Dear Editor,
Coronavirus disease 2019 (COVID‐19) has resulted in an on‐going world‐wide pandemic and is associated with substantial morbidity and mortality. Vaccination against SARS‐CoV‐2 is important to help control the pandemic, and effective vaccines have been rapidly developed and several are now licenced. There are no specific contraindications to COVID‐19 vaccination for people with inherited bleeding disorders.
The COVID vaccine trials demonstrating efficacy have only been performed using an intramuscular (IM) route of administration. Therefore, subcutaneous administration, which is an option for other types of vaccines for people in people with bleeding disorders, 1 is not recommended. However, there is surprisingly little data on the safety of intramuscular (IM) injections in people with bleeding disorders, with only two retrospective surveys based in paediatric populations identified in the literature. 2 , 3
Expert consensus guidelines were rapidly published to guide management of COVID vaccination in people with haemophilia 4 and all bleeding disorders, 5 with the latter representing consensus from the WFH and other major medical and patient organisations which advise to use the smallest gauge needle available (25–27 gauge) and apply pressure to the site for at least 10 min. Both guidelines state that patients with severe or moderate haemophilia should receive prophylactic replacement therapy prior to IM vaccination if available, but for patients with a basal factor level of greater than 10 IU/ml, or patients on Emicizumab, no haemostatic precautions are required. For those patients with factor activity between 05 and 10 IU/ml individual consideration is advised. 4 Patients with Type 3 VWD, should receive VWF concentrate prophylaxis prior to IM vaccination, and for patients with Type 1 or 2 VWD, depending on VWF activity levels therapies such as DDAVP or tranexamic acid can be considered in consultation with their haemophilia treatment centre. Haemostatic support for patients with rare bleeding disorders should depend on the severity of the disorder and be decided in consultation with their treatment centre. 5
The haemostatic advice given to people with bleeding disorders at Bristol and Oxford UK Haemophilia Comprehensive care centres in December 2020 was in line with these published guidelines. We designed a short retrospective survey and asked patients to complete the survey after they received their COVID‐19 vaccination in order to assess if patients were experiencing bleeding complications. Collected data were type of bleeding disorder, age category, usual prophylactic treatment (if any), vaccine type, precautionary measures taken prior to vaccination, length of time pressure was applied at the time of vaccination, complications of vaccine and how these were managed. The survey was distributed via email to patients registered at our haemophilia centres and also via the Haemophilia Society UK website. The survey was registered and approved as a service evaluation project at University Hospitals Bristol and Weston NHS Trust.
One‐hundred and seventy seven patients responded between January 2021 and May 2021 and their responses are outlined below:
Bleeding disorder. The most frequent bleeding disorders were Haemophilia with 54.8% (97) of respondents and VWD with 21.5% (38) of respondents. 4% (seven) respondents had a platelet function disorder and 4.5% (eight) had a rare bleeding disorder. 15.2% (27) had an unclassified bleeding disorder.
Age. The median age category was aged 50–59 years old with 22% (39) of respondents. 2% (three) of respondents were under 20 years old, 10% (18) were aged 20–29, 14% (24) were 30–39 years, 16% (28) were 40–49 years, 18% (32) 60–69 years,16% (29) 70–79 years, and 2% (four) were 80–89 years old.
Type of vaccine. 62% (106) of respondents received the AstraZeneca (AZ) vaccination, 35% (61) received the Pfizer‐BioNTech (PZ) vaccine, .5% (one) received the Moderna vaccine and 2.5% (nine) were unsure which Covid vaccine they received.
Haemostatic Treatment. 46% (81) of respondents reported that they were on regular treatment for their bleeding disorder and 43% (76) of respondents reported that they took treatment (including tranexamic acid) prior to having the Covid 19 vaccination although the majority of patients did not state exactly what treatment this was. Of note, of the 20 patients with Haemophilia A taking Emicizumab, four of these patients took additional TXA.
Local pressure. 9.2% (16) respondents reported that pressure was applied for a minimum of 10 min, 18% (33) reported pressure was applied for 5 min and 65% (114) of respondents reported that pressure was applied for less than 5 min.
Side‐effects. 73% (129) reported that they had no side effects from the vaccination, 8% (14) reported bruising and 4% (seven) reported mild swelling around the vaccination site (Figure 1). Unexpectedly 2% (three) reported bleeding complications at another site: all three patients had an unclassified bleeding disorder and reported increased menorrhagia; two sought advice from their general practitioner and another from a gynaecologist. Table 1 highlights bleeding complication by diagnosis. Further clinical details, including of any blood tests done, are not known. 13% (23) of respondents reported general flu‐like symptom side effects. There was a similar risk of reporting any side effect in the AZ group compared to the PZ group with a risk of .23 and .28, respectively.
FIGURE 1.
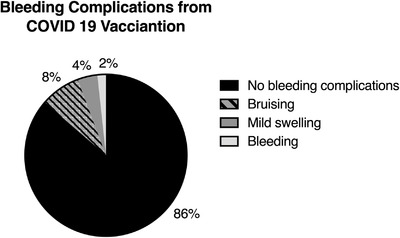
Breakdown of 177 respondents bleeding complications
TABLE 1.
Respondents divided by diagnosis and whether they received prophylactic concentrate prior to vaccination
| Diagnosis | Number of respondents | Reported Bleeding complications |
|---|---|---|
| Haemophilia | ||
| No prophylaxis | 50 |
|
| Prophylaxis | ||
| Emicizumab | 20 | Bruising (n = 1) |
| Factor concentrate | 27 | Bruising (n = 3), Haematoma, spontaneously resolved (n = 1) |
| Von Willebrand disease | ||
| Factor prophylaxis | 4 | Nil |
| No factor prophylaxis | 34 | Bruising (n = 4) |
| Platelet function disorder | 7 | Bruising (n = 1) |
| Rare bleeding disorder | 8 | Haematoma, spontaneously resolved (n = 2) |
| Unclassified Bleeding Disorder | 27 |
|
Overall, only a minority of patients applied pressure for at least 10 min as per the WFH guidelines, and 65% applied pressure for less than 5 min. The rate of self‐reported general flu‐like symptoms and local swelling around the vaccination site is similar to a large prospective observational study in the general population which found self‐reported systemic side‐effects of 13.5%; local swelling of 6.4% and local bruising of 2.4%. 6 Our survey reports a higher rate of bruising around the vaccination site (8%).
The limitations of our survey are that it relied on people retrospectively self‐reporting bleeding disorder, haemostatic treatments and side effects. People with more significant side effects may have been more likely to complete and return the survey. Some respondents did not answer all the questions and in particular data on bleeding disorder severity was therefore not fully captured, and the exact haemostatic management is not known. Thus, the results of the survey are limited by recall bias.
The survey demonstrates that in adults with bleeding disorders that 10 min of pressure following IM COVID vaccine is generally not applied, and that self‐reported bruising is slightly higher than expected for the general population. In this heterogenous group of 177 patients, serious local haematoma requiring intervention did not occur.
In conclusion, our survey suggests that intramuscular vaccination in people with bleeding disorders following general haemostatic guidance from haemophilia centres was not associated with significant local bleeding complications. Specifically, it supports the guidance that people with factor levels more than .10IU/ml, or on Emicizumab, do not need additional haemostatic support. For those patients with factor levels less than .10IU/ml this survey was not able to reliably inform this decision and thus individual consideration is advised in line with published guidelines.
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
REFERENCES
- 1. Srivastava A, Santagostino E, Dougall A, et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020;26(Suppl 6):1–158. [DOI] [PubMed] [Google Scholar]
- 2. Hochart A, Falaise C, Huguenin Y, Meunier S. Intramuscular vaccination of haemophiliacs: is it really a risk for bleeding?. Haemophilia. 2019;25(5):e322–e3. [DOI] [PubMed] [Google Scholar]
- 3. Evans DI, Shaw A. Safety of intramuscular injection of hepatitis B vaccine in haemophiliacs. BMJ. 1990;300(6741):1694–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pfrepper C, Holstein K, Konigs C, et al. Consensus recommendations for intramuscular COVID‐19 vaccination in patients with hemophilia. Hamostaseologie. 2021;41(3):190–196. [DOI] [PubMed] [Google Scholar]
- 5. Kaczmarek R, El Ekiaby M, Hart DP, et al. Vaccination against COVID‐19: rationale, modalities and precautions for patients with haemophilia and other inherited bleeding disorders. Haemophilia. 2021;27(4):515–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Menni C, Klaser K, May A, et al. Vaccine side‐effects and SARS‐CoV‐2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis. 2021;21:939–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from the authors.


