Abstract
Objectives
Antiviral adaptive immunity involves memory B cells (MBC) and memory T cells (MTC). The dynamics of MBC and MTC in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) convalescents warrant further investigation.
Methods
In this cross-sectional and longitudinal study, blood-derived MBC and MTC responses were evaluated in 68 anti-spike IgG-positive mild coronavirus disease 2019 (COVID-19) convalescents at visit 1, between 1 and 7 months (median 4.1 months) after disease onset. SARS-CoV-2 anti-spike IgG was determined by ELISA, MBC by SARS-CoV-2-specific receptor binding domain (RBD) ELISpot, and interferon gamma (IFN-γ)-, interleukin 2 (IL2)-, and IFN-γ+IL2-secreting MTC by IFN-γ and IL2 SARS-CoV-2 FluoroSpot. For 24 patients sampled at the first visit, the IgG, MBC, and MTC analyses were also performed 3 months later at the second visit.
Results
Seventy-two percent of convalescents were both MBC- and MTC-positive, 18% were MBC-positive and MTC-negative, and 10% were MTC-positive and MBC-negative. The peak MBC response level was detected at 3 months after COVID-19 onset and persisted up to 7 months post infection. Significant MTC levels were detected 1 month after onset in response to S1, S2_N, and SNMO peptide pools. The frequency and magnitude of the MTC response to SNMO was higher than those to S1 and S2_N. Longitudinal analysis demonstrated that even when specific humoral immunity declined, the cellular immunity persisted.
Conclusions
The study findings demonstrate the durability of adaptive cellular immunity at least for 7 months after SARS-CoV-2 infection, suggesting long-lasting protection.
Keywords: SARS-CoV-2, Convalescents, Memory B cells, Memory T cells, IgG
Abbreviations: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; MBC, memory B cells; MTC, memory T cells; COVID-19, coronavirus disease 2019; IgG, immunoglobulin G; RBD, receptor binding domain; SFU, spot-forming units; IFN-γ, interferon gamma; IL2, interleukin 2
1. Introduction
The progressive decline in anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody levels in convalescent patients after primary infection (Achiron et al., 2021; Hartley et al., 2020; Rodda et al., 2021; Sakharkar et al., 2021) raises the possibility of SARS-CoV-2 re-infection, once the humoral immunity is diminished. As a result, attention has been drawn to longstanding immunity against SARS-CoV-2 involving memory B and memory T cells (MBC and MTC, respectively). The presence of specific anti-SARS-CoV-2 MBC and MTC has been demonstrated in convalescent patients (Forthal, 2021; Hartley et al., 2020; Peng et al., 2006; Peng et al., 2020; Rodda et al., 2021; Schwarzkopf et al., 2021; Sekine et al., 2020; Sherina et al., 2021; Thieme et al., 2020).
Several recent studies have shown that SARS-CoV-2-specific IgG persists and is accompanied by simultaneously increased proportions of MBC and MTC for up to 8–9 months after disease onset (Dan et al., 2021; Sandberg et al., 2021; Sherina et al., 2021), while others have shown a decline in SARS-CoV-2 IgG levels accompanied by persistence and even an increase in MBC for at least 5 months after disease onset (Hartley et al., 2020; Rodda et al., 2021; Sakharkar et al., 2021).
In view of the reported decline in IgG levels during coronavirus disease 2019 (COVID-19) convalescence, the long-lasting adaptive immunity is most relevant for the re-call immune response in the case of re-infection. The recently reported low frequency of SARS-CoV-2 re-infection in seronegative individuals (Leidi et al., 2021; Murchu et al., 2021) has raised the possibility of anti-SARS-CoV-2 defense by re-call adaptive immunity. Therefore, this study was performed to profile SARS-CoV-2 MBC and MTC responses and the MTC repertoire in anti-spike IgG-positive mild COVID-19 convalescent patients. In particular, the MBC and MTC responses in COVID-19 convalescents with a declined IgG level were assessed.
2. Methods
2.1. Participants
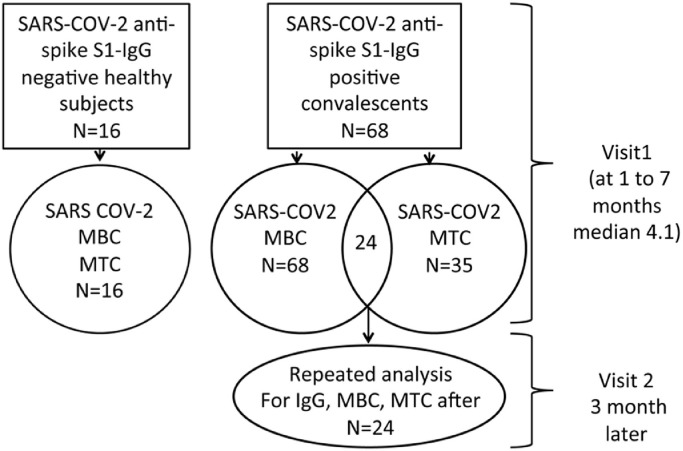
SARS-CoV-2 convalescents were included in the study according to the following criteria: (1) positive RT-PCR SARS-CoV-2 RNA nasopharyngeal swab; (2) positive anti-SARS-CoV-2 IgG test. None of the patients required hospitalization and the disease severity was mild, with the patients presenting at least one of the following symptoms: fatigue, weakness, muscle pain, cough, and fever. Healthy individuals with no history of SARS-CoV-2 symptoms, no household contact with an infected patient, and who were seronegative to anti-spike SARS-CoV-2 were included as a negative control group. Blood samples for immune assessments were obtained from all participants (N = 84), including 68 convalescents and 16 healthy subjects. The MBC response was determined in all 68 convalescents, and 41 were evaluated for MTC at the first visit. For the longitudinal study, 24 patients characterized by a reduced anti-spike IgG test 3 months later (second visit), were again analyzed by MBC and MTC tests.
2.2. Ethics statement
This study was approved by Sheba Institutional Review Board (SMC-750320) and informed consent was obtained from all participants.
2.3. Detection of SARS‐CoV‐2 anti‐spike S1 IgG antibodies
The immunoassay for the detection of SARS-CoV-2 IgG antibodies in blood samples was performed using the Euroimmun (EI, Lubeck, Germany) anti-SARS-CoV-2 IgG quantitative ELISA kit based on a recombinant S1 subunit of the SARS-CoV-2 spike protein. The tests were performed in accordance with the manufacturer's instructions using an Agility automated ELISA analyzer (DYNEX Technologies Inc., Chantilly, VA, USA). Optical density (OD) was detected at 450 nm, and the ratio of the reading of each sample to the reading of the calibrator, included in the kit, was calculated for each sample (referred to as the IgG score below). IgG score values higher than 1.0 were considered positive.
2.4. COVID‐19 memory B cell and memory T cell responses
The analysis of SARS-CoV-2-specific MBC was performed using the FluoroSpot assay for the detection of receptor-binding domain (RBD)-specific MBC-derived IgG-secreting cells, following polyclonal B cell stimulation. The human IgG SARS-CoV-2 receptor-binding domain (RBD)-specific ELISpot PLUS kit (ALP kit; Mabtech, Sweden) was used, according to the manufacturer's instructions. Briefly, peripheral blood mononuclear cells (PBMC) were incubated (250 000 cells/well) on the anti-IgG FluoroSpot plate after stimulation with a mixture of R848 (1 µg/ml) and interleukin 2 (IL2) (10 ng/ml) (B-Cell stimpack; Mabtech, Sweden). The number of SARS-CoV-2-specific IgG-secreting B cells was measured in spot-forming units (SFU) using a Mabtech IRIS reader. The results were expressed as the number of SFU per 250 000 seeded cells after subtracting the background of unstimulated cells. The positive cutoff value was set above the 90% confidence interval in healthy subjects (HS).
The interferon gamma (IFN-γ)-, IL2-, and IFN-γ+IL2-secreting MTC were detected using human IFN-γ and IL2 SARS-CoV-2 FluoroSpot PLUS kits according to the manufacturer's protocol (Mabtech AB, Sweden). The pre-coated plates with monoclonal anti-IFN-γ/IL2 were incubated overnight in RPMI-1640 medium containing 10% Fetal calf serum (FCS) supplemented with SARS-CoV-2 peptide pools or non-specific anti-CD3 stimuli (0.1 µg/ml) in the presence of anti-CD28 (0.1 µg/ml) and 300 000 cells per well in a humidified incubator (5% CO2, 37°C).
The SARS-CoV-2 S1 scanning pool contains 166 peptides from the human SARS-CoV-2 virus (#3629-1; Mabtech AB). The peptides are 15-mers overlapping with 11 amino acids, covering the S1 domain of the S protein (amino acids 13–685). The S1 pool is supplied as S1_1 (1–83) and S1_2 (84–166) peptides. The SARS-CoV-2 SNMO defined peptide pool contains 47 synthetic peptides binding to human leukocyte antigen (HLA), derived from the S, N, M, ORF3a, and ORF7a proteins (#3622-1; Mabtech AB) (Peng et al., 2020). The SARS-CoV-2 S2_N defined peptide pool contains 41 synthetic peptides binding to HLA derived from the S and N proteins of the SARS-CoV-2 virus (#3620-1, Mabtech AB) (Ahmed et al., 2020).
The results of the ELISpot and FluoroSpot assays were evaluated using an IRIS reader and analyzed using IRIS software version 1.1.9 (Mabtech AB). Peptide pool specific responses were quantified by measuring IFN-γ-, IL2-, or IFN-γ+IL2-producing MTC. Measurements of at least 5.0 SFU, that were at least 2-fold higher than the SFU background in culture media, were considered positive. The background SFU were subtracted from the measurements and expressed as SFU/250 000 cells. To determine the overall IFN-γ-, IL2-, or IFN-γ+IL2-producing MTC response as positive or negative, the subjects with at least one positive response for either IFN-γ, IL2, or IFN-γ+IL2 to at least one of the SARS-CoV-2 peptide pools were taken into consideration. To assess the continued overall MTC response in relation to one of the analyzed cytokines, the positive SFU of the specific cytokine-secreting MTC in response to S1, S2_N, and SNMO peptides was summarized as described previously (Sherina et al., 2021). The positive cutoff value was set above the 90% confidence interval of the number of specific MTC in the healthy controls.
2.5. Statistical analysis
All continuous characteristics are described as the mean ± standard deviation (SD); non-continuous characteristics are described as the median with 25–75% interquartile range (IQR). Differences were determined by Mann–Whitney U-test, Fisher's exact test, and the Chi-square test; correlation was assessed by Spearman (rank) test. A P-value <0.05 indicated significance.
3. Results
Sixty-eight anti-spike-IgG positive COVID-19 convalescents, 25 female and 43 male, mean age 39.3 ± 13.2 years, with a median time from disease onset (TFO) at first visit of 4.1 months (IQR 2.6–6.1 months), were included in the study. The MBC analysis was performed for all of the convalescents, while the MTC analysis was performed for 35 convalescents (mean age 42.0 ± 12.8 years; 11 female, 24 male). From this cohort, 24 individuals (mean age 34.6 ± 18.3 years; eight female, 16 male) were tested twice: at first visit (TFO median 4.1 months, IQR 2.1-5.5 months) and 3 months later at the second visit (TFO median 7.1 months, IQR 6.7–8.3 months). The study design is presented in Figure 1 and representative images of MTC and MBC detection are presented in Figure 2 .
Figure 1.
Study design.
MBC, memory B cells; MTC, memory T cells.
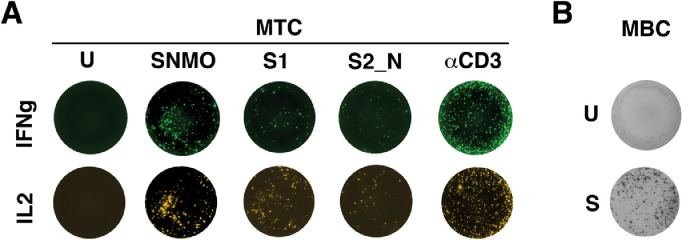
Figure 2.
Representative images for the MTC and MBC tests. (A) MTC test: PBMCs (250 000 cells/well) were stimulated for 48 h with S1, S2_N, and SNMO of the Mabtech SARS-CoV-2 peptide pools or αCD3 as positive control. The MTC were analyzed on the Mabtech IRIS reader using an IFN-γ or IL2 FluoroSpot assay. (B) MBC test: PBMCs were stimulated with R848 and IL2 for 96 h (S). After pre-stimulation, 250 000 cells/well were incubated on an anti-IgG FluoroSpot plate for 18 h. RBD-specific MBC were detected using RBD–streptactin-550 conjugate. The analysis was performed on the Mabtech IRIS reader.
U, unstimulated cells; S, stimulated cells; MTC, memory T cells; MBC, memory B cells.
3.1. Longitudinal dynamics of humoral and adaptive immune responses in SARS‐CoV‐2 convalescents
At the first visit, all convalescents were positive for anti-spike IgG, with an average titer of 4.33 ± 2.89 (OD ratio); 72% were positive for both MBC and MTC, 18% were positive for MBC and negative for MTC, and 10% were positive for MTC and negative for MBC.
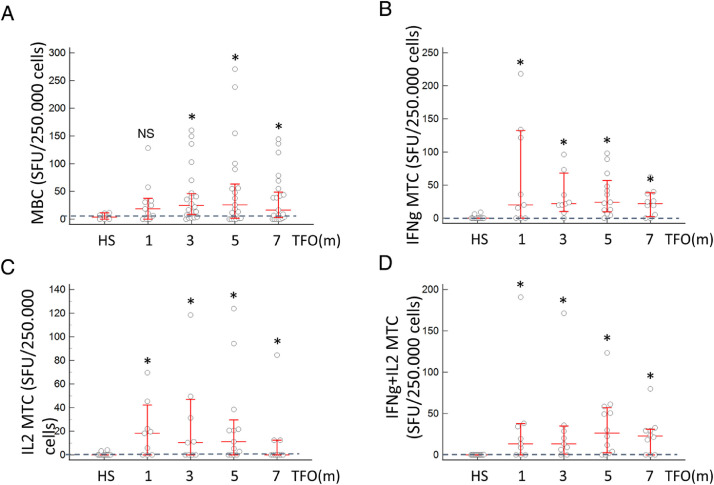
The level of SARS-CoV-2-specific anti-spike IgG in HS was negligible (median 0), while in convalescents the level of IgG was significantly higher at 1 month TFO (median 2.3; P < 0.05) and remained persistent and significantly higher than in HS for the next 6 months (Supplementary Material Figure S1). Similarly, anti-RBD-specific MBC gradually increased and reached a significantly higher level as compared with HS at 3 months (median 24.8 and 6.8, respectively; P < 0.02), and remained significantly higher than in HS at 5 and 7 months, while no difference was observed between the different time points (Figure 3 A). While the MBC were significantly elevated at 3 months TFO, MTC were characterized by an earlier response. The IFN-γ-, IL2-, and poly-functional IFN-γ+IL2-secreting MTC were significantly increased already at 1 month TFO and persisted for up to 7 months (P < 0.005; Figure 3B–D). Notably, while anti-spike IgG levels, MBC, and MTC were significantly higher than in the HS, no statistical differences were observed in convalescents at the different time points.
Figure 3.
Dynamics of the humoral and adaptive immune response in convalescent patients. (A) Dynamics of anti-SARS-CoV-2 MBC. The number of subjects per group was as follows: HS, n = 16; 1 month, n = 13; 3 months, n = 21; 5 months, n = 19; 7 months, n = 15. The dashed line denotes the cutoff level in HS (6.8 SFU), calculated as 90% of confidence interval. (B) Dynamics of IFN-γ MTC. (C) Dynamics of IL2 MTC. (D) Dynamics of IFN-γ+IL2 MTC. For graphs B, C, and D, the number of subjects per group was as follows: HS, n = 12; 1 month, n = 9; 3 months, n = 9; 5 months, n = 13; 7 months, n = 10. The dashed line denotes the cutoff level in HS (0.0 SFU) calculated as 90% of confidence interval.
HS, healthy subject controls; MBC, memory B cells; IFN-γ MTC, overall IFN-γ-producing memory T cells calculated as the sum of the positive SFU of IFN-γ-secreting cells in response to S1, S2_N, and SNMO; IL2 MTC, overall IL2-producing memory T cells calculated as the sum of the positive SFU of IL2-secreting cells in response to S1, S2_N, and SNMO; IFN-γ+IL2 MTC, overall IFN-γ+IL2-producing memory T cells calculated as the sum of the positive SFU of IFN-γ+IL2-secreting cells in response to S1, S2_N, and SNMO.
*P < 0.03 by Mann–Whitney U-test as compared to HS. NS is non-significant as compared to HS. In graphs A, B, C, and D, no significant difference in SFU levels between the different time points was observed.
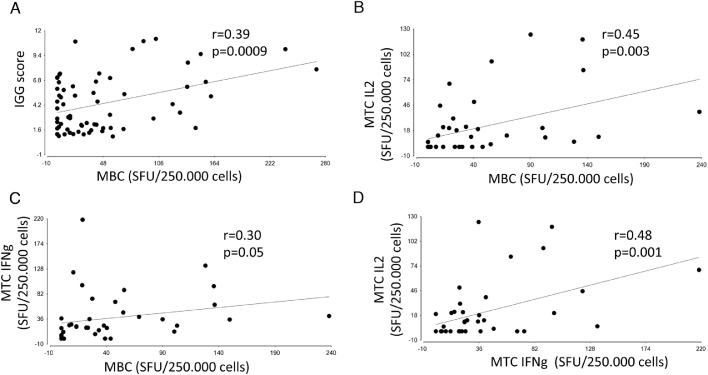
Significant correlations were observed between the number of anti-RBD MBC and the anti-spike IgG level (r = 0.39, P = 0.0009) and IL2-secreting MTC (r = 0.45, P = 0.004), but less with IFN-γ-secreting MTC (r = 0.3, P = 0.05) (Figure 4 A–C), while IFN-γ- and IL2-secreting MTC were correlated as well (r = 0.48, P = 0.001; Figure 4D). No significant correlations were observed between IgG and MTC and between MBC and MTC with TFO.
Figure 4.
Correlation between adaptive immune memory components. (A) Correlation (by Spearman rank test) between anti-S1 spike IgG and MBC levels. (B) Correlation between overall IL2-secreting MTC and MBC levels. (C) Correlation between overall IFN-γ-secreting MTC and MBC. (D) Correlation between overall IFN-γ and IL2-secreting MTC.
SFU, spot-forming units; MBC, memory B cells; IFN-γ MTC, overall IFN-γ-producing memory T cells calculated as the sum of the positive SFU of IFN-γ-secreting cells in response to S1, S2_N, and SNMO; IL2 MTC, overall IL2-producing memory T cells calculated as the sum of the positive SFU of IL2-secreting cells in response to S1, S2_N, and SNMO.
3.2. SARS‐CoV‐2‐specific T cell memory repertoire
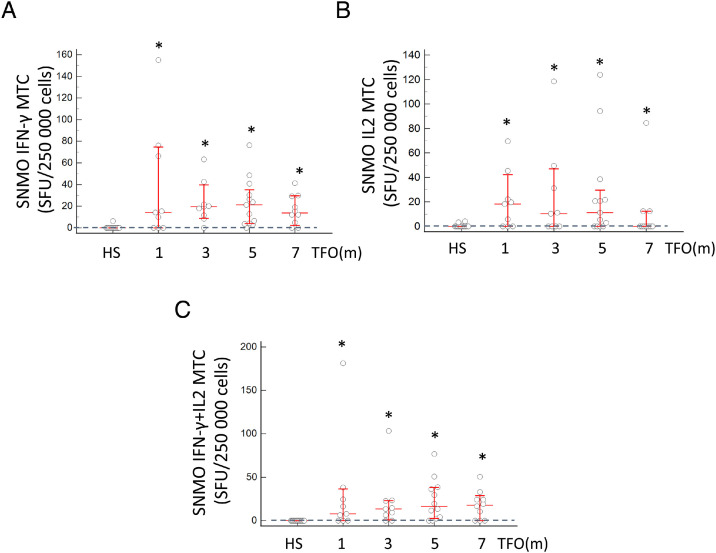
To determine whether MTC were generated and sustained in COVID-19 convalescents, the number of IFN-γ-, IL2-, and IFN-γ+IL2-secreting MTC in response to stimulation with SARS-CoV-2 S1, S2_N, and SNMO peptide pools were measured simultaneously. The significantly highest frequencies of individuals with positive IFN-γ-, IL2-, and IFN-γ+IL2-secreting MTC were observed in response to the SNMO pool (82.9%, 57.5%, and 72.3%, respectively) as compared to S1 (56.6%, 34.1%, and 43.0%, respectively; P < 0.0001) and S2_N (31.7%, 7.3%, and 17.5%, respectively; P < 0.0001) (Supplementary Material Table S1). In addition, the SFU levels of at least IFN-γ-producing MTC in response to SNMO were significantly higher than in response to S1 or S2_N (Supplementary Material Table S2).
As demonstrated by the SFU number, stimulation with the SNMO pool was characterized by an earlier immediate and more persistent response of IFN-γ-, IL2-, and IFN-γ+IL2-secreting MTC than stimulation with the S1 and S2_N peptides. After stimulation with SNMO, IFN-γ-, IL2-, and IFN-γ+IL2-secreting MTC were significantly increased already after 1 month TFO and were still elevated for up to 7 months of follow-up (P < 0.05) (Figure 5 ). The response to the S1 peptides was delayed and significantly different from those of HS at 5 and 7 months in either cytokine-producing MTC (Supplementary Material Figure S2). The response to the S2_N peptide pool was minor and observed in IFN-γ-secreting MTC at 5 months TFO (Supplementary Material Figure S3). No statistical differences were observed in the levels of IFN-γ-, IL2-, and IFN-γ+IL2-secreting MTC in convalescents at the different time points.
Figure 5.
Dynamics of SARS-CoV-2 IFN-γ-, IL2-, and IFN-γ+IL2-secreting MTC cells in response to SNMO peptides. (A) Dynamics of IFN-γ-secreting MTC in response to the SNMO peptide pool. (B) Dynamics of IL2-secreting MTC in response to the SNMO peptide pool. (C) Dynamics of IFN-γ+IL2-secreting MTC in response to the SNMO peptide pool. The number of subjects per group was as follows: HS, n = 12; 1 month, n = 9; 3 months, n = 9; 5 months, n = 13; 7 months, n = 10. The dashed line denotes the cutoff level in the HS (0.0 SFU) calculated as 90% of confidence interval.
HS, healthy subjects; SFU, spot-forming units; TFO, time from COVID-19 onset; SNMO IFN-γ MTC, IFN-γ-secreting memory T cells in response to SNMO; SNMO IL2 MTC, IL2-secreting memory T cells in response to SNMO; SNMO IFN-γ+IL2 MTC, IFN-γ+IL2-secreting memory T cells in response to SNMO.
*P < 0.05 by Mann–Whitney U-test as compared to HS. NS is non-significant as compared to HS. In graphs A, B, and C, no significant difference in SFU levels between the different time points were observed.
3.3. Longitudinal analysis
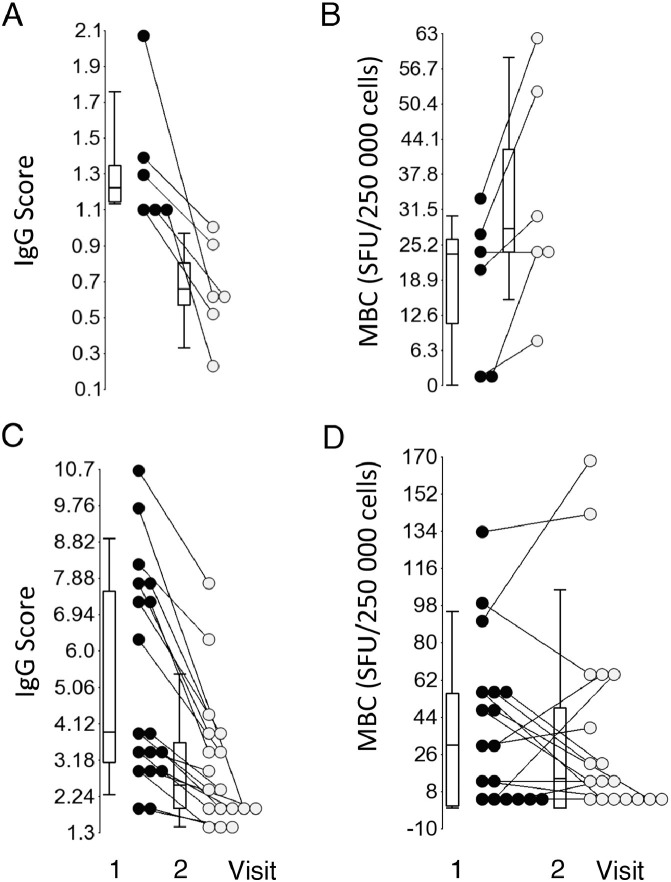
As demonstrated previously, the level of SARS-CoV-2 anti-spike IgG decreased by 50% but still prevailed over a period of 9 months (Achiron et al., 2021). To better understand the longitudinal memory cell responses, the dynamics of IgG, MBC, and MTC from the same patient at two repeated visits were analyzed. Specifically, cellular memory was characterized in 24 convalescents who were anti-spike IgG-positive at the first visit and had a declined IgG level at the second visit. Six of the 24 convalescent patients with positive IgG at the first time point (median 1.22, IQR 1.14–1.58) switched to IgG-negative at the second time point (median 0.66, IQR 0.40–0.96; P = 0.008) (Figure 6 A). All patients in this subgroup were characterized by an increasing or no change in number of MBC: median 23.5 (IQR 0–28.9) and median 28.1 (IQR 19.0–56.3), respectively (P = 0.002) (Figure 6B). However, the overall IFN-γ-secreting, IL2-secreting, and IFN-γ+IL2-secreting MTC remained persistent at both time points: median 17.7 (IQR 3.22–45.2) and 5.85 (IQR 0–41.9), median 9.5 (IQR 0–24.4) and 7.7 (IQR 0–34.0), and median 20.9 (IQR 1.65–34.6) and 8.95 (IQR 4.75–18.5), respectively (P > 0.05) (Supplementary Material Figure S4A–D).
Figure 6.
MBC levels in patients with decreased anti-spike IgG. (A) Convalescent patients switching from positive to negative IgG level between visit 1 (black dots) and visit 2 (gray dots). (B) MBC changes in convalescent patients switching from positive to negative IgG level between visit 1 (black dots) and visit 2 (gray dots). (C) Patients with a decreased IgG level, yet remaining IgG-positive between the first (black dots) and second (gray dots) visits. (D) MBC changes in patients with a decreased IgG level, yet remaining IgG-positive.
MBC, memory B cells.
In contrast, the 18 patients with decreased IgG yet remaining IgG-positive (median 3.91 (IQR 2.81–7.73) and 2.53 (IQR 1.89–3.88); P = 0.01) (Figure 6C) displayed no significant changes in MBC (median 30.5 (IQR 1.73–56.3) and 12.1 (IQR 0–60.8); P = 0.2) (Figure 6D) and in the overall IFN-γ-secreting, IL2-secreting, and IFN-γ+IL2-secreting MTC (median 15.0 (IQR 0.68–38.6) and 25.7 (IQR 0.9–38.5), median 4.8 (IQR 0–22.4) and 8.45 (IQR 0–30.5), and median 5.4 (IQR 0–23.9) and 19.9 (IQR 0–42.5), respectively) (P > 0.05) (Supplementary Material Figure S4E–H).
4. Discussion
The prevention of re-infection involves cellular memory components programmed to rapidly respond to antigen encounter generating memory B and T cells (Cromer et al., 2021; Forthal, 2021; Mazzoni et al., 2021; Turner et al., 2021; Ueffing et al., 2020). This study demonstrated the simultaneous presence of robust SARS-CoV-2 anti-spike antibodies and durable memory B and T cell responses specific for SARS-CoV-2 peptides in patients recovered from COVID-19 up to 7 months post-infection. It was demonstrated that the levels of MBC and MTC increased shortly after onset. Notably, the MTC response preceded RBD-specific MBC, as has been reported previously (Dan et al., 2021). The MTC response was characterized by IFN-γ-, IL2-, and IFN-γ+IL2-secreting MTC in response to S1, S2_N, and SNMO peptide pools.
The observed dynamics of the humoral and adaptive immune response corroborated previous publications reporting that anti-spike IgG remained stable up to 6 months after diagnosis (Dan et al., 2021; Gaebler et al., 2021; Hartley et al., 2020; Sherina et al., 2021). The observed humoral response detected against the spike and the recall memory B cell response against RBD, support the long-lasting protection from SARS-CoV-2 infection. Moreover, the study results suggest that even when the SARS-CoV-2 antibody level switches to seronegative, the presence of MBC and MTC potentially provides defense in the case of re-infection. Numerous studies have described around 1% of re-infections (Leidi et al., 2021; Murchu et al., 2021). This low frequency of re-infection cases could probably be explained by the presence and involvement of adaptive immunity components, i.e., memory B and T cells. This notion is further strengthened by a recent study (McMahan et al., 2021), which showed that relatively low antibody titers are sufficient for protection against SARS-CoV-2 in rhesus macaques, and that cellular immune responses may contribute to protection if antibody responses are suboptimal.
The findings of IFN-γ-secreting effector MTC suggest an immediate, but not sustained protection at pathogen sites of entry, whereas central MTC characterized by IL2 or IFN-γ+IL2 secretion suggests a sustained long-lasting response by proliferating in the secondary lymphoid organs and producing a supply of new effectors (Forthal, 2021).
IL2 is a growth factor for cellular expansion of specific T cells and the generation of effector and memory T cells (Abbas et al., 2018), as has previously been detected upon in vitro stimulation of PBMCs from both acute and convalescent COVID-19 patients (Braun et al., 2020; Grifoni et al., 2020; Peng et al., 2020; Sekine et al., 2020; Thieme et al., 2020; Weiskopf et al., 2020). Intracellular staining revealed that both CD8+ T cells and CD4+ T cells are sources of this cytokine, but CD4+ T cells appear to be the main producers (Braun et al., 2020; Grifoni et al., 2020; Peng et al., 2020; Sekine et al., 2020). Based on the production of IFN-γ and IL2, SARS-CoV-2-specific CD4+ T cells could be divided into three distinct populations: single IFN-γ-secreting cells, double IFN-γ+IL2-secreting cells, and single IL2-secreting cells (Yang et al., 2006). This classification further demonstrates the heterogeneity of MTC and also indicates that distinct subpopulations of memory CD4+ T cells have different capacities to develop into short/effector IFN-γ-secreting and long-lived/central IL2-secreting and IFN-γ+IL2-secreting MTC (Yang et al., 2006).
As expected, the study results demonstrated a T cell response for all SARS-CoV-2 peptide pools. Particularly in convalescents, the SNMO peptide pool elicited the strongest memory T cell responses, that likely reflect the exposure to whole viral particle components including spike (S), nucleoprotein (N), membrane protein (M), and ORF (ORF)-3a and ORF-7a (Peng et al., 2020). Although it seems evident that the SNMO pool will create higher responses when compared to S1 or S2, previous studies have shown that the S1 pool has significantly higher reactivity in COVID-19 recovered individuals than in healthy controls and that SFU fold changes for the S1 pool are higher than those for SNMO (Long et al., 2021). Furthermore, significantly more poly-functional T cells respond to the S1 peptide pool as compared to the N peptide pool in convalescents (Sandberg et al., 2021). Therefore the finding of a higher SNMO SFU response over those of the S1 pool is not easily straightforward.
Nevertheless, we expect that the response of vaccinated subjects (with the mRNA-based vaccine; Pfizer BNT162b2) will be elicited dominantly to the spike protein peptide pool.
In view of recently developed anti-SARS-CoV-2 vaccines and the reported decline in antibody titers in vaccinated individuals (Bates et al., 2021; Sariol et al., 2021) and convalescent patients (Gaebler et al., 2021), it was interesting to follow MBC and MTC levels in patients switching to seronegative status. The critical question in patients with a decline in circulating antibodies is whether the other elements of immunological resistance against SARS-CoV-2 infection still exist.
The study results showed that robust T and B cell responses in such patients not only persisted but even increased, similar to the results of a previous study in which disappearing SARS-CoV-2-specific IgG was associated with a durable memory T cell response as compared to convalescents who remained IgG-positive (Schwarzkopf et al., 2021). The present study results are in line with previous publications related to Middle East respiratory syndrome coronavirus (MERS-CoV) and severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) infections in which a memory T cell response was observed when the antibody response waned (Abayasingam et al., 2021; Alshukairi et al., 2016; Channappanavar et al., 2014; Zhao et al., 2017). Similarly, Sandberg et al. (2021) and Sherina et al. (2020) (Sandberg et al., 2021; Sherina et al., 2021) showed robust MBC and MTC responses in patients with gradually decreased antibody levels at 8–9 months after SARS-CoV-2 onset (Dan et al., 2021). This suggests that although titers of antibody wane, an effective immune response to future infections could be developed by cellular memory components. Considering the high mutation rate of SARS-CoV-2 and potential immune escape, and that different spike mutations impair SARS-CoV-2 antigenicity (Harvey et al., 2021), it remains an open question whether the original memory cells will cross-react with SARS-CoV-2 variants. Although the existing evidence that the spike amino acid changes affect antibody neutralization (Harvey et al., 2021) and some variants can partially escape humoral immunity induced by SARS-CoV-2 infection or BNT162b2 vaccination, yet S-specific CD4+ T cell activation is not affected by the mutations at least in the B.1.1.7 and B.1.351 variants (Geers et al., 2021). SARS-CoV-2 variants analyzed by Tarke et al. in 2021 did not significantly disrupt the total SARS-CoV-2 T cell reactivity; however, the decreases observed in responses highlight the importance of active monitoring of T cell reactivity in the context of SARS-CoV-2 evolution (Tarke et al., 2021).
In conclusion, (1) durable immune humoral and adaptive responses were observed in SARS-CoV-2 convalescent patients with mild symptoms for up to 7 months, suggesting the longevity of humoral and adaptive immunity in convalescent individuals. (2) In convalescent patients, the T cell responses to S1, S2_N, and SNMO peptide pools were characterized by presenting IFN-γ- and IL2-secreting T cells and poly-functional IFN-γ+IL2-secreting T cells. (3) In convalescent patients, a waning anti-spike IgG titer could be accompanied by robust MBC and IFN-γ-producing MTC, suggesting a potential long-lasting recall memory response and defense against re-infection.
Declarations
Funding
The authors did not receive any specific grant from any funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
The authors declare that they obtained informed consent from the patients included in the article and that this report does not contain any personal information that could lead to their identification.
Conflict of interest
The authors have no conflicts of interest to report.
Footnotes
Michael Gurevich and Rina Zilkha-Falb contributed equally.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2021.12.309.
Appendix. Supplementary materials
Figure S1 Dynamics of SARS-CoV-2 anti-spike IgG in SARS-CoV-2 convalescents. Temporal dynamics of the SARS-CoV-2 anti-spike IgG level in serum from convalescent subjects at 1, 3, 5, and 7 months after disease onset.
TFO, time from onset (months). An IgG score higher than 1.0 was considered positive. The results are presented as the median with interquartile range. The number of subjects per group was as follows: HS, n = 16; 1 month, n = 13; 3 months, n = 21; 5 months, n = 19; 7 months, n = 15.
Figure S2 Dynamics of SARS-CoV-2 IFN-γ-, IL2-, and IFN-γ+IL2-secreting MTC in response to S1 peptides. (A) Dynamics of IFN-γ-secreting MTC in response to the S1 peptide pool. (B) Dynamics of IL2-secreting MTC in response to the S1 peptide pool. (C) Dynamics of IFN-γ+IL2-secreting MTC in response to the S1 peptide pool.
HS, healthy subjects; SFU, spot-forming units; TFO, time from COVID-19 onset; S1 IFN-γ MTC, IFN-γ-secreting memory T cells in response to S1; S1 IL2 MTC, IL2-secreting memory T cells in response to S1; S1 IFN-γ+IL2 MTC, IFN-γ+IL2-secreting memory T cells in response to S1.
*P < 0.05 by Mann–Whitney U-test as compared to HS. NS is non-significant as compared to HS. In graphs A, B, and C, no significant difference in SFU levels between the different time points was observed. The number of subjects per group was as follows: HS, n = 12; 1 month, n = 9; 3 months, n = 9; 5 months, n = 13; 7 months, n = 10. The dashed line denotes the cutoff level in the HS (0.0 SFU) calculated as 90% of confidence interval.
Figure S3 Dynamics of SARS-CoV-2 IFN-γ-, IL2-, and IFN-γ+IL2-secreting MTC in response to S2_N peptides. (A) Dynamics of IFN-γ-secreting MTC in response to the S2_N peptide pool. (B) Dynamics of IL2-secreting MTC in response to the S2_N peptide pool. (C) Dynamics of IFN-γ+IL2-secreting MTC in response to the S2_N peptide pool.
HS, healthy subjects; SFU, spot-forming units; TFO, time from COVID-19 onset; S2_N IFN-γ MTC, IFN-γ-secreting memory T cells in response to S2_N; S2_N IL2 MTC, IL2-secreting memory T cells in response to S2_N; S2_N IFN-γ+IL2 MTC, IFN-γ+IL2-secreting memory T cells in response to S2_N.
*P < 0.05 by Mann–Whitney U-test as compared to HS. . In graphs A, B, and C, no significant difference in SFU levels between the different time points was observed. The number of subjects per group was as follows: HS, n = 12; 1 month, n = 9; 3 months, n = 9; 5 months, n = 13; 7 months, n = 10. The dashed line denotes the cutoff level in the HS (0.0 SFU) calculated as 90% of confidence interval.
References
- Abayasingam A, Balachandran H, Agapiou D, Hammoud M, Rodrigo C, Keoshkerian E, Li H, Brasher NA, Christ D, Rouet R, Burnet D, Grubor-Bauk B, Rawlinson W, Turville S, Aggarwal A, Stella AO, Fichter C, Brilot F, Mina M, Post JJ, Hudson B, Gilroy N, Dwyer D, Sasson SC, Tea F, Pilli D, Kelleher A, Tedla N, Lloyd AR, Martinello M, Bull RA, Group CS. Long-term persistence of RBD(+) memory B cells encoding neutralizing antibodies in SARS-CoV-2 infection. Cell Rep Med. 2021;2 doi: 10.1016/j.xcrm.2021.100228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbas AK, Trotta E, RS D, Marson A, Bluestone JA. Revisiting IL-2: Biology and therapeutic prospects. Sci Immunol. 2018;3 doi: 10.1126/sciimmunol.aat1482. [DOI] [PubMed] [Google Scholar]
- Achiron A, Gurevich M, Falb R, Dreyer-Alster S, Sonis P, Mandel M. SARS-COV-2 antibody dynamics and B-cell memory response over-time in COVID-19 convalescent subjects. Clin Microbiol Infect. 2021 doi: 10.1016/j.cmi.2021.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed SF, Quadeer AA, McKay MR. Preliminary Identification of Potential Vaccine Targets for the COVID-19 Coronavirus (SARS-CoV-2) Based on SARS-CoV Immunological Studies. Viruses. 2020:12. doi: 10.3390/v12030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshukairi AN, Khalid I, Ahmed WA, Dada AM, Bayumi DT, Malic LS, Althawadi S, Ignacio K, Alsalmi HS, Al-Abdely HM, Wali GY, Qushmaq IA, Alraddadi BM, Perlman S. Antibody Response and Disease Severity in Healthcare Worker MERS Survivors. Emerg Infect Dis. 2016;22 doi: 10.3201/eid2206.160010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates TA, Leier HC, Lyski ZL, McBride SK, Coulter FJ, Weinstein JB, Goodman JR, Lu Z, Siegel SAR, Sullivan P, Strnad M, Brunton AE, Lee DX, Curlin ME, Messer WB, Tafesse FG. Neutralization of SARS-CoV-2 variants by convalescent and vaccinated serum. medRxiv 2021. [DOI] [PMC free article] [PubMed]
- Braun J, Loyal L, Frentsch M, Wendisch D, Georg P, Kurth F, Hippenstiel S, Dingeldey M, Kruse B, Fauchere F, Baysal E, Mangold M, Henze L, Lauster R, Mall MA, Beyer K, Rohmel J, Voigt S, Schmitz J, Miltenyi S, Demuth I, Muller MA, Hocke A, Witzenrath M, Suttorp N, Kern F, Reimer U, Wenschuh H, Drosten C, Corman VM, Giesecke-Thiel C, Sander LE, Thiel A. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature. 2020;587:270–274. doi: 10.1038/s41586-020-2598-9. [DOI] [PubMed] [Google Scholar]
- Channappanavar R, Zhao J, Perlman S. T cell-mediated immune response to respiratory coronaviruses. Immunol Res. 2014;59:118–128. doi: 10.1007/s12026-014-8534-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromer D, Juno JA, Khoury D, Reynaldi A, Wheatley AK, Kent SJ, Davenport MP. Prospects for durable immune control of SARS-CoV-2 and prevention of reinfection. Nat Rev Immunol. 2021;21:395–404. doi: 10.1038/s41577-021-00550-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, Grifoni A, Ramirez SI, Haupt S, Frazier A, Nakao C, Rayaprolu V, Rawlings SA, Peters B, Krammer F, Simon V, Saphire EO, Smith DM, Weiskopf D, Sette A, Crotty S. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021:371. doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forthal D. Adaptive immune responses to SARS-CoV-2. Adv Drug Deliv Rev. 2021;172:1–8. doi: 10.1016/j.addr.2021.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaebler C, Wang Z, Lorenzi JCC, Muecksch F, Finkin S, Tokuyama M, Cho A, Jankovic M, Schaefer-Babajew D, Oliveira TY, Cipolla M, Viant C, Barnes CO, Bram Y, Breton G, Hagglof T, Mendoza P, Hurley A, Turroja M, Gordon K, Millard KG, Ramos V, Schmidt F, Weisblum Y, Jha D, Tankelevich M, Martinez-Delgado G, Yee J, Patel R, Dizon J, Unson-O'Brien C, Shimeliovich I, Robbiani DF, Zhao Z, Gazumyan A, Schwartz RE, Hatziioannou T, Bjorkman PJ, Mehandru S, Bieniasz PD, Caskey M, Nussenzweig MC. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591:639–644. doi: 10.1038/s41586-021-03207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geers D, Shamier MC, Bogers S, den Hartog G, Gommers L, Nieuwkoop NN, Schmitz KS, Rijsbergen LC, van Osch JAT, Dijkhuizen E, Smits G, Comvalius A, van Mourik D, Caniels TG, van Gils MJ, Sanders RW, Oude Munnink BB, Molenkamp R, de Jager HJ, Haagmans BL, de Swart RL, Koopmans MPG, van Binnendijk RS, de Vries RD, GeurtsvanKessel CH. SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees. Sci Immunol. 2021;6 doi: 10.1126/sciimmunol.abj1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifoni A, Weiskopf D, Ramirez SI, Mateus J, Dan JM, Moderbacher CR, Rawlings SA, Sutherland A, Premkumar L, Jadi RS, Marrama D, de Silva AM, Frazier A, Carlin AF, Greenbaum JA, Peters B, Krammer F, Smith DM, Crotty S, Sette A. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell. 2020;181:1489–1501. doi: 10.1016/j.cell.2020.05.015. e1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley GE, Edwards ESJ, Aui PM, Varese N, Stojanovic S, McMahon J, Peleg AY, Boo I, Drummer HE, Hogarth PM, O'Hehir RE, van Zelm MC. Rapid generation of durable B cell memory to SARS-CoV-2 spike and nucleocapsid proteins in COVID-19 and convalescence. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abf8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidi A, Koegler F, Dumont R, Dubos R, Zaballa ME, Piumatti G, Coen M, Berner A, Darbellay Farhoumand P, Vetter P, Vuilleumier N, Kaiser L, Courvoisier D, Azman AS, Guessous I, Stringhini S. Risk of reinfection after seroconversion to SARS-CoV-2: A population-based propensity-score matched cohort study. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long QX, Jia YJ, Wang X, Deng HJ, Cao XX, Yuan J, Fang L, Cheng XR, Luo C, He AR, Tang XJ, Hu JL, Hu Y, Tang N, Cai XF, Wang DQ, Hu J, Qiu JF, Liu BZ, Chen J, Huang AL. Immune memory in convalescent patients with asymptomatic or mild COVID-19. Cell Discov. 2021;7:18. doi: 10.1038/s41421-021-00250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni A, Maggi L, Capone M, Vanni A, Spinicci M, Salvati L, Tekle Kiros S, Semeraro R, Pengue L, Colao MG, Magi A, Rossolini GM, Liotta F, Cosmi L, Bartoloni A, Annunziato F. Heterogeneous magnitude of immunological memory to SARS-CoV-2 in recovered individuals. Clin Transl Immunology. 2021;10:e1281. doi: 10.1002/cti2.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahan K, Yu J, Mercado NB, Loos C, Tostanoski LH, Chandrashekar A, Liu J, Peter L, Atyeo C, Zhu A, Bondzie EA, Dagotto G, Gebre MS, Jacob-Dolan C, Li Z, Nampanya F, Patel S, Pessaint L, Van Ry A, Blade K, Yalley-Ogunro J, Cabus M, Brown R, Cook A, Teow E, Andersen H, Lewis MG, Lauffenburger DA, Alter G, Barouch DH. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2021;590:630–634. doi: 10.1038/s41586-020-03041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchu EO, Byrne P, Carty PG, De Gascun C, Keogan M, O'Neill M, Harrington P, Ryan M. Quantifying the risk of SARS-CoV-2 reinfection over time. Rev Med Virol. 2021:e2260. doi: 10.1002/rmv.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Yang LT, Wang LY, Li J, Huang J, Lu ZQ, Koup RA, Bailer RT, Wu CY. Long-lived memory T lymphocyte responses against SARS coronavirus nucleocapsid protein in SARS-recovered patients. Virology. 2006;351:466–475. doi: 10.1016/j.virol.2006.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Mentzer AJ, Liu G, Yao X, Yin Z, Dong D, Dejnirattisai W, Rostron T, Supasa P, Liu C, Lopez-Camacho C, Slon-Campos J, Zhao Y, Stuart DI, Paesen GC, Grimes JM, Antson AA, Bayfield OW, Hawkins D, Ker DS, Wang B, Turtle L, Subramaniam K, Thomson P, Zhang P, Dold C, Ratcliff J, Simmonds P, de Silva T, Sopp P, Wellington D, Rajapaksa U, Chen YL, Salio M, Napolitani G, Paes W, Borrow P, Kessler BM, Fry JW, Schwabe NF, Semple MG, Baillie JK, Moore SC, Openshaw PJM, Ansari MA, Dunachie S, Barnes E, Frater J, Kerr G, Goulder P, Lockett T, Levin R, Zhang Y, Jing R, Ho LP, Oxford Immunology Network Covid-19 Response TcC. Investigators IC. Cornall RJ, Conlon CP, Klenerman P, Screaton GR, Mongkolsapaya J, McMichael A, Knight JC, Ogg G, Dong T. Broad and strong memory CD4(+) and CD8(+) T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat Immunol. 2020;21:1336–1345. doi: 10.1038/s41590-020-0782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodda LB, Netland J, Shehata L, Pruner KB, Morawski PA, Thouvenel CD, Takehara KK, Eggenberger J, Hemann EA, Waterman HR, Fahning ML, Chen Y, Hale M, Rathe J, Stokes C, Wrenn S, Fiala B, Carter L, Hamerman JA, King NP, Gale M, Jr., Campbell DJ, Rawlings DJ, Pepper M. Functional SARS-CoV-2-Specific Immune Memory Persists after Mild COVID-19. Cell. 2021;184 doi: 10.1016/j.cell.2020.11.029. 169-183.e117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakharkar M, Rappazzo CG, Wieland-Alter WF, Hsieh CL, Wrapp D, Esterman ES, Kaku CI, Wec AZ, Geoghegan JC, McLellan JS, Connor RI, Wright PF, Walker LM. Prolonged evolution of the human B cell response to SARS-CoV-2 infection. Sci Immunol. 2021;6 doi: 10.1126/sciimmunol.abg6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg JT, Varnaitė R, Christ W, Chen P, Muvva JR, Maleki KT, García M, Dzidic M, Folkesson E, Skagerberg M, Ahlén G, Frelin L, Sällberg M, Eriksson LI, Rooyackers O, Sönnerborg A, Buggert M, Björkström NK, Aleman S, Strålin K, Klingström J, Ljunggren H-G, Blom K, Gredmark-Russ S. Longitudinal characterization of humoral and cellular immunity in hospitalized COVID-19 patients reveal immune persistence up to 9 months after infection. bioRxiv 2021:2021.2003.2017.435581.
- Sariol CA, Pantoja P, Serrano-Collazo C, Rosa-Arocho T, Armina A, Cruz L, Stone ET, Arana T, Climent C, Latoni G, Atehortua D, Pabon-Carrero C, Pinto AK, Brien JD, Espino AM. Function is more reliable than quantity to follow up the humoral response to the Receptor Binding Domain of SARS- CoV-2 Spike protein after natural infection or COVID-19 vaccination. medRxiv. 2021 doi: 10.3390/v13101972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzkopf S, Krawczyk A, Knop D, Klump H, Heinold A, Heinemann FM, Thummler L, Temme C, Breyer M, Witzke O, Dittmer U, Lenz V, Horn PA, Lindemann M. Cellular Immunity in COVID-19 Convalescents with PCR-Confirmed Infection but with Undetectable SARS-CoV-2-Specific IgG. Emerg Infect Dis. 2021:27. doi: 10.3201/2701.203772. [DOI] [PubMed] [Google Scholar]
- Sekine T, Perez-Potti A, Rivera-Ballesteros O, Stralin K, Gorin JB, Olsson A, Llewellyn-Lacey S, Kamal H, Bogdanovic G, Muschiol S, Wullimann DJ, Kammann T, Emgard J, Parrot T, Folkesson E, Karolinska C-SG, Rooyackers O, Eriksson LI, Henter JI, Sonnerborg A, Allander T, Albert J, Nielsen M, Klingstrom J, Gredmark-Russ S, Bjorkstrom NK, Sandberg JK, Price DA, Ljunggren HG, Aleman S, Buggert M. Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19. Cell. 2020;183 doi: 10.1016/j.cell.2020.08.017. 158-168 e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherina N, Piralla A, Du L, Wan H, Kumagai-Braesch M, Andrell J, Braesch-Andersen S, Cassaniti I, Percivalle E, Sarasini A, Bergami F, Di Martino R, Colaneri M, Vecchia M, Sambo M, Zuccaro V, Bruno R, Sachs M, Oggionni T, Meloni F, Abolhassani H, Bertoglio F, Schubert M, Byrne-Steele M, Han J, Hust M, Xue Y, Hammarstrom L, Baldanti F, Marcotte H, Pan-Hammarstrom Q. Persistence of SARS-CoV-2-specific B and T cell responses in convalescent COVID-19 patients 6-8 months after the infection. Med (N Y) 2021;2 doi: 10.1016/j.medj.2021.02.001. 281-295 e284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarke A, Sidney J, Methot N, Yu ED, Zhang Y, Dan JM, Goodwin B, Rubiro P, Sutherland A, Wang E, Frazier A, Ramirez SI, Rawlings SA, Smith DM, da Silva Antunes R, Peters B, Scheuermann RH, Weiskopf D, Crotty S, Grifoni A, Sette A. Impact of SARS-CoV-2 variants on the total CD4(+) and CD8(+) T cell reactivity in infected or vaccinated individuals. Cell Rep Med. 2021;2 doi: 10.1016/j.xcrm.2021.100355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thieme CJ, Anft M, Paniskaki K, Blazquez-Navarro A, Doevelaar A, Seibert FS, Hoelzer B, Konik MJ, Berger MM, Brenner T, Tempfer C, Watzl C, Meister TL, Pfaender S, Steinmann E, Dolff S, Dittmer U, Westhoff TH, Witzke O, Stervbo U, Roch T, Babel N. Robust T Cell Response Toward Spike, Membrane, and Nucleocapsid SARS-CoV-2 Proteins Is Not Associated with Recovery in Critical COVID-19 Patients. Cell Rep Med. 2020;1 doi: 10.1016/j.xcrm.2020.100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JS, Kim W, Kalaidina E, Goss CW, Rauseo AM, Schmitz AJ, Hansen L, Haile A, Klebert MK, Pusic I, O'Halloran JA, Presti RM, Ellebedy AH. SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans. Nature. 2021 doi: 10.1038/s41586-021-03647-4. [DOI] [PubMed] [Google Scholar]
- Ueffing M, Bayyoud T, Schindler M, Ziemssen F. [Basic principles of replication and immunology of SARS-CoV-2] Ophthalmologe. 2020;117:609–614. doi: 10.1007/s00347-020-01155-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskopf D, Schmitz KS, Raadsen MP, Grifoni A, Okba NMA, Endeman H, van den Akker JPC, Molenkamp R, Koopmans MPG, van Gorp ECM, Haagmans BL, de Swart RL, Sette A, de Vries RD. Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abd2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LT, Peng H, Zhu ZL, Li G, Huang ZT, Zhao ZX, Koup RA, Bailer RT, Wu CY. Long-lived effector/central memory T-cell responses to severe acute respiratory syndrome coronavirus (SARS-CoV) S antigen in recovered SARS patients. Clin Immunol. 2006;120:171–178. doi: 10.1016/j.clim.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Alshukairi AN, Baharoon SA, Ahmed WA, Bokhari AA, Nehdi AM, Layqah LA, Alghamdi MG, Al Gethamy MM, Dada AM, Khalid I, Boujelal M, Al Johani SM, Vogel L, Subbarao K, Mangalam A, Wu C, Ten Eyck P, Perlman S, Zhao J. Recovery from the Middle East respiratory syndrome is associated with antibody and T-cell responses. Sci Immunol. 2017;2 doi: 10.1126/sciimmunol.aan5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Dynamics of SARS-CoV-2 anti-spike IgG in SARS-CoV-2 convalescents. Temporal dynamics of the SARS-CoV-2 anti-spike IgG level in serum from convalescent subjects at 1, 3, 5, and 7 months after disease onset.
TFO, time from onset (months). An IgG score higher than 1.0 was considered positive. The results are presented as the median with interquartile range. The number of subjects per group was as follows: HS, n = 16; 1 month, n = 13; 3 months, n = 21; 5 months, n = 19; 7 months, n = 15.
Figure S2 Dynamics of SARS-CoV-2 IFN-γ-, IL2-, and IFN-γ+IL2-secreting MTC in response to S1 peptides. (A) Dynamics of IFN-γ-secreting MTC in response to the S1 peptide pool. (B) Dynamics of IL2-secreting MTC in response to the S1 peptide pool. (C) Dynamics of IFN-γ+IL2-secreting MTC in response to the S1 peptide pool.
HS, healthy subjects; SFU, spot-forming units; TFO, time from COVID-19 onset; S1 IFN-γ MTC, IFN-γ-secreting memory T cells in response to S1; S1 IL2 MTC, IL2-secreting memory T cells in response to S1; S1 IFN-γ+IL2 MTC, IFN-γ+IL2-secreting memory T cells in response to S1.
*P < 0.05 by Mann–Whitney U-test as compared to HS. NS is non-significant as compared to HS. In graphs A, B, and C, no significant difference in SFU levels between the different time points was observed. The number of subjects per group was as follows: HS, n = 12; 1 month, n = 9; 3 months, n = 9; 5 months, n = 13; 7 months, n = 10. The dashed line denotes the cutoff level in the HS (0.0 SFU) calculated as 90% of confidence interval.
Figure S3 Dynamics of SARS-CoV-2 IFN-γ-, IL2-, and IFN-γ+IL2-secreting MTC in response to S2_N peptides. (A) Dynamics of IFN-γ-secreting MTC in response to the S2_N peptide pool. (B) Dynamics of IL2-secreting MTC in response to the S2_N peptide pool. (C) Dynamics of IFN-γ+IL2-secreting MTC in response to the S2_N peptide pool.
HS, healthy subjects; SFU, spot-forming units; TFO, time from COVID-19 onset; S2_N IFN-γ MTC, IFN-γ-secreting memory T cells in response to S2_N; S2_N IL2 MTC, IL2-secreting memory T cells in response to S2_N; S2_N IFN-γ+IL2 MTC, IFN-γ+IL2-secreting memory T cells in response to S2_N.
*P < 0.05 by Mann–Whitney U-test as compared to HS. . In graphs A, B, and C, no significant difference in SFU levels between the different time points was observed. The number of subjects per group was as follows: HS, n = 12; 1 month, n = 9; 3 months, n = 9; 5 months, n = 13; 7 months, n = 10. The dashed line denotes the cutoff level in the HS (0.0 SFU) calculated as 90% of confidence interval.