LINKED CONTENT
This article is linked to Paul et al paper. To view this article, visit https://doi.org/10.1111/apt.16133
LINKED CONTENT
This article is linked to Paul et al paper. To view this article, visit https://doi.org/10.1111/apt.16133
AUTHORSHIP
Guarantor of the article: None.
Author contributions: LBT: Data facilitation and analysis, prepared manuscript and literature review. NCB, SRB: Project supervision and logistical support, edited manuscript. YJC, NS, SS, NS, AS, AZ, MI: Data collection and initial analysis. SPP: Project concept, supervision, edited manuscript and provided expert opinion. All authors have approved the uploaded draft.
We read with interest the articles by Fuchs et al and Paul et al describing the validity of a no‐biopsy pathway (NBP) for coeliac disease in adult patients with IgA‐based anti‐tissue transglutaminase (tTG‐IgA) titres of ≥10‐times upper limit of normal (ULN) whose duodenal biopsy had corroborating histological changes diagnostic of coeliac disease. 1 , 2 Interim COVID‐19 British Society of Gastroenterology (BSG) guidance (reflecting long‐established paediatric practice) advised an NBP for adults with tTG‐IgA ≥10 × ULN and no other alarm symptoms (https://www.bsg.org.uk/covid‐19‐advice/covid‐19‐specific‐non‐biopsy‐protocol‐guidancefor‐those‐with‐suspected‐coeliac‐disease/). A retrospective case note audit study was done in 2021 to evaluate:
The accuracy of NBP in adults with suspected coeliac disease (tTG‐IgA ≥10 × ULN) for the local coeliac serology
To identify cases with the suspected coeliac disease based on a positive tTG‐IgA who were not referred for gastroscopy
We identified 1055 patients with positive tTG‐IgA from January 2013 to December 2020 using our laboratory database; children aged <16 years (n = 179) were excluded. Figure 1 details the diagnostic pathway. Seventy‐two of 876 adult patients with positive tTG‐IgA either did not tolerate gastroscopy, or the clinicians decided electively to avoid it due to frailty or other co‐morbidities. Concerningly, 325/876 (37%) were not referred for gastroscopy; the underlying reasons are being investigated.
FIGURE 1.
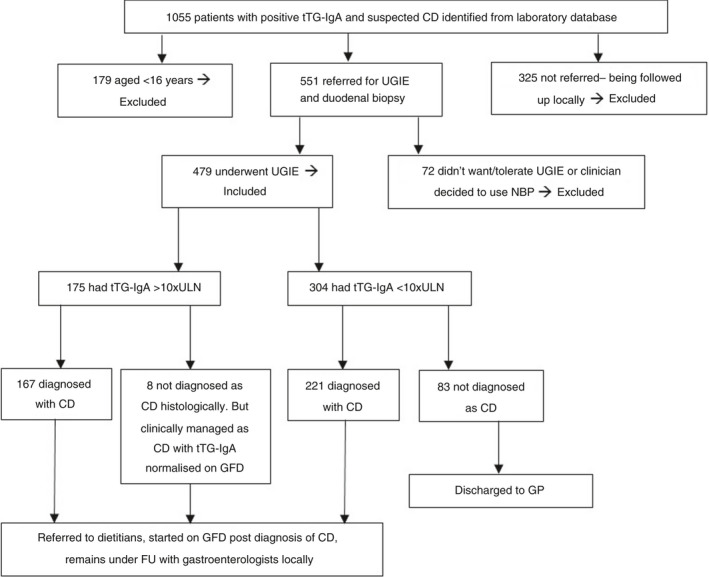
Flow chart showing tTG‐IgA and histological correlation for adult patients with suspected coeliac disease. CD, coeliac diseases; FU follow‐up; GFD, gluten‐free diet; GP, general practitioner; NBP, no‐biopsy pathway; tTG‐IgA, IgA‐based anti‐tissue transglutaminase antibodies; UGIE, upper gastrointestinal endoscopy; ULN, upper limit of normal
Of 479 patients who underwent gastroscopy, 388 had coeliac disease; 167 of 175 patients (95.5%) with tTG‐IgA ≥10 × ULN were histologically confirmed (Marsh 2‐3c); 157/167 had positive anti‐endomysial antibody. There were 83/304 (27%) patients with tTG‐IgA <10 × ULN who had normal histology, indicating the need for continuing histological assessment in this range.
Median age at coeliac disease diagnosis was 47 (range: 16‐88) years; 255 females (66%). Symptoms were documented for only 180/388 coeliac patients. There was an equal distribution of gastrointestinal symptoms (n = 90), extra‐intestinal manifestations (n = 83), mixed (n = 5) and asymptomatic from high‐risk groups (first‐degree relatives with coeliac disease) (n = 2).
There has been concern regarding omitting biopsy to diagnose adult coeliac disease due to worry about missing significant concomitant conditions, notably malignancy in the over 50s. However, a recent study from Italy reassuringly reported no such concerns. 3 One prospective and two retrospective studies from England evaluating the feasibility of an NBP in adults with tTG‐IgA ≥10 × ULN, revealed no other co‐existing organic pathologies, and definite histological correlation with coeliac disease was reported in >95% cases across all three studies. 2 , 4 , 5
Our study echoes the findings of two recently published English studies in which 33% and 17% patients, respectively, were not referred for gastroscopy following a positive coeliac serology. 4 , 5
We provide further evidence that an NBP can be safely implemented in adults with tTG‐IgA ≥10 × ULN in accordance with the interim BSG coeliac disease guidance. Considering the challenges posed by the COVID‐19 pandemic, corroborative evidence across different local services should be encouraged to strengthen the case for secure, non‐invasive coeliac diagnosis. Local teams should monitor and manage the diagnostic pathways appropriately as a continuous audit. Worryingly, a third of tTG‐IgA‐positive patients were not referred. This is being increasingly identified and should be addressed as a potential reason for suboptimal diagnostic rates.
ACKNOWLEDGEMENTS
The authors thank Dr Paul Heaton, Consultant Paediatrician, Yeovil District Hospital, for his great support and valuable input in the study and editing the manuscript. The authors thank the help from Mr John Siewruk, Microbiology and Cellular Pathology Systems Manager based at the Derriford Hospital, Plymouth for his support with this project.
Declaration of personal interests: None.
Declaration of funding interests: None.
REFERENCES
- 1. Fuchs V, Kurppa K, Huhtala H, et al. Serology‐based criteria for adult coeliac disease have excellent accuracy across the range of pre‐test probabilities. Aliment Pharmacol Ther. 2019;49:277‐284. [DOI] [PubMed] [Google Scholar]
- 2. Paul SP, Lau WS, Khan ZH, Heaton PA. Letter: no‐biopsy pathway for diagnosing adult coeliac disease. Aliment Pharmacol Ther. 2021;53:357‐358. [DOI] [PubMed] [Google Scholar]
- 3. Maimaris S, Schiepatti A, Gabrielli GM, et al. Low prevalence of upper endoscopic gastrointestinal findings despite high frequency of alarm symptoms at the time of diagnosis in adult coeliac disease. Eur J Gastroenterol Hepatol. 2020;32:1447‐1451. [DOI] [PubMed] [Google Scholar]
- 4. Penny HA, Raju SA, Lau MS, et al. Accuracy of a no‐biopsy approach for the diagnosis of coeliac disease across different adult cohorts. Gut. 2021;70:876‐883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johnston RD, Chan YJ, Mubashar T, Bailey JR, Paul SP. A no‐biopsy pathway following the interim BSG guidance reliably diagnoses adult coeliac disease in a UK district general hospital. Frontline Gastroenterol. 2020;1‐4. 10.1136/flgastro-2020-101624 [DOI] [PMC free article] [PubMed] [Google Scholar]


