENCES
- 1. WHO Coronavirus Disease (COVID‐19) Dashboard. Available online: https://covid19.who.int
- 2. Koch‐Heier J, Hoffmann H, Schindler M, Lussi A, Planz O. Inactivation of SARS‐CoV‐2 through treatment with the mouth rinsing solutions ViruProX® and BacterX® Pro. Microorganisms. 2021;9:521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Triple mutation Covid variant in India? Here's what we know so far. https://timesofindia.indiatimes.com/india/triple‐mutation‐covid‐variant‐in‐india‐heres‐what‐we‐know‐so‐far/articleshow/82183386.cms
- 4. Peng X, Xu X, Li Y, Cheng L, Zhou X, Ren B. Transmission routes of 2019‐nCoV and controls in dental practice. Int J Oral Sci. 2020;12(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Trougakos IP, Stamatelopoulos K, Terpos E, et al. Insights to SARS‐CoV‐2 life cycle, pathophysiology, and rationalized treatments that target COVID‐19 clinical complications. J Biomed Sci. 2021;28(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yoon JG, Yoon J, Song JY, et al. Clinical significance of a high SARS‐CoV‐2 viral load in the saliva. J Korean Med Sci. 2020;35(20):e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cavalcante‐Leão BL, de Araujo CM, Basso IB, et al. Is there scientific evidence of the mouthwashes effectiveness in reducing viral load in Covid‐19? A systematic review. J Clin Exp Dent. 2021;13(2):e179‐189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carrouel F, Gonçalves LS, Conte MP, et al. Antiviral activity of reagents in mouth rinses against SARS‐CoV‐2. J Dent Res. 2021;100(2):124‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Patel J, Woolley J. Necrotizing periodontal disease: oral manifestation of COVID‐19. Oral Dis. 2021;27(3):768‐769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Corchuelo J, Ulloa FC. Oral manifestations in a patient with a history of asymptomatic COVID‐19: case report. Int J Infect Dis. 2020;100:154‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meyers C, Robison R, Milici J, et al. Lowering the transmission and spread of human coronavirus. J Med Virol. 2021;93(3):1605‐1612. [DOI] [PubMed] [Google Scholar]
- 12. Rahban C, Ailianou A, Jacot E, Landis BN. Anosmie et agueusie: è propos d'un cas [Concomitant anosmia and ageusia: a case report]. Rev Med Suisse. 2015;11(488):1787‐1790. [PubMed] [Google Scholar]
- 13. Cantuti‐Castelvetri L, Ojha R, Pedro LD, et al. Neuropilin‐1 facilitates SARS‐CoV‐2 cell entry and infectivity. Science. 2020;370(6518):856‐860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cirillo N, Colella G. Self‐reported smell and taste alteration as the sole clinical manifestation of SARS‐CoV‐2 infection. Oral Surg Oral Med Oral Pathol Oral Radiol. 2021;131(4):e95‐e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhu Y, Feron G, Von Koskull D, Neiers F, Brignot H, Hummel T. The association between changes of gustatory function and changes of salivary parameters: a pilot study. Clin Otolaryngol. 2021;46(3):538‐545. [DOI] [PubMed] [Google Scholar]
- 16. Mahmoud MM, Abuohashish HM, Khairy DA, Bugshan AS, Khan AM, Moothedath MM. Pathogenesis of dysgeusia in COVID‐19 patients: a scoping review. Eur Rev Med Pharmacol Sci. 2021;25(2):1114‐1134. [DOI] [PubMed] [Google Scholar]
- 17. Vergara‐Buenaventura A, Castro‐Ruiz C. Use of mouthwashes against COVID‐19 in dentistry. Br J Oral Maxillofac Surg. 2020;58(8):924‐927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Testori T, Wang HL, Basso M, et al. COVID‐19 and Oral Surgery: a narrative review of preoperative mouth rinses. Acta Stomatol Croat. 2020;54(4):431‐441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reis INR, do Amaral GCLS, Mendoza AAH, et al. Can preprocedural mouthrinses reduce SARS‐CoV‐2 load in dental aerosols? Medical Hypotheses. 2021;146:110436. 10.1016/j.mehy.2020.110436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Van Der Weijden GA, Dell’Acqua F, Slot DE. Alveolar bone dimensional changes of post‐extraction sockets in humans: a systematic review. J Clin Periodontol. 2009;36(12):1048‐1058. [DOI] [PubMed] [Google Scholar]
- 22. Gottsauner MJ, Michaelides I, Schmidt B, et al. A prospective clinical pilot study on the effects of a hydrogen peroxide mouthrinse on the intraoral viral load of SARS‐CoV‐2. Clin Oral Investig. 2020;24(10):3707‐3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ortega KL, Rech BO, El Haje GLC, Gallo CB, Pérez‐Sayáns M, Braz‐Silva PH. Do hydrogen peroxide mouthwashes have a virucidal effect? A Systematic Review. J Hosp Infect. 2020;106(4):657‐662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. da Mota Santana LA, Andrade Pinho JN, de Albuquerque HIM, de Almeida Souza LM. Virucidal potential of H2O2‐based spray against SARS‐CoV‐2 and biosafety in a dental environment. Oral Dis. 2021;1‐2. 10.1111/odi.13778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goyal SM, Chander Y, Yezli S, Otter JA. Evaluating the virucidal efficacy of hydrogen peroxide vapour. J Hosp Infect. 2014;86(4):255‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bidra AS, Pelletier JS, Westover JB, Frank S, Brown SM, Tessema B. Comparison of in vitro inactivation of SARS CoV‐2 with hydrogen peroxide and povidone‐iodine oral antiseptic rinses. J Prosthodont. 2020;29(7):599‐603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Seneviratne CJ, Balan P, Ko KKK, et al. Efficacy of commercial mouth‐rinses on SARS‐CoV‐2 viral load in saliva: randomized control trial in Singapore. Infection. 2021;49(2):305‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ramalingam S, Graham C, Dove J, Morrice L, Sheikh A. Hypertonic saline nasal irrigation and gargling should be considered as a treatment option for COVID‐19. J Glob Health. 2020;10(1):e010332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sette‐de‐Souza PH, Soares Martins JC, Martins‐de‐Barros AV, Rodrigues Vieira B, Fernandes Costa MJ, da Costa Araújo FA. A critical appraisal of evidence in the use of preprocedural mouthwash to avoid SARS‐CoV‐2 transmission during oral interventions. Eur Rev Med Pharmacol Sci. 2020;24(19):10222‐10224. [DOI] [PubMed] [Google Scholar]
- 30. Capetti AF, Borgonovo F, Morena V, et al. Short‐term inhibition of SARS‐CoV‐2 by hydrogen peroxide in persistent nasopharyngeal carriers. J Med Virol. 2021;93(3):1766‐1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pelletier JS, Tessema B, Frank S, Westover JB, Brown SM, Capriotti JA. Efficacy of povidone‐iodine nasal and oral antiseptic preparations against severe acute respiratory syndrome‐coronavirus 2 (SARS‐CoV‐2). Ear Nose Throat J. 2021;100(2):192S‐196S. [DOI] [PubMed] [Google Scholar]
- 32. Hassandarvish P, Tiong V, Mohamed NA, et al. In vitro virucidal activity of povidone iodine gargle and mouthwash against SARS‐CoV‐2: implications for dental practice. Br Dent J. 2020;2:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Martínez Lamas L, Diz Dios P, Pérez Rodríguez MT, et al. Is povidone iodine mouthwash effective against SARS‐CoV‐2? First in vivo tests. Oral Dis. 2020. 10.1111/odi.13526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mateos Moreno MV, Obrador AM, Márquez VA, Ferrer García MD. Oral antiseptics against coronavirus: in vitro and clinical evidence. J Hosp Infect. 2021;113:30‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Anderson DE, Sivalingam V, Kang AEZ, et al. Povidone‐iodine demonstrates rapid in vitro virucidal activity against SARS‐CoV‐2, the virus causing COVID‐19 disease. Infect Dis Ther. 2020;9(3):669‐675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Elzein R, Abdel‐Sater F, Fakhreddine S, et al. In vivo evaluation of the virucidal efficacy of Chlorhexidine and Povidone‐iodine mouthwashes against salivary SARS‐CoV‐2. A randomized‐controlled clinical trial. J Evid Based Dent Pract. 2021;21(3):101584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zou X, Xu ZQ, Wang HR, Wang BX, He JF, Wang JZ. Study on the COVID‐19 infection status, prevention and control strategies among people entering Shenzhen. BMC Public Health. 2021;21:551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guenezan J, Garcia M, Strasters D, et al. Povidone iodine mouthwash, gargle, and nasal spray to reduce nasopharyngeal viral load in patients with COVID‐19: A randomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2021;147(4):400‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Frank S, Brown SM, Capriotti JA, Westover JB, Pelletier JS, Tessema B. In vitro efficacy of a povidone‐iodine nasal antiseptic for rapid inactivation of SARS‐CoV‐2. JAMA Otolaryngol Head Neck Surg. 2020;146(11):1054‐1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tsuda S, Soutome S, Hayashida S, Funahara M, Yanamoto S, Umeda M. Topical povidone iodine inhibits bacterial growth in the oral cavity of patients on mechanical ventilation: a randomized controlled study. BMC Oral Health. 2020;20(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gray PE, Katelaris CH, Lipson D. Recurrent anaphylaxis caused by topical povidone‐iodine (Betadine). J Pediatr Child Health. 2013;49(6):506‐507. [DOI] [PubMed] [Google Scholar]
- 42. Bernstein D, Schiff G, Echler G, Prince A, Feller M, Briner W. In vitro virucidal effectiveness of a 0.12%‐chlorhexidine gluconate mouthrinse. J Dent Res. 1990;69(3):874‐876. [DOI] [PubMed] [Google Scholar]
- 43. Herrera D, Serrano J, Roldán S, Sanz M. Is the oral cavity relevant in SARS‐CoV‐2 pandemic? Clin Oral Investig. 2020;24(8):2925‐2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Avhad SK, Bhanushali M, Sachdev SS, Save SS, Kalra D, Kamala DN. Comparison of effectiveness of chlorine dioxide mouthwash and chlorhexidine gluconate mouthwash in reduction of oral viral load in patients with COVID‐19. Indian J Public Health Res Develop. 2020;11(11):27‐32. [Google Scholar]
- 45. Huang YH, Huang JT. Use of chlorhexidine to eradicate oropharyngeal SARS‐CoV‐2 in COVID‐19 patients. J Med Virol. 2021;93(7):4370‐4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Eduardo FP, Corrêa L, Heller D, et al. Salivary SARS‐CoV‐2 load reduction with mouthwash use: a randomized pilot clinical trial. Heliyon. 2021;7(6):e07346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chaudhary PP, Melkonyan A, Meethil A, et al. Estimating salivary carriage of SARS‐CoV‐2 in non‐symptomatic individuals and efficacy of mouthwash in reducing viral load: a randomized controlled trial. J Am Dent Assoc. 2021. S0002‐8177(21)00355‐X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Baker N, Williams AJ, Tropsha A, Ekins S. Repurposing quaternary ammonium compounds as potential treatments for COVID‐19. Pharm Res. 2020;37(6):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pitten FA, Kramer A. Efficacy of cetylpyridinium chloride used as oropharyngeal antiseptic. Arzneimittelforschung. 2001;51(7):588‐595. [DOI] [PubMed] [Google Scholar]
- 50. Ellinger B, Bojkova D, Zaliani A, et al. A SARS‐CoV‐2 cytopathicity dataset generated by high‐content screening of a large drug repurposing collection. Sci Data. 2021;8(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. O'Donnell VB, Thomas D, Stanton R, et al. Potential role of oral rinses targeting the viral lipid envelope in SARS‐CoV‐2 infection. Function (Oxf). 2020;1(1):zqaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zimmer S, Korte P, Verde P, Ohmann C, Naumova E, Jordan RA. Randomized controlled trial on the efficacy of new alcohol‐free chlorhexidine mouthrinses after 8 weeks. Int J Dent Hyg. 2015;13(2):110‐116. [DOI] [PubMed] [Google Scholar]
- 53. da Fonseca OB, Vilhena FV, Cardoso de Oliveira R, et al. A phthalocyanine derivate mouthwash to gargling/rinsing as an option to reduce clinical symptoms of COVID‐19: Case series. Clin Cosmet Investig Dent. 2021;13:47‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Meiller TF, Silva A, Ferreira SM, Jabra‐Rizk MA, Kelley JI, DePaola LG. Efficacy of listerine antiseptic in reducing viral contamination of saliva. J Clin Periodontol. 2005;32(4):341‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Meister TL, Brüggemann Y, Todt D, et al. Virucidal efficacy of different oral rinses against severe acute respiratory syndrome coronavirus 2. J Infect Dis. 2020;222(8):1289‐1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Abdelli I, Hassani F, Bekkel Brikci S, Ghalem S. In silico study the inhibition of angiotensin converting enzyme 2 receptor of COVID‐19 by Ammoides verticillata components harvested from Western Algeria. J Biomol Struct Dyn. 2020;1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Thuy BTP, My TTA, Hai NTT, et al. Investigation into SARS‐CoV‐2 resistance of compounds in garlic essential oil. ACS Omega. 2020;5(26):8312‐8320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jackwood MW, Rosenbloom R, Petteruti M, Hilt DA, McCall AW, Williams SM. Avian coronavirus infectious bronchitis virus susceptibility to botanical oleoresins and essential oils in vitro and in vivo. Virus Res. 2010;149(1):86‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sardari S, Mobaiend A, Ghassemifard L, Kamali K, Khavasi N. Therapeutic effect of thyme (Thymus vulgaris) essential oil on patients with COVID19: A randomized clinical trial. J Adv Med Biomed Res. 2021;29(133):83‐91. [Google Scholar]
- 60. Senthil Kumar KJ, Gokila Vani M, Wang CS, et al. Geranium and lemon essential oils and their active compounds downregulate Angiotensin‐Converting Enzyme 2 (ACE2), a SARS‐CoV‐2 spike receptor‐binding domain, in epithelial cells. Plants. 2020;9(6):770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Carrouel F, Viennot S, Ottolenghi L, Gaillard C, Bourgeois D. Nanoparticles as anti‐microbial, anti‐inflammatory, and remineralizing agents in oral care cosmetics: a review of the current situation. Nanomaterials (Basel). 2020;10(1):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Carrouel F, Valette M, Gadea E, et al. Use of an antiviral mouthwash as a barrier measure in the SARS‐CoV‐2 transmission in adults with asymptomatic to mild COVID‐19: a multicentre, randomized, double‐blind controlled trial. Clin Microbiol Infect. 2021;24:S1198‐743X(21)00268‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1‐S2
Supplementary Material
Data Availability Statement
The data that support the findings of this study are openly available in [repository name] at [DOI].


