Abstract
This study was undertaken to assess whether SARS‐CoV‐2 causes a persistent central nervous system infection. SARS‐CoV‐2–specific antibody index and SARS‐CoV‐2 RNA were studied in cerebrospinal fluid following COVID‐19. Cerebrospinal fluid was assessed between days 1 and 30 (n = 12), between days 31 and 90 (n = 8), or later than 90 days (post–COVID‐19, n = 20) after COVID‐19 diagnosis. SARS‐CoV‐2 RNA was absent in all patients, and in none of the 20 patients with post–COVID‐19 syndrome were intrathecally produced anti–SARS‐CoV‐2 antibodies detected. The absence of evidence of SARS‐CoV‐2 in cerebrospinal fluid argues against a persistent central nervous system infection as a cause of neurological or neuropsychiatric post–COVID‐19 syndrome. ANN NEUROL 2022;91:150–157
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection primarily targets the upper and lower respiratory tract, causing dry cough and fever. Neurological and neuropsychiatric manifestations have been associated with coronavirus disease 19 (COVID‐19), ranging from mild to fatal at all disease stages irrespective of disease severity. 1 , 2 Interestingly, immunofluorescence and polymerase chain reaction (PCR) analyses of intestinal biopsies obtained from asymptomatic individuals at 4 months after the onset of COVID‐19 revealed persistent detection of SARS‐CoV‐2 RNA and specific immunoreactivity in the small bowel in 50% of individuals. 3 It appears reasonable to assume that SARS‐CoV‐2 may also reach the central nervous system (CNS) via several routes, including the transcribial, hematogenous, and lymphatic routes, or via axonal transport or trans‐synaptic transfer. 4 Histopathological data revealing viral RNA transcripts and particles by transmission electron microscopy in brain tissue may suggest CNS infection. 5 Therefore, symptoms such as cognitive impairment or fatigue persisting for >90 days (post–COVID‐19) following acute respiratory COVID‐19 might be caused by SARS‐CoV‐2 persistence in the CNS.
Systematic studies of SARS‐CoV‐2 RNA detection in cerebrospinal fluid (CSF) from patients with neurological symptoms early during COVID‐19 and in patients with post–COVID‐19 may help address the question of an acute and/or persistent CNS infection with SARS‐CoV‐2. SARS‐CoV‐2 RNA was infrequently detected in the CSF in single cases and case series, 6 , 7 , 8 , 9 , 10 with all these cases reported within the first 90 days of the respiratory infection.
In addition to molecular assays, the SARS‐CoV‐2–specific CSF antibody index (AISARS‐CoV‐2) allows calculation of an intrathecally produced antibody fraction and might provide indirect evidence of CNS infection. The AI is in clinical use for chronic CNS infections such as herpes virus encephalitis, subacute sclerosing panencephalitis, and neuroborreliosis, 11 and is under investigation for progressive multifocal leukoencephalopathy. 12
This study aimed to clarify whether SARS‐CoV‐2 persistently infects the CNS, with SARS‐CoV‐2 RNA from CSF and the AISARS‐CoV‐2 as outcome measures.
Materials and Methods
Participants
The data and biomaterial were derived from a prospective cohort study at baseline, collected at 2 tertiary university hospitals in Germany (Cologne/Berlin) between April 2020 and April 2021 from patients hospitalized or presenting at the specialized post–COVID‐19 outpatient clinic. The study was approved by the institutional review board of the University of Cologne (20‐1501) and Berlin (EA2/066/20) and registered in the German Clinical Trials Register (DRKS00024434). Patients between 18 and 99 years of age and with neurological or neuropsychiatric symptoms during or after PCR‐confirmed COVID‐19 were eligible for the study following written informed consent.
Detection of SARS‐CoV‐2 RNA in CSF
Viral nucleic acids were extracted from CSF and serum samples (200μl) using the innuPREP Virus DNA/RNA Kit‐IPC16 and the automated platform InnoPure C16 touch (20μl eluate volume; Analytik Jena, Jena, Germany). To assess SARS‐CoV‐2 (N and E gene) RNA reverse transcriptase (RT)‐PCR cycle threshold (Ct) levels, samples were analyzed using the LightMix SarbeccoV E gene plus EAV control (TIB Molbiol, Berlin, Germany) and N gene (inhouse primer sets in multiplex PCR) as previously described. 13 Assays were carried out on LightCycler 480 (Roche Diagnostics, Mannheim, Germany). Samples with a weak signal in the RT‐PCR assay were reanalyzed using a 1‐step RT droplet digital (dd) PCR multiplex assay targeting SARS‐CoV‐2 E, RdRp, and N with a limit of detection of 5 viral RNA copies per reaction as previously described, 14 and 2 additional commercial tests. The Xpert Xpress SARS‐CoV‐2 (Cepheid, Sunnyvale, CA) with a limit of detection of 0.005 PFU/ml for N gene and 0.02 PFU/ml for E gene (PFU is defined as plaque‐forming unit), and the Cobas SARS‐CoV‐2 assay on the automated Cobas 6800 (Roche Diagnostics) with a limit of detection of 0.0063 50% tissue culture infectious dose (TCID50)/ml for SARS‐CoV‐2 ORF1a/b and 0.0082 TCID50/ml for E gene were used.
Assessment of SARS‐CoV‐2–Specific AI
To determine the AISARS‐CoV‐2, SARS‐CoV‐2 immunoglobulin class G (IgG) was quantified in diluted CSF and serum samples using the Anti‐SARS‐CoV‐2 QuantiVac ELISA (IgG) targeting the S1 domain of the spike protein (Euroimmun Diagnostik, Lübeck, Germany). Results were expressed semiquantitatively as the ratio of extinction probe and extinction calibrator. CSF samples were generally diluted at 1:2; if antibody concentration exceeded the standards provided, additional 1:20, 1:40, or 1:80 dilutions were required. Serum samples were diluted at 1:101, 1:404, and 1:1010; a few samples required further 1:2020 and 1:4040 dilutions. AISARS‐CoV‐2 was calculated based on SARS‐CoV‐2 IgG in serum and CSF, and albumin and total IgG to estimate specific intrathecal antibody synthesis as previously described. 11 According to the manufacturer's recommendations, serum SARS‐CoV‐2–specific IgG values were chosen for calculations for which the optical density (OD) was closest to 1 and closest to the OD detected for the corresponding CSF sample.
Results
Characteristics of Study Participants
We analyzed 40 patients after PCR‐confirmed SARS‐CoV‐2 infection treated for neuropsychiatric manifestations of COVID‐19, and an available matching CSF–serum pair (Fig 1). CSF was assessed between days 1 and 30 (acute COVID‐19, n = 12), between days 31 and 90 (ongoing COVID‐19, n = 8), or later than 90 days (post–COVID‐19, n = 20) after the COVID‐19 diagnosis. Patients in the acute COVID‐19 group were older (p < 0.001), and the frequency with a severe or critical COVID‐19 disease course was higher as compared to during ongoing and post–COVID‐19 (10 of 12, 83.3% vs 7 of 28, 25.0%). A majority of the patients in the post–COVID‐19 group complained of cognitive deficits (17 of 20, 85.0%), verified using a screening test in 4 of 15 tested patients (26.7%), and confirmed in 5 of 5 patients (100%) when applying multidomain cognitive testing (Tables 1A and 1B).
FIGURE 1.
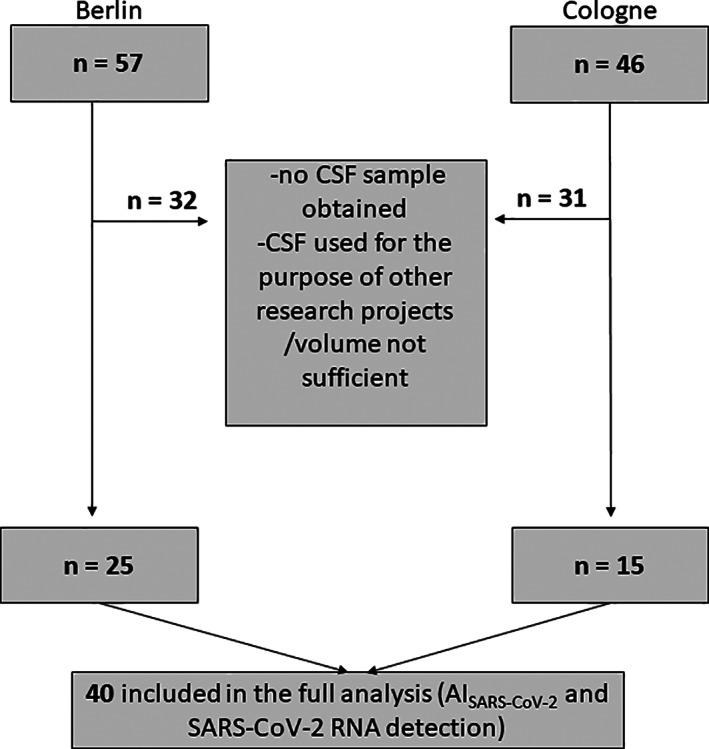
Patient enrollment flow chart. The total number of screened patients at 2 tertiary university hospitals in Germany (Cologne/Berlin) between April 2020 and April 2021 are shown as well as the patients excluded, resulting in the total of 40 patients analyzed for the purpose of this study. AI = antibody‐specific index; CSF = cerebrospinal fluid.
TABLE 1A.
Demographics of Acute and Ongoing Patients
| Case | Sex | Age, Decade | Symptom Onset, Days | COVID‐19 Severity a | Neuropsychiatric Symptoms | MMST b | MOCA b | NPT b |
|---|---|---|---|---|---|---|---|---|
| Acute COVID‐19 | ||||||||
| Case 1 | M | 81–90 | 0 | Mild | Headache, gait disturbance | |||
| Case 2 | M | 61–70 | 6 | Severe | Flaccid paraparesis, delirium, inflammatory neuropathy | |||
| Case 3 | M | 61–70 | 13 | Critical | Delirium | |||
| Case 4 | F | 81–90 | 9 | Critical | Delirium, myoclonus, transient hemiparesis | |||
| Case 5 | F | 31–40 | 21 | Mild | Cognitive deficits, headache, dizziness, fatigue | 27 | ||
| Case 6 | F | 71–80 | 21 | Critical | Delirium, aphasia, impaired consciousness | |||
| Case 7 | F | 71–80 | 20 | Severe | Cognitive deficits, delirium, change in personality | |||
| Case 8 | M | 51–60 | 28 | Critical | Delirium, generalized seizure, critical illness weakness | |||
| Case 9 | M | 61–70 | 13 | Critical | Gaze saccades, ataxia, delirium | |||
| Case 10 | F | 61–70 | 1 | Critical | Delirium | |||
| Case 11 | M | 51–60 | 29 | Critical | PRES, intracranial hemorrhage | |||
| Case 12 | F | 81–90 | 30 | Critical | Paresis left arm | |||
| Median (range) | 63 (56–86) | 16.5 (0–30) | ||||||
| Ongoing COVID‐19 | ||||||||
| Case 13 | M | 21–30 | 43 | Critical | Cognitive deficits, delirium, delayed polyneuropathy | 29 | ||
| Case 14 | F | 51–60 | 55 | Mild | Myelitis with paraparesis | |||
| Case 15 | F | 63 | Mild | Myelitis with paraparesis | ||||
| Case 16 | F | 41–50 | 43 | Mild | Dizziness, limb weakness | |||
| Case 17 | M | 71–80 | 39 | Severe | Cognitive deficits, delirium, ocular motility dysfunction | |||
| Case 18 | M | 31–40 | 53 | Mild | Cognitive deficits, fatigue, depression | 30 | ||
| Case 19 | F | 71–80 | 66 | Mild | Transient ischemic attack, dizziness | |||
| Case 20 | M | 71–80 | 37 | Severe | Guillain–Barré syndrome | |||
| Median (range) | 48 (24–77) | 48.0 (37–66) | ||||||
COVID‐19 severity: mild: any of the various signs and symptoms of COVID‐19 but no shortness of breath, dyspnea, or abnormal chest imaging; moderate: evidence of lower respiratory disease during clinical assessment or imaging and an oxygen saturation (SpO2) ≥ 94% on room air at sea level; severe: SpO2 < 94% on room air at sea level, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen < 300mmHg, respiratory frequency > 30 breaths/min, or lung infiltrates >50%; critical: respiratory failure, septic shock, and/or multiple organ dysfunction (https://www.covid19treatmentguidelines.nih.gov/, accessed October 3, 2021).
Blank: testing not performed.
F = female; M = male; MMST = Mini‐Mental Status Test; MOCA = Montreal Cognitive Assessment, considered pathological for values < 26; NPT = neuropsychological testing battery covering several cognitive domains including learning and memory, attention, executive functioning, language, and visuoconstruction; PRES: posterior reversible encephalopathy syndrome.
TABLE 1B.
Demographics of Post–COVID‐19 Patients
| Case | Sex | Age, Decade | Symptom Onset, Days | COVID‐19 Severity a | Neuropsychiatric Symptoms | MMST b | MOCA b | NPT b |
|---|---|---|---|---|---|---|---|---|
| Post–COVID‐19 | ||||||||
| Case 21 | M | 31–40 | 175 | Mild | Cognitive deficits, fatigue | 29 | ● | |
| Case 22 | F | 21–30 | 119 | Mild | Cognitive deficits, fatigue, depression, anxiety, myalgia | 26 | ||
| Case 23 | M | 51–60 | 284 | Severe | Cognitive deficits, fatigue, anxiety | 26 | ||
| Case 24 | F | 21–30 | 244 | Mild | Cognitive deficits, fatigue, headache | 26 | ||
| Case 25 | F | 51–60 | 255 | Mild | Cognitive deficits, fatigue, depression | 29 | ||
| Case 26 | F | 31–40 | 286 | Mild | Cognitive deficits, hypoesthesia of left arm, left face, right leg | 25 | ||
| Case 27 | F | 61–70 | 113 | Mild | Rapid progression of preexisting polyneuropathy | 27 | ||
| Case 28 | F | 41–50 | 329 | Mild | Fatigue | 26 | ||
| Case 29 | F | 51–60 | 349 | Mild | Cognitive deficits | 26 | ||
| Case 30 | F | 21–30 | 138 | Mild | Cognitive deficits | 28 | ||
| Case 31 | M | 41–50 | 100 | Mild | Cognitive deficits, myalgia | 27 | ||
| Case 32 | M | 61–70 | 143 | Mild | Cognitive deficits, headache, parkinsonian syndrome | 24 | ||
| Case 33 | F | 41–50 | 138 | Mild | Cognitive deficits, fatigue, dizziness | 30 | ● | |
| Case 34 | M | 51–60 | 226 | Mild | Cognitive deficits, fatigue | 29 | ● | |
| Case 35 | F | 41–50 | 133 | Mild | Cognitive deficits, fatigue, myalgia, sensory deficit, insomnia | 29 | ● | |
| Case 36 | M | 51–60 | 120 | Severe | Cognitive deficits, fatigue | 20 | ||
| Case 37 | F | 41–50 | 303 | Mild | Fatigue | 28 | ||
| Case 38 | M | 51–60 | 387 | Mild | Cognitive deficits | 24 | ||
| Case 39 | F | 51–60 | 340 | Severe | Cognitive deficits | 28 | ||
| Case 40 | F | 51–60 | 324 | Severe | Cognitive deficits, depression | 30 | ● | |
| Median (range) | 50.5 (23–70) | 225.3 (100–387) | ||||||
● indicates patients with pathological findings in at least 1 NPT domain.
COVID‐19 severity: mild: any of the various signs and symptoms of COVID‐19 but no shortness of breath, dyspnea, or abnormal chest imaging; moderate: evidence of lower respiratory disease during clinical assessment or imaging and an oxygen saturation (SpO2) ≥ 94% on room air at sea level; severe: SpO2 < 94% on room air at sea level, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen < 300mmHg, respiratory frequency > 30 breaths/min, or lung infiltrates >50%; critical: respiratory failure, septic shock, and/or multiple organ dysfunction (https://www.covid19treatmentguidelines.nih.gov/, accessed October 3, 2021).
Blank: testing not performed.
F = female; M = male; MMST = Mini‐Mental Status Test; MOCA = Montreal Cognitive Assessment, considered pathological for values < 26; NPT = neuropsychological testing battery covering several cognitive domains including learning and memory, attention, executive functioning, language, and visuoconstruction.
Detection of SARS‐CoV‐2 RNA in CSF
SARS‐CoV‐2 RNA (E and/or N gene) was detected in the CSF of 5 patients at low levels with a median Ct value of 39.21 (37.97–40.00), of whom 3 patients were in the acute phase of COVID‐19, 1 patient had ongoing COVID‐19, and 1 patient had post–COVID‐19. None of these results was confirmed by the RT‐ddPCR assay or the 2 additional commercial diagnostic tests.
Assessment of SARS‐CoV‐2–Specific AI
Comparing SARS‐CoV‐2–specific serum antibodies, 11 patients in the acute or ongoing phase of COVID‐19 with detectable antibodies had higher levels than the 16 patients with post–COVID‐19 (median relative units [RU] = 48,274 vs 3,581, p < 0.001). Anti–SARS‐CoV‐2 antibody levels in serum inversely correlated with time since the detection of SARS‐CoV‐2 RNA in the respiratory tract (Fig 2). Regarding SARS‐CoV‐2–specific antibodies in CSF, 11 patients within the first 90 days of infection and with detectable antibodies had higher levels than 13 patients with post–COVID‐19 (median RU = 84.7 vs 7.4, p < 0.001). Anti–SARS‐CoV‐2 antibody levels in CSF inversely correlated with time since the detection of SARS‐CoV‐2 RNA in the respiratory tract (see Fig 2).
FIGURE 2.
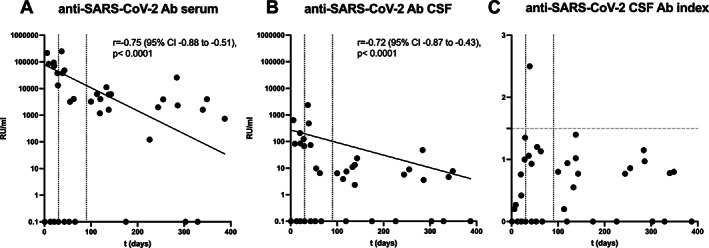
Anti–SARS‐CoV‐2 antibodies (Ab) in serum and cerebrospinal fluid (CSF) over time. (A) Anti–SARS‐CoV‐2 serum and (B) CSF specific antibodies significantly decrease when assessing patients within the first 30 days (acute COVID‐19, n = 12), between days 31 and 90 (ongoing COVID‐19, n = 8) and later than 90 days (post–COVID‐19, n = 20) after their SARS‐CoV‐2 infection. (C) Intrathecally produced antibodies could not be identified for any of the post–COVID‐19 patients. CI = confidence interval; RU = relative units.
In 1 patient, an intrathecally produced anti–SARS‐CoV‐2 antibody fraction was determined as assessed by AISARS‐CoV‐2. This was noted 39 days after the detection of SARS‐CoV‐2 RNA in the respiratory tract (Tables 1A and 2A, 2B). In this patient, CSF was taken to further evaluate delirium and ocular motility dysfunction. At the time of sampling, the patient suffered from acute respiratory distress syndrome due to ongoing COVID‐19, complicated by multiple organ dysfunction and septicemia. The same patient showed borderline CSF AIs to measles and rubella (1.42 and 1.37, respectively, negative for varicella‐zoster virus).
TABLE 2A.
CSF Findings in Acute and Ongoing Patients
| Case | CSF Lymphocytes, μl | Total CSF Protein, g/l | Anti–SARS‐CoV‐2 IgG | AISARS‐CoV‐2 | SARS‐CoV‐2 RNA (E or N gene), Ct Value | |
|---|---|---|---|---|---|---|
| CSF | Serum | |||||
| Acute COVID‐19 | ||||||
| Case 1 | 0 | 0.57 | Not det. | Not det. | — | 37.97 a |
| Case 2 | 1 | 0.34 | 638.44 | 217,210.60 | 0.20 | 40.00 a |
| Case 3 | 1 | 0.43 | Not det. | Not det. | ‐ | Not det. |
| Case 4 | 2 | 0.29 | 82.50 | 84,114.82 | 0.27 | Not det. |
| Case 5 | 1 | 0.22 | Not det. | Not det. | — | 39.21 a |
| Case 6 | 1 | 0.21 | 84.71 | 70,504.06 | 0.42 | Not det. |
| Case 7 | 1 | 0.35 | 208.40 | 95,046.05 | 0.76 | Not det. |
| Case 8 | 2 | 0.14 | 124.02 | 37,796.22 | 1.00 | Not det. |
| Case 9 | 1 | 0.52 | Not det. | Not det. | — | Not det. |
| Case 10 | 3 | 0.33 | Not det. | Not det. | — | Not det. |
| Case 11 | 0 | 0.46 | 67.23 | 13,183.33 | 1.35 | Not det. |
| Case 12 | 2 | 0.41 | Not det. | Not det. | — | Not det. |
| Median (range) | 1.00 (0–3) | 0.34 (0.14–0.57) | 104.37 (67.23–638.44) | 77,309.44 (13,183.33 – 217,210.60) | 0.59 (0.20–1.35) | |
| Ongoing COVID‐19 | ||||||
| Case 13 | 1 | 0.24 | 73.41 | 48,273.96 | 0.93 | Not det. |
| Case 14 | 4 | 0.37 | 9.76 | 3,201.80 | 1.20 | Not det. |
| Case 15 | 2 | 0.23 | 6.49 | 4,086.26 | 1.13 | Not det. |
| Case 16 | 1 | 0.21 | Not det. | Not det. | — | 40.00 a |
| Case 17 | 1 | 0.56 | 479.14 | 37,622.50 | 2.50 | Not det. |
| Case 18 | 6 | 0.39 | Not det. | Not det. | — | Not det. |
| Case 19 | 1 | 0.46 | Not det. | Not det. | — | Not det. |
| Case 20 | 0 | 0.88 | 2,350.40 | 249,768.96 | 1.06 | Not det. |
| Median (range) | 1.00 (0–6) | 0.38 (0.21–0.88) | 73.41 (6.49–2,350.40) | 37,622.50 (3,201.80 – 249,768.96) | 1.13 (0.93–2.50) | |
Oligoclonal band status was available in 33 of the 40 patients and 17 of the 20 patients with post–COVID‐19 syndrome, with none of the patients showing type 2 or 3 oligoclonal bands suggestive of intrathecally produced antibodies.
Not confirmed using alternative polymerase chain reaction protocols; for details, see Materials and Methods section.
AI = antibody index; CSF = cerebrospinal fluid; Ct = cycle threshold; IgG = immunoglobulin class G; Not det. = not detected.
TABLE 2B.
CSF Findings in POST–COVID‐19 Patients
| Case | CSF Lymphocytes, μl | Total CSF Protein, g/l | Anti–SARS‐CoV‐2 IgG | AISARS‐CoV‐2 | SARS‐CoV‐2 RNA (E or N gene), Ct Value | |
|---|---|---|---|---|---|---|
| CSF | Serum | |||||
| Post–COVID‐19 | ||||||
| Case 21 | 0 | 0.42 | Not det. | Not det. | — | Not det. |
| Case 22 | 3 | 0.19 | Not det. | 1,182.71 | — | Not det. |
| Case 23 | 1 | 0.25 | 47.76 | 25,994.37 | 1.15 | 38.20 a |
| Case 24 | 5 | 0.36 | 5.74 | 1,983.64 | 0.77 | Not det. |
| Case 25 | 7 | 0.36 | 8.95 | 3,916.38 | 0.86 | Not det. |
| Case 26 | 1 | 0.24 | 3.59 | 2,340.37 | 0.97 | Not det. |
| Case 27 | 2 | 0.26 | 3.88 | 6,233.72 | 0.20 | Not det. |
| Case 28 | 2 | 0.28 | Not det. | Not det. | — | Not det. |
| Case 29 | 1 | 0.33 | 7.69 | 3,975.46 | 0.80 | Not det. |
| Case 30 | 1 | 0.16 | 2.35 | 1,600.14 | 1.40 | Not det. |
| Case 31 | 4 | 0.33 | 6.40 | 3,244.73 | 0.80 | Not det. |
| Case 32 | 4 | 0.55 | 23.50 | 6,144.03 | 0.77 | Not det. |
| Case 33 | 0 | 0.31 | 13.23 | 5,984.96 | 1.02 | Not det. |
| Case 34 | 0 | 0.26 | Not det. | 121.71 | — | Not det. |
| Case 35 | 1 | 0.22 | 10.99 | 11,228.37 | 0.55 | Not det. |
| Case 36 | 0 | 0.39 | 7.41 | 4,023.44 | 0.94 | Not det. |
| Case 37 | 2 | 0.29 | Not det. | Not det. | — | Not det. |
| Case 38 | 1 | 0.25 | Not det. | 739.52 | — | Not det. |
| Case 39 | 8 | 0.41 | 4.63 | 1,598.22 | 0.78 | Not det. |
| Case 40 | 1 | 0.21 | 17.32 | 13,146.16 | 0.95 | Not det. |
| Median (range) | 1.00 (0–8) | 0.29 (0.16–0.55) | 7.55 (2,35–47.76) | 3,916.38 (121.71–25,994.37) | 0.83 (0.20–1.40) | |
Oligoclonal band status was available in 33 of the 40 patients and 17 of the 20 patients with post–COVID19 syndrome, with none of the patients showing type 2 or 3 oligoclonal bands suggestive of intrathecally produced antibodies.
Not confirmed using alternative polymerase chain reaction protocols; for details, see Materials and Methods section.
AI = antibody index; CSF = cerebrospinal fluid; Ct = cycle threshold; IgG = immunoglobulin class G; Not det. = not detected.
Discussion
As the key finding of our study, neither fundamental CSF findings, nor various PCR protocols, nor IgG‐based SARS‐CoV‐2–directed antibody measures were suggestive of replicative CNS infection as the cause of neuropsychiatric symptoms in post–COVID‐19. These post–COVID‐19 patients had suffered from a mild course of the acute infection, and cognitive deficits were among the leading complaints. The median age of 50 years was within the range of published post–COVID‐19 cohorts. 15 , 16 , 17 We noted an elevated AISARS‐CoV‐2 in 1 patient with severe ongoing COVID‐19 infection, possibly explained by polyspecific immune activation, matching the absence of SARS‐CoV‐2 RNA from CSF, and borderline AI indexes toward other viruses.
The current evidence for direct viral brain invasion in COVID‐19 is conflicting; the frequent detection of SARS‐CoV‐2 in brain reported by one group 5 was not confirmed by others. 18 , 19 These autopsy studies included older individuals that deceased from COVID‐19, demographics that substantially differed from our post–COVID‐19 patients. The same is true for published CSF studies assessing only the acute or ongoing phases of COVID‐19, 20 and lacking systematic antibody analyses.
Owing the limitations to our study, we cannot definitely preclude CNS infection; the sample size is small, a CSF PCR may fail to detect virus latently infecting brain tissue, and an IgG‐based AISARS‐CoV‐2 directed against the spike protein may miss other immune responses.
Nevertheless, despite these limitations, CSF studies such as ours are needed to further explore the still elusive pathogenesis of post–COVID‐19. Whereas neuropsychiatric symptoms during acute COVID‐19 could be explained by hyperinflammation, hypoxemia, hypoperfusion, dehydration, glucose dysregulation, and sedation, 1 they remain unexplained in post–COVID‐19. 15 , 16 , 17 Latent infection, viral persistence, virus‐induced autoimmunity, persistent structural, functional, or metabolic changes following infection, and psychosocial stress are among the alternative nonexclusive explanations. 1
Currently, post–COVID‐19 is defined as “signs and symptoms that develop during or after an infection consistent with COVID‐19, continue for more than 12 weeks and are not explained by an alternative diagnosis” (www.nice.org.uk/guidance). Such a definition based on a temporal association with preceding COVID‐19 illustrates the need for biomarker studies to more precisely differentiate post–COVID‐19 from pre‐ or coexisting other conditions, given the relatively young patient population with complaints of cognitive deficits several months after SARS‐CoV‐2 infection.
Author Contributions
F.S., Y.G., C.F., S.S., V.D.C., and C.W. contributed to the conception and design of the study; all authors contributed to the acquisition and analysis of data. F.S., Y.G., and C.W. contributed to drafting the text or preparing the figures; all authors critically revised the manuscript for important intellectual content.
Potential Conflicts of Interest
CW received personal compensation from BioNTech for participating in an educational discussion. The other authors have nothing to report.
Acknowledgments
This study was supported by the German Research Foundation (PR 1274/8–1, FR 4479/1–1, WA4101/2–1).
We thank V. Worm, D. Wilken, and Dr E. Brüsehaber of Euroimmun for technical and material support and assistance with interpreting AI data. Open Access funding enabled and organized by Projekt DEAL.
References
- 1. Schweitzer F, Kleineberg NN, Göreci Y, et al. Neuro‐COVID‐19 is more than anosmia: clinical presentation, neurodiagnostics, therapies, and prognosis. Curr Opin Neurol 2021;34:423–431. [DOI] [PubMed] [Google Scholar]
- 2. Reza‐Zaldívar EE, Hernández‐Sapiéns MA, Minjarez B, et al. Infection mechanism of SARS‐CoV‐2 and its implication on the nervous system. Front Immunol 2020;11:621735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gaebler C, Wang Z, Lorenzi JCC, et al. Evolution of antibody immunity to SARS‐CoV‐2. Nature 2021;591:639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen X, Laurent S, Onur OA, et al. A systematic review of neurological symptoms and complications of COVID‐19. J Neurol 2021;268:392–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Matschke J, Lütgehetmann M, Hagel C, et al. Neuropathology of patients with COVID‐19 in Germany: a post‐mortem case series. Lancet Neurol 2020;19:919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS‐coronavirus‐2. Int J Infect Dis 2020;94:55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kamal YM, Abdelmajid Y, Al Madani AAR. Cerebrospinal fluid confirmed COVID‐19‐associated encephalitis treated successfully. BMJ Case Rep 2020;16:e23737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sattar SBA, Haider MA, Zia Z, et al. Clinical, radiological, and molecular findings of acute encephalitis in a COVID‐19 patient: a rare case report. Cureus 2020;12:e10650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lersy F, Benotmane I, Helms J, et al. Cerebrospinal fluid features in patients with coronavirus disease 2019 and neurological manifestations: correlation with brain magnetic resonance imaging findings in 58 patients. J Infect Dis 2021;223:600–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lewis A, Frontera J, Placantonakis DG, et al. Cerebrospinal fluid in COVID‐19: a systematic review of the literature. J Neurol Sci 2021;421:117316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reiber H, Lange P. Quantification of virus‐specific antibodies in cerebrospinal fluid and serum: sensitive and specific detection of antibody synthesis in brain. Clin Chem 1991;37:1153–1160. [PubMed] [Google Scholar]
- 12. Warnke C, von Geldern G, Markwerth P, et al. Cerebrospinal fluid JC virus antibody index for diagnosis of natalizumab‐associated progressive multifocal leukoencephalopathy. Ann Neurol 2014;76:792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eberhardt KA, Meyer‐Schwickerath C, Heger E, et al. RNAemia corresponds to disease severity and antibody response in hospitalized COVID‐19 patients. Viruses 2020;12:1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Kock R, Baselmans M, Scharnhorst V, et al. Sensitive detection and quantification of SARS‐CoV‐2 by multiplex droplet digital RT‐PCR. Eur J Clin Microbiol Infect Dis 2021;40:807–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Augustin M, Schommers P, Stecher M, et al. Post‐COVID syndrome in non‐hospitalised patients with COVID‐19: a longitudinal prospective cohort study. Lancet Reg Health Eur 2021;6:100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Blomberg B, Mohn G‐IK, Brokstad KA, et al. Long COVID in a prospective cohort of home‐isolated patients. Nat Med 2021;27:1607–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Davis ED, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021;38:101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Poloni TE, Medici V, Moretti M, et al. COVID‐19‐related neuropathology and microglial activation in elderly with and without dementia. Brain Pathol 2021;31:e12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee MH, Perl DP, Nair G, et al. Microvascular injury in the brains of patients with Covid‐19. N Engl J Med 2021;384:481–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Younger DS. Postmortem neuropathology in Covid‐19. Brain Pathol 2021;31:385–386. [DOI] [PMC free article] [PubMed] [Google Scholar]


