Abstract
The COVID‐19 pandemic is one of the most disruptive social, political and economic crises of the modern era. In today's interconnected world, the pandemic shows how quickly infectious disease outbreaks can spread across continents. Since the initial outbreak, the introduction of several vaccines has brought hope to a virus‐weary world. In spite of the remarkable results of approved vaccines, many lower‐middle countries are yet to receive a single vaccine shot. This manuscript highlights the fact that global health inequities have intensified during the pandemic. While many wealthy nations have ramped up vaccination efforts and cautiously opened their borders, many in the developed world are still waiting to be inoculated. With the rise of several resistant variants, this work argues that public health policy experts demand a greater need for global solidarity in vaccine access. This is not only important ethically, but it is also a pragmatic response.
Keywords: coronavirus, COVID‐19, global health, TRIPS waiver, vaccine equity, vaccine nationalism, vaccines
1. INTRODUCTION
The SARS‐CoV‐2 (Severe Acute Respiratory Syndrome 2) hereafter COVID‐19 pandemic has exposed the fragility of global health systems. Over a year has passed since the initial outbreak of COVID‐19 in Wuhan, Hubei province, China. As the world cautiously reopens, several vaccines have been approved based on their antigenic responses to COVID‐19. For instance, the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have approved: Pfizer‐BioNTech, Moderna, Oxford‐AstraZeneca and Janssen COVID‐19 (Johnson & Johnson). Other vaccines such as Gamaleya‐Sputnik, Sinopharm, and Sinovac have been approved for use in some countries. Vaccines such as Novavax, Sanofi–GSK and CanSinoBIO are in Phase III clinical trials and may be approved soon. The different approaches and platforms used for these vaccines have led to differences in performance, storage conditions, prices and availability. Although efficacy rates have been well above expectations, COVID‐19 vaccination rates have been uneven globally. While countries such as the United States and the United Kingdom have made concerted efforts to vaccinate their populations, many countries have yet to receive a single dose of any COVID‐19 vaccine. For instance according to the Centers for Disease Control (CDC), approximately 74.6 percent of the U.S adults have received at least one dose of the vaccine. 1 In the UK, more than 44.5 million people have received at least one dose. However, in Africa out of a continent of 1.3 billion people, only 31 million have received at least one dose of the vaccine. 2 But as precarious as the situation is on the African continent, African countries are not the only ones dealing with outbreaks as India, Nepal, Brazil and many other countries in Latin America and the Caribbean have dealt with successive waves of outbreaks. Global outbreaks—particularly those in lower and middle‐income (LMIC) countries lay bare the shortcomings of the global vaccination initiative.
For many developing countries, vaccine access is wholly reliant on the COVAX program (COVID‐19 Vaccines Global Access) launched in April 2020. COVAX is a collaboration involving the World Health Organization (WHO), Gavi, The Vaccine Alliance, The Coalition for Epidemic Preparedness Innovations (CEPI), UNICEF, The World Bank, the Bill and Melinda Gates Foundation, the European Commission and others. 3 COVAX was created to facilitate equal access to vaccines as well as diagnostics. Since its inception, vaccines have become more available globally, but other problems have arisen. Firstly, the complexity of the vaccine effort often means that countries are at different stages of the pandemic. Secondly, some countries have been overwhelmed by collapsing healthcare systems, limited intensive care beds, oxygen and ventilators. Others have experienced difficulties distributing vaccines internally. For instance, Malawi burned up to 20 thousand doses of the Oxford‐AstraZeneca vaccine after only 18 days of receiving them due to the risk of expiration. South Sudan also announced it would return 72 thousand does of the Oxford‐AstraZeneca vaccine to COVAX because of risk of expiration. 4 While these events have come in conjunction with vaccine hesitancy following rare blood clotting events associated with the Oxford‐AstraZeneca vaccine; it is also the case that many countries lack the resources and infrastructure to mount effective vaccination campaigns. Although the United States recently pledged to donate 500 million Pfizer‐BioNTech vaccines (75 percent through COVAX and 25 percent directly to countries), researchers at Duke University state that about 11 billion doses are needed to vaccinate 70 percent of the world's population. 5
In Africa, vaccine efforts have been guided by the African Vaccine Acquisition Trust (AVAT). AVAT acts as a centralized purchasing agent on the behalf of the continent. Its main purchasing partners are the African Union's (AU) Centers for Disease and Prevention (Africa CDC), the African Export‐Import Bank (Afeximbank) and the United Nations Economic Commission. Despite AVAT's purchasing efforts, research states that African countries have been charged more for their vaccines. 6 Many countries have paid significantly more for the same vaccine than countries in the European Union (EU) bloc and the United States. These deficiencies highlight vaccine deficiencies and price gouging. Tedros Adhanom the Director of the WHO stated that many LMIC's could ‘miss out on vaccines because they have less bargaining power than big countries’. 7
According to the WHO, 75 percent of vaccine supplies have been sent to just 10 countries and less than 10 percent have gone to countries with low GDP's. 67 LMICs have made no vaccine purchases of their own and are wholly dependent on COVAX. 8 While funding pledges like those undertaken by the U.S government will help COVAX move closer to their goal of global vaccination equity, it does not go far enough. Data from Johns Hopkins University indicates that Argentina, Colombia and Paraguay are awash with new cases. In India, rapidly spreading variants like the Delta variant have infected over 30 million and killed almost 360 thousand people. In Uganda, Namibia and South Africa, cases have increased and due to limited testing, official tallies are likely much higher than has been reported. 9 Now, as the global community faces vaccine shortages, vaccine equity must become a reality. This article argues the urgency of global solidarity and an urgent need to exacerbate global access to vaccines. Addressing complicated bottlenecks in distribution and delivery will require that production capacities and health infrastructures can ramp up global vaccination efforts.
2. THE NEED FOR A TARGETED APPROACH TO VACCINE EQUITY
Prior outbreaks of diseases such as Severe Acute Respiratory Syndrome (SARS‐CoV‐1), H7N9 Influenza, Middle East Respiratory Syndrome (MERS‐CoV), Ebola Virus Disease (EVD), and H1N1 Influenza illustrate the need for a renewed focus on: (a) pandemic planning and (b) vaccine and medical equity. Vaccination efforts in LMICs have often fallen behind due to complex red tape, cost and access. In the last century, due to a combination of scientific advances and improvements in vaccine production, vaccines against polio (1955), measles (1963) and rubella (1969) were introduced in a global campaign to ramp up global vaccination rates. By the 1970s, smallpox was eradicated due to a successful worldwide inoculation effort. Despite early successes with vaccines, little was actually done to invest in local health systems and by the late 1990s, global vaccination efforts were stalling. Nearly 30 million children in LMICs were not fully immunized against deadly infectious diseases and some were not immunized at all. Vaccine deficiencies arose simply because many countries simply could not afford them. 10
Regarding vaccine price discrepancies, research illustrates that Pfizer‐BioNTech and Moderna are charging governments up to 4 billion dollars more than the estimated cost of production for a single dose. 11 Despite the rapid spread of the devastating Delta variant, the African Union has been charged almost six times the production cost of the Pfizer‐BioNTech vaccine. Likewise, Moderna has charged South Africa almost 4 to 13 times more than the production cost of the vaccine. 7 In addition, published reports illustrates that the Serum Institute of India (producers of the Oxford‐AstraZeneca vaccine) charged countries like Bangladesh and South Africa more per dose of the vaccine than the prices charged to the countries of the EU. When asked about price discrepancies, AstraZeneca stated that high income countries had invested in the research and development of the vaccine—hence the discount in prices. This despite the fact that up to 2 thousand South Africans had participated in clinical trials for the vaccine in 2020. 12
The need for a targeted approach to vaccines and essential medicines underscores the importance of putting up a united front against global health challenges. As such, vaccine disadvantages in LMICs illustrate the weaknesses of the current global health paradigm. Nearly 2 billion people worldwide lack access to essential vaccines and medicines. These deficiencies have led to greater pain and suffering, prolonged illnesses and preventable deaths. Still, essential vaccines and medicines are crucial components of Sustainable Development Goals (SDG) to ensure access to safe, effective and affordable essential medicines and vaccines for all’. 13
Echoing this sentiment, Prime Minister of Trinidad and Tobago Keith Rowley described the problem of ‘price gouging’ and the uneven prices of vaccines which put them out of the reach of people from LMIC's. In an ideal world, the price of a vaccine would be consistent worldwide but unfortunately, that is not the case. 14 While there are significant price differences for some vaccines because they are more expensive to produce, there are discrepancies even for the exact same vaccine. There have been numerous reports of LMIC's paying significantly more than high income nations. For instance, Uganda was being charged up to $8.50 per dose of the Oxford‐AstraZeneca vaccine while the EU was being charged $3.50 per dose. 14 For countries that hosted clinical trials, the same situation persists. Many countries still find it difficult to negotiate deals with the same vaccine companies which they helped in early trials. For instance, Argentina hosted major Phase 3 trials for Pfizer‐BioNTech but has found it difficult to reach an agreement to procure vaccines. Likewise, Peru which conducted clinical trials for Sinopharm found it difficult to get discounts for vaccines once the experimental phase was completed. 11 At the recent G7 summit, global leaders agreed to sharing commitments to provide up to 870 million doses to COVAX in 2021 and 2022. Despite this, that COVAX has only been able to deliver about 78 million doses or about 4 percent of the 1.95 billion doses administered globally. 15 COVAX has struggled to get enough doses at the speed required (given the spread of COVID‐19 variants) because many wealthy countries have bought vaccines—often at the excessive process charged by manufacturers. Without ‘price gouging’, and ‘vaccine hoarding’ the money spent by COVAX should have been able to fully vaccinate people in LMIC and middle‐income countries. At best however, COVAX may only reach 23 percent of its target by the end of 2021. Simply put, the pandemic underscores the urgencies of access to quality‐assured vaccines and medicines. As Ursula von der Leyen, President of the European Commission (EC) recently stated: ‘A global pandemic requires a world effort to end it—none of us will be safe until everyone is safe’. 16
3. BEYOND VACCINE NATIONALISM
The worldwide effort to develop safe and effective vaccines has yielded many effective results—in part due to decisive investments in efforts such as Operation Warp Speed (OWS). OWS was the largest of the global efforts in the development of COVID‐19 vaccines with an investment of approximately U.S $ 1.8 billion in late stage clinical trials primarily for the U.S population. By mid‐August 2020, the United States had secured up to 800 million doses of six vaccines in development. 17 The United Kingdom purchased about 340 million doses and the EU and Japan had also purchased millions of doses. COVAX made deals to purchase about 300 million doses of the Oxford‐AstraZeneca vaccine but fell short of the 18 billion that it needed for donors to purchase and deliver up to 2 billion doses. Despite U.S President Biden's decision to donate millions of surplus vaccines through COVAX, early competitive procurement of vaccines (by the United States and other developed countries) perpetuates the notion of vaccine nationalism. Vaccine nationalism is the process whereby countries secure vaccines and medicines to supply their own populations before they become available to less wealthy nations. Even before the now‐approved COVID‐19 vaccines had completed Phase III clinical trials, wealthy countries such as the United States and the European Union (EU) bloc had procured the ones which seemed most promising. 18 However, as seen in the United States (which leads the world in COVID‐19 deaths) fatalities due to COVID‐19 had reached 600 thousand by June 2021. After devastating death tolls to millions received the first doses of the Pfizer‐BioNTech and Moderna vaccines—later followed by the general population.
The WHO has expressed concerns about unilateral deals made by wealthier countries and raised concerns about accessibility to the rest of the world. Tedros Adhanom stated that: ‘vaccine nationalism is not just morally indefensible; it is epidemiologically self‐defeating and clinically counterproductive’. 19 COVID‐19 is a global problem and given its spread and variants (Alpha, Delta) new mutations may be more resistant and could become dominant strains causing new infections in those vaccinated against older variants. Still, despite the rising death rates across the developing world Pfizer‐BioNTech and Moderna have sold most of their vaccines to wealthy countries—sometimes charging up to 24 times the original costs of production. 14
While Pfizer‐BioNTech announced a partnership with South African company Biovac to manufacture up to 100 million doses by 2022, the immunization campaign is plagued by unequal access. Africa remains the least vaccinated continent. 12 The partnership is noteworthy however because it is the first in the Southern Hemisphere to procure the mRNA technology underlying both the Moderna and Pfizer‐BioNTech vaccines. 15 An analysis of production for mRNA vaccines illustrates that these can be made for as little as $1.20 per dose. Yet COVAX has been paying up to five times more. 14 COVAX has also had difficulties with consistent supply since many wealthy nations have pushed to the front of the line. These bilateral deals between wealthier countries and vaccine manufacturers have raised concerns about the lack of equity and supply for LMIC and middle income countries.
While pharmaceutical companies have stated that they can ‘tweak’ vaccines with booster shots, these are forecasted to cost more as drug makers compete for sales. 11 Yet it is unclear how many people will need boosters and how often. While emerging research suggests that waning antibody levels in vaccinated people (after 6 months) necessitates the need for booster shots, many in the developing world have not gotten their initial shot. Given the seriousness of the pandemic, vaccine nationalism is shortsighted since failing to provide equity in distribution could double worldwide mortality rates.
In response, some activists have suggested that national governments insist vaccine developers licence their vaccines to domestic manufacturers thereby allowing countries to make their own versions of COVID‐19 vaccines. Stakeholders in this argument are at odds about the treatment of intellectual property (IP) rights now that COVID‐19 vaccines are on the market. As a result, the Trade Related Aspects of Intellectual Property Rights (TRIPS) Agreement has been part of a global conversation about patents for COVID‐19 vaccines. 20 TRIPS is a World Trade Organization (WTO) agreement on global property rights. Since October 2020, India and South Africa (with the support of several WTO member states) have proposed a TRIPS waiver that could temporarily wave IP protections needed to make COVID‐19 vaccines. The argument being that high income countries hold most of the vaccine IP and sell vaccines to the developing world. As such, the prices of vaccines—as well as the inability of LMIC's to make their own are partly the reason why vaccines are not reaching LMIC's fast enough. 21
On the other hand, many high income countries such as the United Kingdom, several EU member states, Japan, Canada and Australia have opposed the TRIPS waiver. In May 2021, U.S President Biden announced support for the waiver but has not gone further than the announcement of support. 22 Opposition to the waiver stems from the argument that patent protections for COVID‐19 vaccines are necessary and that pharmaceutical companies will not invest in research and development without them. Additionally, the argument states that removing IP protections will not speed up vaccination rates in LMIC's since many lack the domestic infrastructure to ramp up production.
Under WHO rules, compulsory licensures are permitted and have been used in the creation of antiretroviral drugs for HIV/AIDS. 23 However, these rules only cover patents and not the other proprietaries which often go into making a vaccine and getting it approved. As such, the process can be quite complex and are vastly different from the processes needed to make small molecule drugs. Still, high income and wealthier middle income countries have purchased up to 5.4 billion vaccine doses compared to LMICs which have just 1.2 billion doses.
3.1. Addressing vaccine inequities and global constraints
In spite of initiatives such as COVAX, several challenges exist in vaccine distribution and resource allocation. While some LMICs like Brazil and Indonesia struck advance purchase deals for vaccines like the Sinovac vaccine, majority of LMICs are acquiring vaccines through COVAX. 3 Other countries such as India secured more approved COVID‐19 vaccines than other LMICs due to its partnership with Oxford‐AstraZeneca and the Serum Institute of India one of the world's largest vaccine manufacturers. Through its partnership with the University of Oxford, the institute signed a licence to produce up to one billion doses of its vaccine per year—doses intended for LMICs and countries committed to COVAX. 15 Despite this, complications arose when India's vaccine manufacturing capacity was hit by a raw material shortage when President Biden invoked the Defense Production Act (DPA) in early 2021 giving American vaccine makers' priority access. At the urging of a bipartisan group of politicians, both Presidents Trump and Biden have repeatedly invoked the DPA in order to expand the production of medical goods such as N95 masks and COVID‐19 vaccines.
The DPA has drawn sharp criticism from the rest of the world including the Serum Institute of India. In April 2021, Adar Poonawalla the chief executive of the Serum Institute tweeted to President Biden that: ‘If we are to truly unite in beating this virus, on behalf of the vaccine industry outside the U.S., I humbly request you to lift the embargo of raw material exports out of the U.S.’ 24 But India itself is hard hit by the virus and exports that were originally promised for export were suspended as the Indian government gave priority to its domestic immunization campaign. In the spring, 7‐day incidence rates increased to highs of 200 per 100 thousand in just a few weeks. The decision to suspend exports is likely a blow to COVAX as LMICs face delays in supplies. The Serum Institute of India was Africa's largest source of vaccines prior to the suspension. 24 After weeks of public appeals from the Serum Institute, the US finally supplied filters needed to make COVISHIELD (a licenced version of the Oxford‐AstraZeneca vaccine). While President Biden has recently shown support for waiving patents for COVID‐19 vaccines, waivers would still not address a conspicuous problem: the worldwide shortage of vaccine raw materials and equipment. Essential materials such as filters, tubing, and specialized disposable bags are required and vaccines cannot be made without access to supplies—even with patent waivers. Additionally, many deficiencies complicate COVID‐19 inoculation efforts in LMICs. Some of these deficiencies include: effective diagnostic testing, sound epidemiological data, personal protective equipment (PPE) and systems to report adverse effects after vaccination.
In August 2021, AVAT announced the shipment of over 117 thousand doses of the Johnson and Johnson single shot vaccine to Togo. 25 This shipment is part of about 6.4 million doses to be shipped to the African Union member states. The Johnson and Johnson vaccine was selected because it is a single‐shot vaccine and because it is partly being manufactured in Africa with Aspen Pharmacare. Despite this agreement, Johnson and Johnson was criticized for shipping doses manufactured in Africa to Europe even though European countries have immunized large numbers of their citizens. 26 In contrast, Africa has vaccinated less than 3 percent of its people fully. Ramping up vaccine production on the continent is seen as a way to increase vaccination levels. Amid increased criticism of COVAX, LMIC's have touted the hoarding of vaccines, increased prices and reluctance of high income countries to back a TRIPS waiver.
Given these logistical concerns, social, political and religious unrest can also complicate vaccination efforts. For instance, many LMICs contain large numbers of internally displaced persons (IDP's). In countries such as Burkina Faso and Nigeria, the pandemic has exacerbated the factors that triggered the migration in the first place. Many IDP's remain within the borders of their own countries lacking housing and living in cramped camps which are difficult to practice social distancing. In Nigeria, for instance, thousands of IDP's have been triggered by conflicts with the Islamic militant group Boko Haram. 27 Displacement has also been compounded by recent natural disasters such as flooding from increased rainfall. These floods have led to the establishment of longstanding IDP camps. In other regions such as Brazil's Favela de Rocinha in Rio de Janeiro, about 250 thousand people live in cramped, overcrowded conditions. Most homes do not have running water—thereby making basic hygiene like hand washing with soap and water very difficult. 28 Given that Brazil has become a leader in COVID‐19 mortality, the threat of more deaths remains high.
In this vein, although vaccine hesitancy remains an issue (i.e. as seen in the Democratic Republic of the Congo where concerns about blood clots pushed the country to donate millions of doses of the Oxford‐AstraZeneca vaccine to other African countries before they expired), concerns about access are far more pressing given the rapid spread of the Alpha and Delta variants. All things considered, the scramble for COVID‐19 vaccines illustrates several formidable challenges: (a) restricted exports of vaccines and raw materials have stifled global needs; (b) vaccines have been dominated by nations with wealth, industrial capacities, scientific expertise and diplomatic relationships required to secure exclusive access. On the other hand, countries without such resources have been hard hit by successive COVID‐19 outbreaks overwhelming fragile healthcare systems. Rising cases have meant that health systems have had to deal with vaccine demands as well as shortages of hospital beds, oxygen and ventilators. Modelling by the Duke Global Health Innovation Center suggests that given the current pace of production and access, it could be up to 2023 or 2024 before enough vaccines are produced for the world population. 9 This means that the countries with the greatest needs are not always the ones with the most resources. In a high stakes global pandemic, the supply of vaccines cannot be an afterthought.
4. DISCUSSION
As Figure 1 illustrates, there is a need for an effective global partnership in ending the COVID‐19 pandemic. The figure depicts global partnerships with regards to vaccination efforts—especially as it concerns vaccine manufacturers, wealthy high income countries, and lower middle income countries. The pandemic has revealed how global power differentials have affected access to COVID‐19 testing, as well as vaccine distribution. Hospitals and local governments in many LMICs are scrambling to get the virus and its variants under control. Yet, in any functional health initiative, the most vulnerable should always be prioritized. To achieve vaccine equity, equitable distribution strategies must be sustained. It is important to see the COVID‐19 pandemic as what it is: The spread of an infectious disease embedded in a social and political context defined by unequal access to healthcare. Without support, LMICs will find it very difficult to emerge from this crisis.
FIGURE 1.
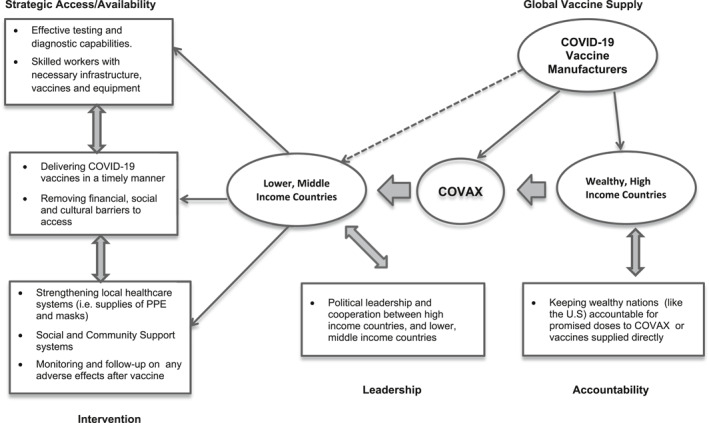
Conceptual model of COVID‐19 global vaccine supply and relationships with key partners
Health is a universal right. Yet, vaccine nationalism and expensive drug prices are prolonging the pandemic. Since the demand for the vaccine has already outpaced supply with high income countries taking a large share of doses, more innovation is needed to address global shortfalls. A main solution is for wealthy countries to stop hoarding vaccines. Another solution is for pharmaceutical companies to stop overcharging for COVID‐19 vaccines. A process is required whereby LMIC's can take some ownership over manufacturing and distribution without bureaucratic red tape. Measures to take ownership should address the building of infrastructure for local vaccine production. These incentives can include constant electricity and water supply (even to remote areas) so that production can be ramped up. A TRIPS waiver may only be a partial solution. Governments need to step up vaccine licencing programs with pharmaceutical companies to facilitate production. In the event that companies are still hesitant, the TRIPS waiver already allows compulsory licencing without any loss of IP.
Other solutions include regional cooperation to allow the pooling of purchasing powers. Regional economic initiatives such as AVAT, the Pan American Health Organization (PAHO) and the newly created Africa Continental Free Trade Area (AfCFTA) can establish regional value chains to enable small economies to be integral partners needed to fight future pandemics. Regional cooperation can also create legal certainty around IP rights when dealing with drug manufacturers. This will be helpful in boosting vaccine production capacities—not only in times of pandemic, but at all times. Again, it remains imperative that high‐income countries donate excess vaccines to parts of the world that need them the most. It is unfair that countries such as the U.S have approved booster shots while LMIC's remain with shortages of the first shot. Vaccinating the world is not only a national issue, or one constrained to a single country. Rather, it is an international one which will need sustained effort to complete. Given the rise of many resistant strains, global cooperation is not just a matter of social justice; it is a requirement for ending the spread of a highly contagious virus that knows no borders.
ETHICS STATEMENT
This manuscript was written based on an analysis of published articles and research. No ethical approval from an institutional review board is needed.
ACKNOWLEDGEMENTS
The author is grateful for all the constructive comments and feedback on previous drafts of the manuscript. The author received no financial support for the research, authorship and/or publication of this article.
Obinna DN. Solidarity across borders: a pragmatic need for global COVID‐19 vaccine equity. Int J Health Plann Mgmt. 2022;37(1):21‐29. doi: 10.1002/hpm.3341
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analysed in this study.
REFERENCES
- 1. Centers for Disease Control and Prevention. Percent of Delivered First Vaccine Doses Administered by U.S. States and Territories. June 28, 2021. Accessed June 30, 2021. https://www.cdc.gov/coronavirus/2019‐ncov/vaccines/distributing/first‐doses.html
- 2. Ledford H. Six months of COVID vaccines: what 1.7 billion doses have taught scientists. June 04, 2021. Accessed June 12, 2021. https://www.nature.com/articles/d41586‐021‐01505‐x [DOI] [PubMed]
- 3. Berkley S. COVAX Explained. Gavi, the Vaccine Alliance; 2020. Accessed May 23, 2021. https://www.gavi.org/vaccineswork/covax‐explained
- 4. Cirino W. South Sudan Returning 72,000 COVID Vaccine Doses. VOA News. May 24, 2021. Accessed June 24, 2021. https://www.voanews.com/africa/south‐sudan‐focus/south‐sudan‐returning‐72000‐covid‐vaccine‐doses
- 5. Shamasunder S, Holmes SM, Goronga T, et al. COVID‐19 reveals weak health systems by design: why we must re‐make global health in this historic moment. Glob Publ Health. 2020;15(7):1083‐1089. doi: 10.1080/17441692.2020.1760915 [DOI] [PubMed] [Google Scholar]
- 6. Kampmann B, Okomo U. COVID‐19 vaccines for children in LMICs: another equity issue. Lancet. 2021;398(10302):731‐732. doi: 10.1016/S0140-6736(21)01748-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Paun C, Furlong A. Poorer countries hit with higher price tag for Oxford/AstraZeneca vaccine. Politico. February 22, 2021. Accessed August 21, 2021. https://www.politico.eu/article/astrazeneca‐vaccine‐cost‐higher‐in‐poorer‐countries‐coronavirus/
- 8. Dyer O. Covid‐19: many poor countries will see almost no vaccine next year, aid groups warn. Br Med J (Online). 2020:371. doi: 10.1136/bmj.m4809 [DOI] [PubMed] [Google Scholar]
- 9. Holder J. Tracking coronavirus vaccinations around the world. New York Times. June 30, 2021. Accessed August 18, 2021. https://www.nytimes.com/interactive/2021/world/covid‐vaccinations‐tracker.html
- 10. Czabanowska K, Kuhlmann E. Public health competences through the lens of the COVID‐19 pandemic: what matters for health workforce preparedness for global health emergencies. Int J Health Plan Manag. 2021;36(S1):14‐19. doi: 10.1002/hpm.3131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Erman MP. Moderna seen reaping billions from COVID‐19 vaccine booster market. Reuters. August 13, 2021. Accessed August 15, 2021. https://www.reuters.com/business/healthcare‐pharmaceuticals/pfizer‐moderna‐seen‐reaping‐billions‐covid‐19‐vaccine‐booster‐market‐2021‐08‐13/
- 12. Sullivan H. South Africa paying more than double EU price for Oxford vaccine. The Guardian. January 22, 2021. Accessed August 10, 2021. https://www.theguardian.com/world/2021/jan/22/south‐africa‐paying‐more‐than‐double‐eu‐price‐for‐oxford‐astrazeneca‐vaccine
- 13. United Nations. About the sustainable development goals [Online]. 2019. Accessed May 2021. https://www.un.org/sustainabledevelopment/sustainable‐development‐goals/
- 14. Beaubien J. Price check: Nations pay wildly different prices for vaccines. NPR. February 19, 2021. Accessed August 15, 2021. https://www.npr.org/sections/goatsandsoda/2021/02/19/969529969/price‐check‐nations‐pay‐wildly‐different‐prices‐for‐vaccines
- 15. Baker SP. The world's best hope to end the pandemic still needs more doses. June 3, 2021. Accessed June 23, 2021. https://www.bloomberg.com/news/features/2021‐06‐03/when‐will‐covid‐pandemic‐really‐end‐covax‐says‐poor‐nations‐need‐vaccines
- 16.WHO. A global pandemic requires a world effort to end it – none of us will be safe until everyone is safe. Accessed June 23, 2021. https://www.who.int/news‐room/commentaries/detail/a‐global‐pandemic‐requires‐a‐world‐effort‐to‐end‐it‐none‐of‐us‐will‐be‐safe‐until‐everyone‐is‐safe
- 17. Katz IT, Weintraub R, Bekker LG, Brandt AM. From vaccine nationalism to vaccine equity—finding a path forward. N. Engl J Med. 2021;384(14):1281‐1283. doi: 10.1056/NEJMp2103614 [DOI] [PubMed] [Google Scholar]
- 18. Correia T. The precariousness of political management of the SARS‐CoV‐2 pandemic in the search for scientific answers: calling for prudence in public health emergencies. Int J Health Plan Manag. 2021;36:1387‐1391. doi: 10.1002/hpm.3253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. United Nations. Unequal vaccine distribution self‐defeating, World Health Organization Chief tells Economic and Social Council's Special Ministerial Meeting. April 6, 2021. Accessed June 23, 2021. https://www.un.org/press/en/2021/ecosoc7039.doc.htm
- 20. Labonté R, Johri M, Plamondon K, Murthy S. Canada, global vaccine supply, and the TRIPS waiver. Can J Public Health. 2021:1‐5. doi: 10.17269/s41997-021-00541-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rubin H, Saidel N. Innovation beyond patent waivers: Achieving global vaccination goals through public‐private partnerships. Brookings Institute. August 31, 2021. Accessed September 1, 2021. https://www.brookings.edu/blog/up‐front/2021/08/31/innovation‐beyond‐patent‐waivers‐achieving‐global‐vaccination‐goals‐through‐public‐private‐partnerships/
- 22. Gupta V, Namboodiri S. America and the TRIPS Waiver: You Can Talk The Talk, But Will You Walk The Walk? Health Affairs. July 13, 2021. Accessed August 12, 2021. https://www.healthaffairs.org/do/10.1377/hblog20210712.248782/full/
- 23. Ogunkola IO, Adebisi YA, Imo UF, Odey GO, Esu E, Lucero‐Prisno DE, III . Rural communities in Africa should not be forgotten in responses to COVID‐19. Int J Health Plan Manag. 2020;35(6):1302‐1305. doi: 10.1002/hpm.3039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reuters staff. Indian vaccine maker Serum Institute appeals to Biden to lift embargo on raw material exports. April 16, 2021. Accessed June 3, 2021. https://www.reuters.com/article/us‐health‐coronavirus‐india‐serum/indian‐vaccine‐maker‐serum‐institute‐appeals‐to‐biden‐to‐lift‐embargo‐on‐raw‐material‐exports‐idUSKBN2C30ZS
- 25.African Vaccine Acquisition Trust (AVAT) announces 117,600 doses of vaccines arriving in Togo as part of the first monthly shipment of Johnson & Johnson vaccines. Africa Medical Supplies Platform. Press Release. August 7, 2021. https://amsp.africa/african‐vaccine‐acquisition‐trust‐avat‐announces‐117600‐doses‐of‐vaccines‐arriving‐in‐togo‐as‐part‐of‐the‐first‐monthly‐shipment‐of‐johnson‐johnson‐vaccines/
- 26. Muhumuza R. Vaccines made in South Africa to stay in Africa, says envoy. Associated Press. September 2, 2021. Accessed September 5, 2021. https://apnews.com/article/europe‐africa‐business‐health‐coronavirus‐pandemic‐b2797c07c6233c28bdd43827b55789bf
- 27. Ajayi TF. Women, internal displacement and the Boko Haram conflict: broadening the debate. Afr Secur. 2020;13(2):171‐194. doi: 10.1080/19392206.2020.1731110 [DOI] [Google Scholar]
- 28. Fernandes LA, Silva CA, Dameda C, Bicalho PP. Covid‐19 and the Brazilian reality: the role of favelas in combating the Pandemic. Front. Sociol. 2020;5:117. doi: 10.3389/fsoc.2020.611990 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.


