Abstract
Aims
Fluvoxamine, a selective serotonin reuptake inhibitor (SSRI) and sigma‐1 receptor agonist, has so far shown promise in the prevention of COVID‐19 progression as an early treatment option in three trials. The aim of this study was to evaluate the safety and efficacy of fluvoxamine in COVID‐19 patients if administered later in the course of the disease.
Methods
The study was designed as an open‐label, prospective cohort trial with matched controls. In April and May 2021, 51 ICU COVID‐19 patients hospitalised in the University Hospital Dubrava and University Hospital Centre Zagreb, Croatia, were treated with fluvoxamine 100 mg three times daily for 15 days in addition to standard therapy and they were prospectively matched for age, gender, vaccination against COVID‐19, disease severity and comorbidities with 51 ICU controls.
Results
No statistically significant differences between groups were observed regarding the number of days on ventilator support, duration of ICU or total hospital stay. However, overall mortality was lower in the fluvoxamine group, 58.8% (n = 30/51), than in the control group, 76.5% (n = 39/51), HR 0.58, 95% CI (0.36–0.94, P = .027).
Conclusion
Fluvoxamine treatment in addition to the standard therapy in hospitalised ICU COVID‐19 patients could have a positive impact on patient survival. Further studies on the effects of fluvoxamine in COVID‐19 patients are urgently required.
Keywords: COVID‐19, fluvoxamine, ICU, intensive care, SARS‐CoV‐2; SSRI, clinical trial

What is already known about this subject
Fluvoxamine is known to reduce inflammation and cytokine production in a mouse sepsis model through interactions with the sigma‐1 receptor.
Early outpatient treatment of COVID‐19 patients with fluvoxamine has been demonstrated as effective in preventing clinical deterioration and hospitalisation in three studies performed thus far.
What this study adds
It seems fluvoxamine treatment in addition to standard therapy in hospitalised ICU COVID‐19 patients could have a positive impact on patient survival.
Administration of fluvoxamine to ICU COVID‐19 patients was well tolerated.
1. INTRODUCTION
The first cases of coronavirus disease 2019 (COVID‐19) were reported in December 2019, in Wuhan, China. The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) has since spread worldwide causing a pandemic with more than 215 714 824 confirmed cases and 4 490 753 deaths by 31 August 2021. 1
Multiple SARS‐CoV‐2 vaccines have been developed in record time, but the problems of vaccine hesitancy, 2 global vaccine distribution inequalities, 3 emergence of new variants 4 and fading postvaccination immunity 5 , 6 make the goal of reaching global herd immunity against SARS‐CoV‐2 unlikely. This emphasises the need for effective, cheap and widely available pharmacological treatment options. New small molecular anti‐SARS‐CoV‐2 drugs are currently being developed by several pharmaceutical companies, but by the time these new compounds successfully pass clinical trials and reach the market as new pharmacological entities, their price and low availability will once again limit worldwide use and broaden inequalities between the poor and the rich countries. Therefore, since the start of the pandemic, the repurposing of already existing and approved drugs for COVID‐19 has been deemed a worthwhile approach.
Many potential drugs have already been tested for repurposing against COVID‐19, but the results of such trials have mostly been disappointing. The WHO Solidarity Trial interim results showed that hydroxychloroquine, lopinavir, remdesivir and interferon beta‐1a had no impact on mortality, hospitalisation duration or initiation of ventilation support in COVID‐19 patients. 7 Among other drugs considered for repurposing, neither azithromycin, 8 metformin 9 nor favipiravir 10 demonstrated significant benefit in the treatment of COVID‐19 patients. Large controversies currently exist around the use of ivermectin in COVID‐19, 11 but both the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) advise against its use for the prevention or treatment of COVID‐19 outside of randomised clinical trials.
Despite many proposed repurposing drugs failing, fluvoxamine, an old, widely available and cheap selective serotonin reuptake inhibitor (SSRI) has shown promise in treating COVID‐19 as an early outpatient therapy in three reported trials.
First, Lenze et al. 12 conducted a double‐blind, placebo‐controlled, randomised clinical trial of fluvoxamine in adult outpatients with confirmed SARS‐CoV‐2 infection focusing on its efficacy in preventing clinical deterioration (defined as hypoxia, <92% oxygen saturation, with either shortness of breath or hospitalisation). The final analysis included 80 patients in the intervention group, who received up to 3 × 100 mg fluvoxamine for up to 15 days post PCR‐confirmed SARS‐CoV‐2 infection, and 72 patients in the placebo group. None of the 80 patients in the treatment group experienced clinical deterioration, while in the placebo group, six patients (8.3%) clinically deteriorated, which resulted in a between‐group difference of 8.7% (95% CI, 1.8%–16.4%, P = .009).
Second, Seftel and Boulware 13 conducted an open‐label prospective cohort study on 113 SARS‐COV‐2 antigen‐positive patients who were offered 2 × 50 mg fluvoxamine as a treatment option. Out of 113 patients, 65 opted for fluvoxamine treatment and 48 declined therapy. No hospitalisations occurred in the fluvoxamine group, while six of the 48 control patients required hospital admission (P = .005).
Finally, the TOGETHER trial, 14 conducted on symptomatic SARS‐CoV‐2‐positive adult patients with a known risk factor for severe disease progression, allocated 739 patients to its fluvoxamine arm and 733 patients received placebo. The intervention group received 2 × 100 mg fluvoxamine for 10 days, with the primary endpoint being emergency room observation for >6 hours or hospitalisation from COVID‐19. The primary endpoint was reached in 10.4% (77/739) of patients in the fluvoxamine group and 14.7% (108/733) in the control group, resulting in a significant 29% relative risk reduction. There were no significant effects on viral clearance, mortality, hospitalisation duration, or number of days on mechanical ventilation.
Fluvoxamine's mechanism of action in COVID‐19 is still unknown, but it is presumed to be multifactorial (see Figure 1). 15 , 16 , 17 Besides being an SSRI, fluvoxamine also has high affinity for the sigma‐1 receptor (S1R) which has been shown to mediate fluvoxamine's anti‐inflammatory and immunomodulatory effects in an animal model of lipopolysaccharide (LPS) induced sepsis. 18 The same study also reported that fluvoxamine significantly reduced the production of interleukin (IL)‐6, IL‐1 beta, IL‐12 and IL‐8 induced by LPS in human heparinised peripheral blood. 18 In light of COVID‐19, S1R activation is believed to act immunomodulatorily by reducing endoplasmic reticulum stress due to SARS‐CoV‐2 replication resulting in a reduction of proinflammatory cytokine production. 17 , 19 Fluvoxamine is also known to inhibit cyclooxygenase 2 expression in LPS‐stimulated macrophages. 20
FIGURE 1.
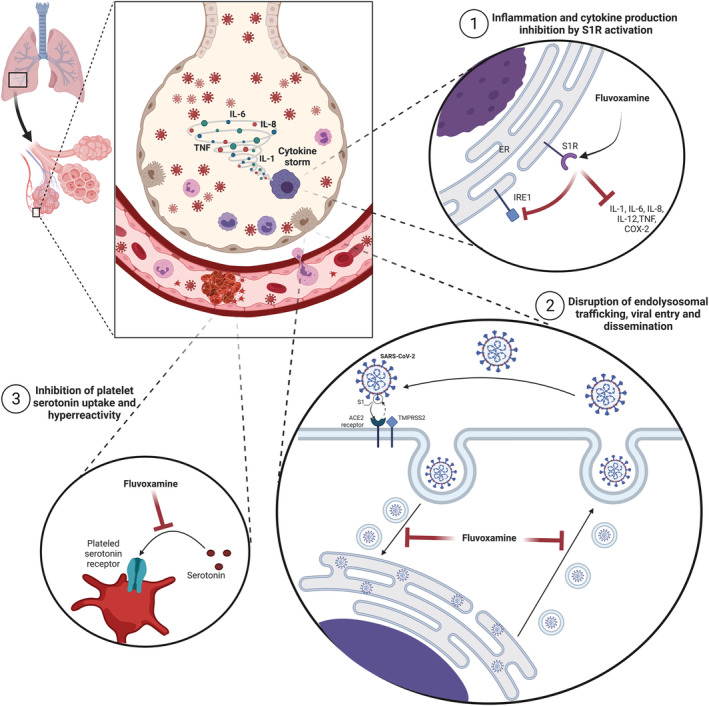
Proposed mechanisms of action of fluvoxamine in COVID‐19. Created with BioRender.com
Additionally, fluvoxamine was also proposed as a lysosomotropic agent which, by influencing intravesical pH levels, could disrupt intracellular endolysosomal trafficking, viral fusion and thereby dissemination. 21 The lysosomotropic properties of fluvoxamine could be tightly connected with its functional inhibition of acid sphingomyelinase (FIASMA). Additional research suggested that fluvoxamine could be a FIASMA agent. 22 It seems that SARS‐CoV‐2 cell viral entry depends on activation of the ASM/ceramide system which leads to the formation of ceramide‐enriched membrane domains and clustering of ACE2 SARS‐CoV‐2 viral entry receptors. 23 Inhibition of ASM by antidepressants fluoxetine and amitriptyline, which are also FIASMA agents, was shown to inhibit the entry and propagation of SARS‐CoV‐2 in cell culture models. 24 , 25
Other suggested mechanisms possibly contributing to fluvoxamine protective effects in COVID‐19 include prevention of platelet hyperreactivity and serotonin depletion, 15 increases in plasma melatonin levels through CYP1A2 and CYP2C19 inhibition 26 and inhibition of mast cell histamine release. 15
Trials conducted so far regarding the use of fluvoxamine in COVID‐19 evaluated its efficacy and safety in outpatients, early in the course of the disease. This is the first study to investigate the safety and potential efficacy of fluvoxamine when administered to COVID‐19 patients treated in the ICU.
2. METHODS
In April and May of 2021, 51 COVID‐19 patients who met criteria for severe disease were admitted into the intensive care units (ICUs) of the University Hospital Dubrava and the University Hospital Centre Zagreb, Croatia, and started on fluvoxamine 100 mg three times daily for 15 days in addition to standard therapy immediately following ICU admission. The standard therapy included conventional oxygen therapy or invasive mechanical ventilation, when indicated. Cardiovascular complications (myocardial injury, myocarditis, thromboembolic events, cardiogenic shock, etc.) were treated according to guidelines while continuous renal replacement therapy (CRRT) was used in acute renal failure. COVID‐19 pharmacotherapy included administration of remdesivir and corticosteroids. Other therapies such as tocilizumab, anakinra or other anti‐cytokine drugs were not used. After Day 15, the patients were tapered off fluvoxamine with a reduced dose of 50 mg two times daily for 7 days before complete treatment cessation. The dosing regimen has been chosen in accordance with the fluvoxamine COVID‐19 trials conducted previously, and fluvoxamine 100 mg three times daily was selected as a dosing scheme that could potentially be more effective in critically ill COVID‐19 patients treated in the ICU.
The study was designed as an open‐label, prospective cohort trial with matched controls. The fluvoxamine patient cohort was prospectively matched for age, gender, vaccination against COVID‐19, disease severity and comorbidities with 51 ICU controls.
The primary study endpoint was the difference in survival between the treatment group and the control group, and the secondary study endpoint was the duration of hospital stay. Considering the earlier observations of COVID‐19 ICU patient mortality in Croatia of up to 80%, the sample size was estimated at 38 patients in each group in order to detect a difference in mortality of 30% (study power: 80%, alpha: 0.05, beta: 0.2). The trial was ended in May 2021 due to an improving COVID‐19 epidemiological situation within the country and a lack of ICU COVID‐19 patients. The study flow chart is provided in the Appendix.
The key study inclusion criteria were: (1) patients over the age of 18, (2) positive laboratory confirmed SARS‐CoV‐2 PCR test, (3) acute clinical condition consistent with COVID‐19 requiring ICU admission, and (4) capacity to understand, willingness to participate in and sign the study informed consent form.
Exclusion criteria for the study were: (1) immunocompromised patients (e.g., having a solid organ or bone marrow transplant, AIDS or having been on high dose corticosteroid therapy), (2) underlying chronic conditions: severe pulmonary disease (oxygen‐dependant COPD, interstitial lung disease, pulmonary hypertension), decompensated liver cirrhosis or congestive heart failure, (3) involvement in other interventional COVID‐19 trials or concomitant therapy with chloroquine, hydroxychloroquine, azithromycin or colchicine, (4) inability or unwillingness to sign the study informed consent form, (5) known prior hypersensitivity reaction to the interventional drug, and (6) SSRI use in chronic therapy preceding hospitalisation.
Ethical approval for the study was provided by the Institutional ethics board of the University Hospital Centre Zagreb, Croatia. Informed consent was obtained from all patients participating in the study.
R programming language v. 4.1.1 was used for statistical data analysis and descriptive statistics. Fisher's exact test was used for categorical variables and independent t‐test for continuous variables. Cox univariate and multivariate regressions were used for survival analysis and hazard ratio estimates.
2.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20. 27
3. RESULTS
A total of 102 ICU COVID‐19 patients were included in the study: 51 in the fluvoxamine group and 51 standard therapy patients were matched as controls. Patient characteristics are provided in Table 1. There were no significant differences between the groups with regard to age, gender, body mass index (BMI), chronic comorbidities, disease duration prior to ICU admission, COVID‐19 vaccination status, Apache II or SOFA score.
TABLE 1.
Patient characteristics
| Group | Fluvoxamine + standard therapy | Standard therapy | P‐value | ||
|---|---|---|---|---|---|
| Male | Female | Male | Female | ||
| No. of patients | 34 (66.7%) | 17 (33.3%) | 34 (66.7%) | 17 (33.3%) | |
| Age, years (mean ± SD) | 65 ± 12 | 66.82 ± 11.52 | 65.59 ± 11.9 | 66.71 ± 11.49 | 0.987 |
| Age >65 years | 20 (39%) | 12 (70.6%) | 20 (39%) | 12 (70.6%) | |
| Age 50–64 years | 10 (20%) | 3 (17.7%) | 10 (20%) | 3 (17.7%) | |
| Race/ethnicity | |||||
| Caucasian | 34 (100%) | 17 (100%) | 34 (100%) | 17 (100%) | |
| BMI (mean ± SD) | 28.70 ± 5.39 | 29.15 ± 3.95 | 30.13 ± 4.36 | 31.28 ± 8.48 | 0.129 |
| Chronic comorbidity | |||||
| Diabetes | 9 (27%) | 3 (17.7%) | 9 (27%) | 5 (29%) | 0.653 |
| Hypertension, treated | 19 (56%) | 12 (70.6%) | 25 (74%) | 13 (76.5%) | 0.141 |
| Coronary artery disease | 4 (11.8%) | 3 (17.6%) | 2 (5.9%) | 0 | 0.08 |
| COPD | 2 (6%) | 0 | 4 (12%) | 1 (6%) | 0.245 |
| Cerebrovascular disease | 1 (3%) | 1 (6%) | 0 | 0 | 0.159 |
| Chronic renal insufficiency | 3 (9%) | 0 | 2 (6%) | 1 (6%) | 1 |
| Hepatic cirrhosis | 1 (3%) | 0 | 0 | 2 (11.8%) | 0.563 |
| Apache II score (mean ± SD) | 13.3 ± 5.1 | 13.9 ± 5.9 | 0.545 | ||
| SOFA score (mean ± SD) | 3.9 ± 2.3 | 4.1 ± 2.5 | 0.651 | ||
| COVID‐19 vaccination | 6 (11.8%) | 6 (11.8%) | 1 | ||
Treatment outcomes are shown in Table 2. No significant differences between the groups were observed in the number of days on ventilator support, duration of ICU or total hospital stay. In contrast, the overall mortality was significantly lower in the fluvoxamine group, 58.8% (n = 30/51), than in the control group, 76.5% (n = 39/51), HR 0.58, 95% CI (0.36–0.94, P = .027). Kaplan–Meier survival curves are depicted in Figure 2.
TABLE 2.
Treatment outcomes
| Group | Fluvoxamine + standard therapy | Standard therapy | P‐value | ||
|---|---|---|---|---|---|
| Male | Female | Male | Female | ||
| Disease duration in days prior to ICU admission (mean ± SD) | 12.82 ± 10.4 | 10.47 ± 8.8 | 12.74 ± 6.30 | 10.44 ± 7.27 | 0.952 |
| Days of ICU stay | 14.35 ± 10.5 | 13.94 ± 12.5 | 11.59 ± 12.37 | 12.4 ± 11.1 | 0.304 |
| Days of hospital stay | 21.03 ± 13.73 | 20.88 ± 14.25 | 17.12 ± 12.99 | 16.9 ± 12.8 | 0.139 |
| Days on ventilator support | 10.03 ± 10.82 | 9.16 ± 10.63 | 10.21 ± 11.84 | 12.3 ± 10.9 | 0.544 |
| Mortality males | 23/34 (68%) | – | 25/34 (74%) | – | HR 0.69, 95% CI (0.39–1.24, P = .22) |
| Mortality females | – | 7/17 (41.2%) | – | 14/17 (82.4%) | HR 0.40, 95% CI (0.16–0.99, P = .047) |
| Total mortality | 30/51 (58.8%) | 39/51 (76.5%) | HR 0.58, 95% CI (0.36–0.94, P = .027) | ||
FIGURE 2.

Kaplan–Meier survival curves of COVID‐19 ICU patients (n = 51) treated with fluvoxamine + standard therapy in comparison to patients (n = 51) treated with standard therapy alone; HR 0.58, 95% CI (0.36–0.94, P = .027)
Additionally, no statistically significant differences in mortality were observed between men, with 68% (n = 23/34) deaths in the fluvoxamine group vs 74% (n = 25/34) deaths in the control group, HR 0.69, 95% CI (0.39–1.24, P = .22). However, among women, there were significantly fewer deaths in the fluvoxamine group, 41.2% (n = 7/17) vs 82.4% (n = 14/17) deaths in the control group; HR 0.40, 95% CI (0.16–0.99, P = .047).
Complications comparison between the groups is shown in Table 3. There were no significant differences between groups, except for CRRT, which was administered to 41.2% (n = 21/51) of fluvoxamine patients in comparison to 11.8% (n = 6/51) of standard therapy patients (P < .001).
TABLE 3.
Comparison of complications
| Complication | Fluvoxamine + standard therapy | Standard therapy | P‐value |
|---|---|---|---|
| Acute renal failure | 19 (37.3%) | 13 (25.5%) | 0.2 |
| Acute myocardial infarction | 1 (1.9%) | 3 (5.9%) | 0.31 |
| Bacterial pneumonia | 32 (62.8%) | 34 (67%) | 0.68 |
| Bleeding | 6 (11.8%) | 8 (15.7%) | 0.56 |
| CRRT | 21 (41.2%) | 6 (11.8%) | <0.001 |
| ECMO | 2 (3.9%) | 3 (5.9%) | 0.65 |
| Inotropic support | 13 (25.5%) | 6 (11.8%) | 0.08 |
| Pneumomediastinum | 5 (9.8%) | 6 (11.8%) | 0.75 |
| Pneumothorax | 7 (13.7%) | 5 (9.8%) | 0.54 |
| Sepsis | 28 (54.9%) | 32 (62.7%) | 0.42 |
| Stroke | 1 (1.9%) | 0 | 0.31 |
| Thromboembolism | 2 (3.9%) | 2 (3.9%) | 1 |
| UTI | 13 (25.5%) | 18 (35.3%) | 0.28 |
| Vasopressor | 36 (70.6%) | 40 (78.4%) | 0.36 |
A multivariate Cox regression analysis of time to death was conducted on time‐constant covariates for all 102 patients (see Table 4). The analysis demonstrated four variables with statistically significant effects: (1) coronary artery disease, HR 4.06, 95% CI (1.33 ± 12.35; P = .014); (2) CRRT, HR 2.26, 95% CI (1.14 ± 4.49; P = .019); (3) age, HR 1.06, 95% CI (1.02 ± 1.1; P = .000451) and (4) fluvoxamine treatment, HR 0.42, 95% CI (0.22 ± 0.8; P = .009).
TABLE 4.
Multivariate Cox regression analysis of time to death on the time‐constant covariates
| Covariate | Hazard ratio (HR) |
|---|---|
| Age | HR 1.06, 95% CI (1.02 ± 1.1; P = .000451) |
| APACHE II score | HR 1.07, 95% CI (0.99 ± 1.15; P = .058) |
| Arterial hypertension | HR 1.83, 95% CI (0.89 ± 3.78; P = .097) |
| Body mass index | HR 1.04, 95% CI (0.98 ± 1.1; P = .17) |
| Cerebrovascular disease | HR 1.24, 95% CI (0.11 ± 14.5; P = .86) |
| Chronic renal insufficiency | HR 1.39, 95% CI (0.28 ± 6.98; P = .68) |
| Chronic obstructive pulmonary disease | HR 0.52, 95% CI (0.17 ± 1.63; P = .265) |
| Coronary artery disease | HR 4.06, 95% CI (1.33 ± 12.35; P = .014) |
| CRRT | HR 2.26, 95% CI (1.14 ± 4.49; P = .019) |
| Diabetes mellitus type 2 | HR 0.72, 95% CI (0.37 ± 1.38; P = .31) |
| Fluvoxamine | HR 0.42, 95% CI (0.22 ± 0.8; P = .009) |
| Gender | HR 1.05, 95% CI (0.57 ± 1.9; P = .87) |
| Hepatic cirrhosis | HR 1.22, 95% CI (0.25 ± 5.87; P = .8) |
| Hyperlipidaemia | HR 1.31, 95% CI (0.65 ± 2.67; P = .44) |
| SOFA score | HR 1.11, 95% CI (0.97 ± 1.26; P = .126) |
4. DISCUSSION
This open‐label, prospective cohort trial is the first study to investigate the safety and efficacy of fluvoxamine in ICU COVID‐19 patients. Fluvoxamine has shown efficacy as an early outpatient treatment option, 12 , 13 , 14 but it was unknown if it would have any beneficial effect if started later in the disease course. Our study results indicate that fluvoxamine treatment in addition to standard therapy in ICU patients could have potential for significant mortality reduction, HR 0.58, 95% CI (0.36–0.94, P = .027).
Concerning the protective effects of COVID‐19 vaccination status on patient outcomes, only one patient in each group was fully vaccinated, while five more patients in each study arm were partially vaccinated; however, we observed no significant association between vaccination status and mortality reduction.
Looking at mortality according to gender, it seems the effect of fluvoxamine was more significant among female COVID‐19 ICU patients. Gender differences in mortality in ICU COVID‐19 patients have been described before, with significantly lower mortality rates among women. 28 Also, gender‐specific differences in fluvoxamine pharmacokinetics have been described in the published literature, 29 as well as gender differences in CYP2D6 30 and CYP1A2 31 enzyme activity. Whether the same gender‐specific differences which impact COVID‐19 mortality also play a role in the response to fluvoxamine treatment, or whether biological sex‐specific differences in cytochrome P450 enzymes CYP2D6 and/or CYP1A2 could be responsible for improved efficacy in female patients and consequently better outcomes remains to be investigated in additional studies.
Moreover, there were roughly 40% more diabetic women in the control arm as compared to the treatment arm. As diabetes is a well‐known poor prognostic factor in severely ill COVID‐19 patients, it is possible that this fact contributed to an increased mortality among diabetic women in the control group, 80% (n = 4/5) vs 67% (n = 2/3) in the treatment arm. Importantly, univariate Cox regression analysis performed for diabetes mellitus covariate among females was not significant, HR 1.55, 95% CI (0.59 ± 4.01; P = .4), most likely due to the small sample size.
Additionally, mortality among men with coronary artery disease was 100% (n = 4/4) in the treatment as well as in the control arm (n = 2/2). It was 33% (n = 1/3) among women in the treatment group, while no women with coronary artery disease were present in the control group. History of coronary artery disease was a covariate most strongly associated with poor prognosis in this study, HR 4.06, 95% CI (1.33 ± 12.35; P = .014) (see Table 4).
Regarding the safety of fluvoxamine in COVID‐19 ICU patients, the drug was well tolerated with no statistically significant differences in complications between the two study groups except for more common use of CRRT in the fluvoxamine group. As fluvoxamine is one of the oldest SSRIs, extensive post‐marketing safety studies 32 , 33 exist. In our view, the higher prevalence of CRRT in the intervention group is probably not a direct consequence of fluvoxamine treatment, but more likely a consequence of acute kidney injury (AKI). This was shown to be 1.46 times more frequent in the treatment group (n = 19), than in the control group (n = 13), despite this difference being statistically nonsignificant (P = .2). It is worth noting that CRRT can sometimes be used in sepsis 34 and for CAR‐T therapy 35 associated cytokine release syndrome with an aim of reducing circulating cytokine levels. Such a rationale might indicate that the higher incidence of CRRT in the fluvoxamine group was a confounding factor which could have assisted in removal of excess cytokines in intervention group patients thus improving their outcomes. However, mortality among men on CRRT in the treatment arm (n = 15/15) as well as in the control arm (n = 5/5) was 100%. The mortality among women on CRRT in the treatment arm was 50% (n = 3/6), while there were no deaths among women in the control group (n = 1). The multivariate Cox regression analysis demonstrated CRRT to be statistically significantly associated with increased mortality in our patients, HR 2.26, 95% CI (1.14 ± 4.49; P = .019) (see Table 4). Moreover, cytokine removal by means of CRRT remains nonselective, as both pro‐ and anti‐inflammatory cytokines are eliminated, thereby limiting the usefulness of this treatment approach in COVID‐19 patients.
Fluvoxamine also inhibits the uptake of serotonin into platelets, thus leading to an increase in bleeding risk; however, we identified no difference in the incidence of severe bleeding between the groups. While the safety profile of fluvoxamine in ICU COVID‐19 patients appears good, physicians prescribing it should be aware of possible drug–drug interactions, as it is a strong CYP1A2 and less potent CYP2C and CYP3A4 inhibitor. 32 An increased risk of bleeding and potentially other drug–drug interactions in critically ill COVID‐19 ICU patients were the reasons behind our decision to avoid randomisation and for choosing an open‐label study design.
Some of the limitations of this trial include its relatively small sample size, lack of randomisation and the open‐label design. Additionally, although meticulous care was taken to match the patients with controls of the same age, gender, disease severity, vaccination status and comorbidity, selection bias still cannot entirely be ruled out. Also, all patients were of Caucasian race, thus future larger trials should include a more racially diverse patient population, ideally in a double‐blind randomised controlled trial design.
Finally, efficacy of fluvoxamine in COVID‐19 patients could be dependent on the timing of treatment, where increased effectiveness could be achieved if treatment is initiated earlier during SARS‐CoV‐2 infection. If fluvoxamine is unequivocally proven effective, an improvement in treatment outcomes could also potentially be achieved by combining fluvoxamine therapy with other, newly developed antivirals active against SARS‐CoV‐2, such as molnupiravir.
5. CONCLUSION
It appears fluvoxamine treatment in hospitalised ICU COVID‐19 patients could have had a positive effect on mortality in the treatment group, without impacting the number of days on ventilator support, duration of ICU or total hospital stay. The safety profile of fluvoxamine in ICU COVID‐19 patients remained good. Additional clinical trials are required to confirm the results of this study.
COMPETING INTERESTS
All authors declare no conflict of interests. Robert Likic is an executive editor of the British Journal of Clinical Pharmacology.
CONTRIBUTORS
M.C., L.L., I.J. and N.K. had direct clinical responsibility for the patients, collected the data and matched the cases with controls. S.M., R.M., R.L. and M.C. analysed the data and wrote the manuscript. R.L. and S.M. designed the study and performed a critical revision of the manuscript. All authors approved the final version of the manuscript.
ACKNOWLEDGEMENTS
We would like to acknowledge the help and dedication of the following colleagues who participated in the care of our patients or have otherwise supported us in the conduct of this study: Nikola Blazevic (University Hospital Centre Zagreb), Robert Bojcic (University Hospital Centre Zagreb), Ivana Cegec (University Hospital Centre Zagreb), Filip Culek (University Hospital Centre Zagreb), Ela Curic (University Hospital Centre Zagreb), Viktor Jonke (University Hospital Centre Zagreb), Nikica Karkovic (University Hospital Centre Zagreb), Steve Kirsch (COVID‐19 Early Treatment Fund), Katica Kodzoman (University Hospital Centre Zagreb), Josip Kovacevic (University Hospital Centre Zagreb), Tin Kranjec (University Hospital Centre Zagreb), Martin Lackovic (University Hospital Centre Zagreb), Ivica Luksic (University Hospital Dubrava), Josip Ljevak (University Hospital Centre Zagreb), Alka Makovsek (University Hospital Centre Zagreb), Nedo Marcinkovic (University Hospital Centre Zagreb), William Migo (University of Zagreb), Marijan Pasalic (University Hospital Centre Zagreb), Ivan Peric (University Hospital Centre Zagreb), Linda Perica (University Hospital Centre Zagreb), Jasminka Persec (University Hospital Dubrava), Ivan Pristas (Croatian Institute of Public Health), Lucija Svetina (University Hospital Centre Zagreb), Marko Tripkovic (University Hospital Centre Zagreb), Igor Virag (University Hospital Centre Zagreb), Valentina Zulic (University Hospital Centre Zagreb).
No funding support was provided for this study.
APPENDIX A.
The study flow chart
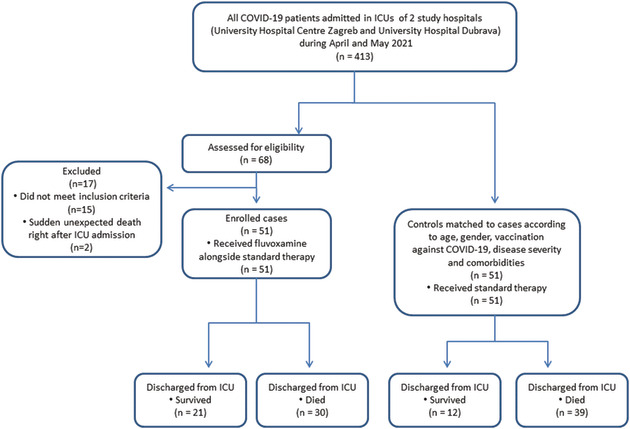
Calusic M, Marcec R, Luksa L, et al. Safety and efficacy of fluvoxamine in COVID‐19 ICU patients: An open label, prospective cohort trial with matched controls. Br J Clin Pharmacol. 2022;88(5):2065-2073. doi: 10.1111/bcp.15126
Slobodan Mihaljevic and Robert Likic contributed equally to the article.
The authors confirm that the Principal Investigator for this paper is Martina Calusic, MD and that she had direct clinical responsibility for the patients.
DATA AVAILABILITY STATEMENT
The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. WHO . COVID‐19 Weekly Epidemiological Update 55. 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---31-august-2021. Published August 31, 2021. Accessed November 5, 2021.
- 2. Marcec R, Majta M, Likic R. Will vaccination refusal prolong the war on SARS‐CoV‐2? Postgrad Med J. 2021;97(1145):143‐149. 10.1136/postgradmedj-2020-138903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saha S, Tanmoy AM, Tanni AA, et al. New waves, new variants, old inequity: a continuing COVID‐19 crisis. BMJ Glob Health. 2021;6(8):1‐5. 10.1136/bmjgh-2021-007031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Planas D, Veyer D, Baidaliuk A, et al. Reduced sensitivity of SARS‐CoV‐2 variant delta to antibody neutralization. Nature. 2021;596(7871):276‐280. 10.1038/s41586-021-03777-9 [DOI] [PubMed] [Google Scholar]
- 5. Shrotri M, Navaratnam AMD, Nguyen V, et al. Spike‐antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet. 2021;398(10298):385‐387. 10.1016/S0140-6736(21)01642-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Israel A, Shenhar Y & Green I et al. Large‐scale study of antibody titer decay following BNT162b2 mRNA vaccine or SARS‐CoV‐2 infection. medRxiv. 2021:2021.08.19.21262111. 10.1101/2021.08.19.21262111v1 [DOI] [PMC free article] [PubMed]
- 7. WHO Solidarity Trial Consortium . Repurposed antiviral drugs for Covid‐19—interim WHO Solidarity Trial results. N Engl J Med. 2021;384(6):497‐511. 10.1056/nejmoa2023184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hinks TSC, Cureton L, Knight R, et al. Azithromycin versus standard care in patients with mild‐to‐moderate COVID‐19 (ATOMIC2): an open‐label, randomised trial. Lancet Respir Med. 2021;19(21):1‐11. 10.1016/s2213-2600(21)00263-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Effect of Early Treatment with Metformin on Risk of Emergency Care and Hospitalization Among Patients with COVID‐19: The TOGETHER Randomized Platform Clinical Trial. https://www.togethertrial.com/results [DOI] [PMC free article] [PubMed]
- 10. Hassanipour S, Arab‐Zozani M, Amani B, Heidarzad F, Fathalipour M, Martinez‐de‐Hoyo R. The efficacy and safety of favipiravir in treatment of COVID‐19: a systematic review and meta‐analysis of clinical trials. Sci Rep. 2021;11(1):1‐11. 10.1038/s41598-021-90551-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reardon S. Flawed ivermectin preprint highlights challenges of COVID drug studies. Nature. 2021;596(7871):173‐174. 10.1038/d41586-021-02081-w [DOI] [PubMed] [Google Scholar]
- 12. Lenze EJ, Mattar C, Zorumski CF, et al. Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID‐19: a randomized clinical trial. JAMA. 2020;324(22):2292‐2300. 10.1001/jama.2020.22760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Seftel D, Boulware DR. Prospective cohort of fluvoxamine for early treatment of Coronavirus Disease 19. Open Forum Infect Dis. 2021;8(2):1‐3. 10.1093/ofid/ofab050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reis G, Moreira Silva EA, Medeiros Silva DC, et al. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID‐19: the TOGETHER randomised, platform clinical trial. Lancet Glob Health. 2021:1‐10. 10.1016/s2214-109x(21)00448-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sukhatme VP, Reiersen AM, Vayttaden SJ, Sukhatme VV. Fluvoxamine: a review of its mechanism of action and its role in COVID‐19. Front Pharmacol. 2021;12:1‐9. 10.3389/fphar.2021.652688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marčec R, Likić R. Could fluvoxamine keep COVID‐19 patients out of hospitals and intensive care units? Croat Med J. 2021;62(1):95‐100. 10.3325/CMJ.2021.62.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hashimoto K. Repurposing of CNS drugs to treat COVID‐19 infection: targeting the sigma‐1 receptor. Eur Arch Psychiatry Clin Neurosci. 2021;271(2):249‐258. 10.1007/s00406-020-01231-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rosen DA, Seki SM, Fernández‐Castañeda A, et al. Modulation of the sigma‐1 receptor‐IRE1 pathway is beneficial in preclinical models of inflammation and sepsis. Sci Transl Med. 2019;11(478):eaau5266. 10.1126/scitranslmed.aau5266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hashimoto Y, Suzuki T, Hashimoto K. Old drug fluvoxamine, new hope for COVID‐19. Eur Arch Psychiatry Clin Neurosci. 2021:1‐3. 10.1007/s00406-021-01326-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Esfahani HN, Rafiee L, Javanmard SH. Evaluation of the effect of antidepressant drug, fluvoxamine, on cyclooxygenase‐2 protein expression in lipopolysaccharide‐stimulated macrophages. Adv Biomed Res. 2019;(8):1‐4. 10.4103/abr.abr_141_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Homolak J, Kodvanj I. Widely available lysosome targeting agents should be considered as potential therapy for COVID‐19. Int J Antimicrob Agents. 2020;56(2):106044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kornhuber J, Muehlbacher M, Trapp S, et al. Identification of novel functional inhibitors of acid sphingomyelinase. PLoS ONE. 2011;6(8):e23852. 10.1371/journal.pone.0023852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hoertel N, Sánchez‐Rico M, Cougoule C, et al. Repurposing antidepressants inhibiting the sphingomyelinase acid/ceramide system against COVID‐19: current evidence and potential mechanisms. Mol Psychiatry. 2021:1‐2. 10.1038/s41380-021-01254-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schloer S, Brunotte L, Goretzko J, et al. Targeting the endolysosomal host‐SARS‐CoV‐2 interface by clinically licensed functional inhibitors of acid sphingomyelinase (FIASMA) including the antidepressant fluoxetine. Emerg Microbes Infect. 2020;9(1):2245‐2255. 10.1080/22221751.2020.1829082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Carpinteiro A, Edwards MJ, Hoffmann M, et al. Pharmacological inhibition of acid sphingomyelinase prevents uptake of SARS‐CoV‐2 by epithelial cells. Cell Reports Med. 2020;1(8):100142. 10.1016/j.xcrm.2020.100142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Anderson GM. Fluvoxamine, melatonin and COVID‐19. Psychopharmacology (Berl). 2021;238(2):611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alexander SPH, Kelly E, Mathie A, et al. The Concise Guide to PHARMACOLOGY 2021/22: Introduction and other protein targets. Br J Pharmacol. 2021;178(S1):S1‐S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moiseev S, Brovko M, Tao E, Bulanov N, Akulkina L, Fomin V. Sex differences in mortality in the intensive care unit patients with severe COVID‐19. J Infect. 2021;82(2):282‐327. 10.1016/j.jinf.2020.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Soldin OP, Mattison DR. Sex differences in pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2009;48(3):143‐157. 10.2165/00003088-200948030-00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hägg S, Spigset O, Dahlqvist R. Influence of gender and oral contraceptives on CYP2D6 and CYP2C19 activity in healthy volunteers. Br J Clin Pharmacol. 2001;51(2):169‐173. 10.1046/j.1365-2125.2001.01328.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ou‐Yang DS, Huang SL, Wang W, et al. Phenotypic polymorphism and gender‐related differences of CYP1A2 activity in a Chinese population. Br J Clin Pharmacol. 2000;49(2):145‐151. 10.1046/j.1365-2125.2000.00128.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Buchberger R, Wagner W. Fluvoxamine: safety profile in extensive post‐marketing surveillance. Pharmacopsychiatry. 2002;35(3):101‐108. 10.1055/s-2002-31522 [DOI] [PubMed] [Google Scholar]
- 33. Asakura S, Koyama T, Hosokai T, Kawano H, Kajii Y. Post‐marketing surveillance of fluvoxamine maleate used long‐term in patients with social anxiety disorder in Japan. Drugs Real World Outcomes. 2014;1(1):7‐19. 10.1007/s40801-014-0005-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Servillo G, Vargas M, Pastore A, et al. Immunomodulatory effect of continuous venovenous hemofiltration during sepsis: preliminary data. Biomed Res Int. 2013;2013:1‐6. 10.1155/2013/108951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Elbahlawan L, Bissler J, Morrison RR. Continuous renal replacement therapy: a review of use and application in pediatric hematopoietic stem cell transplant recipients. Front Oncol. 2021;11:1‐10. 10.3389/fonc.2021.632263 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are not publicly available due to privacy or ethical restrictions.


