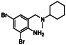
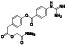
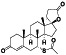
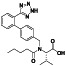
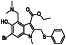
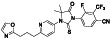
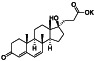
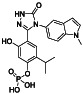
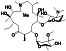
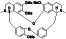
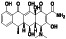
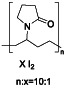
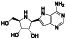
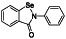
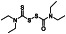
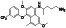
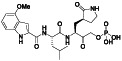
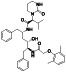
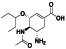
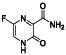
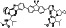
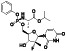
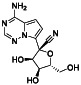
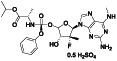
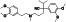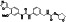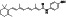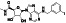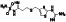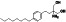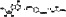Abstract
The coronavirus disease‐19 (COVID‐19) pandemic has become a global threat since its first outbreak at the end of 2019. Several review articles have been published recently, focusing on the aspects of target biology, drug repurposing, and mechanisms of action (MOAs) for potential treatment. This review gathers all small molecules currently in active clinical trials, categorizes them into six sub‐classes, and summarizes their clinical progress. The aim is to provide the researchers from both pharmaceutical industries and academic institutes with the handful information and dataset to accelerate their research programs in searching effective small molecule therapy for treatment of COVID‐19.
Keywords: anti‐viral agent, COVID‐19, small molecules
1. INTRODUCTION
Since its first large‐scale outbreak in central China in December 2019, the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has caused the coronavirus disease‐19 (COVID‐19) pandemic globally (Sahin et al., 2020; Wang, Hu, et al., 2020), and has led to severe damage to human lives and the economy of more than 200 countries worldwide. By the time this manuscript is submitted, 143,445,675 people have been infected by this virus, and the death toll mounts up to 3,051,736 globally (World Health Organization website, n.d.). Besides SARS‐CoV‐2, six more coronaviruses have been characterized by now: human CoV229E (HCoV‐229E, 1966), human CoV OC43 (HCoV‐OC43, 1967), human CoV HKU1 (HCoV‐HKU1, 2004), human CoV NL63 (HCoV‐NL63, 2004), severe acute respiratory syndrome CoV (SARS‐CoV, 2003), and Middle East respiratory syndrome CoV (MERS‐CoV, 2013). Chronological analysis of the commence time and severity of these viruses suggests that the outbreak cycle of the coronaviruses is getting shorter and shorter, and the impact of the viruses is getting worse and worse. To treat current SARS‐CoV‐2 infection, and more importantly to prepare for unforeseeable new coronaviruses in the future, scientists from the globe respond quickly in attempt to identify suitable solutions such as small molecules for the potential therapy or vaccines for the prevention.
SARS‐CoV‐2 belongs to a class of coronaviruses featured with positive‐sense, enveloped, single‐stranded RNA (Zeidler & Karpinski, 2020). The spike proteins (S proteins) on the viral envelope include two subunits, S1 and S2, which are the key surface proteins that participate in the interaction between the viruses and the host cells, and eventually promote the virus to enter the host cell (Walls et al., 2020).SARS‐CoV‐2 uses angiotensin‐converting enzyme 2 (ACE2) receptors to enter the lung cells (Figure 1a). After the attachment of the virus to the host cells, the S protein of the virus interacts with protease enzymes from the host cells, enabling the virus fusing to the host cell membrane (Cascella et al., 2021). This process relies on transmembrane serine protease (TMPRSS2) activating S proteins (Hoffmann et al., 2020; Valencia, 2020). After the genomic RNA released into the cytoplasm and then translated to produce polyproteins, which is facilitated by virally encoded chymotrypsin‐like protease (3CLpro) or main protease (Mpro) (Cascella et al., 2021). Polyproteins cleavage affords non‐structural proteins for the viral RNA replicase‐transcriptase complex. Viral nucleocapsids are assembled with the structural proteins after the viral replication and transcription. Upon encasing viral RNA, these nucleocapsids form the new virions and are then released from the cells via exocytosis (Valencia, 2020). Clinical studies indicate that several cytokines from severe patients' tissue undergo extensive changes, which play a crucial role in the COVID‐19 pathogenesis (Liu, Zhang, Huang, Yang, et al., 2020; Mehta, McAuley, et al., 2020; Wan et al., 2020). Hypercytokinemia (also known as “cytokine storm”) may play as a pivotal role in life‐threatening pathological processes (Figure 1b) (Xu, Shi, Li, & Zhou, 2020; Xu, Shi, Wang, et al., 2020). It has been proven that CD4+ T cells are rapidly activated to secret inflammatory cytokines upon the infection with SARS‐CoV‐2, which further lead to CD14+ CD16+ monocyte activation with high interleukin expression (such as IL‐1, IL‐6 etc. see Figure 1b) (Zhou, Fu, et al., 2020). Thus, blocking the IL‐1 or the IL‐6 receptors could potentially alleviate immunopathology caused by SARS‐CoV‐2. The pulmonary renin‐angiotensin system (RAS) is composed by two pathways, whose balance is crucial for pulmonary homeostasis (Figure 1c). Angiotensin II (Ang II), generated by endothelial ACE, acts on angiotensin II receptor type 1 (AT1) to promote pro‐inflammatory effects and vasoconstriction, whereas epithelial ACE2 cleaves Ang II into Ang (1–7), which acts on the MAS1 (MAS proto‐oncogene) oncogene to exert anti‐inflammatory and vasodilatory effects. The ACE‐dependent Ang II formation is a vital pathophysiological mechanism in different forms of acute respiratory distress syndrome (ARDS) (South et al., 2020).
FIGURE 1.
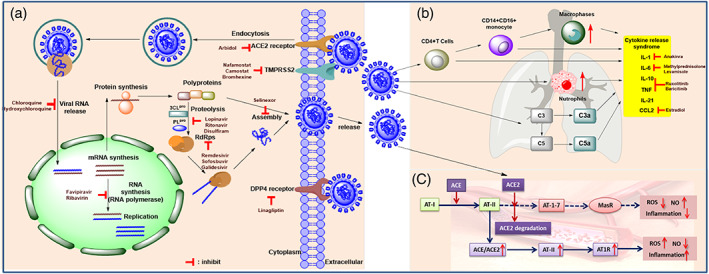
(a) Schematic illustration of the replication cycle of SARS‐CoV‐2 and the biological targets for the potential treatment. (b) the inhibition of excessive inflammatory response. (c) SARS‐CoV‐2 infects type II alveolar epithelial cells (type II AEC) via the interaction between its S proteins and the ACE2 receptor by promoting internalization and degradation of ACE2 and pulmonary ACE/ACE2 imbalance. In turn, the degradation of angiotensin II (AT II) into angiotensin 1–7 (AT‐1‐7) is prevented (dotted arrows), reducing anti‐inflammatory signaling through the mas receptor (MasR), and promoting pro‐inflammatory AT II signaling through the angiotensin receptor type I (AT1R) in vascular endothelial cells. Complex interactions involving the renin‐angiotensin system, oxidative state, endothelial interaction, and immune activation lead to alveola edema, lung inflammation, microvascular thrombosis, and acute respiratory distress syndrome. Potential targets have been identified as possible pharmacotherapies for the prevention, treatment, or management of COVID‐19
SARS‐CoV‐2 interacts with ACE2, triggers ACE2 degradation and the ratio of ACE/ACE2 imbalance, further drives Ang II‐mediated vascular inflammation and pulmonary injury which lead to severe COVID‐19 symptoms (Figure 1c) (South et al., 2020).
Global scientific communities and pharmaceutical industries have been racing for success to cope with the COVID‐19 pandemic in two major approaches: vaccines for the prevention (Buchholz et al., 2004; Cheng et al., 2020) and small molecule drugs for the potential treatment (Clinical trial ID: NCT04252885, n.d.; Cava et al., 2020; Kadam & Wilson, 2017; Lu, 2020; Pant et al., 2021; Sheahan, Sims, Leist, et al., 2020; Wu et al., 2020; Zhou, Hou, et al., 2020). The vaccine approach might ultimately be the most effective solution to the current pandemic (Karpiński et al., 2021). However, the small molecule approach is also pivotally important and desperately needed. Such approach not only could treat the patients at high risk or in critical condition in the current pandemic but also could prepare for diseases caused by unforeseeable new coronaviruses in the future.
Since 2020, several review articles have been published regarding the advances of coronavirus treatment in different aspects. For example, Pillaiyar et al. (2020) reviewed the small molecule inhibitors for the potential treatment of coronavirus by focusing on the drug repurposing and drug discovery stage. Zhu et al. (2021), Zeng et al. (2020) used the deep learning approach to identify 41 old drugs potentially repurposable for treatment of COVID‐19. Jean et al. (2020) reviewed the treatment reality and challenges for COVID‐19. Zhang & Penninger et al. (2020) reviewed the ACE2 as a potential therapeutic target for COVID‐19. Ghosh et al. (2020) summarized the drug development and medicinal chemistry aspect of COVID‐19 therapeutics. Naujokat et al.(2020) concisely summarized the candidate drugs against COVID‐19 by discussing some of the recent clinical studies, but not in a comprehensive manner. Monpara et al. (2020) focused on COVID‐19 associated complications and biological targets for potential treatment. Most recently, Alahari et al. reviewed the mechanisms of action (MOAs) for repurposing drugs in the treatment of COVID‐19 (Yousefi et al., 2021). In addition to above reviews, other approaches such as drug repurposing strategy (DRS) (Sahoo et al., 2021), system biology (Jaiswal et al., 2020), and computation practices (Ojha et al., 2021) were also reviewed recently.
Focusing on the small molecule approach, this article systematically summarizes those small molecules in current clinical trials for the potential treatment of COVID‐19 in a comprehensive manner. By the time this review is submitted (April 22, 2021), there are 5441 COVID‐19 related clinical studies listed on the NIH Clinical Trials website (http://ClinicalTrials.gov), ranging from evaluation of small molecule pharmacotherapies, mesenchymal stem cells or T‐cell‐based therapies, convalescent plasma therapies, and immunoglobulins to medical devices in the treatment of COVID‐19. Most of the clinical studies are in Phases II‐IV stages. Drug repurposing, defined as finding new indications for existing approved drugs (Tobinick, 2009), is of particular interest in the response to the COVID‐19 pandemic emergency and urgency. Historic data suggest that de novo drug development typically takes 10 to 17 years and costs 800 million USD (Tobinick, 2009). Therefore, de novo drugs approach for combating COVID‐19 might not turn out to be effective and satisfactory in the current pandemic emergency. On the other hand, the drug repurposing approach has been proven to be practical and successful in several cases. This approach significantly shortens the development time and reduces the cost. Repurposing existing drugs to treat COVID‐19 is biologically feasible as SARS‐CoV‐2 shares some similarities with other coronaviruses, such as SARS‐CoV and MERS‐CoV (Chen, Tian, et al., 2020), and there are many successful precedents in repurposing antivirals for new virus targets (Mercorelli et al., 2018). Indeed, most of the drugs currently in clinical trials for COVID‐19 are repurposed from approved antiviral drugs.
Data mining on more than four thousand clinical trials related to COVID‐19 provided us with a pretty large dataset related to small molecules approach. To better discuss them, those small molecules were categorized into six classes based on the clinical features of COVID‐19 and possible MOAs. The purpose of this review is to provide the researchers from both pharmaceutical industries and academic institutes with a comprehensive summary in this field so that to save their time in drug discovery research and to accelerate the finding of effective therapy for COVID‐19.
2. TYPES OF AGENTS IN CLINICAL TRAILS
To facilitate the interity of the review, we manually sorted out the drugs currently in clinical trials by querying the drug database from the FDA. Thus, for each identified small molecular drug, we searched for clinical trials on NIH Clinical Trials website using the name of drug plus “COVID‐19” and screened thoroughly the results obtained. As of April 22, 2021, there are 126 small molecule drugs in at least 777 clinical trials with various stages for the potential treatment of COVID‐19. These agents are classified into six categories based on their probable MOAs (Figures 2, 3, 4): (1) blocking virus‐cell membrane fusion and entry (25 agents, accounting for 20%); (2) inhibiting viral replication (29 agents, accounting for 23%); (3) addressing cytokine storm syndrome (CSS) (51 agents, accounting for 40%); (4) modulating immune system (10 agents, accounting for 8%); (5) anticoagulant therapy (7 agents, accounting for 6%); and (6) antioxidant supplement (4 agents, accounting for 3%). On the other hand, all clinical candidates are grouped into four small sub‐classes based on their clinical stages. Thus, there are 4 agents in Phase I, 42 agents in Phase II, 46 agents in Phase III, and 34 agents in Phase IV (Figures 2, 3, 4).
FIGURE 2.
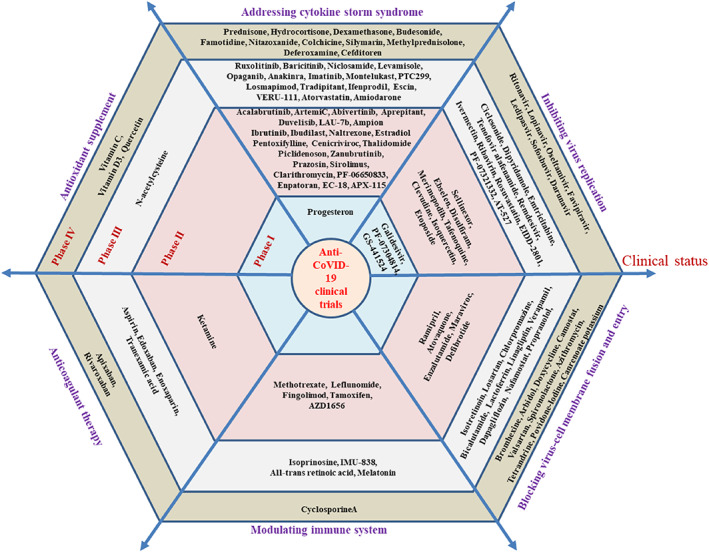
All of the small molecules in COVID‐19 clinical trials as of April 22, 2021: Categorizing these trials depending upon the clinical features of COVID‐19 and their probable MOAs. The hexagon contains small molecular compounds in different clinical trial stages: Phase I to phase IV from the inside to the outside
FIGURE 3.
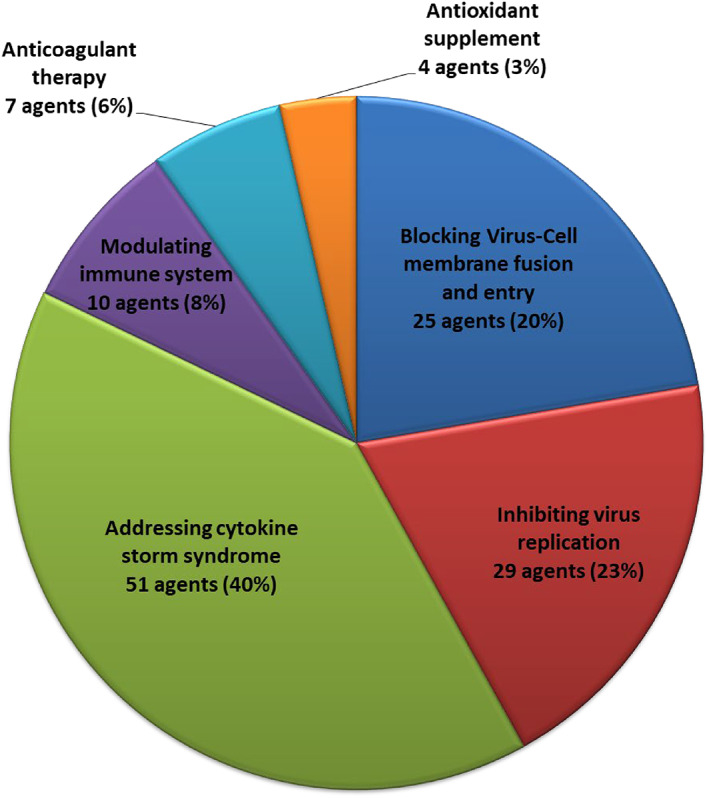
The distribution of different categories of small molecules in COVID‐19 clinical trials as of April 22, 2021. Sub‐classes are made based on the clinical features of COVID‐19 and their possible MOAs
FIGURE 4.
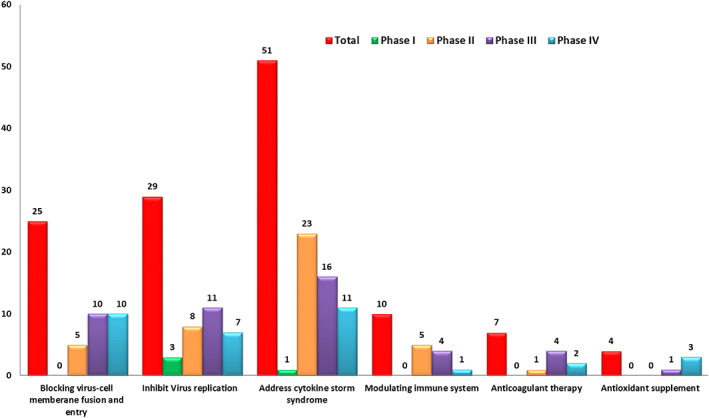
Mechanisms of action of agents in phase I‐IV
2.1. Blocking virus‐cell membrane fusion and entry
Mounting studies suggest that SARS‐CoV‐2 invades into the membrane of the host cells mainly through the endocytosis after the S protein binding to ACE2 on the surface of the host cells (Yang & Shen, 2020). Then the S protein with the altered configuration facilitates the viral envelope to fuse with the host cell membrane via the endosome pathway. At present, agents such as bromhexine, camostat, arbidol, linagliptin, chlorpromazine, etc. are being tested to block endocytosiswhile hydroxychloroquine and chloroquine are being investigated to block the viral genome released into the cytoplasm. However, the data from clinical trials indicate that antimalarial drug hydroxychloroquine neither helps COVID‐19 patients improve the recovery or reduce their symptoms nor prevents coronavirus infection in healthy people (Self et al., 2020). Both the World Health Organization (WHO) and NIH have suspended the clinical trials related to chloroquine class of medicines. The ongoing clinical trials of potential drugs targeting to block viral entry into the host cells are summarized in Table 1.
TABLE 1.
Blocking virus‐cell membrane fusion and entry
| Drug name and structure | Original mechanism | FDA‐approved indication(s) | Possible mechanism for anti‐COVID‐19 | Clinical trials ID (stage) | The highest anti‐COVID‐19 phase/ID/status/start–(estimated)end dates | References |
|---|---|---|---|---|---|---|
|
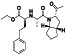
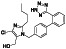
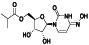
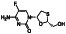
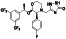
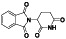
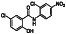
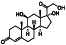
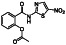
Ramipril
|
ACE inhibitor | Type 2 diabetes, vascular disease, hypertension, metabolic syndrome X, peripheral arterial disease, kidney transplant | ACE inhibitor | NCT04366050 (Phase 2) | Phase 2/NCT04366050/enrolling by invitation/May Huaier Granule 11, 2020–May 2021 | (Amat‐Santos et al., 2020) |
|
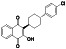
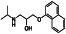
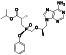
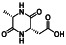
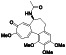
Atovaquone
|
Mitochondrial cytochrome bc1 complex inhibitor, antimalarial agent | HIV infections, malaria, plasmodium falciparum malaria, rabies | Elevating endosomal pH and interfere with ACE2 glycosylation |
NCT04339426 (Phase 2) NCT04456153 (Phase 2) |
Phase 2/NCT04456153/completed/July 22, 2020–January 31, 2021 | (Clinical trial ID: NCT04456153, n.d.; Farag et al., 2020; Sachdeva et al., 2020) |
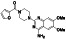
|
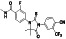
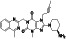
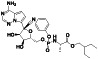
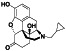
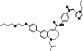
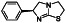
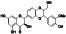
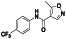
Enzalutamide
|
Androgen receptor (AR) antagonist | Prostate cancer | Blocking TMPRSS2 |
NCT04456049 (Phase 2) NCT04475601 (Phase 2) |
Phase 2/NCT04475601/recruiting/July 15, 2020–July 8, 2021 | (Cattrini et al., 2020) |
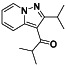
|
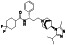
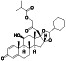
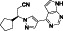
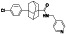
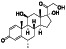
Maraviroc
|
CCR5 antagonist | HIV infection, endothelial dysfunction, | Inhibiting s‐protein mediated cell fusion and SARS‐CoV‐2 multiplication |
NCT04441385 (Phase 2) NCT04475991 (Phase 2) NCT04710199 (Phase 2) NCT04435522 (Phase 1) |
Phase 2/NCT04441385/recruiting/June 26, 2020–November 30, 2020 |
(Koenig et al., 2020; Risner et al., 2020; Shamsi et al., 2020) |
|
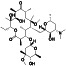
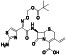
Losartan
|
AT II receptor antagonist | Hypertension, kidney disease, proteinuria, emphysema, colitis, etc. | ACE2 blocker preventing virus entry into the host cells |
Approximately 8 clinical trials, some of the examples are: NCT04335123 (Phase 1) NCT04447235 (Phase 2) NCT04428268 (Phase 2) NCT04643691 (Phase 2) NCT04311177 (Phase 2) NCT04312009 (Phase 2) NCT04606563 (Phase 3) NCT04343001 (Phase 3) |
Phase3/NCT04606563/recruiting/October 9, 2020–June 30, 2021 | |
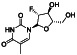
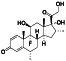
|
Isotretinoin (13‐cis‐retinoic acid)
|
Anti‐apoptosis | Severe acne | Down‐regulates ACE2 receptors, combination with all‐trans retinoic acid may enhances neutralizing antibodies in COVID‐19 infected Patients |
Approximately 9 clinical trials, some of the examples are: NCT04353180 (Phase 3) NCT04396067 (Phase 2) NCT04577378 (Phase 2) NCT04389580 (Phase 2) NCT04578236 (Phase 2) NCT04361422 (Phase 3) NCT04382950 (Phase 1) |
Phase 3/NCT04353180/not yet recruiting/October 2020–June 2021 |
(Hamouda Elgarhy, 2020) |
|
Lactoferrin (protein) |
Anti‐microbial, anti‐inflammatory, and immunomodulatory |
Anti‐gram‐negative, anti‐gram‐positive bacteria, fungi and viruses |
Inhibiting the union of ACE2 with SARS‐CoV‐2 spike protein, blocking the heparan sulfate proteoglycan receptor |
NCT04526821 (Phase 2) NCT04421534 (Phase 3) NCT04412395 (Phase 3) NCT04427865 (Phase 3) NCT04847791(not applicable) NCT04475120 (Phase 3) NCT04713735 (not applicable) |
Phase 3/NCT04475120/completed/April 15, 2020–July 2, 2020 | |
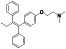
|
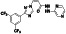
Propranolol
|
Non‐selective β1/β2‐blocker |
Hyperalgesia, social anxiety disorder, migraine headache, cigarette smoking, asthma, etc. | Blocking entry of SARS‐COV‐2 through downregulation of the ACE2 receptor and CD147 | NCT04467086 (Phase 3) | Phase 3/NCT04467086/not yet recruiting/December 2020–April 2021 |
(Vasanthakumar, 2020) |
|
Linagliptin
|
DPP‐4 inhibitor | Diabetes mellitus, type 2 | Inhibiting DPP4, blocking DPP4 inteacting with spike protein of the MERS‐Co‐V | NCT04371978 (Phase 3) | Phase 3/NCT04371978/recruiting/October 1, 2020–June 30, 2021 |
(Solerte et al., 2020) |
|
Verapamil
|
Calcium channel blocker and P‐gp inhibitor, CYP3A4 inhibitor | Heart arrhythmias, high blood pressure and angina research |
A slow‐channel calcium‐blocking agent. Blocking ion channels to inhibit coronavirus entry. |
NCT04351763 (Phase 3) |
Phase 3/NCT04351763/recruiting/April 27, 2020–March 2, 2021 |
(Clinical trial ID: NCT04351763, n.d.) |
|
Chlorpromazine
|
Antagonist of dopamine D2, 5‐HT2A, potassium channel and sodium channel. | Psychotic disorders such as schizophrenia. Glioblastoma multiforme | K+/Na+ channel inhibitor, inhibits clathrin‐mediated endocytosis, |
NCT04366739 (Phase 3) NCT04354805 (Phase 3) |
Phase 3/NCT04366739/not yet recruiting/April 29, 2020–August 30, 2020 |
(Plaze et al., 2020) |
|
Dapagliflozin
|
Competitive SGLT2 inhibitor | Diabetes mellitus (DM) | Reducing the viral load and preventing the lowering of cytosolic pH | NCT04350593 (Phase 3) | Phase 3/NCT04350593/active, not recruiting/April 22, 2020–April 2021 |
(Cure & Cumhur Cure, 2020) |
|
Nafamostat
|
Serine protease inhibitor, | Acute kidney injury | TMPRSS2 inhibitor |
NCT04390594 (Phase 3) NCT04352400 (Phase 3) NCT04418128 (Phase 3) |
Phase 3/NCT04390594/recruiting/August 13, 2020–February 12, 2021 |
(Fernandez‐Fernandez et al., 2020) |
|
Bicalutamide
|
Non‐steroidal AR antagonist | Prostate cancer, neoplasms, prostate, | Blocking TMPRSS2 |
NCT04509999 (Phase 3) NCT04652765 (Phase 1) |
Phase 3/NCT04509999/recruiting/October 26, 2020–September 2022 |
(Cattrini et al., 2020) |
|
Bromhexine
|
Clearing mucus from respiratory tract, antioxidant | Chronic bronchitis, asthma and other causes of phlegm not easy to cough out | TMPRSS2 inhibitor |
NCT04340349 (Early Phase1) NCT04424134 (Phase 3) NCT04355026 (Phase 4) NCT04273763 (not applicable) |
Phase 4/NCT04355026/recruiting/April 10, 2020–June 30, 2020 | |
|
Camostat
|
Serine protease inhibitor, | Corona virus infection, COVID‐19, coagulopathy cardiovascular complication COVID‐19 | TMPRSS2 inhibitor |
Approximately 19clinical trials, some of the examples are: NCT04353284 (Phase 2) NCT04524663 (Phase 2) NCT04583592 (Phase 2) NCT04608266 (Phase 3) NCT04455815 (Phase 3) NCT04657497 (Phase 3) NCT04530617 (Phase 2) |
Phase 4/NCT04338906/withdrawn (lack of public funding)/June 1, 2020–December 2021 |
(Huang et al., 2020) |
|
Spironolactone
|
AR antagonist | Heart failure, cardiomyopathy, alcoholic, alcoholism, osteoarthritis, Hypoglycemia, healthy | Inhibiting the androgen‐dependent expression of TMPRSS2 |
NCT04643691 (Phase 2) NCT04424134 (Phase 3) NCT04826822 (Phase 3) NCT04345887 (Phase 4) |
Phase 4/NCT04345887/not yet recruiting/April 21, 2020–July 21, 2020 | |
|
Valsartan (diovan)
|
AT II receptor antagonist | Hypertension stage 2 systolic hypertension |
ACE2 inhibitor, inhibition of TMPRSS2 |
NCT04335786 (Phase 4) | Phase 4/NCT04335786/recruiting/April 17, 2020–December 2020 |
(Vitiello et al., 2020) |
|
Arbidol (umifenovir)
|
Antiviral agent | Influenza |
Inhibition of ACE2/S protein interaction, blocking membrane fusion |
NCT04350684 (Phase 4) NCT04260594 (Phase 4) NCT04476719 (Phase 1) |
Phase 4/NCT04350684/enrolling by invitation/April 15, 2020–April 22, 2020 |
(Clinical trial ID: NCT04260594, n.d.; Clinical trial ID: NCT04252885, n.d.; Huang et al., 2020) |
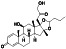
|
Proxalutamide (GT0918)
|
Androgen receptor (AR) antagonist | COVID‐19, Prostate cancer |
ACE2 and TMPRSS2 levels in lung and cardiac cells are reduced by ant androgens |
NCT04853134 (Phase 3) NCT04728802 (Phase 3) NCT04853927 (Phase 3) NCT05009732 (Phase 3) NCT04870606 (Phase 3) NCT04446429 (not applicable) |
Phase 3/NCT04728802/completed/January 28, 2021–April 15, 2021 | (McCoy et al., 2021) |
|
Canrenoate potassium
|
Competitive mineralocorticoid receptor (aldosterone receptor) antagonist | Brain‐dead organ donors, Cirrhosis, COVID‐19 pneumonia |
Pleiotropic effects with favorable renin–angiotensin–aldosterone system (RAAS) and ACE2 expression, reduction in transmembrane serine protease 2 (TMPRSS2) activity and antiandrogenic action |
NCT04977960 (Phase 2) NCT04912011 (Phase 4) |
Phase 4/NCT04912011/recruiting/June 3, 2021–August 31, 2021 | (Kotfis & Lechowicz, 2021:) |
|
Defibrotide
|
Thrombotic microangiopathies, Kawasaki disease, Sickle cell disease | Coagulopathy | Mediated by the p38 MAPK pathway, which is upregulated as a result of the binding of SARSCoV2 on ACE2 receptors on the surface of endothelial cells and, in turn, activates the transcription of the proinflammatory cytokines. |
NCT04335201 (Phase 2) NCT04652115 (Phase 2) NCT04530604 (Ohase 1) NCT04348383 (Phase 2) |
Phase 2/NCT04335201/recruiting/April 6, 2020–June 20, 2021 |
(Macciò et al., 2020) |
|
Azithromycin
|
Inhibit translation via interacting with 50S subunit of the bacterial ribosome | Acute bacterial, community‐acquired pneumonia, uncomplicated skin infections, et al |
Inhibiting viral invasion via interact with spike protein/CD147 interaction or blocking CD147 expression |
Approximately 52 clinical trials, some of the examples are: NCT04334382 (Phase 3) NCT04371406 (Phase 3) NCT04359316 (Phase 4) NCT04339816 (Phase 3) NCT04332107 (Phase 3) NCT04621461 (Phase 4) NCT03871491 (Phase 3) |
Phase 4/NCT04621461/completed/November 2020–February 8, 2021 | |
|
Tetrandrine
|
Voltage‐gated Ca2+ current (ICa) and Ca2+‐activated K+ current inhibitor | Corona virus disease 2019, COVID‐19 | Inhibiting voltage‐gated Ca2+ current (ICa) and Ca2+‐activated K+ current. | NCT04308317 (Phase 4) | Phase 4/NCT04308317/enrolling by invitation/March 5, 2020–March 1, 2021 |
(Clinical trial ID: NCT04308317, n.d.; Heister & Poston, 2020) |
|
Doxycycline
|
Broad‐spectrum metalloproteinase (MMP) inhibitor | Anal chlamydia infection, rosacea, overactive bladder, HIV infections, sinusitis, acne vulgaris | Inhibition of the E2 envelope glycoprotein involved in virus entry |
Approximately 10 clinical trials, some of the examples are: NCT04591600 (Phase 2) NCT04523831 (Phase 3) NCT04371952 (Phase 3) NCT04370782 (Phase 4) NCT04407130 (Phase 2) NCT04551755 (Phase 2) NCT04584567 (Phase 3) |
Phase 4/NCT04370782/completed/28, 2020–September 30, 2020 | |
|
Povidone‐iodine
|
Antibacterial agent (MRSA and MSSA strains) | External uses‐infection; cesarean section, vaginal surgeries, arrhythmia |
Interacting with surface proteins of enveloped viruses, destabilize membrane fatty acids, inducing cell apoptosis |
Approximately 12clinical trials, some of the examples are: NCT04410159 (Phase 2) NCT04549376 (Phase 2) NCT04510402 (Phase 2) NCT04371965 (Phase 2) NCT04872686 (Phase 3) NCT04344236 (Phase 2) NCT04603794 (Phase 4) |
Phase 4/NCT04603794/recruiting/October 1, 2020–November 2020 |
(Pattanshetty et al., 2021) |
2.2. Inhibiting the virus replication
There are two mainly targets inhibiting virus replication: (1) the protease (3CLpro and PLpro); and (2) the RNA‐dependent RNA polymerase (RdRP). Anti‐HIV agents (lopinavir or ritonavir) inhibit protease 3CLpro, thereby blocking the formation of non‐structural proteins. In July 2020, the WHO announced the suspension of the lopinavir/ritonavir combination clinical trial, citing little or no effect in reducing the death rate of hospitalized COVID‐19 patients in the branch trial (News press from WHO website, 2021). PF‐07321332 and PF‐07304814 are potent and orally active SARS‐CoV 3C‐like protease (3CLpro) inhibitors currently in multiple clinical trials including Phase III (Vandyck & Deval, 2021). On the other hand, RdRP inhibitors such as remdesivir (Grein et al., 2020), sofosbuvir, and galidesivir could block the replication of the viral genome and the formation of structural proteins. Discovered by Gilead Sciences, remdesivir is the first drug to receive emergency FDA approval as sympathy medication for COVID‐19 patients and is currently under various forms of approval in several countries. Especifically, molnupiravir (aka EIDD‐2801 and MK‐4482) induced RNA mutagenesis by the viral RNA‐dependent RdRp. Molnupiravir showed an exciting experimental results in phase 2 clinical trials, and was one of the most promising small molecule anti‐coronavirus drugs at present (Imran et al., 2021; Kabinger & Stiller, 2021; Malone & Campbell, 2021). In October 2021, Merck Co. submitted an Emergency Use Authorization (EUA) application to the US Food and Drug Administration (FDA) for molnupiravir based on the promising results from the Phase 3 MOVeOUT clinical trial (Press releas, Merck Company website, 2021). If approved, molnupiravir will be the first oral drug to treat COVID‐19. Clinical trials for inhibiting virus replication are summarized in Table 2.
TABLE 2.
Inhibiting virus replication
| Drug | Original mechanism | FDA‐approved indication(s) | Possible mechanism for anti‐COVID‐19 | Clinical trials ID (stage) | The highest anti‐COVID‐19 phase/ID/status/start–(estimated)end dates | References |
|---|---|---|---|---|---|---|
|
Galidesivir
|
Viral RNA‐dependent RNA polymerase (RdRP) inhibitor | Not yet approved | Inhibiting RNA dependent RNA polymerase | NCT03891420 (Phase 1) | Phase 1/NCT03891420/recruiting/April 9, 2020–May 31, 2021 | |
|
Ebselen (PZ‐51, SPI‐1005, CCG‐39161,)
|
Potent voltage‐dependent calcium channel (VDCC) blocker | Not yet approved | Inhibiting main protease via binding to Mpro from forming selenosulfide |
NCT04484025 (Phase 2) NCT04483973 (Phase 2) |
Phase 2/NCT04484025/not yet recruiting/ May 2021–June 2022 |
|
|
Disulfiram (tetraethylthiuram disulfide; TETD)
|
Aldehyde‐dehydrogenase (ALDH1) inhibitor | Cocaine addictive, chronic alcoholism |
Blocking the Mpro protease, inhibiting cytokine release induced by NF‐kB and NLRP inflammasome |
NCT04485130 (Phase 2) NCT04594343 (Phase 2) | Phase 2/NCT04594343/recruiting/November 20, 2020 –July 20, 2021 | |
|
Tafenoquine
|
Anti‐malarial prophylactic agent. | Not yet approved | Inhibiting virus infection via down regulating Mpro activity | NCT04533347 (Phase 2) | Phase 2/NCT04533347/recruiting/February 19, 2021–June 8, 2021 | |
|
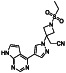
EIDD‐2801 (Molnupiravir, MK‐4482)
|
Virus RNA mutation | Antivirus, not yet approved | Induced RNA mutagenesis by the viral RNA‐dependent RNA polymerase (RdRp) | NCT04405739 (Phase 2) NCT04392219 (Phase 1) NCT04575584 (Phase 3) NCT04405570 (Phase 2) | Phase 3/NCT04575584/active, not recruiting/October 5, 2020–August 10, 2021 | (Sheahan, Sims, Zhou, et al., 2020; PubChem, National Library of Medicine, n.d.; Kabinger & Stiller, 2021) |
|
Merimepodib(MMPD, VX‐497)
|
Noncompetitive inosine monophosphate dehydrogenase (IMPDH) inhibitor | Not yet approved | Inhibiting Zika viral RNA replication | NCT04410354 (Phase 2) | Phase 2/NCT04410354/terminated (Failure to meet primary endpoint)/June 16, 2020–December 1, 2020 |
(X. Tong et al., 2018) |
|
Clevudine (L‐FMAU)
|
Nucleoside analog of the unnatural L‐configuration, non‐competitive inhibitor that is not incorporated into the viral DNA but rather binds to the polymerase. | Chronic hepatitis B | Inhibition of viral RNA synthesis |
NCT04891302 (Phase 2) NCT04347915 (Phase 2) |
Phase 2/NCT04347915/recruiting/April 15, 2020–January 30, 2021 | (Hui & Lau, 2005; Jang et al., 2011) |
|
Selinexor
|
Selective nuclear transport (SINE) inhibitor | Multiple myeloma | Interference nuclear export of vRNPs, viral mRNAs mediated by XPO1, blocking late‐stage viral assembly processes | NCT04349098(Phase 2) NCT04355676(Phase 2) | Phase 2/NCT04349098/completed/April 17, 2020–October 5, 2020 |
(Uddin et al., 2020) |
|
Emtricitabine
|
Nucleoside reverse transcriptase inhibitor (NRTI). | HIV infection, PrEP adherence monitoring, healthy, hepatitis B | Reverse transcriptase inhibitor |
NCT04712357 (not applicable) NCT04519125 (Phase 2) NCT04334928 (Phase 3) NCT04405271 (Phase 3) |
Phase 3/NCT04405271/recruiting/July 31, 2020–April 1, 2021 |
(Ayerdi et al., 2020) |
|
Tenofovir alafenamide
|
HIV‐1 nucleotide reverse transcriptase inhibitor | HIV infections, hepatitis B. | Reverse transcriptase inhibitor |
NCT04519125 (Phase NCT04334928 (Phase 3) NCT04405271 (Phase 3) |
Phase 3/NCT04405271/not yet recruiting/July 31, 2020–November 15, 2020 |
(Duan et al., 2020) |
|
Remdesivir
|
Nucleoside analog with effective antiviral activity | Not yet approved |
Viral RNA‐dependent RNA polymerase (RdRP) inhibitor, reducing viral multiplication |
Approximately 33 clinical trials, some of the examples are: NCT04539262 (Phase 2) NCT04610541 (Phase 3) NCT04501952 (Phase 3) NCT04292730 (Phase 3) NCT04560231 (Phase 1) NCT04672564 (Phase 3) NCT04480333 (Phase 1) |
Phase3/NCT04292899/completed/March 6, 2020–April 9, 2020 | |
|
Ciclesonide
|
Glucocorticoid receptor inhibitor | Obstructive airway diseases. Asthma, Allergic Rhinitis, Perennial | Inhibiting the replication of SARS‐CoV‐2 genomic RNA via targeting viral endonuclease NSP15 |
NCT04330586 (Phase 2) NCT04435795 (Phase 3) NCT04377711 (Phase 3) NCT04381364 (Phase 2) NCT04356495 (Phase 3) |
Phase 3/NCT04377711/completed/June 8, 2020–January 5, 2021 |
(Kimura et al., 2020) |
|
Ribavirin
|
Nucleoside inhibitor | HIV infections, hepatitis C | RNA‐dependent RNA polymerase inhibitor |
Approximately 8 clinical trials, some of the examples are: NCT04828564(Phase 3) NCT04563208 (Phase 2) NCT04494399 (Phase 2) NCT04460443 (Phase 3) NCT04356677 (Phase 1) NCT04392427 (Phase 3) |
Phase 3/NCT04392427/not yet recruiting/October 2020–May 2022 | |
|
Dipyridamole
|
Phosphodiesterase inhibitor | Cirrhosis, hypertension, status; splenectomy, venous thrombosis, brain ischemia, transient ischemic attack, arteriosclerosis, | Suppressing HCoV‐19 replication |
NCT04391179 (Phase 2) NCT04424901 (Phase 2) NCT04410328 (Phase 3) |
Phase 3/NCT04410328/recruiting/October 21, 2020–March 15, 2021 |
(Liu, Li, Liu, Chen, & Luo, 2020; Liu, Zheng, Huang, Shan, & Huang, 2020; Ma & Wang, 2021) |
|
Ivermectin
|
Anti‐parasite agent, impα/β1‐mediated nuclear import inhibitor | Healthy, rosacea, lymphatic filariasis, loiasis |
Blocking α/β1‐mediated nuclear import |
Approximately 56 clinical trials, some of the examples are: NCT04529525 (Phase 3) NCT04381884 (Phase 2) NCT04523831 (Phase 3) NCT04391127 (Phase 3) NCT04602507 (Phase 2) NCT04422561 (Phase 3) NCT04530474 (Phase 3) |
Phase 3/NCT04523831/completed/June 1, 2020–August 22, 2020 | |
|
Rosuvastatin
|
HMG‐CoA reductase enzyme inhibitor, cholesterol level inhibitor, block hERG current |
Dyslipidemias, non‐ST‐elevation acute coronary syndromes, cardiovascular disease, myocardial infarction, hypercholesterolemia | Targeting COVID‐19 virus Mpro | NCT04472611 (Phase 3) | Phase 3/NCT04362137/not yet recruiting/August 1, 2020–August 1, 2021 |
(Farag et al., 2020) |
|
Isoquercetin (Quercetin 3‐glucoside, IQC‐950AN)
|
Regulates the expression of nitric oxide synthase 2 (NO2) via modulating the nuclear factor‐κB (NF‐κB) transcription regulation system | Cardiovascular disease, chronic kidney disease, Covid19 | Mpro inhibitor |
NCT04536090 (Phase 2) NCT04733651 (Phase 2) NCT04622865 (Phase 2) |
Phase 2/NCT04622865/recruiting/November 10, 2020–June 2021 | (Saeedi‐Boroujeni & Mahmoudian‐Sani, 2021) |
|
Ritonavir
|
HIV type 1aspartate protease inhibitor | HIV infection | Inhibit the coronaviral 3CLpro protease |
Approximately 24 clinical trials, some of the examples are: NCT04403100 (Phase 3) NCT04321174 (Phase 3) NCT04291729 (Phase 4) NCT04345276 (Phase 4) NCT04261270 (Phase 3) NCT04386876 (Phase 1) NCT04466241 (Phase 2) |
Phase 4/NCT04291729/completed/February 17, 2020–March 19, 2020 |
(Cao et al., 2020; Chu et al., 2004; Li & De Clercq, 2020; Ma et al., 2021; Sheahan, Sims, Leist, et al., 2020; Zhang, Lin, et al., 2020) |
|
Etoposide (VP‐16; VP‐16‐213)
|
Topoisomerase II inhibitor | Adult acute lymphocytic leukemia, NK + T Cell lymphoma, mantle cell lymphoma, small cell lung cancer | SARS‐3CL protease inhibitor | NCT04356690 (Phase 2) | Phase 2/NCT04356690/Active, not recruiting/April 22, 2020–December 2021 | (Rashid et al., 2022) |
|
PF‐07321332
|
SARS‐CoV 3C‐like protease (3CLPRO) inhibitor | COVID‐19 | 3CLpro inhibitor |
NCT04960202 (Phase 3) NCT04962230 (Phase 1) NCT05005312 (Phase 1) NCT04909853 (Phase 1) NCT04962022 (Phase 1) NCT05032950 (Phase 1) NCT04756531 (Phase 1) NCT05011513 (Phase 3) |
Phase 3/NCT04960202/recruiting/July 13, 2021–October 16, 2021 | (Vandyck & Deval, 2021) |
|
PF‐07304814
|
Potent 3CLpro protease (Mpro) inhibitor | Viral disease | 3CLpro proteaseinhibitor |
NCT04535167 (Phase 1) NCT04627532 (Phase 1) |
Phase 1/NCT04627532/completed/November 13, 2020–December 17, 2020 | (Boras, 2021; Vandyck & Deval, 2021) |
|
Lopinavir
|
HIV type 1 aspartate protease inhibitor | HIV infection |
Inhibit the viral proteases: 3CLpro or Plpro, increasing t1/2 via inhibiting cytochrome P450 when combined with lopinavir |
Approximately 18 clinical trials, some of the examples are: NCT04403100 (Phase 3) NCT04307693 (Phase 2) NCT04321174 (Phase 3) NCT04455958 (Phase 2) NCT04255017 (Phase 4) NCT04330690 (Phase 2) NCT04386876 (Phase 1) |
Phase 4/NCT04255017/recruiting/February 1, 2020–June 1, 2020 |
(Cao et al., 2020; Chu et al., 2004; Li & De Clercq, 2020; Ma et al., 2021; Sheahan, Sims, Leist, et al., 2020; Zhang, Lin, et al., 2020) |
|
Oseltamivir
|
Virus neuraminidase enzyme inhibitor | Influenza A and B viruses | Virus‐release blockers | NCT04303299 (Phase 3) NCT04558463 (Phase 3) NCT04516915 (Phase 3) NCT04255017 (Phase 4) NCT02735707 (Phase 4) NCT04338698 (Phase 3) | Phase 4/NCT04255017/recruiting/February 1, 2020–June 1, 2020 |
(Akram et al., 2020; Clinical trial ID: NCT04255017, n.d.; Whitley, 2007) |
|
Favipiravir
|
Viral RNA polymerase inhibitor | Influenza |
RNA dependent RNA polymerase inhibitor, reducing virus replication |
Approximately 38 clinical trials, some of the examples are: NCT04402203 (Phase 3) NCT04464408 (Phase 3) NCT04359615 (Phase 4) NCT04303299 (Phase 3) NCT04529499 (Phase 3) NCT04349241 (Phase 3) NCT04600999 (Phase 3) NCT04613271 (Phase 3) |
Phase 4/NCT04359615/not yet recruiting/April 20, 2020–May 3, 2020 | |
|
Ledipasvir
|
Inhibitor of the hepatitis C virus NS5A |
Hepatitis C, hepatocellular carcinoma | RdRP inhibitor | NCT04498936 (Phase 4) NCT04530422 (Phase 3) |
Phase 4/NCT04498936/ sofosbuvir/ledipasvir and nitazoxanide for treatment of COVID‐19 |
|
|
Sofosbuvir
|
HCV RNA replication inhibitor | Hepatitis C, liver cirrhosis | RdRP inhibitor |
Approximately 8 clinical trials, some of the examples are: NCT04530422 (Phase 3) NCT04498936 (Phase 4) NCT04497649 (Phase 3) NCT04532931 (Phase 2) NCT04535869 (Phase 3) NCT04561063 (Phase 2) NCT04460443 (Phase 3) |
Phase 4/NCT04498936/completed/July 15, 2020–October 30, 2020 | |
|
GS‐441524
|
Inhibits feline infectious peritonitis virus (FIPV), parent nucleoside of remdesivir | COVID‐19 |
Interferes with the SARS‐CoV‐2 RNA‐dependent RNA polymerase (RdRp) |
NCT04859244 (Phase 1) | Phase 1/NCT04859244/completed/April 26, 2021–May 1, 2021 | (Yan & Muller, 2020) |
|
AT‐527 (R07496998)
|
HCV viral replication inhibitor | HCV Infection | Inhibit the RdRp for RNA synthesis |
NCT04889040 (Phase 3) NCT04709835 (Phase 2) NCT04396106 (Phase 2) NCT04877769 (Phase 1) |
Phase 3/NCT04889040/recruiting/May 17, 2021–August 3, 2021 | (Good et al., 2021) |
|
Darunavir
|
HIV protease inhibitor | HIV infections, healthy | Protease inhibitor |
NCT04252274 (Phase 3) NCT04435587 (Phase 4) NCT04303299 (Phase 3) |
Phase 4/NCT04435587/recruiting/July 13, 2020–June 2021 |
2.3. Addressing CSS
CSS refers to a group of related medical conditions in which the immune system releases excess of inflammatory signals (interferons, interleukins, tumor necrosis factors (TNF), chemokines, and several other mediators), resulting in a clinical presentation of unremitting high fever, hyperferritinemia, hepatosplenomegaly, cytopaenia, lymphadenopathy and central nervous system abnormalities, and, if untreated, could lead to the organ failure and even the death (Fajgenbaum & June, 2020; Gao et al., 2021). Abundant studies have revealed that majority of COVID‐19 patients are often have higher levels of inflammatory mediators in blood. Those inflammatory mediators include cytokines and chemokines such as interleukin (IL)‐1 IL‐6, IL‐7, IL‐10, IL‐21, TNF, and monocyte chemoattractant protein‐1 (MCP1, also known as CCL2) etc. (Chen, Wu, et al., 2020; Gorbalenya et al., 2020; Hojyo et al., 2020; Tay et al., 2020). Therefore, COVID‐19 could be featured as a cytokine release syndrome (CRS) and the fatality could be caused by the high mortality cytokine storm. In fact, the approach on addressing the CSS for the potential treatment of COVID‐19 is currently investigated in clinics. For example, physicians apply medicines such as corticosteroids (prednisone, methylprednisolone, and hydrocortisone), interleukin inhibitors (anakinra, levamisole, and colchicine), and JAK–STAT inhibitors (ruxolitinib and baricitinib) to patients to see whether these medicines could alleviate the symptoms of COVID‐19. Studies suggest that suppressing the release of cytokine could probably improve the clinical outcomes (L. Lu et al., 2020). However, the adverse consequences were also observed in patients when corticosteroid therapy was applied during COVID‐19 infection. Thereby, it is important to strictly control the time and dosage of glucocorticoids administration in the disease prognosis (Sanders et al., 2020). Glucocorticoids are mainly used in severe patients with CSS (Ye et al., 2020). The WHO currently does not recommend the routine use of corticosteroids in COVID‐19 patients, due to the potential delay in viral clearance. Hence, corticosteroids should be given to patient with certain symptoms such as severe acute respiratory distress syndrome or refractory septic shock (Mehta, Mazer‐Amirshahi, et al., 2020). Agents in clinical trials for potential inhibiting cytokine storm in COVID‐19 are summarized in Table 3.
TABLE 3.
Addressing the cytokine storm syndrome
| Drug name and structure | Original mechanism | FDA‐approved indication(s) | Possible mechanism for anti‐COVID‐19 | Clinical trials ID (stage) | The highest anti‐COVID‐19 phase/ID/status/start–(estimated)end dates | References |
|---|---|---|---|---|---|---|
|
Progesteron
|
Steroid hormone regulating menstrual cycle | Suicidal ideation, infertility, hormone replacement, concussion | Inhibiting pro‐inflammatory cytokines IL‐1β and IL 12 | NCT04365127 (Phase 1) | Phase1/NCT04365127/completed/April 27, 2020–August 20, 2020 |
(Mauvais‐Jarvis et al., 2020) |
|
Acalabrutinib
|
BTK inhibitor | Not yet approved | BTK inhibitor, decreased inflammatory cell infiltration and proinflammatory cytokines | NCT04380688(Phase 2) NCT04346199(Phase 2) NCT04497948(Phase 1) NCT04564040(Phase 1) | Phase 2/NCT04346199/completed/June 12, 2020–November 17, 2020 |
(Roschewski et al., 2020) |
|
Ibrutinib
|
Irreversible BTK inhibitor |
Lymphocytic, waldenstrom macroglobulinemia, mantle‐cell, B‐cell, etc. |
BTK inhibitor, decreasing inflammatory cell infiltration and proinflammatory cytokines |
NCT04375397 (Phase 2) NCT04665115 (Phase 2) NCT04439006 (Phase 2) |
Phase 2/NCT04439006/recruiting/July 22, 2020–December 31, 2021 |
(Treon et al., 2020) |
|
Zanubrutinib
|
Selective BTK inhibitor | Not approved yet | BTK inhibitor, decreased inflammatory cell infiltration and proinflammatory cytokines | NCT04382586 (Phase 2) | Phase 2/NCT04382586/active, not recruiting/July 6, 2020–February 2, 2021 |
(Chong et al., 2020) |
|
Duvelisib
|
Selectivite p100δ inhibitor | Not approved yet |
Inhibiting phosphatidylinositol 3‐kinase(PI3K) which plays acrucial role in eliciting immune response. |
NCT04372602 (Phase 2) NCT04487886 (Phase 2) |
Phase 2/NCT04372602/recruiting/October 12, 2020–November 30, 2021 |
(Clinical trial ID: NCT04487886, n.d.) |
|
Sirolimus (rapamycin)
|
mTOR inhibitor | Kidney transplantation, blue rubber bleb nevus syndrome, venous malformation, kidney transplantation, uterine fibroids |
Alleviating cytokine strom and T‐cell senescence, preventing severe COVID‐19 progression |
Approximately 7 clinical trials, some of the examples are: NCT04461340 (Phase 2) NCT04371640 (Phase 1) NCT04341675 (Phase 2) NCT04409327 (Phase 2) |
Phase 2/NCT04341675/recruiting/April 24, 2020–July 2020 |
(Omarjee et al., 2020) |
| ArtemiC (artemisinin, curcumin, frankincense and vitamin C) | Anti‐inflammation | Not currently approved | Diminishing activity of TNF‐α and IL‐6 levels | NCT04382040 (Phase 2) | Phase 2/NCT04382040/completed/May 8, 2020–November 2020/MGC pharmaceuticals d.o.o) |
(Clinical trial ID: NCT04382040, n.d.) |
|
Abivertinib
|
Tyrosine kinase inhibitor (TKI), BTK Inhibitor, third‐generation EGFR tyrosine kinase inhibitor | Not yet approved | Inhibition of vital pro‐inflammatory cytokine production, such as IL‐6, IL‐1β and TNF‐α |
NCT04528667 (Phase 2) NCT04440007 (Phase 2) |
Phase 2/NCT04528667/recruiting/September 2020–May 2021 |
(Clinical trial ID: NCT04440007, n.d.; Sorrento Therapeutics Inc, News Release, 2020) |
|
Estradiol
|
Enhancing expression of interleukin 6 via the estrogen receptor β (ERβ) pathway |
Menorrhagia, vaginal atrophy, healthy, suicidal ideation |
Anti‐inflammatory and immunomodulatory, blocking the proinflammatory cytokines production, such as IL‐6, IL‐1β, TNF‐α and chemokine CCL2 |
NCT04359329 (Phase 2) | Phase 2/NCT04359329/recruiting/April 20, 2020–November 15, 2020 |
(Mauvais‐Jarvis et al., 2020) |
|
Pentoxifylline
|
Competitive nonselective phosphodiesterase inhibitor | Type 2 diabetes, chronic renal failure, severe alcoholic hepatitis, lupus nephritis, irritable bowel syndrome, diabetic kidney disease et al. |
Anti‐inflammatory, blocking the proinflammatory cytokines production, such as IL‐6, IL‐1, TNF‐α, C‐reactive protein and other immunoregulators. |
NCT04433988 (Phase 2) NCT04570254 (not applicable) |
Phase 2/NCT04433988/not yet recruiting/December 13, 2020–December 30, 2021 |
(Assimakopoulos et al., 2020; González‐Pacheco et al., 2020) |
|
Deferoxamine
|
Iron chelator | Iron overload, diabetic foot ulcer, acute ischemic stroke, Beta‐Thalassemia, |
Inhibiting IL‐6 synthesis through decreasing NF‐kB. |
NCT04333550 (Phase 2) NCT04361032 (Phase 3) NCT04389801 (Phase 4) |
Phase 4/NCT04389801/Not yet recruiting/May 15, 2020–September 30, 2020 | (Clinical trial ID: NCT04389801, n.d.; Dalamaga et al., 2020) |
|
Cefditoren (Pivoxil, ME 1207)
|
New‐third generation cephalosporin antibiotic, broad spectrum of activity against Gram‐positive and gram‐negative bacteria | Rhinosinusitis, COVID‐19 pneumonia, urinary tract infections | Decrease IL‐6 level and other pro‐inflammatory cytokines | NCT04709172 (Phase 4) | Phase 4/NCT04709172/recruiting/January 14, 2021–April 5, 2021 |
(Clinical trial ID: NCT04709172, n.d.) |
|
Aprepitant (cinvanti)
|
Neurokinin 1 receptor antagonist | Postoperative nausea and vomiting, healthy volunteers, emesis, breast cancer, leukemia |
Anti‐inflammatory, blocking inflammatory cytokines release mediated by the binding of substance P to NK1 receptors |
NCT04470622 (Phase 2) |
Phase 2/NCT04470622/recruiting/July 20, 2020–June 2021/Heron Therapeutics |
(Clinical trial ID: NCT04470622, n.d.; Mehboob et al., 2020) |
|
Prazosin
|
Selective alpha 1‐adrenergic blocking agent. | PTSD, panic disorder, hypertension and anxiety and anxiety | α‐1 adrenergic receptor antagonist, prevent cytokine storm | NCT04365257 (Phase 2) | Phase 2/NCT04365257/recruiting/May 13, 2020–June 30, 2021 |
(Konig et al., 2020) |
|
Ampion (DA‐DKP, aspartyl‐alanyl diketopiperazine)
|
Modulating inflammatory immune response via molecular pathways related to T lymphocyte energy deficiency |
Inflammation associated with osteoarthritis | Anti‐inflammatory, blocking the proinflammatory cytokines production in T‐cells |
NCT04456452 (Phase 1) NCT04839965 (Phase 2) NCT04606784(Phase 1) |
Phase2/NCT04839965/recruiting/April 2021–October 2021 |
(Clinical trial ID: NCT04414618, n.d.) |
|
Ibudilast
|
Nonselective phosphodiesterase inhibitor | Asthma, multiple sclerosis | Anti‐inflammatory | NCT04429555 (Phase 2) | Phase 2/NCT04429555/recruiting/November 2020–June 30, 2021 |
(Clinical trial ID: NCT04429555, n.d.) |
|
LAU‐7b (fenretinide [4‐HPR])
|
Retinoic acid receptors (RAR) inhibitor | Not approved yet | Anti‐inflammatory and antiviral activities | NCT04417257 (Phase 2) | Phase 2/NCT04417257/recruiting/June 29, 2020–May 15, 2021 |
(Orienti et al., 2020) |
|
Naltrexone
|
Blockade of opioid receptors, κ‐opioid receptor antagonist | Alcoholism, opioid use, obesity | Interrupt the inflammation |
NCT04365985 (Phase 2) NCT04756128 (Phase 2) NCT04604678 (Phase 2) NCT04604704 (Phase 2) NCT04708327 (Phase 2) |
Phase 2/NCT04365985/recruiting/April 29, 2020–May 2021 |
(Choubey et al., 2020) |
|
Piclidenoson
|
Adenosine A3 receptor agonist | Not approved yet | Anti‐inflammatory | NCT04333472 (Phase 2) | Phase 2/NCT04333472/recruiting/January 6, 2021–March 6, 2022 |
(David & Shweta, 2020) |
|
Clarithromycin
|
Macrolide antibiotic and a CYP3A4 inhibitor | Antibiotics‐bacterial infection, nontuberculous mycobacteria, mycobacterium avium complex, crohn's disease, ulcer, peptic ulcer, periodontitis | Anti‐inflammatory nature and enhance the immunity | NCT04398004 (Phase 2) NCT04622891 (not applicable) | Phase 2/NCT04398004/completed/May 6, 2020–November 30, 2020 | |
|
Thalidomide
|
Cereblon (CRBN) inhibitor, sedative, part of the cullin‐4 E3 ubiquitin ligase complex CUL4‐RBX1‐DDB1 |
Mantle cell lymphoma, NSCLC, congestive heart failure, multiple myeloma, gastric cancer | Anti‐inflammatory and anti‐fibrosis | NCT04273581 (Phase 2) NCT04273529 (Phase 2) | Phase 2/NCT04273581/ot yet recruiting/February 18, 2020–April 30, 2020 |
(Khalil et al., 2020) |
|
Cenicriviroc
|
Dual CCR2/CCR5 antagonist | Not yet approved | Anti‐inflammatory and immunomodulatory effects | NCT04500418 (Phase 2) | Phase 2/NCT04500418/recruiting/August 25, 2020–September 30, 2021 |
(Okamoto et al., 2020) |
|
Ruxolitinib
|
Selective JAK1/2 inhibitor | Thalassemia major, splenomegaly | Inhibit the downstream IFNg pathway targeting JAK kinase receptor |
Approximately 19 clinical trials, some of the examples are: NCT04414098 (Phase 2) NCT04477993 (Phase 3) NCT04581954 (Phase 2) NCT04362137 (Phase 3) NCT04348071 (Phase 3) NCT04377620 (Phase 3) NCT04338958 (Phase 2) |
Phase 3/NCT04362137/completed/May 2, 2020–October 17, 2020 | |
|
Baricitinib
|
JAK1 and JAK2 inhibitor | Rheumatoid arthritis |
JAK–STAT signal blocking |
Approximately 11 clinical trials, some of the examples are: NCT04358614 (Phase 3) NCT04320277 (Phase 3) NCT04340232 (Phase 3) NCT04373044 (Phase 2) NCT04421027 (Phase 3) NCT04346147 (Phase 2) NCT04832880 (Phase 3) |
Phase 3/NCT04421027/recruiting/June 12, 2020–May 19, 2021 | |
|
Niclosamide
|
Anti‐parasitic drug, STAT3 inhibitor, DNA replication inhibitor | Not yet approved |
Blocking signaling pathways including mTORC1, IL‐6‐JAK1‐STAT3 signaling |
Approximately 13 clinical trials, some of the examples are: NCT04558021 (Phase 3) NCT04399356 (Phase 2) NCT04542434 (Phase 2) NCT04372082 (Phase 3) NCT04603924 (Phase 3) NCT04436458 (Phase 2) NCT04524052 (Phase 1) |
Phase 3/NCT04558021/recruiting/October 8, 2020–January 30, 2021 |
(Pindiprolu & Pindiprolu, 2020; Xu, Shi, Wang, et al., 2020) |
|
Levamisole
|
Anthelmintic and immunomodulator | Broad‐spectrum insect repellent |
Down regulating the level of IL‐6 and TNF‐α |
NCT04360122 (Phase 3) NCT04383717 (Phase 3) NCT04331470 (Phase 3) |
Phase 3/NCT04331470/recruiting/April 4, 2020–April 20, 2020 |
(Uyaroğlu et al., 2020) |
|
Opaganib
|
Selective, competitive sphingosine kinase 2 (SK2) inhibitor | Not yet approved |
Down regulating the level of IL‐6 and TNF‐α, inhibiting viral replication |
NCT04414618 (Phase 2) NCT04467840 (Phase 3) NCT04502069 (Phase 2) |
Phase 3/NCT04467840/recruiting/August 21, 2020–June 2021 |
(Kurd et al., 2020) |
|
Anakinra
|
Interleukin‐1 receptor (IL‐1) antagonist | Rheumatoid arthritis, knee injuries | Interleukin‐1 (IL‐1) blockage |
Approximately 17 clinical trials, some of the examples are: NCT04826588 (Phase 3) NCT04364009 (Phase 3) NCT04443881 (Phase 3) NCT04603742 (Phase 2) NCT04357366 (Phase 2) NCT04339712 (Phase 2) NCT04412291 (Phase 2) |
Phase 3/NCT04443881/recruiting/May 8, 2020–December 2020 |
(Gallelli et al., 2020) |
|
PF‐06650833
|
Interleukin‐1 receptor associated kinase 4 (IRAK4) inhibitor | Rheumatoid arthritis, lupus, lymphomas | Inhibition of interleukin‐1 receptor associated kinase 4 (IRAK4) in ameliorating the proinflammatory state and improving outcomes in severe COVID‐19. | NCT04933799 (Phase 2) | Phase 2/NCT04933799/recruiting/June 22, 2021–March 6, 2022 | (Clinical trial ID: NCT04933799, n.d.) |
|
Atorvastatin
|
HMG‐CoA reductase inhibitor | Stable angina, acute coronary syndrome, hepatocellular carcinoma, cardiovascular disease, ischemia et al. | lower levels of inflammatory cytokines |
NCT04466241 (Phase 2) NCT04380402 (Phase 2) NCT04486508 (Phase 3) |
Phase 3/NCT04486508/active, not recruiting/July 30, 2020–April 4, 2021 |
(Ghati et al., 2020; Li, 2020; Tan et al. 2020) |
|
PTC299
|
Dihydroorotate dehydrogenase (DHODH) inhibitor | Not yet approved |
Blocking the proinflammatory cytokines production such as IL‐6, IL‐17, IL‐17F |
NCT04439071 (Phase 3) | Phase 3/NCT04439071/recruiting/July 9, 2020–January 30, 2021 |
(Luban et al., 2021) |
|
Amiodarone
|
ATP‐sensitive potassium channel inhibitor | Atrial fibrillation, atrial fibrillation, new oonset atrial fibrillation, paroxysmal atrial fibrillation, ventricular Tachycardia et al. |
NCT04351763 (Phase 3) |
Phase 3/NCT04351763/recruiting/April 27, 2020–March 2, 2021 |
(Sanchis‐Gomar et al., 2020) |
|
|
VERU‐111
|
Orally bioavailable α and β tubulin inhibitor | Metastatic castration resistant prostate cancer | Anti‐inflammatory action against the key cytokines involved in the cytokine storm | NCT04388826 (Phase 2) NCT04842747 (Phase 3) | Phase 3/NCT04842747/not yet recruiting/April 30, 2021–January 5, 2022 |
(Clinical trial ID: NCT04388826, n.d.) |
|
Escin
|
Vasoprotective anti‐inflammatory, anti‐nociceptive and anti‐edematous agent. | Chronic venous insufficiency | Vasoprotective anti‐inflammatory | NCT04322344 (Phase 2) | Phase 3/NCT04322344/recruiting/March 23, 2020–June 30, 2020 |
(Clinical trial ID: NCT04322344, n.d.; Gallelli et al., 2020) |
|
Imatinib
|
Tyrosine kinases inhibitor |
Breast cancer, gastrointestinal stromal tumors, chronic myeloid leukemia |
Tyrosine kinase inhibitors in the regulation of inflammation |
NCT04794088 (Phase 2) NCT04422678 (Phase 3) NCT04394416 (Phase 3) NCT04357613 (Phase 2) NCT04346147 (Phase 2) |
Phase 3/NCT04394416/recruiting/June 2, 2020–June 1, 2022 |
(Sisk et al., 2018) |
|
Montelukast
|
Cysteinyl leukotriene receptor 1 (Cysltr1) antagonist | Asthma and liver injury, reduce cardiac damage |
Anti‐inflammatory, reducing production of cytokine |
NCT04714515 (Phase 3) NCT04389411 (Phase 3) NCT04695704 (Phase 3) NCT04718285 (Phase 2) |
Phase 3/NCT04714515/completed/February 20, 2020–March 30, 2020 | |
|
Losmapimod
|
p38 MAPK inhibitor | Not yet approved | Reduce the acute exaggerated pro‐inflammatory responses | NCT04511819 (Phase 3) | Phase 3/NCT04511819/active, not recruiting/August 28, 2020–June 2021 |
(Clinical trial ID: NCT04511819, n.d.) |
|
Tradipitant
|
Neurokinin‐1 (NK‐1) antagonist | Eczema, pruritus, gastroparesis, chronic pruritus, and atopic dermatitis | NK‐1 antagonist involved in neuroinflammatory processes | NCT04326426 (Phase 3) | Phase 3/NCT04326426/enrolling by invitation/April 13, 2020–August 1, 2020 |
(Clinical trial ID: NCT04326426, n.d.) |
|
Ifenprodil (NP‐120)
|
Noncompetitive N‐methyl‐d‐aspartate (NMDA) receptor antagonist | Posttraumatic stress disorders |
NDMA receptor‐type subunit 2B antagonist and the subunit receptor mainly expressed on T cells and neutrophils |
NCT04382924 (Phase 3) | Phase 3/NCT04382924/active, not recruiting/August 5, 2020–January 2022 |
(Clinical trial ID: NCT04382924, n.d.) |
|
Prednisone
|
Synthetic corticosteroid agent, upregulating natriuretic peptide receptor type A | Comparative study, immunosuppressive agents, inflammatory bowel disease, multiple sclerosis |
Agonist of glucocorticosteroid, blocking inflammatory and immune responses. |
NCT04534478 (Phase 4) NCT04344288 (Phase 2) |
Phase 4/NCT04534478//not yet recruiting/September 7, 2020–May 2, 2021 |
(Herman et al., 2020) |
|
Hydrocortisone
|
Human endogenous metabolite, steroid hormone or glucocorticoid secreted by the adrenal cortex | Coronary artery disease, healthy, septic shock, asthma |
Agonist of glucocorticosteroid, blocking inflammatory and immune responses. |
NCT04348305 (Phase 3) NCT02735707 (Phase 4) NCT02517489 (Phase 3) NCT04599959 (not applicable) NCT04563676 (not applicable) | Phase 4/NCT02735707/recruiting/April 11, 2016–December 2021 | |
|
Dexamethasone
|
Glucocorticoid receptor agonist | Cervical radiculopathy, cancer, inflammatory response, hip fracture, pain |
Agonist of glucocorticosteroid, reducing CD11b, CD18, and CD62L |
Approximately 31 clinical trials, some of the examples are: NCT04834375 (Phase 4) NCT04603729 (Phase 3) NCT04499313 (Phase 3) NCT04325061 (Phase 4) NCT04325061 (Phase 4) NCT04347980 (Phase 3) NCT04563494 (Phase 4) NCT03170882 (Phase 2) |
Phase 4/NCT04834375/recruiting/March 19, 2021–March 19, 2022 |
(Horby et al., 2021) |
|
Budesonide
|
Orally active glucocorticoid receptor agonist | Asthma | Glucocorticoid receptor agonist, Suppress the immune reaction locally in the respiratory system. | NCT04331470 (Phase 3) NCT04374474 (Phase 4) NCT04361474 (Phase 3) | Phase 4/NCT04374474/active, not recruiting/May 18, 2020–November 24, 2020 |
(De León‐Rendón et al., 2020) |
|
Famotidine
|
Competitive histamine H2‐receptor antagonist | Peptic ulcer, HIV infections, aspirin, dyspepsia, stomach ulcer, heartburn | Anti‐inflammatory action | NCT04836806 (Phase 4) NCT04504240 (Phase 3) NCT04545008 (Phase 1) NCT04565392 (Phase 4) NCT04724720 (Phase 2) NCT04370262 (Phase 3) NCT04389567 (not applicable) | Phase 4/NCT04565392/not yet recruiting/May 1, 2021–November 1, 2021 |
(Ennis & Tiligada, 2021) |
|
Nitazoxanide
|
Antiprotozoal agent |
Irritable bowel syndrome, hepatitis C, diabetes mellitus type 2 |
Control excessive inflammatory immune responses |
Approximately 24 clinical trials, some of the examples are: NCT04406246 (Phase 4) NCT04552483 (Phase 2) NCT04498936 (Phase 4) NCT04463264 (Phase 3) NCT04486313 (Phase 3) NCT04532931 (Phase 2) NCT04563208 (Phase 2) |
Phase 4/NCT04498936/completed/July 15, 2020–October 30, 2020 |
(Kelleni, 2020; Mahmoud et al., 2020; Pepperrell et al., 2020) |
|
Colchicine
|
Tubulin inhibitor and microtubule disrupting agent | Myocardial infarction, intercritical gout | Anti‐inflammatory effects, an inhibitor of NLRP3 inflammasomes and mitigating interleukin activation. |
Approximately 27 clinical trials, some of the examples are: NCT04818489 (Phase 4) NCT04392141 (Phase 2) NCT04416334 (Phase 3) NCT04472611 (Phase 3) NCT04322565 (Phase 2) NCT04724629 (Phase 3) NCT04492358 (Phase 3) |
Phase 4/NCT04818489/recruiting/March 25, 2021–May 25, 2021 | |
|
Silymarin
|
p38 MAPK pathway inhibitor | Non‐alcoholic fatty liver disease, metastatic colorectal cancer, hepatitis | Anti‐inflammatory and anti‐oxidant effects |
NCT04394208 (Phase 3) NCT04816682 (Phase 4) |
Phase 4/NCT04816682/recruiting/March 17, 2021–June 30, 2021 |
(Clinical trial ID: NCT04394208, n.d.) |
|
Methylprednisolone
|
Synthetic corticosteroid, anti‐inflammatory and immunomodulating properties | COPD, purpura, schoenlein‐henoch, postoperative pain, drug interaction potentiation, et al. |
Anti‐inflammatory, decreasing level of IL‐6, ACE2 activation |
Approximately 15 clinical trials, some of the examples are: NCT04499313 (Phase 3) NCT04485429 (Phase 3) NCT04603729 (Phase 3) NCT04826588 (Phase 3) NCT03708718 (Phase 2) NCT04780581 (Phase 4) NCT04765371 (Phase 3) |
Phase 4/NCT04780581/recruiting/February 1, 2021–December 1, 2021 |
(Edalatifard et al., 2020; Liu, Zheng, Huang, Shan, & Huang, 2020) |
|
Enpatoran (M5049)
|
Dual TLR7/8 inhibitor | Systemic lupus erythematosus, Coronavirus Disease 2019 | Blocks the activation of toll‐like receptor (TLR)7 and TLR8, reduction in the inflammatory response | NCT04448756 (Phase 2) | Phase 2/NCT04448756/Active, not recruiting/June 26, 2020–August 22, 2021 | (Clinical trial ID: NCT04448756, n.d.) |
|
EC‐18
|
Anti‐inflammation | Stomatitis, chemotherapy‐Induced neutropenia, febrile neutropenia | Anti‐inflammatory, prevention of COVID‐19 infection to severe pneumonea or ARDS |
NCT04500132 (Phase 2) NCT04569227 (Phase 2) |
Phase 2/NCT04569227/recruiting/September 29, 2020–September 2021 | (Clinical trial ID: NCT04569227, n.d.) |
|
APX‐115 (Ewha‐18,278)
|
pan NADPH oxidase (Nox) inhibitor | Healthy, diabetic nephropathies |
NADPH‐dependent generation of superoxide and secondary reactive oxygen species (ROS). ROS are often generated during virus infection, thus promoting apoptosis, lung injury, and inflammation/allergy. |
NCT04880109 (Phase 2) | Phase 2/NCT04880109/not yet recruiting/May 10, 2021–February 2022 |
(Clinical trial ID: NCT04880109, n.d.) |
2.4. Modulating immune system
Special cells (such as white blood cells B‐ and T‐lymphocytes), organs (such as bone marrow, spleen, and thymus), and chemicals (antibodies) mainly compose the immune system (Sompayrac, 2019). The well‐working immune system produces antibodies to kill pathogens and protects the body against viruses and diseases. The immune system in human body plays a fundamental role in maintaining health (Chowdhury et al., 2020). After invasion into the human body, SARS‐CoV‐2 will encounters a robust innate immune response when it moves to the respiratory tract (Rokni et al., 2020; Tang et al., 2005). The immune system plays a critical role in the body's defenses against SARS‐CoV‐2 infection. Immune system modulation prevents tissue damage resulting from an excessive response. Under some circumstances, drugs modulating immune system could be an efficient strategy for fighting against COVID‐19. Agents such as cyclosporine A, isoprinosine, all‐trans retinoic acid are in clinical trials aiming to suppress the virus infection through modulation of the immune system (Table 4).
TABLE 4.
Modulating immune system
| Drug name and structure | Original mechanism | FDA‐approved indication(s) | Possible mechanism for anti‐COVID‐19 | Clinical trials ID (stage) | The highest anti‐COVID‐19 phase/ID/status/start–(estimated)end dates | References |
|---|---|---|---|---|---|---|
|
Methotrexate
|
Enzyme dihydrofolate reductase inhibitor, an immunosuppressant and antineoplastic agent | Rheumatoid arthritis, ophthalmopathy, thyroid‐associated, psoriatic arthritis |
Immunomodulatory agent, preventing excessive immunereaction |
NCT04352465 (Phase 2) NCT04610567 (Phase 2) |
Phase 2/NCT04610567/recruiting/October 27, 2020–March 15, 2021 |
(Abuo‐Rahma et al., 2020) |
|
Leflunomide
|
Dihydroorotate dehydrogenase inhibitor | Rheumatoid arthritis, psoriatic arthritis, immunoglobulin G4 related sclerosing disease, juvenile idiopathic arthritis |
Immunomodulatory agent, preventing excessive immunereaction |
NCT04532372 (Phase 2) NCT04361214 (Phase 1) |
Phase 2/NCT04532372/recruiting/January 7, 2021–September 18, 2022 |
(Abuo‐Rahma et al., 2020) |
|
Fingolimod
|
Sphingosine 1‐phosphate (S1P) antagonist, pak1 activator, a immunosuppressant | Multiple sclerosis, relapsing–remitting |
Immune modulator, S1P1 receptors antagonist |
NCT04280588 (Phase 2) |
Phase 2/NCT04280588/withdrawn (no participants enrolled)/February 22, 2020–July 1, 2020 |
|
|
Tamoxifen
|
Selective estrogen receptor modulator (SERM), Hsp90 activator | Breast cancer, infertility, menorrhagia, endometrium | Modulation of NK cells activity and reduce viral replication, through reducing PGE2 production |
NCT04389580 (Phase 2)NCT04568096 (Phase 2) |
Phase 2/NCT04568096/not yet recruiting/November 2020–December 2020 | |
|
Isoprinosine
|
Immunostimulant | Immunostimulant | Immunostimulant |
NCT04360122 (Phase 3) NCT04383717 (Phase 3) |
Phase 3/NCT04360122/not yet recruiting/May 20, 2020–November 1, 2020 |
(Clinical trial ID: NCT04360122, n.d.; Kumar et al., 2020) |
|
IMU‐838 (vidofludimus calcium)
|
Immunomodulatory agent, DHODH inhibitor, inhibiting IL‐17 secreting |
Not yet approved | Selective immunomodulatory effect against highly activated immune cells | NCT04516915 (Phase 2) NCT04379271 (Phase 3) | Phase 3/NCT04379271/recruiting/June 11, 2020–September 2020 |
(Clinical trial ID: NCT04379271, n.d.; Immunic Therapeutics website) |
|
All‐trans retinoic acid
|
Neutrophil elastase inhibitor, RAR nuclear receptor agonist | Acute promyelocytic leukemia | Enhance neutralizing antibodies |
NCT04396067 (Phase 2) NCT04568096 (Phase 2) NCT04730895 (Phase 2) NCT04578236 (Phase 2) NCT04353180 (Phase 3) |
Phase 3/NCT04353180/not yet recruiting/April 2021–June 2021 |
(Clinical trial ID: NCT04396067, n.d.) |
|
Melatonin
|
Selective ATF‐6 inhibitor | Parkinson's disease, infertility, immediate dental implant, immediate dental implant, postoperative pain, anxiety, acute ischemic stroke | Modulate the immune response and neuroinflammation |
Approximately 8 clinical trials, some of the examples are: NCT04474483 (Phase 2) NCT04470297 (Phase 2) NCT04568863 (Phase 2) NCT04353128 (Phase 3) NCT04409522 (not applicable) NCT04531748 (Phase 2) NCT04530539 (not applicable) |
Phase 3/NCT04353128/recruiting/April 20, 2020–October 2020 |
(Bahrampour Juybari et al., 2020) |
|
Cyclosporinea(Synonyms: cyclosporine; Ciclosporin)
|
Immunosuppressant, inhibiting CD11a/CD18 adhesion | End‐stage renal disease, renal function and chronic allograft vasculopathy, kidney transplantation, etc |
Immunomodulatory agent acting on T cells |
NCT04540926 (Phase 2) NCT04412785 (Phase 1) NCT04492891 (Phase 2) NCT04392531 (Phase 4) NCT04451239 (not applicable) | Phase 4/NCT04392531/recruiting/April 16, 2020–March 2021 | |
|
AZD1656
|
Glucokinase activator | Type II diabetes | Activate the migration of T regulatory cells to sites of inflammation | NCT04516759 (Phase 2) | Phase 2/NCT04516759/completed/August 18, 2020–April 25, 2021 | (Clinical trial ID: NCT04516759, n.d.) |
2.5. Anticoagulant therapy
SARS‐CoV‐2 can invade cells lining blood vessels and cause a series of consequences related to cardiovascular diseases and thrombosis. More specifically, the virus invasion could induce endothelial dysfunction which further leads to pulmonary thrombi (Carfora et al., 2021; Poissy et al., 2020). Applying anticoagulant therapy in COVID 19 patients has scientific support and is in line with the COVID‐19 global pandemic emergency. Agents such as rivaroxaban and apixaban are in ongoing clinical trials to combat COVID‐19 associated hypercoagulation (Figure 2 and Table 5). The efficacy and effectiveness of using anticoagulant therapy for COVID‐19 await clinical data.
TABLE 5.
Anticoagulant therapy
| Drug name and structure | Original mechanism | FDA‐approved indication(s) | Possible mechanism for anti‐COVID‐19 | Clinical trials ID (stage) | The highest anti‐COVID‐19 phase/ID/status/start–(estimated)end dates | References |
|---|---|---|---|---|---|---|
|
Ketamine
|
Antagonist of N‐methyl D‐aspartate receptor | Anesthesia, pain relief, sedation, and memory loss | Anesthesia, pain relief | NCT04365985 (Phase 2) | Phase 2/NCT04365985/recruiting/April 29, 2020–May 2021 |
(Suri & Sindwani, 2020) |
|
Aspirin
|
Non‐selective and irreversible COX‐1 and COX‐2 inhibitor | Common cold, healthy, acute coronary syndrome, et al. |
Interfering with normal platelet aggregation, COX‐1 inhibitor |
NCT04365309 (Phase 3) NCT04363840 (Phase 2) NCT04343001 (Phase 3) NCT04410328 (Phase 3) NCT04324463 (Phase 3) NCT04808895 (Phase 3) | Phase 3/NCT04410328/recruiting/October 21, 2020–March 15, 2021 |
(Clinical trial ID: NCT04365309, n.d.; Bianconi et al., 2020) |
|
Rivaroxaban
|
Selective and direct Factor Xa (FXa) inhibitor | Atherosclerosis, mitral valve stenosis|atrial fibrillation | Adequate antithrombotic therapy | NCT04504032 (Phase 2) NCT04508023 (Phase 3) NCT0441604 (Phase 3) NCT04324463 (Phase 3) | Phase 3/NCT04324463/recruiting/April 21, 2020–December 31, 2020 |
(Di Tano et al., 2020) |
|
Enoxaparin
|
Anticoagulant medication |
Pulmonary embolism (PE), deep vein thrombosis (DVT) |
Adequate antithrombotic therapy |
Approximately 8 clinical trials, some of the examples are: NCT04427098 (Phase 2) NCT04366960 (Phase 3) NCT04400799 (Phase 3) NCT04540926 (Phase 2) NCT04492254 (Phase 3) NCT04408235 (Phase 3) NCT04640181 (Phase 2) |
Phase 3/NCT04400799/recruiting/June 15, 2020–March 14, 2021 |
(Drago et al., 2020) |
|
Tranexamic acid
|
Antifibrinolytic agent, blocking lysine‐binding sites of plasmin and elastase‐derived plasminogen fragments |
Prevent blood loss in surgical procedure | Antifibrinolytic agent, that competitively inhibits activation of plasminogen to plasmin, an enzyme that degrades fibrin clots. | NCT04338126 (Phase 2) NCT04550338 (Phase 3) NCT04338074 (Phase 2) | Phase 3/NCT04550338/not yet recruiting/December 1, 2020–December 31, 2022 |
(Ogawa & Asakura, 2020) |
|
Edoxaban
|
FXa inhibitor | Atrial fibrillation (AF), venous thrombosis|neoplasms|anticoagulant, ischemic stroke, blood coagulation disorder, coronary artery disease | Anticoagulant activity of direct FXa inhibitors |
NCT04516941 (Phase 3) NCT04542408 (Phase 3) |
Phase 3/NCT04542408/recruiting/November 12, 2020–May 31, 2021 |
(Al‐Horani, 2020) |
|
Apixaban
|
FXa inhibitor |
Deep vein thrombosis|pulmonary embolism, diabetes, venous thromboembolism, etc. |
Anticoagulant properties | NCT04512079 (Phase 4) | Phase 4/NCT04512079/recruiting/September 8, 2020–March 2021 |
(Al‐Horani, 2020) |
2.6. Antioxidant supplement
Antioxidant agents help prevent free radicals or reactive oxygen species (ROS) from harming healthy cells in human body. The effects of antioxidants on heart disease, cancer, and arthritis have been widely studied. As a powerful antioxidant, vitamin‐C might protect the human body against SARS‐CoV‐2 infection through its important role in enhancing human body immunity (Carr & Maggini, 2017). A high‐dose vitamin C in coping with the oxidative stress in COVID‐19 patients is currently being studied (Colunga Biancatelli et al., 2020; Hunt et al., 1994). In addition, vitamin D supplementation might lower the odds of developing respiratory infections according to the data from observational studies (Table 6) (Martineau et al., 2017; McCartney & Byrne, 2020)
TABLE 6.
Antioxidant supplement
| Drug name and structure | Original mechanism | FDA‐approved indication(s) | Possible mechanism for anti‐COVID‐19 | Clinical trials ID (stage) | The highest anti‐COVID‐19 phase/ID/status/start–(estimated)end dates | References |
|---|---|---|---|---|---|---|
|
N‐acetylcysteine
|
ROS inhibitor, mucolytic agent reducing thickness of mucus, | Gastric mucosal lesion, polycystic ovary syndrome, etc. |
Antioxidant precursor to glutathione (γ‐glutamylcysteinylglycine), mucolytic agent |
NCT04374461 (Phase 2) NCT04792021 (Phase 3) NCT04545008 (Phase 1) NCT04458298 (Phase 2) NCT04419025 (Phase 2) NCT04703036 (Phase 1) NCT04755972 (not applicable) | Phase 3/NCT04792021/recruiting/March 9, 2021–June 2021 | |
|
Vitamin C (ascorbic acid)
|
Effective reducing agent and donor antioxidant | Scurvy, upper respiratory tract infections, reduces the risk of lung cancer. |
Upregulating phagocytes and lymphocytes activity, modulating immune system |
Approximately 24 clinical trials, some of the examples are: NCT04401150 (Phase 3) NCT04468139 (Phase 4) NCT04363216 (Phase 2) NCT04264533 (Phase 2) NCT02735707 (Phase 4) NCT04780061 (Phase 3) NCT04828538 (not applicable) |
Phase 4/NCT02735707/recruiting/11, 2016–December 2021 |
(Baladia et al., 2020) |
|
Vitamin D3 (Cholecalciferol)
|
Inducing cell differentiation and preventing the proliferation of cancer cells | Epilepsy, healthy, vitamin D deficiency, osteoporosis | Protect respiratory infections |
Approximately 14 clinical trials, some of the examples are: NCT04344041 (Phase 3) NCT04536298 (Phase 3) NCT04411446 (Phase 4) NCT04482673 (Phase 4) NCT04407286 (Phase 1) NCT04386850 (Phase 3) NCT04395768 (Phase 2) |
Phase 4/NCT04482673/recruiting/July 31, 2020–December 31, 2021 |
(Weir et al., 2020) |
|
Quercetin
|
Stimulator of recombinant SIRT1, PI3K inhibitor | Menopause related conditions, mountain sickness, flushing | Prophylactic, antioxidant |
NCT04853199 (early Phase 1) NCT04578158 (Phase 2) NCT04468139 (Phase 4) NCT04536090 (Phase 2) NCT04377789 (not applicable) |
Phase 4/NCT04468139/recruiting/June 20, 2020–July 20, 2020 |
(Diniz et al., 2020) |
3. AGENTS IN THE DRUG DEVELOPMENT PHASES OF THE PIPELINE
Approved drugs or clinical candidates in COVID‐19 clinical trials are arranged and analyzed based on their highest stage of clinical trial. A summary of the drug name, structure, class, original mechanism, FDA‐approved indication(s), possible mechanism for COVID‐19 treatment, the highest anti‐COVID‐19 phase (clinical trial ID) etc. are presented in this section (Figure 4 and Tables 1, 2, 3, 4, 5, 6).
3.1. Phase I/II clinical candidates
The primary goal of Phase I study is to assess the safety, the tolerability, the pharmacokinetic parameters, and the pharmacodynamics effects of a drug candidate in healthy volunteers. At the end of this phase, the optimum dose and formulation will be determined. The information obtained could be used for subsequent phases. There are four agents in Phase I clinical trials (Figure 4). PF‐07304814 is an inhibitor of 3CLpro currently in several clinical stages including Phase I. Galidesivir and GS‐441524, RdRP inhibitors, are being tested in Phase I for potential blocking viral replication. Progesterone, a steroid hormone, aims to address the CSS. No small molecule in other categories is currently in phase I trials. It takes a long time to develop a new drug from scratch, so researchers focus their main efforts on drug repurposing.
A phase II trial is designed to obtain the therapeutic effects and side effects of the drug candidate in patients with the disease. In contrast to only two trials in phase I, there are significantly more trials in phase II. The drugs for phase II trials are all FDA‐approved drugs for indications other than COVID‐19. In fact, there are 42 agents in approximately 85 phase II trials (Figure 4).
There are 5 small molecules in phase II trials aiming to block virus‐cell membrane fusion and entry, 8 small molecules inhibiting the viral replication, 23 small molecules addressing cytokine storm, 5 small molecules modulating the immune system, and 1 small molecule used as anticoagulant therapy. There is no small molecule phase II trial using antioxidant supplement.
Of the drugs blocking virus‐cell membrane fusion and entry, there are three agents (ramipril and atovaquone and defibrotide) targeting ACE, one agent (enzalutamide) acting as androgen receptor (AR) antagonist aiming to block TMPRSS2, the other agent (maraviroc) acting as CCR5 antagonist inhibiting cell fusion. Of the drugs targeting viral replication, there were four Mpro protease inhibitors (ebselen, disulfiram, tafenoquine and isoquercetin. Ebselen and disulfiram are nonspecific promiscuous SARS‐CoV‐2 main protease inhibitors), one 3CLpro inhibitor (etoposide), two agents (, merimepodib and clevudine) targeting RNA, one agent (selinexor) inhibiting virus assembly processes. Of the drugs addressing CSS, there were three Bruton's tyrosine kinase (BTK) inhibitors (zanubrutinib, acalabrutinib, and ibrutinib), one phosphatidylinositol 3‐kinase (PI3K) inhibitor (duvelisib), one mTOR inhibitor (sirolimus), one agent (enpatoran) blocking the activation of Toll‐like receptor 7 (TLR7) and TLR8, 8 agents (artemiC, abivertinib, estradiol, pentoxifylline, aprepitant, prazosin, PF‐06650833 and ampion) mediating the release of inflammatory cytokines, 9 agents (ibudilast, LAU‐7b, naltrexone, piclidenoson, clarithromycin, thalidomide, EC‐18, APX‐115 and cenicriviroc) are anti‐inflammatory drugs. Of the drugs modulating the innate immune system, there are four agents: methotrexate, leflunomide, fingolimod, and tamoxifen. There is only one agent (ketamine) used for symptomatic control.
3.2. Phase III clinical candidates
Phase III clinical trials are the studies in the efficacy confirmatory stage. Its goal is to further verify the therapeutic effectiveness and the safety of a drug candidate on patients with specific indications, and to assess the overall risk–benefit relationships. The results from this stage trial will ultimately provide substantial ground for the new drug registration applications. In this stage of the trial, a larger array of trials and more patients will be required and assessed than those tirals in phase II or phase I. There are 46 agents in approximately 294 trials in total (Figure 4). Among them, 10 agents aim at blocking virus‐cell membrane fusion and entry, 11 small molecules inhibit viral replication, 16 small molecules address CSS, four small molecules modulate the innate immune system, four small molecules are used as anticoagulant therapy, and one small molecule is from the category of antioxidant supplement.
Of the drugs blocking virus‐cell membrane fusion and entry, there are four agents (losartan, isotretinoin, lactoferrin, and propranolol) interacting with ACE2, one agent (linagliptin) inhibiting DPP4, two compounds (verapamil, chlorpromazine) targeting on ion channel, one agent (dapagliflozin) reducing the viral load, and two agents (nafamostat and bicalutamide) blocking TMPRSS2. Of the drugs targeting virus replication, there are two reverse transcriptase inhibitors (emtricitabine and tenofovir alafenamide), five RNA‐targeting agents (remdesivir, ciclesonide, EIDD‐2801, AT‐527 and ribavirin), one phosphodiesterase inhibitor (dipyridamole), one Impα/β1‐mediated nuclear import inhibitor (ivermectin), one Mpro inhibitor (rosuvastatin) and one 3CLpro inhibitor (PF‐07321332). In the category of the drugs addressing CSS, there are three JAK inhibitors (ruxolitinib, baricitinib, and niclosamide), five interleukin modulators (levamisole, opaganib, anakinra, atorvastatin, and PTC299), two modulators targeting inflammatory cytokines release (amiodarone, and VERU‐111), one inflammatory inhibitor (escin), one tyrosine kinase inhibitor (imatinib), one cysteinyl leukotriene (cysLT) receptor antagonist (montelukast), one p38 MAPK pathway inhibitors (losmapimod), one neurokinin‐1 (NK‐1) antagonist (tradipitant), and one N‐methyl‐d‐aspartate (NMDA) receptor antagonist (ifenprodil). There are four agents (isoprinosine, IMU‐838, all‐trans retinoic acid, and melatonin) modulating the innate immune system and four agents (aspirin, enoxaparin, tranexamic acid, and edoxaban) used as anticoagulant therapy. There is only one agent (N‐acetylcysteine) belonging to antioxidant supplement sub‐class. Dipyridamole does not inhibit the SARS‐CoV‐2 main protease. Its antiviral activity might involve other mechanisms (Ma & Wang, 2021).
3.3. Phase IV clinical candidates
Phase IV clinical trial is conducted by the applicant after the new drug has already been approved by FDA. The main objective in this phase is to assess the long‐term benefits and risks of using the drug in general population groups or in special population groups. In category, there are 34 agents in approximately 386 trials (Figure 4). Among them, 10 agents aim at blocking virus‐cell membrane fusion and entry, 7 small molecules inhibit viral replication, 11 small molecules address CSS, 1 small molecule modulates immune system. In addition, there are currently 2 agents used as anticoagulant therapy and 3 agents identified as antioxidant supplements.
Of those drugs blocking virus‐cell membrane fusion and entry, three agents are TMPRSS2 inhibitors (bromhexine, camostat, and spironolactone), three agents (valsartan, arbidol and canrenoate potassium) interact with ACE2, one agent (azithromycin) interfers with S protein/CD147 interaction, one agent (tetrandrine) inhibits voltage‐gated Ca2+ current (ICa) and Ca2+‐activated K+ current, one agent (doxycycline) inhibits the E2 envelope glycoprotein involved in virus entry, and one binder of surface proteins of enveloped viruses (povidone‐iodine). Of those drugs targeting virus replication, two agents (ritonavir and lopinavir) inhibit the coronaviral 3CLpro protease, one compound (oseltamivir) blocks virus‐release, three agents (favipiravir, ledipasvir, and sofosbuvir) target RdRp, and 1 agent (darunavir) inhibits HIV protease. Of those drugs addressing CSS, there are four glucocorticosteroid agonists (prednisone, hydrocortisone, dexamethasone, and budesonide), one competitive histamine H2‐receptor antagonist (famotidine), 1 antiprotozoal agent (nitazoxanide), four interleukin modulators (colchicine, methylprednisolone, deferoxamine and cefditoren), one mTOR inhibitor (sirolimus), and one p38 MAPK pathway inhibitor (silymarin). In addition, there is 1 immunosuppressive drug (cyclosporine A) which could modulate the immune system. Apixaban and rivaroxaban belong to anticoagulant therapy. vitamin C, vitamin D3, and quercetin are used as antioxidant supplements.
4. CONCLUSIONS AND OUTLOOK
At present, several COVID‐19 vaccines have been approved for clinical use in many countries worldwide. The vaccine approach aims at the preventive management, which will increase antibody level against the virus and reduce the probability for viral infection in a certain extend. However, the vaccine approach is not suitable for those people already infected with the virus. As SARS‐CoV‐2 continues to worsen global health conditions and economic situations, the number of people infected with this virus continues climbing. Therefore, seeking effective medicines to treat COVID‐19 is urgently needed. This review gathers all small molecules currently in active clinical trials, categorizes them into six sub‐classes, and analyzes possible therapeutical treatments and their underlying mechanisms against COVID‐19. Among all of the clinical trials, the antioxidant supplement sub‐class accounts for the smallest percentile, while addressing CSS sub‐class accounts for the largest percentile. Blocking virus‐cell membrane fusion/entry and inhibiting virus replication are two sub‐classes next to addressing CSS sub‐class, and together these two sub‐classes account for 42% of all clinical agents. The agents from the above two sub‐classes are all belonged to repurposing drugs. Some preliminary results from the studies using repurposing antiviral drugs in clinic have demonstrated the efficacy in blocking virus‐cell membrane fusion and entry or inhibiting virus replication. In addition, given the hyperinflammatory response mediated by addressing CSS agents or modulating immune system agents are expected to work in preventing body deterioration. Finally, Anticoagulant therapy and antioxidant supplement are also playing important roles in combating COVID‐19. However, their effectiveness, safety/side‐effects remain to be watched. In summary, repurposing antiviral and anticancer drugs for treatment of COVID‐19 show promising. The races of small molecules for the treatment of COVID‐19 are intense, and is likely to show some promise in the future.
CONFLICT OF INTEREST
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
- 3CLpro
3C‐like protease
- ACE
angiotensin‐converting enzyme
- ACE2
angiotensin‐converting enzyme 2
- ALDH1
aldehyde dehydrogenase 1
- Ang II
angiotensin II
- AR
androgen receptor
- ARDS
acute respiratory distress syndrome
- AT
angiotensin II receptor type
- AT1R
angiotensin receptor type I
- ATF‐6
activating transcription factor 6
- ATP
adenosine triphosphate
- BTK
Bruton's tyrosine kinase
- CCL2
C‐C Motif Chemokine Ligand 2
- CCR2
C‐C chemokine receptor type 2
- CCR5
C‐C chemokine receptor type 5
- CD11b
cluster of differentiation molecule 11b
- CD147
cluster of differentiation 147
- CD16
cluster of differentiation 16
- CD18
integrin beta chain‐2
- CD4
cluster of differentiation 4
- CD62L
L‐selectin
- COPD
chronic obstructive pulmonary disease
- COVID‐19
coronavirus disease 2019
- COX‐1
cyclooxygenase‐1
- COX‐2
cyclooxygenase‐2
- CRS
cytokine release syndrome
- CSS
cytokine storm syndrome
- CUL4‐RBX1‐DDB1
cullin 4‐ ring box proteins‐ DNA binding protein 1
- CYP3A4
cytochrome P450 3A4
- cysLT
cysteinyl leukotriene
- DHODH
dihydroorotate dehydrogenase
- DPP4
dipeptidyl peptidase‐4
- EGFR
epidermal growth factor receptor
- FDA
food and drug administration
- GLP‐1
glucagon‐like peptide‐1
- G‐CSF
granulocyte colony‐stimulating factor
- HCoV‐19
human coronavirus 2019
- HCoV229E
human coronavirus 229E
- HCoV‐HKU1
human coronavirus HKU1
- HCoV‐NL63
human coronavirus NL63
- HCoV‐OC43
human coronavirus OC43
- HCV
hepatitis C virus
- hERG
the human Ether‐à‐go‐go‐Related Gene
- HIV
human immunodeficiency viruses
- HMG‐CoA
β‐Hydroxy β‐methylglutaryl‐CoA
- HSV
herpes simplex virus
- IL
Interleukin
- IMPDH
Inosine‐5′‐monophosphate dehydrogenase
- JAK–STAT
Janus kinase‐signal transducer and activator of transcription protein
- MAPK
mitogen‐activated protein kinase
- MAS1
mas‐related G‐protein coupled receptor
- MasR
mas receptor
- MCP1
monocyte chemoattractant protein‐1
- MERS‐CoV
middle East respiratory syndrome‐related coronavirus
- MOA
mechanism of action
- Mpro
main protease
- mRNA
messenger ribonucleic acid
- MRSA
methicillin‐resistant Staphylococcus aureus
- mTOR
mechanistic target of rapamycin
- NF‐κB
nuclear factor kappa‐light‐chain‐enhancer of activated B cell
- NIH
National Institutes of Health
- NK‐1
neurokinin‐1
- NLRP3
NLR family pyrin domain containing 3
- NMDA
N‐methyl‐d‐aspartate
- NS5A
nonstructural protein 5A
- NSCLC
non‐small‐cell lung carcinoma
- NSP15
nonstructural protein 15
- PGE2
prostaglandin E2
- P‐gp
P‐glycoprotein
- PI3K
phosphatidylinositol 3‐kinase
- PTSD
post‐traumatic stress disorder
- RAR
retinoic acid receptor
- RAS
pulmonary renin‐angiotensin system
- RdRP
RNA‐dependent RNA polymerase
- RNA
ribonucleic acid
- ROS
reactive oxygen species
- S1P1
sphingosine‐1‐phosphate receptor 1
- SARS‐CoV
severe acute respiratory syndrome coronavirus
- SARS‐CoV‐2
severe Acute Respiratory Syndrome Coronavirus 2
- SGLT2
sodium/glucose cotransporter 2
- SV‐SMC
saphenous vein‐ structural maintenance of chromosomes protein
- TKI
tyrosine kinase inhibitor
- TLR7/9
toll‐like receptor 7/9
- TMPRSS2
transmembrane protease, serine 2
- TNF
tumor necrosis factor
- VDCC
voltage‐dependent calcium channel
- vRNP
viral ribonucleoprotein
- WHO
world health organization
- XPO1
exportin 1
ACKNOWLEDGMENTS
This work was funded by the National Natural Science Foundation of China (82073686, 81730108, 81973635, and 81803340), Hangzhou “115” overseas intelligence program (2020C001), Hangzhou Normal University startup fund (4125C5021920419), Hangzhou Normal University, School of Medicine Teaching Reform Fund (4125b30100112).
Hu, S. , Jiang, S. , Qi, X. , Bai, R. , Ye, X.‐Y. , & Xie, T. (2022). Races of small molecule clinical trials for the treatment of COVID‐19: An up‐to‐date comprehensive review. Drug Development Research, 83(1), 16–54. 10.1002/ddr.21895
Funding information Hangzhou “115” overseas intelligence program, Grant/Award Number: 2020C001; Hangzhou Normal University startup fund, Grant/Award Number: 4125C5021920419; Hangzhou Normal University, School of Medicine Teaching Reform Fund, Grant/Award Number: 4125b30100112; National Natural Science Foundation of China, Grant/Award Numbers: 81730108, 81803340, 81973635, 82073686
Contributor Information
Renren Bai, Email: renrenbai@hznu.edu.cn.
Xiang‐Yang Ye, Email: xyye@hznu.edu.cn.
Tian Xie, Email: xbs@hznu.edu.cn.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- Abuo‐Rahma, G. E.‐D. A. , Mohamed, M. F. A. , Ibrahim, T. S. , Shoman, M. E. , Samir, E. , & Abd El‐Baky, R. M. (2020). Potential repurposed SARS‐CoV‐2 (COVID‐19) infection drugs. RSC Advances, 10(45), 26895–26916. 10.1039/D0RA05821A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akram, J. , Azhar, S. , Shahzad, M. , Latif, W. , & Khan, K. S. (2020). Pakistan randomized and observational trial to evaluate coronavirus treatment (PROTECT) of Hydroxychloroquine, Oseltamivir and azithromycin to treat newly diagnosed patients with COVID‐19 infection who have no comorbidities like diabetes mellitus: A structured summary of a study protocol for a randomized controlled trial. Trials, 21(1), 702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Horani, R. A. (2020). Potential therapeutic roles for direct factor Xa inhibitors in coronavirus infections. American Journal of Cardiovascular Drugs, 20(6), 525–533. 10.1007/s40256-020-00438-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almosawey, H. , Al‐Khikani, F. , Hameed, R. M. , Abdullah, Y. J. , & Alasadi, A. A. (2020). Tamoxifen from chemotherapy to antiviral drug: Possible activity against COVID19. Biomedical and Biotechnology Research Journal (BBRJ), 4(2), 108–116. [Google Scholar]
- Amat‐Santos, I. J. , Santos‐Martinez, S. , López‐Otero, D. , Nombela‐Franco, L. , Gutiérrez‐Ibanes, E. , Del Valle, R. , … San Román, J. A. (2020). Ramipril in high‐risk patients with COVID‐19. Journal of the American College of Cardiology, 76(3), 268–276. 10.1016/j.jacc.2020.05.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreou, A. , Trantza, S. , Filippou, D. , Sipsas, N. , & Tsiodras, S. (2020). COVID‐19: The potential role of copper and N‐acetylcysteine (NAC) in a combination of candidate antiviral treatments against SARS‐CoV‐2. In Vivo, 34(3 Suppl), 1567–1588. 10.21873/invivo.11946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assimakopoulos, S. F. , Seintis, F. , & Marangos, M. (2020). Pentoxifylline and complicated COVID‐19: A pathophysiologically based treatment proposal. Medical Hypotheses, 143, 109926. 10.1016/j.mehy.2020.109926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayerdi, O. , Puerta, T. , Clavo, P. , Vera, M. , Ballesteros, J. , Fuentes, M. E. , … Del Romero, J. (2020). Preventive efficacy of Tenofovir/Emtricitabine against severe acute respiratory syndrome coronavirus 2 among pre‐exposure prophylaxis users. Open Forum Infectious Diseases, 7(11), ofaa455. 10.1093/ofid/ofaa455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrampour Juybari, K. , Pourhanifeh, M. H. , Hosseinzadeh, A. , Hemati, K. , & Mehrzadi, S. (2020). Melatonin potentials against viral infections including COVID‐19: Current evidence and new findings. Virus Research, 287, 198108. 10.1016/j.virusres.2020.198108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baladia, E. , Pizarro, A. B. , Ortiz‐Muñoz, L. , & Rada, G. (2020). Vitamin C for COVID‐19: A living systematic review. Medwave, 20(6), e7978. 10.5867/medwave.2020.06.7978 [DOI] [PubMed] [Google Scholar]
- Barzegar, M. , Mirmosayyeb, O. , Nehzat, N. , Sarrafi, R. , Khorvash, F. , Maghzi, A. H. , & Shaygannejad, V. (2020). COVID‐19 infection in a patient with multiple sclerosis treated with fingolimod. Neurology: Neuroimmunology & Neuroinflammation, 7(4), e753. 10.1212/nxi.0000000000000753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianconi, V. , Violi, F. , Fallarino, F. , Pignatelli, P. , Sahebkar, A. , & Pirro, M. (2020). Is acetylsalicylic acid a safe and potentially useful choice for adult patients with COVID‐19 ? Drugs, 80(14), 1383–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boras, B. (2021). Discovery of a Novel Inhibitor of Coronavirus 3CL Protease for the Potential Treatment of COVID‐19. 10.1101/2020.09.12.293498. [DOI]
- Bozek, A. , & Winterstein, J. (2020). Montelukast's ability to fight COVID‐19 infection. The Journal of Asthma, 1‐2, 1348–1349. 10.1080/02770903.2020.1786112 [DOI] [PubMed] [Google Scholar]
- Buchholz, U. J. , Bukreyev, A. , Yang, L. , Lamirande, E. W. , Murphy, B. R. , Subbarao, K. , & Collins, P. L. (2004). Contributions of the structural proteins of severe acute respiratory syndrome coronavirus to protective immunity. Proceedings of the National Academy of Sciences of the United States of America, 101(26), 9804–9809. 10.1073/pnas.0403492101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadegiani, F. A. , Goren, A. , & Wambier, C. G. (2020). Spironolactone may provide protection from SARS‐CoV‐2: Targeting androgens, angiotensin converting enzyme 2 (ACE2), and renin‐angiotensin‐aldosterone system (RAAS). Medical Hypotheses, 143, 110112. 10.1016/j.mehy.2020.110112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantini, F. , Niccoli, L. , Matarrese, D. , Nicastri, E. , Stobbione, P. , & Goletti, D. (2020). Baricitinib therapy in COVID‐19: A pilot study on safety and clinical impact. The Journal of Infection, 81(2), 318–356. 10.1016/j.jinf.2020.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, B. , Wang, Y. , Wen, D. , Liu, W. , Wang, J. , Fan, G. , … Wang, C. (2020). A trial of Lopinavir‐ritonavir in adults hospitalized with severe Covid‐19. The New England Journal of Medicine, 382(19), 1787–1799. 10.1056/NEJMoa2001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carfora, V. , Spiniello, G. , Ricciolino, R. , Di Mauro, M. , Migliaccio, M. G. , Mottola, F. F. , … Coppola, N. (2021). Anticoagulant treatment in COVID‐19: A narrative review. Journal of Thrombosis and Thrombolysis, 51(3), 642–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr, A. C. , & Maggini, S. (2017). Vitamin C and immune function. Nutrients, 9(11), 1211. 10.3390/nu9111211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascella, M. , Rajnik, M. , Cuomo, A. , Dulebohn, S. C. , & Di Napoli, R. (2021). In StatPearls I. & Island T. (Eds.), Features, evaluation, and treatment of coronavirus (COVID‐19). StatPearls Publishing. [PubMed] [Google Scholar]
- Cattrini, C. , Bersanelli, M. , Latocca, M. M. , Conte, B. , Vallome, G. , & Boccardo, F. (2020). Sex hormones and hormone therapy during COVID‐19 pandemic: Implications for patients with cancer. Cancers, 12(8), 2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cava, C. , Bertoli, G. , & Castiglioni, I. (2020). In Silico discovery of candidate drugs against Covid‐19. Viruses, 12(4), 404. 10.3390/v12040404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalichem, N. S. S. , Bethapudi, B. , & Mundkinajeddu, D. (2020). Aminoglycosides can be a better choice over macrolides in COVID‐19 regimen: Plausible mechanism for repurposing strategy. Medical Hypotheses, 144, 109984. 10.1016/j.mehy.2020.109984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, R. , Ng, T. B. , & Sun, W. Z. (2020). Lactoferrin as potential preventative and adjunct treatment for COVID‐19. International Journal of Antimicrobial Agents, 56(3), 106118. 10.1016/j.ijantimicag.2020.106118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, B. , Tian, E. K. , He, B. , Tian, L. , Han, R. , Wang, S. , … Cheng, W. (2020). Overview of lethal human coronaviruses. Signal Transduction and Targeted Therapy, 5(1), 89. 10.1038/s41392-020-0190-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, G. , Wu, D. , Guo, W. , Cao, Y. , Huang, D. , Wang, H. , … Ning, Q. (2020). Clinical and immunological features of severe and moderate coronavirus disease 2019. The Journal of Clinical Investigation, 130(5), 2620–2629. 10.1172/jci137244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. , Yang, W.‐H. , Huang, L.‐M. , Wang, Y.‐C. , Yang, C.‐S. , Liu, Y.‐L. , … Hung, M.‐C. (2020). Inhibition of severe acute respiratory syndrome coronavirus 2 main protease by tafenoquine in vitro. bioRxiv, 250258. 10.1101/2020.08.14.250258 [DOI] [Google Scholar]
- Cheng, H. , Wang, Y. , & Wang, G. Q. (2020). Organ‐protective effect of angiotensin‐converting enzyme 2 and its effect on the prognosis of COVID‐19. Journal of Medical Virology, 92(7), 726–730. 10.1002/jmv.25785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju, J. , Li, X. , Kumar, S. , Jockusch, S. , Chien, M. , Tao, C. , … Morozova, I. (2020). Nucleotide analogues as inhibitors of SARS‐CoV‐2 polymerase. Pharmacology Research & Perspectives. 8(6), e00674. 10.1101/2020.03.18.997585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong, E. A. , Roeker, L. E. , Shadman, M. , Davids, M. S. , Schuster, S. J. , & Mato, A. R. (2020). BTK inhibitors in cancer patients with COVID‐19: "the winner will be the one who controls that chaos" (Napoleon Bonaparte). Clinical Cancer Research, 26(14), 3514–3516. 10.1158/1078-0432.ccr-20-1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choubey, A. , Dehury, B. , Kumar, S. , Medhi, B. , & Mondal, P. (2020). Naltrexone a potential therapeutic candidate for COVID‐19. Journal of Biomolecular Structure & Dynamics, 1‐8, 1–8. 10.1080/07391102.2020.1820379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury, M. A. , Hossain, N. , Kashem, M. A. , Shahid, M. A. , & Alam, A. (2020). Immune response in COVID‐19: A review. Journal of Infection and Public Health, 13(11), 1619–1629. 10.1016/j.jiph.2020.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, C. M. , Cheng, V. C. , Hung, I. F. , Wong, M. M. , Chan, K. H. , Chan, K. S. , … Yuen, K. Y. (2004). Role of lopinavir/ritonavir in the treatment of SARS: Initial virological and clinical findings. Thorax, 59(3), 252–256. 10.1136/thorax.2003.012658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical trial ID: NCT04252885 . Retrieved from https://clinicaltrials.gov/ct2/show/NCT04252885. Accessed August 15, 2021.
- Clinical trial ID: NCT04255017 . Retrieved from https://www.clinicaltrials.gov/ct2/show/NCT04255017. Accessed August 15, 2021.
- Clinical trial ID: NCT04260594 . Retrieved from https://clinicaltrials.gov/ct2/show/NCT04260594. Accessed August 15, 2021.
- Clinical trial ID: NCT04308317 . Retrieved from https://clinicaltrials.gov/ct2/show/NCT04308317. Accessed August 15, 2021.
- Clinical trial ID: NCT04322344 . Retrieved from https://clinicaltrials.gov/ct2/show/NCT04322344. Accessed August 15, 2021.
- Clinical trial ID: NCT04326426 . Retrieved from https://clinicaltrials.gov/ct2/show/NCT04326426. Accessed August 15, 2021.
- Clinical trial ID: NCT04351763 . Retrieved from https://clinicaltrials.gov/ct2/show/NCT04351763. Accessed August 15, 2021.
- Clinical trial ID: NCT04360122 . Retrieved from https://clinicaltrials.gov/ct2/show/NCT04360122. Accessed August 15, 2021.
- Clinical trial ID: NCT04365309 . Retrieved from https://clinicaltrials.gov/ct2/show/NCT04365309. Accessed August 15, 2021.
- Clinical trial ID: NCT04379271 . Retrieved from https://www.clinicaltrials.gov/ct2/show/NCT04379271. Accessed August 15, 2021.
- Clinical trial ID: NCT04382040 . Retrieved from https://clinicaltrials.gov/ct2/show/NCT04382040. Accessed August 15, 2021.
- Clinical trial ID: NCT04382924 . Retrieved from https://clinicaltrials.gov/ct2/show/NCT04382924. Accessed August 15, 2021.
- Clinical trial ID: NCT04388826 . Retrieved from https://clinicaltrials.gov/ct2/show/NCT04388826. Accessed August 15, 2021.
- Clinical trial ID: NCT04394208 . Retrieved from https://clinicaltrials.gov/ct2/show/NCT04394208. Accessed August 15, 2021.
- Clinical trial ID: NCT04396067 . Retrieved from https://clinicaltrials.gov/ct2/show/NCT04396067. Accessed August 15, 2021.
- Clinical trial ID: NCT04414618 . Retrieved from https://clinicaltrials.gov/ct2/show/NCT04414618. Accessed August 15, 2021.
- Clinical trial ID: NCT04429555 . Retrieved from https://clinicaltrials.gov/ct2/show/NCT04429555. Accessed August 15, 2021.
- Clinical trial ID: NCT04440007 . Retrieved from https://clinicaltrials.gov/ct2/show/NCT04440007. Accessed August 15, 2021.
- Clinical trial ID: NCT04448756 . Retrieved from https://clinicaltrials.gov/ct2/show/NCT04448756. Accessed August 15, 2021.
- Clinical trial ID: NCT04456153 . Retrieved from https://clinicaltrials.gov/ct2/show/NCT04456153. Accessed August 15, 2021.
- Clinical trial ID: NCT04470622 . Retrieved from https://clinicaltrials.gov/ct2/show/NCT04470622. Accessed August 15, 2021.
- Clinical trial ID: NCT04487886 . Retrieved from https://www.clinicaltrials.gov/ct2/show/NCT04487886. Accessed August 15, 2021.
- Clinical trial ID: NCT04511819 . Retrieved from https://clinicaltrials.gov/ct2/show/NCT04511819. Accessed August 15, 2021.
- Clinical trial ID: NCT04516759 . Retrieved from https://clinicaltrials.gov/ct2/show/NCT04516759. Accessed August 15, 2021.
- Clinical trial ID: NCT04569227 . Retrieved from https://clinicaltrials.gov/ct2/show/NCT04569227. Accessed August 15, 2021.
- Clinical trial ID: NCT04880109 . Retrieved from https://clinicaltrials.gov/ct2/show/NCT04880109. Accessed August 15, 2021.
- Clinical trial ID: NCT04709172 . Retrieved from https://clinicaltrials.gov/ct2/show/NCT04709172. Accessed August 15, 2021.
- Clinical trial ID: NCT04389801 . Retrieved from https://clinicaltrials.gov/ct2/show/NCT0404389801. Accessed August 15, 2021.
- Clinical trial ID: NCT04933799 . Retrieved from https://clinicaltrials.gov/ct2/show/NCT04933799. Accessed August 15, 2021.
- Colunga Biancatelli, R. M. L. , Berrill, M. , Catravas, J. D. , & Marik, P. E. (2020). Quercetin and vitamin C: An experimental, synergistic therapy for the prevention and treatment of SARS‐CoV‐2 related disease (COVID‐19). Frontiers in Immunology, 11, 1451. 10.3389/fimmu.2020.01451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coomes, E. A. , & Haghbayan, H. (2020). Favipiravir, an antiviral for COVID‐19? The Journal of Antimicrobial Chemotherapy, 75(7), 2013–2014. 10.1093/jac/dkaa171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copin, M. C. , Parmentier, E. , Duburcq, T. , Poissy, J. , & Mathieu, D. (2020). Time to consider histologic pattern of lung injury to treat critically ill patients with COVID‐19 infection. Intensive Care Medicine, 46(6), 1124–1126. 10.1007/s00134-020-06057-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cure, E. , & Cumhur Cure, M. (2020). Can dapagliflozin have a protective effect against COVID‐19 infection? A hypothesis. Diabetes and Metabolic Syndrome: Clinical Research and Reviews, 14(4), 405–406. 10.1016/j.dsx.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalamaga, M. , Karampela, I. , & Mantzoros, C. S. (2020). Commentary: Could iron chelators prove to be useful as an adjunct to COVID‐19 treatment regimens? Metabolism, 108, 154260. 10.1016/j.metabol.2020.154260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damle, B. , Vourvahis, M. , Wang, E. , Leaney, J. , & Corrigan, B. (2020). Clinical pharmacology perspectives on the antiviral activity of azithromycin and use in COVID‐19. Clinical Pharmacology and Therapeutics, 108(2), 201–211. 10.1002/cpt.1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder, D. A. , & Mehta, S. (2020). Docking adenosine receptor ligands to SARS‐CoV2 mRNA cap 2'‐O‐Methyltransferase. ChemRxiv. https://chemrxiv.org/engage/chemrxiv/article-details/60c74d1d567dfe014cec52c9 [Google Scholar]
- De León‐Rendón, J. L. , Hurtado‐Salazar, C. , & Yamamoto‐Furusho, J. K. (2020). Aspects of inflammatory bowel disease during the COVID‐19 pandemic and general considerations. Revista de Gastroenterología de México, 85(3), 295–302. 10.1016/j.rgmx.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deftereos, S. , Giannopoulos, G. , Vrachatis, D. A. , Siasos, G. , Giotaki, S. G. , Cleman, M. , … Stefanadis, C. (2020). Colchicine as a potent anti‐inflammatory treatment in COVID‐19: Can we teach an old dog new tricks? European Heart Journal ‐ Cardiovascular Pharmacotherapy, 6(4), 255. 10.1093/ehjcvp/pvaa033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Tano, G. , Moschini, L. , Loffi, M. , Testa, S. , & Danzi, G. B. (2020). Late pulmonary embolism after COVID‐19 pneumonia despite adequate rivaroxaban treatment. European Journal of Case Reports in Internal Medicine, 7(7), 001790. 10.12890/2020_001790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz, L. R. L. , de Santana Souza, M. T. , Duarte, A. B. S. , & de Sousa, D. P. (2020). Mechanistic aspects and therapeutic potential of Quercetin against COVID‐19‐associated acute kidney injury. Molecules, 25(23), 5772. 10.3390/molecules25235772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow, G. S. , Luttick, A. , Fenner, J. , Wesche, D. , Yeo, K. R. , & Rayner, C. (2020). Tafenoquine inhibits replication of SARS‐Cov‐2 at pharmacologically relevant concentrations in vitro. bioRxiv. 10.1101/2020.07.12.199059 [DOI] [Google Scholar]
- Drago, F. , Gozzo, L. , Li, L. , Stella, A. , & Cosmi, B. (2020). Use of enoxaparin to counteract COVID‐19 infection and reduce thromboembolic venous complications: A review of the current evidence. Frontiers in Pharmacology, 11, 579886. 10.3389/fphar.2020.579886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan, Y. , Zhu, H. L. , & Zhou, C. (2020). Advance of promising targets and agents against COVID‐19 in China. Drug Discovery Today, 25(5), 810–812. 10.1016/j.drudis.2020.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edalatifard, M. , Akhtari, M. , Salehi, M. , Naderi, Z. , Jamshidi, A. , Mostafaei, S. , … Rostamian, A. (2020). Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID‐19 patients: Results from a randomised controlled clinical trial. The European Respiratory Journal, 56(6), 2002808. 10.1183/13993003.02808-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfiky, A. A. (2020). Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS‐CoV‐2 RNA dependent RNA polymerase (RdRp): A molecular docking study. Life Sciences, 253, 117592. 10.1016/j.lfs.2020.117592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis, M. , & Tiligada, K. (2021). Histamine receptors and COVID‐19. Inflammation Research, 70(1), 67–75. 10.1007/s00011-020-01422-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajgenbaum, D. C. , & June, C. H. (2020). Cytokine Storm. The New England Journal of Medicine, 383(23), 2255–2273. 10.1056/NEJMra2026131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag, A. , Wang, P. , Ahmed, M. , & Sadek, H. (2020). Identification of FDA Approved Drugs Targeting COVID‐19 Virus by Structure‐Based Drug Repositioning.
- Favalli, E. G. , Biggioggero, M. , Maioli, G. , & Caporali, R. (2020). Baricitinib for COVID‐19: A suitable treatment? The Lancet Infectious Diseases, 20(9), 1012–1013. 10.1016/s1473-3099(20)30262-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez‐Fernandez, B. , D'Marco, L. , Górriz, J. L. , Jacobs‐Cachá, C. , Kanbay, M. , Luis‐Lima, S. , Ortiz, A. , & (2020). Exploring sodium glucose co‐Transporter‐2 (SGLT2) inhibitors for organ protection in COVID‐19. Journal of Clinical Medicine, 9(7), 2030. 10.3390/jcm9072030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidan, C. , & Aydoğdu, A. (2020). As a potential treatment of COVID‐19: Montelukast. Medical Hypotheses, 142, 109828. 10.1016/j.mehy.2020.109828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francini, E. , Miano, S. T. , Fiaschi, A. I. , & Francini, G. (2020). Doxycycline or minocycline may be a viable treatment option against SARS‐CoV‐2. Medical Hypotheses, 144, 110054. 10.1016/j.mehy.2020.110054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallelli, L. , Zhang, L. , Wang, T. , & Fu, F. (2020). Severe acute lung injury related to COVID‐19 infection: A review and the possible role for Escin. Journal of Clinical Pharmacology, 60(7), 815–825. 10.1002/jcph.1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, Y. M. , Xu, G. , Wang, B. , & Liu, B. C. (2021). Cytokine storm syndrome in coronavirus disease 2019: A narrative review. Journal of Internal Medicine, 289(2), 147–161. 10.1111/joim.13144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghati, N. , Roy, A. , Bhatnagar, S. , Bhati, S. , Bhushan, S. , Mahendran, M. , … Deepti, S. (2020). Atorvastatin and aspirin as adjuvant therapy in patients with SARS‐CoV‐2 infection: A structured summary of a study protocol for a randomised controlled trial. Trials, 21(1), 902. 10.1186/s13063-020-04840-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh, A. K. , Brindisi, M. , Shahabi, D. , Chapman, M. E. , & Mesecar, A. D. (2020). Drug development and medicinal chemistry efforts toward SARS‐coronavirus and Covid‐19 therapeutics. ChemMedChem, 15(11), 907–932. 10.1002/cmdc.202000223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goker Bagca, B. , & Biray Avci, C. (2020). The potential of JAK/STAT pathway inhibition by ruxolitinib in the treatment of COVID‐19. Cytokine & Growth Factor Reviews, 54, 51–62. 10.1016/j.cytogfr.2020.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González‐Pacheco, H. , Amezcua‐Guerra, L. M. , Sandoval, J. , & Arias‐Mendoza, A. (2020). Potential usefulness of pentoxifylline, a non‐specific phosphodiesterase inhibitor with anti‐inflammatory, anti‐thrombotic, antioxidant, and anti‐fibrogenic properties, in the treatment of SARS‐CoV‐2. European Review for Medical and Pharmacological Sciences, 24(13), 7494–7496. 10.26355/eurrev_202007_21921 [DOI] [PubMed] [Google Scholar]
- Good, S. S. , Westover, J. , Jung, K. H. , Zhou, X. J. , Moussa, A. , La Colla, P. , … Sommadossi, J. P. (2021). AT‐527, a double Prodrug of a Guanosine nucleotide analog, is a potent inhibitor of SARS‐CoV‐2 in vitro and a promising Oral antiviral for treatment of COVID‐19. Antimicrobial Agents and Chemotherapy, 65(4), e02479‐20. 10.1128/aac.02479-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya, A. E. , Baker, S. C. , Baric, R. S. , Groot, R. , Ziebuhr, J. , Gulyaeva, A. A. , … John, Z. (2020). The species severe acute respiratory syndrome‐related coronavirus: Classifying 2019‐nCoV and naming it SARS‐CoV‐2. Nature Microbiology, 5(4), 536–544. 10.1038/s41564-020-0695-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grein, J. , Ohmagari, N. , Shin, D. , Diaz, G. , Asperges, E. , Castagna, A. , … Flanigan, T. (2020). Compassionate use of Remdesivir for patients with severe Covid‐19. The New England Journal of Medicine, 382(24), 2327–2336. 10.1056/NEJMoa2007016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurwitz, D. (2020). Angiotensin receptor blockers as tentative SARS‐CoV‐2 therapeutics. Drug Development Research, 81(5), 537–540. 10.1002/ddr.21656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habtemariam, S. , Nabavi, S. F. , Ghavami, S. , Cismaru, C. A. , Berindan‐Neagoe, I. , & Nabavi, S. M. (2020). Possible use of the mucolytic drug, bromhexine hydrochloride, as a prophylactic agent against SARS‐CoV‐2 infection based on its action on the Transmembrane serine protease 2. Pharmacological Research, 157, 104853. 10.1016/j.phrs.2020.104853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamouda Elgarhy, L. (2020). Could patients taking isotretinoin therapy be immune against SARS‐CoV‐2? Dermatologic Therapy, 33(4), e13573. 10.1111/dth.13573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haritha, C. V. , Sharun, K. , & Jose, B. (2020). Ebselen, a new candidate therapeutic against SARS‐CoV‐2. International Journal of Surgery, 84, 53–56. 10.1016/j.ijsu.2020.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidary, F. , & Gharebaghi, R. (2020). Ivermectin: A systematic review from antiviral effects to COVID‐19 complementary regimen. Journal of Antibiotics (Tokyo), 73(9), 593–602. 10.1038/s41429-020-0336-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heister, P. M. , & Poston, R. N. (2020). Pharmacological hypothesis: TPC2 antagonist tetrandrine as a potential therapeutic agent for COVID‐19. Pharmacology Research & Perspectives, 8(5), e00653. 10.1002/prp2.653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman, P. , Vincent, C. , Parietti Winkler, C. , Loundon, N. , Couloigner, V. , Tankere, F. , … Schmerber, S. (2020). Consensus statement. Corticosteroid therapy in ENT in the context of the COVID‐19 pandemic. European Annals of Otorhinolaryngology, Head and Neck Diseases, 137(4), 315–317. 10.1016/j.anorl.2020.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, M. , Kleine‐Weber, H. , Schroeder, S. , Krüger, N. , Herrler, T. , Erichsen, S. , … Pöhlmann, S. (2020). SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell, 181(2), 271–280.e278. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojyo, S. , Uchida, M. , Tanaka, K. , Hasebe, R. , Tanaka, Y. , Murakami, M. , & Hirano, T. (2020). How COVID‐19 induces cytokine storm with high mortality. Inflammation and Regeneration, 40, 37. 10.1186/s41232-020-00146-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horby, P. , Lim, W. S. , Emberson, J. R. , Mafham, M. , Bell, J. L. , Linsell, L. , … Faust, S. N. (2021). Dexamethasone in hospitalized patients with Covid‐19. The New England Journal of Medicine, 384(8), 693–704. 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, Y. , Meng, X. , Zhang, F. , Xiang, Y. , & Wang, J. (2021). The in vitro antiviral activity of lactoferrin against common human coronaviruses and SARS‐CoV‐2 is mediated by targeting the heparan sulfate co‐receptor. Emerging Microbes & Infections, 10(1), 317–330. 10.1080/22221751.2021.1888660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, J. , Song, W. , Huang, H. , & Sun, Q. (2020). Pharmacological therapeutics targeting RNA‐dependent RNA polymerase, proteinase and spike protein: From mechanistic studies to clinical trials for COVID‐19. Journal of Clinical Medicine, 9(4). 1131. 10.3390/jcm9041131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui, C. K. , & Lau, G. K. (2005). Clevudine for the treatment of chronic hepatitis B virus infection. Expert Opinion on Investigational Drugs, 14(10), 1277–1284. 10.1517/13543784.14.10.1277 [DOI] [PubMed] [Google Scholar]
- Hunt, C. , Chakravorty, N. K. , Annan, G. , Habibzadeh, N. , & Schorah, C. J. (1994). The clinical effects of vitamin C supplementation in elderly hospitalised patients with acute respiratory infections. International Journal for Vitamin and Nutrition Research, 64(3), 212–219. [PubMed] [Google Scholar]
- Immunic Therapeutics website Retrieved from https://www.immunic-therapeutics.com. Accessed August 15, 2021.
- Imran, M. , Arora, M. K. , Asdaq, S. M. B. , Khan, S. A. , Alaqel, S. I. , Alshammari, M. K. , … Abida, A. (2021). Discovery, development, and patent trends on molnupiravir: a prospective oral treatment for COVID‐19. Molecules, 26(19), 5795. 10.3390/molecules26195795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal, S. , Kumar, M. , Mandeep, S. , Singh, Y. , & Shukla, P. (2020). Systems biology approaches for therapeutics development against COVID‐19. Frontiers in Cellular and Infection Microbiology, 10, 560240. 10.3389/fcimb.2020.560240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang, J. H. , Kim, J. W. , Jeong, S. H. , Myung, H. J. , Kim, H. S. , Park, Y. S. , … Lee, D. H. (2011). Clevudine for chronic hepatitis B: Antiviral response, predictors of response, and development of myopathy. Journal of Viral Hepatitis, 18(2), 84–90. 10.1111/j.1365-2893.2010.01281.x [DOI] [PubMed] [Google Scholar]
- Jean, S. S. , Lee, P. I. , & Hsueh, P. R. (2020). Treatment options for COVID‐19: The reality and challenges. Journal of Microbiology, Immunology, and Infection, 53(3), 436–443. 10.1016/j.jmii.2020.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabinger, F. , & Stiller, C. (2021). Mechanism of molnupiravir‐induced SARS‐CoV‐2 mutagenesis. Nature Structural & Molecular Biology, 28(9), 740–746. 10.1038/s41594-021-00651-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadam, R. U. , & Wilson, I. A. (2017). Structural basis of influenza virus fusion inhibition by the antiviral drug Arbidol. Proceedings of the National Academy of Sciences of the United States of America, 114(2), 206–214. 10.1073/pnas.1617020114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpiński, T. M. , Ożarowski, M. , Seremak‐Mrozikiewicz, A. , Wolski, H. , & Wlodkowic, D. (2021). The 2020 race towards SARS‐CoV‐2 specific vaccines. Theranostics, 11(4), 1690–1702. 10.7150/thno.53691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleni, M. T. (2020). Nitazoxanide/azithromycin combination for COVID‐19: A suggested new protocol for early management. Pharmacological Research, 157, 104874. 10.1016/j.phrs.2020.104874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil, A. , Kamar, A. , & Nemer, G. (2020). Thalidomide‐revisited: Are COVID‐19 patients going to be the latest victims of yet another theoretical drug‐repurposing? Frontiers in Immunology, 11, 1248. 10.3389/fimmu.2020.01248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalili, J. S. , Zhu, H. , Mak, N. S. A. , Yan, Y. , & Zhu, Y. (2020). Novel coronavirus treatment with ribavirin: Groundwork for an evaluation concerning COVID‐19. Journal of Medical Virology, 92(7), 740–746. 10.1002/jmv.25798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, H. , Kurusu, H. , Sada, M. , Kurai, D. , Murakami, K. , Kamitani, W. , … Ryo, A. (2020). Molecular pharmacology of ciclesonide against SARS‐CoV‐2. The Journal of Allergy and Clinical Immunology, 146(2), 330–331. 10.1016/j.jaci.2020.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig, L. M. , Boehmer, D. F. R. , Metzger, P. , Schnurr, M. , Endres, S. , & Rothenfusser, S. (2020). Blocking inflammation on the way: Rationale for CXCR2 antagonists for the treatment of COVID‐19. The Journal of Experimental Medicine, 217(9), e20201342. 10.1084/jem.20201342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig, M. F. , Powell, M. , Staedtke, V. , Bai, R. Y. , Thomas, D. L. , Fischer, N. , … Bettegowda, C. (2020). Preventing cytokine storm syndrome in COVID‐19 using α‐1 adrenergic receptor antagonists. The Journal of Clinical Investigation, 130(7), 3345–3347. 10.1172/jci139642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotfis, K. , & Lechowicz, K. (2021). COVID‐19‐the potential beneficial therapeutic effects of spironolactone during SARS‐CoV‐2. Infection, 14(1), 71. 10.3390/ph14010071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, R. , Gupta, N. , Kodan, P. , Mittal, A. , Soneja, M. , & Wig, N. (2020). Battling COVID‐19: Using old weapons for a new enemy. Tropical Diseases, Travel Medicine and Vaccines, 6, 6. 10.1186/s40794-020-00107-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurd, R. , Ben‐Chetrit, E. , Karameh, H. , & Bar‐Meir, M. (2020). Compassionate use of opaganib for patients with severe COVID‐19. Journal of Emerging Diseases and Virology, 5(3). 10.16966/2473-1846.159 [DOI] [Google Scholar]
- Kutlu, Ö. , & Metin, A. (2020). A case of exacerbation of psoriasis after oseltamivir and hydroxychloroquine in a patient with COVID‐19: Will cases of psoriasis increase after COVID‐19 pandemic? Dermatologic Therapy, 33(4), e13383. 10.1111/dth.13383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C. (2020) Application of atorvastatin calcium in preparation of drug for treating new coronary pneumonia. CN111588720A
- Li, G. , & De Clercq, E. (2020). Therapeutic options for the 2019 novel coronavirus (2019‐nCoV). Nature Reviews. Drug Discovery, 19(3), 149–150. 10.1038/d41573-020-00016-0 [DOI] [PubMed] [Google Scholar]
- Liaudet, L. , & Szabo, C. (2020). Blocking mineralocorticoid receptor with spironolactone may have a wide range of therapeutic actions in severe COVID‐19 disease. Critical Care, 24(1), 318. 10.1186/s13054-020-03055-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , Zheng, X. , Huang, Y. , Shan, H. , & Huang, J. (2020). Successful use of methylprednisolone for treating severe COVID‐19. The Journal of Allergy and Clinical Immunology, 146(2), 325–327. 10.1016/j.jaci.2020.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Li, Z. , Liu, S. , Chen, Z. , & Luo, H. B. (2020). Therapeutic effects of dipyridamole on COVID‐19 patients with coagulation dysfunction. 10.1101/2020.02.27.20027557. [DOI]
- Liu, X. , Li, Z. , Liu, S. , Sun, J. , Chen, Z. , Jiang, M. , … Luo, H. B. (2020). Potential therapeutic effects of dipyridamole in the severely ill patients with COVID‐19. Acta Pharmaceutica Sinica B, 10(7), 1205–1215. 10.1016/j.apsb.2020.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Zhang, C. , Huang, F. , Yang, Y. , Wang, F. , Yuan, J. , … Jiang, C. (2020). Elevated plasma levels of selective cytokines in COVID‐19 patients reflect viral load and lung injury. National Science Review, 7(6), 1003–1011. 10.1093/nsr/nwaa037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, H. (2020). Drug treatment options for the 2019‐new coronavirus (2019‐nCoV). Bioscience Trends, 14(1), 69–71. 10.5582/bst.2020.01020 [DOI] [PubMed] [Google Scholar]
- Lu, L. , Zhang, H. , Zhan, M. , Jiang, J. , Yin, H. , Dauphars, D. J. , … He, Y. W. (2020). Preventing mortality in COVID‐19 patients: Which cytokine to target in a raging storm? Frontiers in Cell and Development Biology, 8, 677. 10.3389/fcell.2020.00677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luban, J. , Sattler, R. A. , Mühlberger, E. , Graci, J. D. , Cao, L. , Weetall, M. , … Peltz, S. W. (2021). The DHODH inhibitor PTC299 arrests SARS‐CoV‐2 replication and suppresses induction of inflammatory cytokines. Virus Research, 292, 198246. 10.1016/j.virusres.2020.198246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, C. , Hu, Y. , Townsend, J. A. , Lagarias, P. I. , Marty, M. T. , Kolocouris, A. , & Wang, J. (2020). Ebselen, Disulfiram, Carmofur, PX‐12, Tideglusib, and Shikonin are nonspecific promiscuous SARS‐CoV‐2 Main protease inhibitors. ACS Pharmacology & Translational Science, 3(6), 1265–1277. 10.1021/acsptsci.0c00130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, C. , Tan, H. , Choza, J. , Wang, Y. , & Wang, J. (2021). Validation and invalidation of SARS‐CoV‐2 main protease inhibitors using the Flip‐GFP and Protease‐Glo luciferase assays. Acta Pharmaceutica Sinica B, in press, 10.1016/j.apsb.2021.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, C. , & Wang, J. (2021). Dipyridamole, chloroquine, montelukast sodium, candesartan, oxytetracycline, and atazanavir are not SARS‐CoV‐2 main protease inhibitors, 118(8), e2024420118. 10.1073/pnas.2024420118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macciò, A. , Madeddu, C. , Caocci, G. , Oppi, S. , & La Nasa, G. (2020). Defibrotide in the COVID‐19 coagulopathy: What is the timing? Journal of Thrombosis and Haemostasis, 18(11), 3113–3115. 10.1111/jth.15100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio, R. , & Corsini, G. U. (2020). Repurposing the mucolytic cough suppressant and TMPRSS2 protease inhibitor bromhexine for the prevention and management of SARS‐CoV‐2 infection. Pharmacological Research, 157, 104837. 10.1016/j.phrs.2020.104837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud, D. B. , Shitu, Z. , & Mostafa, A. (2020). Drug repurposing of nitazoxanide: Can it be an effective therapy for COVID‐19? Journal, Genetic Engineering & Biotechnology, 18(1), 35. 10.1186/s43141-020-00055-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone, B. , & Campbell, E. A. (2021). Molnupiravir: Coding for catastrophe. Nature Structural & Molecular Biology, 28(9), 706–708. 10.1038/s41594-021-00657-8 [DOI] [PubMed] [Google Scholar]
- Martineau, A. R. , Jolliffe, D. A. , Hooper, R. L. , Greenberg, L. , Aloia, J. F. , Bergman, P. , … Camargo, C. A., Jr. (2017). Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta‐analysis of individual participant data. BMJ, 356, i6583. 10.1136/bmj.i6583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurya, D. K. (2020). A Combination of Ivermectin and Doxycycline Possibly Blocks the Viral Entry and Modulate the Innate Immune Response in COVID‐19 Patients.
- Mauvais‐Jarvis, F. , Klein, S. L. , & Levin, E. R. (2020). Estradiol, progesterone, immunomodulation, and COVID‐19 outcomes. Endocrinology, 161(9), bqaa127. 10.1210/endocr/bqaa127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney, D. M. , & Byrne, D. G. (2020). Optimisation of vitamin D status for enhanced Immuno‐protection against Covid‐19. Irish Medical Journal, 113(4), 58. [PubMed] [Google Scholar]
- McCoy, J. , Goren, A. , Cadegiani, F. A. , Vaño‐Galván, S. , Kovacevic, M. , Situm, M. , … Wambier, C. G. (2021). Proxalutamide reduces the rate of hospitalization for COVID‐19 male outpatients: A randomized double‐blinded placebo‐controlled trial. Frontiers in Medicine (Lausanne), 8, 668698. 10.3389/fmed.2021.668698 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- McKee, D. L. , Sternberg, A. , Stange, U. , Laufer, S. , & Naujokat, C. (2020). Candidate drugs against SARS‐CoV‐2 and COVID‐19. Pharmacological Research, 157, 104859. 10.1016/j.phrs.2020.104859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehboob, R. , Ahmad, F. J. , Qayyum, A. , Rana, M. A. , Gilani, S. A. , Tariq, M. A. , … Akram, J. (2020). Aprepitant as a combinant with dexamethasone reduces the inflammation via Neurokinin 1 receptor antagonism in severe to critical Covid‐19 patients and potentiates respiratory recovery: A novel therapeutic approach. medRxiv, 20166678. preprint, 10.1101/2020.08.01.20166678 [DOI] [Google Scholar]
- Mehta, N. , Mazer‐Amirshahi, M. , Alkindi, N. , & Pourmand, A. (2020). Pharmacotherapy in COVID‐19; a narrative review for emergency providers. The American Journal of Emergency Medicine, 38(7), 1488–1493. 10.1016/j.ajem.2020.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta, P. , McAuley, D. F. , Brown, M. , Sanchez, E. , Tattersall, R. S. , & Manson, J. J. (2020). COVID‐19: Consider cytokine storm syndromes and immunosuppression. Lancet, 395(10229), 1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merck Company website . Retrieved from https://www.businesswire.com/news/home/20211011005110/en/. Accessed October. 11, 2021.
- Mercorelli, B. , Palù, G. , & Loregian, A. (2018). Drug repurposing for viral infectious diseases: How far are we? Trends in Microbiology, 26(10), 865–876. 10.1016/j.tim.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerli, F. H. , Siontis, G. C. M. , & Rexhaj, E. (2020). COVID‐19 and renin angiotensin blockers: Current evidence and recommendations. Circulation, 141(25), 2042–2044. 10.1161/circulationaha.120.047022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mevada, V. , Dudhagara, P. , Gandhi, H. , Vaghamshi, N. , & Patel, R. (2020). Drug repurposing of approved drugs Elbasvir, Ledipasvir, Paritaprevir, Velpatasvir, Antrafenine and Ergotamine for Combating COVID19.
- Millán‐Oñate, J. , Millan, W. , Mendoza, L. A. , Sánchez, C. G. , Fernandez‐Suarez, H. , Bonilla‐Aldana, D. K. , & Rodríguez‐Morales, A. J. (2020). Successful recovery of COVID‐19 pneumonia in a patient from Colombia after receiving chloroquine and clarithromycin. Annals of Clinical Microbiology and Antimicrobials, 19(1), 16. 10.1186/s12941-020-00358-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monpara, J. D. , Sodha, S. J. , & Gupta, P. K. (2020). COVID‐19 associated complications and potential therapeutic targets. European Journal of Pharmacology, 886, 173548. 10.1016/j.ejphar.2020.173548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasiripour, S. , & Zamani, F. (2020). Can colchicine as an old anti‐inflammatory agent be effective in COVID‐19? Journal of Clinical Pharmacology, 60(7), 828–829. 10.1002/jcph.1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubauer, A. , Wiesmann, T. , Vogelmeier, C. F. , Mack, E. , Skevaki, C. , Gaik, C. , … Burchert, A. (2020). Ruxolitinib for the treatment of SARS‐CoV‐2 induced acute respiratory distress syndrome (ARDS). Leukemia, 34(8), 2276–2278. 10.1038/s41375-020-0907-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- News press from WHO website WHO discontinues hydroxychloroquine and lopinavir/ritonavir treatment arms for COVID‐19. Retrieved from https://www.who.int/news/item/04-07-2020-who-discontinues-hydroxychloroquine-and-lopinavir-ritonavir-treatment-arms-for-covid-19. Accessed August 15, 2021.
- Ogawa, H. , & Asakura, H. (2020). Consideration of Tranexamic acid administration to COVID‐19 patients. Physiological Reviews, 100(4), 1595–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojha, P. K. , Kar, S. , Krishna, J. G. , Roy K., & Leszczynski J. (2021). Therapeutics for COVID‐19: from computation to practices—where we are, where we are heading to. Molecular Diversity, 25(1), 625–659. 10.1007/s11030-020-10134-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto, M. , Toyama, M. , & Baba, M. (2020). The chemokine receptor antagonist cenicriviroc inhibits the replication of SARS‐CoV‐2 in vitro. Antiviral Research, 182, 104902. 10.1016/j.antiviral.2020.104902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omarjee, L. , Janin, A. , Perrot, F. , Laviolle, B. , Meilhac, O. , & Mahe, G. (2020). Targeting T‐cell senescence and cytokine storm with rapamycin to prevent severe progression in COVID‐19. Clinical Immunology, 216, 108464. 10.1016/j.clim.2020.108464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orienti, I. , Gentilomi, G. A. , & Farruggia, G. (2020). Pulmonary delivery of Fenretinide: A possible adjuvant treatment in COVID‐19. International Journal of Molecular Sciences, 21(11), 3812. 10.3390/ijms21113812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pant, S. , Singh, M. , Ravichandiran, V. , Murty, U. S. N. , & Srivastava, H. K. (2021). Peptide‐like and small‐molecule inhibitors against Covid‐19. Journal of Biomolecular Structure & Dynamics, 39(8), 2904–2913. 10.1080/07391102.2020.1757510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papic, N. , Radmanic, L. , Dusek, D. , Kurelac, I. , Zidovec Lepej, S. , & Vince, A. (2020). Trends of late presentation to Care in Patients with chronic hepatitis C during a 10‐year period in Croatia. Infectious Disease Reports, 12(3), 74–81. 10.3390/idr12030016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattanshetty, S. , Narayana, A. , & Radhakrishnan, R. (2021). Povidone‐iodine gargle as a prophylactic intervention to interrupt the transmission of SARS‐CoV‐2. Oral Diseases, 27(Suppl 3), 752–753. 10.1111/odi.13378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier, D. , & Hafler, D. A. (2012). Fingolimod for multiple sclerosis. The New England Journal of Medicine, 366(4), 339–347. 10.1056/NEJMct1101691 [DOI] [PubMed] [Google Scholar]
- Pepperrell, T. , Pilkington, V. , Owen, A. , Wang, J. , & Hill, A. M. (2020). Review of safety and minimum pricing of nitazoxanide for potential treatment of COVID‐19. Journal of Virus Eradication, 6(2), 52–60. 10.1016/s2055-6640(20)30017-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen, M. W. , Meyhoff, T. S. , Granholm, A. , Hjortsø, C. J. S. , Helleberg, M. , Kjaer, M. N. , … Rasmussen, B. S. (2020). Low‐dose hydrocortisone in patients with COVID‐19 and severe hypoxia (COVID STEROID) trial‐protocol and statistical analysis plan. Acta Anaesthesiologica Scandinavica, 64(9), 1365–1375. 10.1111/aas.13673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillaiyar, T. , Meenakshisundaram, S. , & Manickam, M. (2020). Recent discovery and development of inhibitors targeting coronaviruses. Drug Discovery Today, 25(4), 668–688. 10.1016/j.drudis.2020.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pindiprolu, S. , & Pindiprolu, S. H. (2020). Plausible mechanisms of Niclosamide as an antiviral agent against COVID‐19. Medical Hypotheses, 140, 109765. 10.1016/j.mehy.2020.109765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaze, M. , Attali, D. , Petit, A. C. , Blatzer, M. , Simon‐Loriere, E. , Vinckier, F. , … Gaillard, R. (2020). Repurposing chlorpromazine to treat COVID‐19: The reCoVery study. Encephale, 46(3), 169–172. 10.1016/j.encep.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poe, F. L. , & Corn, J. (2020). N‐Acetylcysteine: A potential therapeutic agent for SARS‐CoV‐2. Medical Hypotheses, 143, 109862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poissy, J. , Goutay, J. , Caplan, M. , Parmentier, E. , Duburcq, T. , Lassalle, F. , … Susen, S. (2020). Pulmonary embolism in patients with COVID‐19: Awareness of an increased prevalence. Circulation, 142(2), 184–186. [DOI] [PubMed] [Google Scholar]
- PubChem National Library of Medicine. Retrieved from https://pubchem.ncbi.nlm.nih.gov/compound/eidd-2801. Accessed August 15, 2021.
- Rashid, H. U. , Ahmad, N. , Abdalla, M. , Khan, K. , Martines, M. A. U. , & Shabana, S. (2022). Molecular docking and dynamic simulations of Cefixime, Etoposide and Nebrodenside a against the pathogenic proteins of SARS‐CoV‐2. Journal of Molecular Structure, 1247, 131296. 10.1016/j.molstruc.2021.131296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risner, K. H. , Tieu, K. V. , Wang, Y. , Bakovic, A. , Alem, F. , Bhalla, N. , … Narayanan, A. (2020). Maraviroc inhibits SARS‐CoV‐2 multiplication and s‐protein mediated cell fusion in cell culture. bioRxiv . doi: 10.1101/2020.08.12.246389. [DOI]
- Rokni, M. , Ghasemi, V. , & Tavakoli, Z. (2020). Immune responses and pathogenesis of SARS‐CoV‐2 during an outbreak in Iran: Comparison with SARS and MERS. Reviews in Medical Virology, 30(3), e2107. 10.1002/rmv.2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roschewski, M. , Lionakis, M. S. , Sharman, J. P. , Roswarski, J. , Goy, A. , Monticelli, M. A. , … Wilson, A. (2020). Inhibition of Bruton tyrosine kinase in patients with severe COVID‐19. Science Immunology, 5(48), eabd0110. 10.1126/sciimmunol.abd0110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnicka, L. , Glowacka, P. , Goldust, M. , Sikora, M. , Sar‐Pomian, M. , Rakowska, A. , … Olszewska, M. (2020). Cyclosporine therapy during the COVID‐19 pandemic. Journal of the American Academy of Dermatology, 83(2), e151–e152. 10.1016/j.jaad.2020.04.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdeva, C. , Wadhwa, A. , Kumari, A. , Hussain, F. , Jha, P. , & Kaushik, N. K. (2020). In silico potential of approved antimalarial drugs for repurposing against COVID‐19. OMICS, 24(10), 568–580. 10.1089/omi.2020.0071 [DOI] [PubMed] [Google Scholar]
- Saeedi‐Boroujeni, A. , & Mahmoudian‐Sani, M. R. (2021). Anti‐inflammatory potential of Quercetin in COVID‐19 treatment. Journal of Inflammation, 18(1), 3. 10.1186/s12950-021-00268-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin, A. R. , Erdogan, A. , Agaoglu, P. M. , Dineri, Y. , Cakirci, A. Y. , Senel, M. E. , … Tasdogan, A. M. (2020). 2019 novel coronavirus (COVID‐19) outbreak: A review of the current literature. Eurasian Journal of Medicine and Oncology, 4(1), 1–7. 10.14744/ejmo.2020.12220 [DOI] [Google Scholar]
- Sahoo, B. M. , Ravi Kumar, B. V. V. , Sruti, J. , Mahapatra, M. K. , Banik, B. K. , & Borah, P. (2021). Drug repurposing strategy (DRS): Emerging approach to identify potential therapeutics for treatment of novel coronavirus infection. Frontiers in Molecular Biosciences, 8, 628144. 10.3389/fmolb.2021.628144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis‐Gomar, F. , Lavie, C. J. , Morin, D. P. , Perez‐Quilis, C. , Laukkanen, J. A. , & Perez, M. V. (2020). Amiodarone in the COVID‐19 era: Treatment for symptomatic patients only, or drug to prevent infection? American Journal of Cardiovascular Drugs, 20(5), 413–418. 10.1007/s40256-020-00429-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders, J. M. , Monogue, M. L. , Jodlowski, T. Z. , & Cutrell, J. B. (2020). Pharmacologic treatments for coronavirus disease 2019 (COVID‐19): A review. JAMA, 323(18), 1824–1836. 10.1001/jama.2020.6019 [DOI] [PubMed] [Google Scholar]
- Sayad, B. , Sobhani, M. , & Khodarahmi, R. (2020). Sofosbuvir as repurposed antiviral drug against COVID‐19: Why were we convinced to evaluate the drug in a registered/approved clinical trial? Archives of Medical Research, 51(6), 577–581. 10.1016/j.arcmed.2020.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self, W. H. , Semler, M. W. , Leither, L. M. , Casey, J. D. , Angus, D. C. , Brower, R. G. , … Diercks, D. (2020). Effect of Hydroxychloroquine on clinical status at 14 days in hospitalized patients with COVID‐19: A randomized clinical trial. JAMA, 324(21), 2165–2176. 10.1001/jama.2020.22240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamsi, A. , Mohammad, T. , Anwar, S. , AlAjmi, M. F. , Hussain, A. , Rehman, M. T. , … Hassan, M. I. (2020). Glecaprevir and Maraviroc are high‐affinity inhibitors of SARS‐CoV‐2 main protease: Possible implication in COVID‐19 therapy. Bioscience Reports, 40(6), BSR20201256. 10.1042/bsr20201256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharun, K. , Dhama, K. , Patel, S. K. , Pathak, M. , Tiwari, R. , Singh, B. R. , … Leblebicioglu, H. (2020). Ivermectin, a new candidate therapeutic against SARS‐CoV‐2/COVID‐19. Annals of Clinical Microbiology and Antimicrobials, 19(1), 23. 10.1186/s12941-020-00368-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan, T. P. , Sims, A. C. , Leist, S. R. , Schäfer, A. , Won, J. , Brown, A. J. , … Denison, M. R. (2020). Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS‐CoV. Nature Communications, 11(1), 222. 10.1038/s41467-019-13940-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan, T. P. , Sims, A. C. , Zhou, S. , Graham, R. L. , Pruijssers, A. J. , Agostini, M. L. , … Baric, R. S. (2020). An orally bioavailable broad‐spectrum antiviral inhibits SARS‐CoV‐2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Science Translational Medicine, 12(541), abb5883. 10.1126/scitranslmed.abb5883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva Arouche, T. D. , Reis, A. F. , Martins, A. Y. , Costa, J. F. S. , Carvalho Junior, R. N. , & Neto, A. M. J. C. (2020). Interactions between Remdesivir, ribavirin, Favipiravir, Galidesivir, Hydroxychloroquine and Chloroquine with fragment molecular of the COVID‐19 Main protease with inhibitor N3 complex (PDB ID:6LU7) using molecular docking. Journal of Nanoscience and Nanotechnology, 20(12), 7311–7323. 10.1166/jnn.2020.18955 [DOI] [PubMed] [Google Scholar]
- Sisk, J. M. , Frieman, M. B. , & Machamer, C. E. (2018). Coronavirus S protein‐induced fusion is blocked prior to hemifusion by Abl kinase inhibitors. The Journal of General Virology, 99(5), 619–630. 10.1099/jgv.0.001047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solerte, S. B. , Di Sabatino, A. , Galli, M. , & Fiorina, P. (2020). Dipeptidyl peptidase‐4 (DPP4) inhibition in COVID‐19. Acta Diabetologica, 57(7), 779–783. 10.1007/s00592-020-01539-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompayrac, L. M. (2019). How the immune system works (6th ed.). Wiley‐Blackwell. [Google Scholar]
- Sorrento Therapeutics Inc , News Release, Retrieved from: https://investors.sorrentotherapeutics.com/news-releases/news-release-details/fda-clears-abivertinib-phase-2-safety-and-efficacy-study Accessed July 20, 2020.
- South, A. M. , Tomlinson, L. , Edmonston, D. , Hiremath, S. , & Sparks, M. A. (2020). Controversies of renin‐angiotensin system inhibition during the COVID‐19 pandemic. Nature Reviews. Nephrology, 16(6), 305–307. 10.1038/s41581-020-0279-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri, A. , & Sindwani, G. (2020). Ketamine use in the COVID‐19 era: Be cautious! Korean Journal of Anesthesiology, 73(6), 568–569. 10.4097/kja.20318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, W. Y. T. , Young, B. E. , Lye, D. C. , Chew, D. E. K. , & Dalan, R. (2020). Statin use is associated with lower disease severity in COVID‐19 infection. Scientific Reports, 10(1), 17458. 10.1038/s41598-020-74492-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, N. L. , Chan, P. K. , Wong, C. K. , To, K. F. , Wu, A. K. , Sung, Y. M. , … Lam, C. W. (2005). Early enhanced expression of interferon‐inducible protein‐10 (CXCL‐10) and other chemokines predicts adverse outcome in severe acute respiratory syndrome. Clinical Chemistry, 51(12), 2333–2340. 10.1373/clinchem.2005.054460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay, M. Z. , Poh, C. M. , Rénia, L. , MacAry, P. A. , & Ng, L. F. P. (2020). The trinity of COVID‐19: Immunity, inflammation and intervention. Nature Reviews. Immunology, 20(6), 363–374. 10.1038/s41577-020-0311-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, L. , Liu, W. , & Sun, L. (2020). Role of cyclophilin a during coronavirus replication and the antiviral activities of its inhibitors. Sheng Wu Gong Cheng Xue Bao, 36(4), 605–611. 10.13345/j.cjb.200049 [DOI] [PubMed] [Google Scholar]
- Tobinick, E. L. (2009). The value of drug repositioning in the current pharmaceutical market. Drug News & Perspectives, 22(2), 119–125. [DOI] [PubMed] [Google Scholar]
- Tong, S. , Su, Y. , Yu, Y. , Wu, C. , Chen, J. , Wang, S. , & Jiang, J. (2020). Ribavirin therapy for severe COVID‐19: A retrospective cohort study. International Journal of Antimicrobial Agents, 56(3), 106114. 10.1016/j.ijantimicag.2020.106114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong, X. , Smith, J. , Bukreyeva, N. , Koma, T. , Manning, J. T. , Kalkeri, R. , … Paessler, S. (2018). Merimepodib, an IMPDH inhibitor, suppresses replication of Zika virus and other emerging viral pathogens. Antiviral Research, 149, 34–40. 10.1016/j.antiviral.2017.11.004 [DOI] [PubMed] [Google Scholar]
- Treon, S. P. , Castillo, J. J. , Skarbnik, A. P. , Soumerai, J. D. , Ghobrial, I. M. , Guerrera, M. L. , … Yang, G. (2020). The BTK inhibitor ibrutinib may protect against pulmonary injury in COVID‐19‐infected patients. Blood, 135(21), 1912–1915. 10.1182/blood.2020006288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin, M. H. , Zonder, J. A. , & Azmi, A. S. (2020). Exportin 1 inhibition as antiviral therapy. Drug Discovery Today, 25(10), 1775–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich, H. , & Pillat, M. M. (2020). CD147 as a target for COVID‐19 treatment: Suggested effects of azithromycin and stem cell engagement. Stem Cell Reviews and Reports, 16(3), 434–440. 10.1007/s12015-020-09976-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyaroğlu, O. A. , Güven, G. S. , & Güllü, İ. (2020). Can Levamisole be used in the treatment of COVID‐19 patients presenting with diarrhea? Journal of Infection in Developing Countries, 14(8), 844–846. 10.3855/jidc.13101 [DOI] [PubMed] [Google Scholar]
- Valencia, D. N. (2020). Brief review on COVID‐19: The 2020 pandemic caused by SARS‐CoV‐2. Cureus, 12(3), e7386. 10.7759/cureus.7386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandyck, K. , & Deval, J. (2021). Considerations for the discovery and development of 3‐chymotrypsin‐like cysteine protease inhibitors targeting SARS‐CoV‐2 infection. Current Opinion in Virology, 49, 36–40. 10.1016/j.coviro.2021.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasanthakumar, N. (2020). Beta‐adrenergic blockers as a potential treatment for COVID‐19 patients. BioEssays, 42(11), e2000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello, A. , La Porta, R. , & Ferrara, F. (2020). Sacubitril, valsartan and SARS‐CoV‐2. BMJ Evidence‐Based Medicine, 26, 205. 10.1136/bmjebm-2020-111497 [DOI] [PubMed] [Google Scholar]
- Walls, A. C. , Park, Y. J. , Tortorici, M. A. , Wall, A. , McGuire, A. T. , & Veesler, D. (2020). Structure, function, and antigenicity of the SARS‐CoV‐2 spike glycoprotein. Cell, 181(2), 281–292.e286. 10.1016/j.cell.2020.02.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan, S. , Yi, Q. , Fan, S. , Lv, J. , Zhang, X. , Guo, L. , … Chen, Y. (2020). Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP). medRxiv. 10.1101/2020.02.10.20021832 [DOI] [Google Scholar]
- Wang, D. , Hu, B. , Hu, C. , Zhu, F. , Liu, X. , Zhang, J. , … Peng, Z. (2020). Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA, 323(11), 1061–1069. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M. , Cao, R. , Zhang, L. , Yang, X. , Liu, J. , Xu, M. , … Xiao, G. (2020). Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Research, 30(3), 269–271. 10.1038/s41422-020-0282-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W. , McLeod, H. L. , & Cassidy, J. (2003). Disulfiram‐mediated inhibition of NF‐kappaB activity enhances cytotoxicity of 5‐fluorouracil in human colorectal cancer cell lines. International Journal of Cancer, 104(4), 504–511. 10.1002/ijc.10972 [DOI] [PubMed] [Google Scholar]
- Weir, E. K. , Thenappan, T. , Bhargava, M. , & Chen, Y. (2020). Does vitamin D deficiency increase the severity of COVID‐19? Clinical Medicine (London, England), 20(4), e107–e108. 10.7861/clinmed.2020-0301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley, R. J. (2007). The role of oseltamivir in the treatment and prevention of influenza in children. Expert Opinion on Drug Metabolism & Toxicology, 3(5), 755–767. 10.1517/17425255.3.5.755 [DOI] [PubMed] [Google Scholar]
- World Health Organization website . Coronavirus (COVID‐19) data. Retrieved from https://www.who.int/data#reports. Accessed August 15, 2021.
- Wu, R. , Wang, L. , Kuo, H.‐C. D. , Shannar, A. , Peter, R. , Chou, P. J. , … Kong, A.‐N. (2020). An update on current therapeutic drugs treating COVID‐19. Current Pharmacology Reports, 6(3), 56–70. 10.1007/s40495-020-00216-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J. , Shi, P. Y. , Li, H. , & Zhou, J. (2020). Broad Spectrum antiviral agent Niclosamide and its therapeutic potential. ACS Infectious Diseases, 6(5), 909–915. 10.1021/acsinfecdis.0c00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Z. , Shi, L. , Wang, Y. , Zhang, J. , Huang, L. , Zhang, C. , … Wang, F. S. (2020). Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. The Lancet Respiratory Medicine, 8(4), 420–422. 10.1016/s2213-2600(20)30076-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, V. C. , & Muller, F. L. (2020). Advantages of the parent nucleoside GS‐441524 over Remdesivir for Covid‐19 treatment. ACS Medicinal Chemistry Letters, 11(7), 1361–1366. 10.1021/acsmedchemlett.0c00316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, N. , & Shen, H. M. (2020). Targeting the Endocytic pathway and autophagy process as a novel therapeutic strategy in COVID‐19. International Journal of Biological Sciences, 16(10), 1724–1731. 10.7150/ijbs.45498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, Q. , Wang, B. , & Mao, J. (2020). The pathogenesis and treatment of the “cytokine storm” in COVID‐19. The Journal of Infection, 80(6), 607–613. 10.1016/j.jinf.2020.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefi, J. , Mashouri, L. , Okpechi, S. C. , Alahari, N. , & Alahari, S. K. (2021). Repurposing existing drugs for the treatment of COVID‐19/SARS‐CoV‐2 infection: A review describing drug mechanisms of action. Biochemical Pharmacology, 183, 114296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidler, A. , & Karpinski, T. M. (2020). SARS‐CoV, MERS‐CoV, SARS‐CoV‐2 comparison of three emerging coronaviruses. Jundishapur Journal of Microbiology, 13(6), e103744. [Google Scholar]
- Zeng, X. , Song, X. , Ma, T. , Pan, X. , Zhou, Y. , Hou, Y. , … Cheng, F. (2020). Repurpose open data to discover therapeutics for COVID‐19 using deep learning. Journal of Proteome Research, 19(11), 4624–4636. 10.1021/acs.jproteome.0c00316 [DOI] [PubMed] [Google Scholar]
- Zhang, H. , Penninger, J. M. , Li, Y. , Zhong, N. , & Slutsky, A. S. (2020). Angiotensin‐converting enzyme 2 (ACE2) as a SARS‐CoV‐2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Medicine, 46(4), 586–590. 10.1007/s00134-020-05985-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L. , Lin, D. , Sun, X. , Curth, U. , Drosten, C. , Sauerhering, L. , … Hilgenfeld, R. (2020). Crystal structure of SARS‐CoV‐2 main protease provides a basis for design of improved α‐ketoamide inhibitors. Science, 368(6489), 409–412. 10.1126/science.abb3405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y. , Fu, B. , Zheng, X. , Wang, D. , Zhao, C. , Qi, Y. , …, Wei, H. (2020). Aberrant pathogenic GM‐CSF+ T cells and inflammatory CD14+ CD16+ monocytes in severe pulmonary syndrome patients of a new coronavirus. National Science Review, 7(6), 998–1002. 10.1093/nsr/nwaa041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y. , Hou, Y. , Shen, J. , Huang, Y. , Martin, W. , & Cheng, F. (2020). Network‐based drug repurposing for novel coronavirus 2019‐nCoV/SARS‐CoV‐2. Cell Discovery, 6, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Y. , Li, J. , & Pang, Z. (2021). Recent insights for the emerging COVID‐19: Drug discovery, therapeutic options and vaccine development. Asian Journal of Pharmaceutical Sciences, 16(1), 4–23. 10.1016/j.ajps.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.