Abstract
We report a case of endocarditis in a human infected with Bartonella vinsonii subsp. berkhoffii, which causes bacteremia and endocarditis in dogs. Bacterial identification was established by PCR amplification and sequencing of an intergenic spacer region (ITS1), 16S ribosomal DNA, and a gene encoding citrate synthase (gltA). Bartonella antibodies were detected by immunofluorescence.
CASE REPORT
A 35-year-old Portuguese waiter who lived in London was admitted to St. Thomas' Hospital (London, United Kingdom), in mid-September 1998 with a history of feeling unwell for a month and having had shortness of breath and chest pain for a week. The patient had a cat and a dog in London but had spent the previous month in Portugal, where he had also had contact with a pig, a cow, and a cockerel. On admission he was afebrile. His peripheral white blood cell count was 10.4, hemoglobin was 13.6, C-reactive protein was 46, and erythrocyte sedimentation rate was 39. Transthoracic echocardiography showed a dilated left ventricle and a bicuspid aortic valve with thickened cusps and significant regurgitation. Three set of blood cultures collected on admission were negative, but antibodies reactive to chlamydial antigens were detected by immunofluorescence study. No antibiotic treatment was given. Eighteen days later, the patient underwent an aortic valve replacement with a homograft during which he was given vancomycin and gentamicin as peri-operative prophylaxis. A diagnosis of endocarditis was not considered, but the valve had to be changed for hemodynamic reasons. A large (7-mm) vegetation was apparent on the excised valve, but no microorganism could be microscopically observed after Gimenez staining and routine and cell cultures remained sterile.
The genus Bartonella comprises 14 species that cause asymptomatic bacteremia in various mammals including humans. Based on 16S rRNA gene sequence comparison, the Bartonella genus has been classified in the alpha subgroup of the Proteobacteria. Bartonella quintana and Bartonella bacilliformis infect humans. B. bacilliformis has long been recognized as the agent of Carrion's disease, and B. quintana was described as the agent of trench fever over 70 years ago. Far more recently, Bartonella henselae has been identified as the agent of cat scratch disease and peliosis hepatitis, and along with a reemerging B. quintana, has also been implicated in bacillary angiomatosis. Bartonella clarridgeiae has now been demonstrated as an agent of cat scratch disease (5). Two of these species, B. henselae and B. quintana, together with the fifth pathogen, Bartonella elizabethae, have been reported to cause endocarditis in humans (8). We herein implicate a sixth Bartonella species, B. vinsonii subsp. berkhoffii, in human disease. We used molecular methods to detect its presence in an infected bicuspid aortic valve of a patient with endocarditis.
Anti-Bartonella antibodies were detected in serum by immunofluorescence as previously described (7) at the following titers: B. henselae (200), B. quintana (400), and B. vinsonii subsp. vinsonii (100). Valvular material was used as template in a PCR. Genomic DNA was extracted using Qiagen columns (QIAamp tissue kit; QIAGEN, Hilden, Germany). PCR was designed to specifically amplify a fragment of the 16S-23S rRNA intergenic spacer region of Bartonella species (ITS1) using primers QHVE1 and QHVE3 (10). An amplification product was obtained, but when the nucleotide base sequence of this product was determined and compared to Bartonella ITS sequences deposited in GenBank, it was found not to match any of them. Thus, to identify the newly detected organism, valvular material was also incorporated as a template in a Bartonella-specific PCR targeting a fragment of the citrate synthase gene (gltA) and in a PCR targeting a fragment of the 16S rRNA gene as previously described (1). The success of the amplifications was confirmed under UV light following ethidium bromide staining. Sequencing reactions were performed using a DNA sequencing kit (dRhodamine Terminator cycle sequencing ready reaction with Amplitaq Polymerase FS) (PE Applied Biosystem, Warrington, United Kingdom) as described by the manufacturer. Sequence products were resolved on 5% polyacrylamide gels, and electrophoresis was performed with the ABI PRISM 377 DNA Sequencer (Perkin Elmer). The sequences of both strands were determined twice. A total of 929 bp and 1,458 bp were sequenced for the citrate synthase gene and 16S rDNA, respectively. Comparison with sequences deposited in GenBank showed >99.7% homology with 16S ribosomal DNA (rDNA) and 100% homology with a 338-bp gltA fragment of B. vinsonii subsp. berkhoffii. This bacterium was never cultivated and PCR amplified in our laboratory, so PCR contamination was impossible. As previously described for other Bartonella spp., ITS1 contained the genes encoding isoleucine- and alanine-accepting tRNAs. The unique insertion sequence of 16S rRNA reported for isolate 93-CO1 (2) was also found in the 16S DNA sequence of the studied Bartonella. For Bartonella species, Birtles and Raoult were the first to show that the gltA-derived phylogeny was more useful than the phylogeny derived from 16S rDNA sequence comparison (1). This observation was confirmed in previously published articles (2, 6), as B. vinsonii subsp. berkhoffii and B. vinsonii subsp. vinsonii were not included in a monophyletic group when phylogenetic analysis was inferred from 16S rDNA sequences. However, when citrate synthase gene sequences were compared, the two subspecies of B. vinsonii formed a group (Fig. 1).
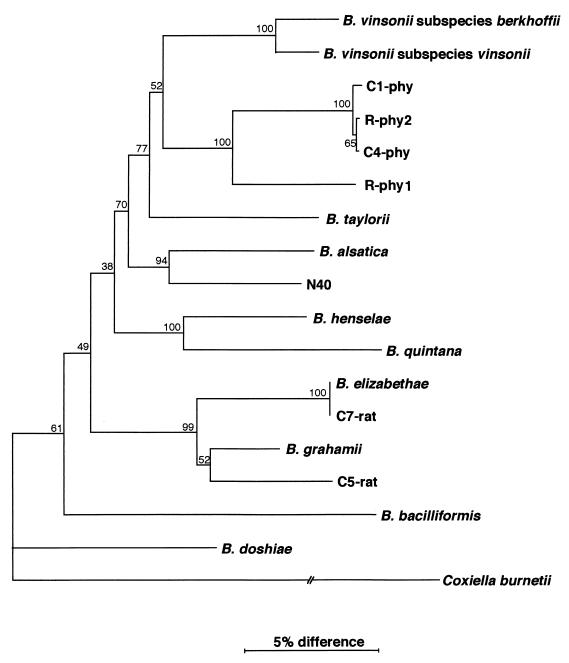
FIG. 1.
Phylogenetic analysis of bacteria included in the genus Bartonella inferred from the citrate synthase gene (gltA) sequence comparison. C1-phy, C4-phy, R-phy1, and R-phy2 were isolated from blood samples from Phyllotis sp. in Peru, C5-rat and C7-rat were isolated from blood samples from Rattus sp. in Peru, N40 was isolated from blood samples from Apodemus sylvaticus in the United Kingdom (1). The evolutionary distance values were determined by the method of Kimura, and the tree was constructed by the neighbor-joining method. The numbers at the nodes are the proportions of 100 bootstrap resamplings that support the topology shown.
The patient had no clinical features of endocarditis and was afebrile. The aortic vegetation found at surgery had not been detected on transthoracic echocardiography. Although the serum antibody titers against Bartonella were significant, the levels were markedly lower than those usually encountered in cases of Bartonella endocarditis (1:1,600 or more) (9). According to the Duke criteria for the diagnosis of infective endocarditis, this case was diagnosed as a possible endocarditis (4) before the histological examination of the valve. We failed to culture Bartonella from the excised valve, possibly because of prophylactic antibiotics prescribed just before surgery, so PCR product sequencing was the only approach that could be used in identifying the agent of infection. Previously, Bartonella vinsonii subsp. berkhoffii had been isolated only from blood samples from a healthy dog and a dog suffering valvular endocarditis (2, 6). Our patient had contact with many animals, including a dog, which may have been the source of his infection, but none of these animals were investigated. Another Bartonella species, B. elizabethae, was isolated only once from a patient with endocarditis, and no exposure to pets or other animals was noted (3). Nevertheless, this bacteria is phylogenetically closely related to other Bartonella spp. isolated from rodents. For the majority of Bartonella species, a nonhuman mammalian reservoir exists, with infections being transmitted among hosts by arthropods (8). Human infections by these species are therefore accidental. However, the exceptional species B. quintana and B. bacilliformis appear to use humans as their primary host (8).
Nucleotide sequence accession numbers.
The nucleotide sequence data reported here have been deposited in GenBank under the following accession numbers: AF143445 for the citrate synthase-encoding gene and AF143446 for the ITS1 and 16S rRNA-encoding genes.
REFERENCES
- 1.Birtles R J, Raoult D. Comparison of partial citrate synthase gene (gltA) sequences for phylogenetic analysis of Bartonella species. Int J Syst Bacteriol. 1996;46:891–897. doi: 10.1099/00207713-46-4-891. [DOI] [PubMed] [Google Scholar]
- 2.Breitschwerdt E B, Kordick D L, Malarkey D E, Keene B, Hadfield T L, Wilson K. Endocarditis in a dog due to infection with a novel Bartonella subspecies. J Clin Microbiol. 1995;33:154–160. doi: 10.1128/jcm.33.1.154-160.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daly J S, Worthington M G, Brenner D J, Moss C W, Hollis D G, Weyant R S, Steigerwalt A G, Weaver R E, Daneshvar M I, O'Connor S P. Rochalimaea elizabethae sp. nov. isolated from a patient with endocarditis. J Clin Microbiol. 1993;31:872–881. doi: 10.1128/jcm.31.4.872-881.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fournier P E, Casalta J P, Habib G, Messana T, Raoult D. Modification of the diagnostic criteria proposed by the Duke endocarditis service to permit improved diagnosis of Q fever endocarditis. Am J Med. 1996;100:629–633. doi: 10.1016/s0002-9343(96)00040-x. [DOI] [PubMed] [Google Scholar]
- 5.Kordick D L, Hilyard E J, Hadfield T L, Wilson K H, Steigerwalt A G, Brenner D J, Breitschwerdt E B. Bartonella clarridgeiae, a newly recognized zoonotic pathogen causing inoculation papules, fever, and lymphadenopathy (cat scratch disease) J Clin Microbiol. 1997;35:1813–1818. doi: 10.1128/jcm.35.7.1813-1818.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kordick D L, Swaminathan B, Greene C E, Wilson K H, Whitney A M, O'Connor S, Hollis D G, Matar G M, Steigerwalt A G, Malcolm G B, Hayes P S, Hadfield T L, Breistschwerdt E B, Brenner D J. Bartonella vinsonii subsp. berkhoffii subsp. nov., isolated from dogs; Bartonella vinsonii subsp. vinsonii; and emended description of Bartonella vinsonii. Int J Syst Bacteriol. 1996;46:704–709. doi: 10.1099/00207713-46-3-704. [DOI] [PubMed] [Google Scholar]
- 7.La Scola B, Raoult D. Serological cross-reactions between Bartonella quintana, Bartonella henselae, and Coxiella burnetii. J Clin Microbiol. 1996;34:2270–2274. doi: 10.1128/jcm.34.9.2270-2274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maurin M, Birtles R J, Raoult D. Current knowledge of Bartonella species. Eur J Microbiol Infect Dis. 1997;16:487–506. doi: 10.1007/BF01708232. [DOI] [PubMed] [Google Scholar]
- 9.Raoult D, Fournier P E, Drancourt M, Marrie T J, Etienne J, Cosserat J, Caboub P, Poinsignon Y, Leclercq P, Sefton A M. Diagnosis of 22 new cases of Bartonella endocarditis. Ann Intern Med. 1996;125:646–652. doi: 10.7326/0003-4819-125-8-199610150-00004. [DOI] [PubMed] [Google Scholar]
- 10.Roux V, Raoult D. Inter- and intraspecies identification of Bartonella (Rochalimaea) species. J Clin Microbiol. 1995;33:1573–1579. doi: 10.1128/jcm.33.6.1573-1579.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]