Severe coronavirus disease 2019 (COVID‐19), due to the severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), may be associated with mild thrombocytopenia, 1 , 2 and some COVID‐19‐induced immune thrombocytopenia (ITP) cases have been reported. 3 , 4 Some reports suggest that patients with pre‐existing ITP may experience a relapse 5 in the case of SARS‐CoV‐2 infection that could be prevented by vaccination. However, a growing number of de novo ITP occurring after anti‐SARS‐CoV‐2 vaccination are being reported with mRNA‐based vaccines. In a recent series, 6 20 patients who received either BNT162b2 (Comirnaty®) or mRNA‐1273 (SpikeVax®) vaccines had new‐onset thrombocytopenia after the first injection (median time: 5 days), usually associated with bleeding and low platelet counts (median: 2 × 109/l). The incidence of vaccine‐induced thrombocytopenia remains difficult to assess precisely as no routine blood count is recommended after vaccination. However, it appears very low, with >20 million people vaccinated in the United States with at least one dose at the time this paper 6 was submitted. However, whether patients with ITP have a risk of relapse after anti‐SARS‐CoV‐2 vaccination remains a matter of debate. In France, the National Reference Centre for Adult Immune Cytopenia proposed that anti‐SARS‐CoV‐2 vaccination should be considered in patients with ITP and that a platelet count should be obtained 1 week after each vaccine injection in order to detect ITP relapse. In the present study, we assessed the evolution of a cohort of patients with ITP that received anti‐SARS‐CoV‐2 vaccination.
We conducted an observational study in four French referral centres from January 2021 to June 2021. Inclusion criteria were patients aged >18 years with a previous ITP diagnosis (primary or secondary) according to international criteria 7 and receiving at least one anti‐SARS‐CoV‐2 vaccine injection. All these patients were already enrolled in the prospective CARMEN‐France registry and were not opposed to real‐world data collection about ITP management. All clinical and biological data used in the present study were already recorded in the registry (authorisation number 2012‐438 from the French national agency regulating data protection).
From January to June 2021, 92 adult patients with ITP (55 women, 59·8%), with a median [interquartile range (IQR), range] age of 69 [19, 24–90] years (Table SI) were selected. In all, 78 patients had primary ITP (85%), and two had a history of COVID‐19‐associated ITP 1 year previously. At the time of first vaccine dose, the median (IQR, range) ITP duration was 90 (164, 2–914) months and 38 patients (41%) had no ongoing treatment (Table SI). Patients received BNT162b2 (Comirnaty®; n = 78, 85%), ChadOx1nCoV‐19 (VAXZEVRIA®; n = 9, 10%), and mRNA‐1273 (SpikeVax®; n = 5, 5%) vaccines.
First, we focussed on the 65 patients (70%) who had a platelet count at 7 ± 3 days after the first shot of vaccine (Fig 1). The last platelet count available (56/65 patients) in the 4 weeks before vaccination was a median (IQR, range) of 116 (137, 12–644) × 109/l, including two patients (3%) with platelet counts of <30 × 109/l, while the median (IQR, range) platelet count at 7±3 days after the first shot was 118 (133, 14–729) × 109/l, among which three patients (4·5%) had a platelet count of <30 × 109/l (one of them also had a platelet count of <30 × 109/l before vaccination; Fig 2). A total of 70 patients (76%) were fully vaccinated (two shots), all of them with the same vaccine (including 46 patients with a platelet count at 7±3 days after the first shot). Reasons for absence of the second injection were lack of follow‐up (16 patients), SARS‐CoV‐2 infection before the second injection (four), patient refusal (one) and ITP relapse (one). Among the 53 patients (75·7%) who had a platelet count available at 7±3 days after the second dose, six (11·3%) had a platelet count of <30 × 109/l.
Fig 1.
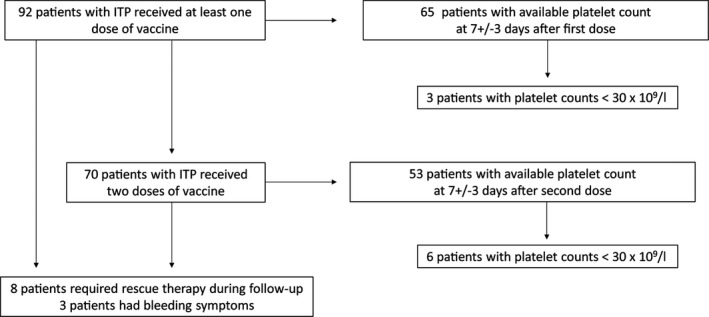
Flow chart of patients with immune thrombocytopenia (ITP).
Fig 2.
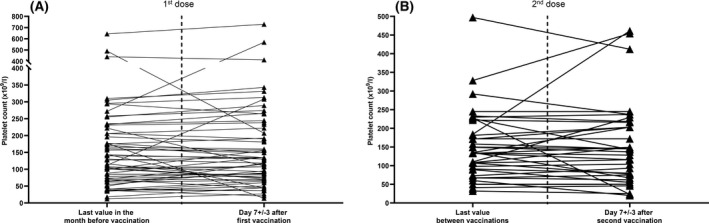
Platelet counts before and after vaccination in the 65 patients with platelet counts available at 7 ± 3 days after the first vaccine dose. A paired t‐test was used to compare continuous variables as appropriate. A P ≤ 0·05 was considered statistically significant.
During the follow‐up [median (IQR, range) time: 59 (35, 15–126) days], after the first vaccination of the entire cohort of 92 patients (Fig S1), eight (8·7%) required a rescue therapy and/or a change of ITP therapy, including three patients with bleeding manifestations (Fig 1 and Fig S2). Among these eight patients, three had a previous stable disease, and platelet counts decreased only after vaccination (Fig S2A–C). One 69‐year‐old man with a history of ITP who had been in remission without any treatment for 7 years, had a decrease in platelet count from 177 × 109/l (18 days before) to 12 × 109/l on day 2 after vaccination with BNT162b2 (Fig S2A). The patient presented with bleeding and received dexamethasone, repeated intravenous immunoglobulin courses, romiplostim and vinblastine with a transient response. After 2 months, he received rituximab and eventually achieved a complete response in combination with romiplostim at 10µg/kg/week. He was not given a second vaccine dose. One 49‐year‐old woman with chronic ITP and long‐term treatment with dapsone and low‐dose corticosteroids had fluctuating platelet counts of between 30 and 60 × 109/l without bleeding. At 1 week after the second injection of mRNA‐1273 vaccine she had mild purpura and 20 × 109/l platelets (Fig S2B). She achieved a complete response 5 days after a transient increase of her corticosteroids dose. One 46‐year‐old man with chronic ITP and long‐term treatment with eltrombopag (Fig S2C) had a decrease in platelet count from 227 × 109/l at 11 days before to 23 × 109/l at 2 days after the second dose of BTN16B2, with no bleeding. He achieved a response (49 × 109/l) 5 days after an increase in the eltrombopag dose combined with a short course of prednisone. The five remaining patients had bleeding symptoms and treatment modifications within the 3 months preceding vaccination, with multiple relapses that were chronologically unrelated to vaccination (Fig S2D–H).
Overall, we observed three clinically significant drops in platelet counts that could be attributed to anti‐SARS‐CoV‐2 vaccine (3·3%). Interestingly, all ITP relapses occurred within the week after vaccination, as previously described for thrombocytopenia in patients with no pre‐existing ITP 6 and in a recent series, 8 and in two out of three patients after the second dose. The low number of events did not allow the identification of risk factors, but one patient had ITP remission for several years, suggesting that ITP relapse may be unpredictable. One of the main limitations of our present study is the relatively few patients and limited follow‐up. Furthermore, the proportion of patients who received non‐RNA‐based vaccines in our present study was too low (nine patients) to draw any definite conclusion on the safety of non‐replicating adenovirus‐based vaccines in patients with ITP. Overall, our present results suggest that ITP relapses after mRNA vaccines are rare and most often benign and support a routine assessment of platelet counts in the week after each vaccination.
Author contributions
Etienne Crickx and Bertrand Godeau designed the study and analysed the data. Etienne Crickx, Guillaume Moulis, Matthieu Mahevas and Marc Michel wrote the manuscript. Other authors included patients in the study.
Conflict of Interest
Guillaume Moulis received meeting attendance grants from Amgen and Novartis, is coordinator of research studies granted by Amgen, CSL Behring, Novartis and Grifols. He participated to educational sessions funded by Amgen and Novartis, and to boards for Amgen, Argenx, Novartis and Sobi. Mikael Ebbo received meeting attendance grants from Novartis, Octapharma and Sobi, and participated to educational sessions for Amgen, Grifols, Novartis and to boards for Grifols and Novartis. Marc Michel participated to educational sessions and boards for Amgen, Argenx, Novartis, Sobi and UCB. Matthieu Mahevas received meeting attendance grants from Amgen and Novartis, is coordinator of research studies granted by GSK. He participated to educational sessions funded by Amgen and Grifols. Bertrand Godeau participated in educational sessions and boards for Amgen, Grifols, Novartis, Roche and Sobi. Louis Terriou participated in educational sessions and boards for Amgen, Novartis and Sobi. The other authors have no conflict of interest to declare.
Supporting information
Fig S1. Platelet counts after vaccination.
Fig S2. Patients with treatment modification during follow‐up after first vaccine (day 0, dotted line).
Table SI. Patients’ characteristics.
Acknowledgments
We thank Marine Cecchi and Odile Gosset for technical assistance.
References
- 1. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maquet J, Lafaurie M, Sommet A, Moulis G. Thrombocytopenia is independently associated with poor outcome in patients hospitalized for COVID‐19. Br J Haematol. 2020;190:e276–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mahévas M, Moulis G, Andres E, Riviere E, Garzaro M, Crickx E, et al. Clinical characteristics, management and outcome of COVID‐19‐associated immune thrombocytopenia: a French multicentre series. Br J Haematol. 2020;190:e224–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Murt A, Eskazan AE, Yılmaz U, Ozkan T, Ar MC. COVID‐19 presenting with immune thrombocytopenia: A case report and review of the literature. J Med Virol. 2021;93:43–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee EJ, Liu X, Hou M, Bussel JB. Immune thrombocytopenia during the COVID‐19 pandemic. Br J Haematol. 2021;193:1093–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee EJ, Cines DB, Gernsheimer T, Kessler C, Michel M, Tarantino MD, et al. Thrombocytopenia following Pfizer and Moderna SARS‐CoV‐2 vaccination. Am J Hematol. 2021;96:534–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Neunert C, Terrell DR, Arnold DM, Buchanan G, Cines DB, Cooper N, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019;3:3829–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuter DJ. Exacerbation of immune thrombocytopenia following COVID‐19 vaccination. Br J Haematol. 2021. 10.1111/bjh.17645 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Platelet counts after vaccination.
Fig S2. Patients with treatment modification during follow‐up after first vaccine (day 0, dotted line).
Table SI. Patients’ characteristics.


