Abstract
Background
There is conflicting evidence regarding the effect of asthma and its different therapeutic options on COVID-19 severity and the clinical outcomes.
Aim
This study aimed to investigate the relationship between using inhaled corticosteroids (ICS) by asthmatic patients and the severity of COVID-19.
Materials and methods
This retrospective observational study was conducted from March 15 to October 23, 2020 and included data of all COVID-19 asthmatic patients (n = 287) at King Abdulaziz Medical City. Twelve patients were excluded due to poor medication history documentation or using ICS for non-asthma indication. Ordinal logistic regression was used to determine the clinical variables that affect COVID-19 severity. The clinical outcomes of ICS and non-ICS users were compared.
Results
Of the sample (n = 275), 198 (72%) were using ICS therapy. No significant difference was found between ICS and non-ICS users in disease severity (P = 0.12), mortality (P = 0.45), ICU admission (P = 0.78), and the occurrence of complications. However, the number of days on ventilation were significantly increased in ICS users (P = 0.006). Being prescribed the ICS/LABA combination (adj OR: 0.72 [0.15,1.2]; P = 0.021), being hypertensive (adj OR: 0.98 [0.28,1.6]; P = 0.006), having cancer (adj OR: 1.49 [0.12, 2.8]; P = 0.033), or having diabetes (adj OR: 0.75 [0.09, 1.4]; P = 0.024) could not increase the risk for more severe disease.
Conclusion
Overall, ICS therapy did not alter the COVID-19 severity or mortality in asthmatic patients. The continued use of ICS during the pandemic should be encouraged to prevent asthma exacerbations.
Keywords: COVID-19, Asthma, Inhaled corticosteroids, Viral infection
Introduction
Coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has become a pandemic. After a median incubation period of five days, the disease occurs in different stages, ranging from mild cases where the disease is limited to the upper and lower airway (80% to 90% of patients) and severe cases with bilateral pneumonia (10% to 20%) [[1], [2], [3]]. Patients with severe COVID-19 may also develop Acute Respiratory Distress Syndrome (ARDS), and require mechanical ventilation in an intensive care [4,5]. COVID-19 patients with pre-existing comorbid conditions have worse disease outcomes, including a higher incidence of hospitalization, ICU admission, and mortality [2,6].
Although respiratory viral infections contribute to the majority of exacerbations in asthmatic patients [7], asthma is underrepresented in literature as a comorbidity in COVID-19 severity [2,3,8]. Currently, moderate to severe asthma are classified by the Centers for Disease Control and Prevention (CDC) as a high-risk group that is vulnerable to severe COVID-19.
Theoretically, long-term treatment with systemic corticosteroids (e.g. in transplant patients) increases the risk and severity of viral infections due to immunosuppression [9]. However, in vitro studies suggested that ICS could have a protective effect against SARS-CoV-2 as they dose-dependently reduce the expression of the Angiotensin-Converting Enzyme type 2 (ACE-2) on the surface of the lung cells, decreasing the viral entry into the cells [10]. The RECOVERY trial reported that the use of dexamethasone reduced the mortality rate in hospitalized COVID-19 patients who required mechanical ventilation or supplemental oxygen [11]. Based on these findings, the World Health Organization (WHO) and the National Institute of Medicine (NIH) recommended the use of dexamethasone, contrary to their initial recommendation against this practice during the early phases of the pandemic, if not clinically indicated for another disease state [12]. The drug now is added to the treatment guidelines in most centers treating COVID-19 patients. In addition, professional societies in their response to the pandemic, urge asthmatic patients to continue their prescribed medication, including ICS [13,14].
These recommendations have caused uncertainty in asthma patients and attending clinicians in terms of maintaining ICS therapy as withholding ICS increases the risk of severe exacerbations. A recent meta-analysis related to COVID-19 outcomes in patients with chronic respiratory diseases using ICS, concluded that there is currently insufficient evidence to abandon ICS treatment in asthma [15].
Today, approximately 300 million individuals globally have asthma [16] and an estimated 11.3% of the Saudi Arabian population are affected by the disease [17]. Acute asthma exacerbations are considered a frequent cause of hospitalization and emergency room visits [18]. More importantly ICS are prescribed in 90.8% of asthma cases [19]. A recent study conducted by Sen et al. disclosed that ICS therapy had no impact on COVID-19 related clinical outcomes or mortality in COPD patients [20]. Asthmatic patients prescribed a low to medium-dose ICS were not at an increased risk of mortality (adjusted HR = 1.14; 95% CI, 0.85–1.54) [21].
However, COVID-19-related mortality was significantly increased in COPD patients using ICS compared to the group who are maintained with long-acting beta agonist (LABA) and the long-acting muscarinic agent (LAMA) combination therapy (adjusted (adj.) HR = 1.39; 95% CI, 1.1–1.76) [21]. COVID-19-related mortality was increased in asthmatic patients prescribed a high-dose ICS compared to patients taking short-acting beta-agonists (SABAs) (adj. HR = 1.55; 95% CI, 1.1–2.18) [21]. Clear evidence regarding the impact of prior or continued use of ICS on the clinical course of COVID-19 in asthma patients is still lacking. It is crucial to understand which asthma patients are particularly at risk of being infected with COVID-19 and how ICS may influence the morbidity and mortality associated with COVID-19. There is also a need to clarify the demographic and clinical characteristics which determine disease severity and outcomes of COVID-19 in asthmatic patients. This is the first study in the Middle East to examine the effect of ICS therapy on asthmatic patients infected with SARS-CoV-2.
This study aimed to investigate the impact of using ICS by asthmatic patients and the latter’s clinical characteristics on the severity of COVID-19. We hypothesized that the antecedent use of ICS complicates the clinical course and severity of COVID-19.
Methods
Design and patients selection
This retrospective cross-sectional study was conducted at King Abdulaziz Medical City, Riyadh from March 15 to October 23, 2020, to investigate the association effect of ICS use and other asthmatic patients’ characteristics on COVID-19 severity. The data management department was initially approached for data on COVID-19 patients with possible asthma diagnosis based on symptoms (i.e., cough and wheezing) (n = 574). Files were then manually checked for documented asthma diagnosis (n = 287) which was based on the hospital’s diagnostic and classification criteria that are based on the NIH guidelines (Appendix I): intermittent, mild persistent, moderate persistent, and severe persistent.
Sample size calculation
Grandbastien et al. reported that 23 (21.7%) of patients admitted with SARS-CoV-2 pneumonia had an established diagnosis of asthma [22]. The sample size was calculated using the OpenEpi calculator and estimated at 262 with a 5% level of significance. The sample size was inflated by 10%–288 patients due to possible missing data.
Outcome measures
The medical records were reviewed and the demographic, clinical, and outcome data were collected. The variables collected included age, weight, body mass index (BMI), gender, comorbidities (i.e., cardiac disease, non-asthmatic pulmonary disease, kidney disease, liver disease, obesity, stroke, and malignancy), and concurrent medications, including the type of ICS. Details of the disease management were also recorded, including the disease severity, level of care (ward-based, high dependency unit (HDU), or Intensive care units (ICU)), hospital length of stay (LOS), ICU length of stay, ventilation days, complications (e.g., acute respiratory distress syndrome (ARDS), acute kidney injury (AKI), sepsis, septic shock), and mortality.
Primary outcomes
The severity of COVID-19, for both ICS and non-ICS users in asthmatic patients, based on the National Institute of Medicine (NIH) categories [12]:
-
1
Asymptomatic type: COVID-19 positive but no symptoms
-
2
Mild type: Mild clinical symptoms with no abnormal findings on the radiograph
-
3
Moderate type: Pneumonia is evident on chest CT along with fever, cough, and other symptoms
-
4Severe type: The disease is classified as severe if one of the following conditions is met:
-
•Respiratory distress, respiratory rate >30/min
-
•Oxygen saturation on room air at rest <93%
-
•Partial pressure of oxygen in arterial blood/FIO2 < 300 mm Hg
-
•
-
5Critical type: One of the following conditions has to be met:
-
•Respiratory failure occurs and mechanical ventilation is required
-
•Shock occurs
-
•Other organ dysfunction is present, requiring ICU monitoring and treatment
-
•
This classification was then modified to three major groups to avoid blank cells when subgrouping patients. The groups included asymptomatic, mild-moderate, and severe-critical cases.
Secondary outcomes
The secondary outcomes included the level of care, hospital LOS, ICU LOS, ventilation days, complications (ARDS, AKI, sepsis, septic shock), and mortality.
Statistical analysis
The sample was analyzed to determine if there was an association between the severity of COVID-19 and ICS use or other clinical variables. Continuous data were summarized as mean (standard deviation) and categorical data as frequency (percentage).
The sample was divided in two groups: ICS users and non-ICS users and compared in terms of the clinical outcomes (e.g., baseline characteristics, hospitalization, ICU admission oxygenation and ventilation status, complications, and mortality) using a χ2 test or fisher’s exact test for the categorical data, or the independent sample t-test for the scale variables (age, height, and weight).
The sample was divided in three groups, based on the disease severity: asymptomatic, mild-moderate, severe-critical. The Mann-Whitney U test or Kruskal-Wallis test was used to analyze the association with the nominal variables, and the categorical data with the χ2 test or fisher’s exact test. A Spearman correlation was done to assess the correlation between the groups and the scale/ordinal variables.
Ordinal logistic regression was then used to adjust for asthma severity and to assess the association between COVID-19 severity and the different patient and clinical variables. All four assumptions were met for creating the model. A McNemar’s test was used to analyze the change in ventilation mode.
All analyses were two-tailed and were performed at a significance level of 0.05. IBM SPSS Statistics version 25.0 was used for statistical analysis.
Results
Patients’ characteristics
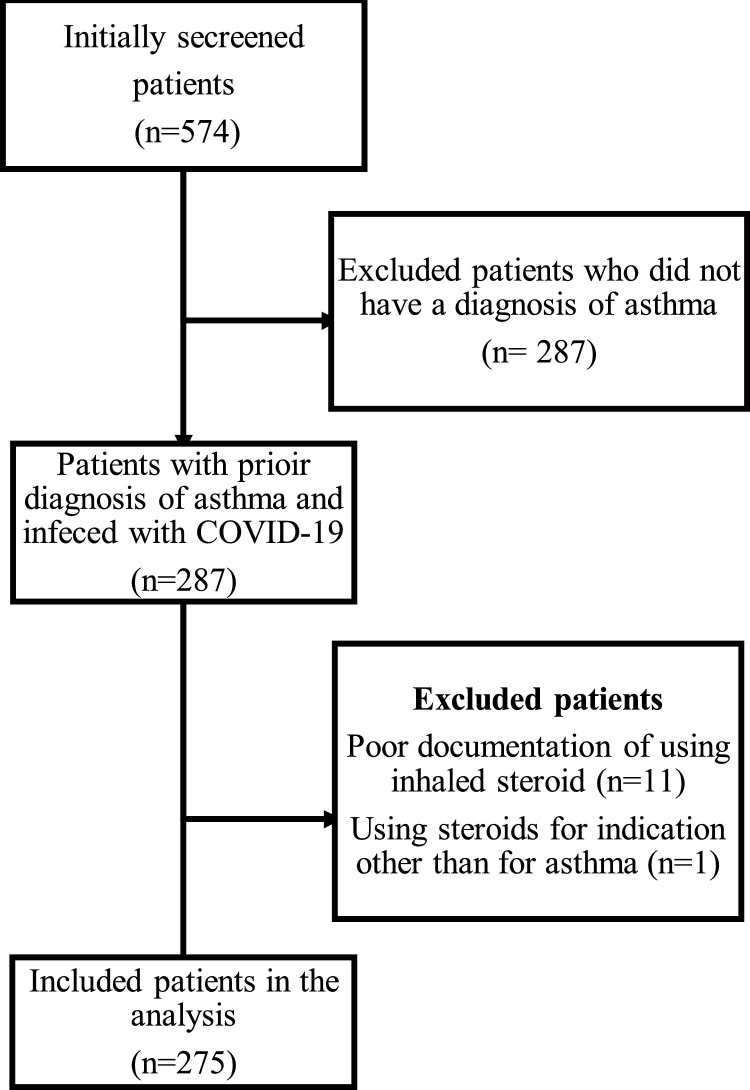
After excluding non-eligible subjects (Fig. 1 ), the sample size realized as 275 patients with a 1:1 gender distribution. However, Most of the ICS users were female (n = 107, 54%) (P = 0.04). The mean age of the participants was 37.7 (23.3) years. The ICS users were significantly older than the non-ICS users (P = 0.002), and had a higher weight, 71.02 (27.9) kg (P = 0.015). There were more obese patients among the ICS user group (n = 109, 55%) compared to non-ICS users group (P = 0.003).
Fig. 1.
Study flow chart.
Related comorbidities
The most prevalent comorbidities in the sample were diabetes (n = 72, 26.2%), hypertension (n = 64, 23.3%), and obesity (n = 62, 22.5%). The ICS user group had more renal failure (P = 0.038), cardiovascular diseases (P = 0.04), and hypertension (P = 0.012). However, more dyslipidemia occurred in the non-ICS user group (P = 0.02). No significant difference was found for any other comorbidities. The majority of the sample had mild-persistent asthma. The asthma severity considerably varied between ICS and non-ICS users, more intermittent cases occurred in the non-ICS users (P < 0.001), with more moderate and severe cases in the ICS users (P < 0.001) (Table 1 ).
Table 1.
Baseline characteristics of asthmatic patients with COVID-19 based on ICS use (N,%) unless otherwise stated.
| ICS Use Status |
|||||
|---|---|---|---|---|---|
| All patients (n = 275) | ICS users (n = 198) | Non-ICS users (n = 77) | P value | ||
| Demographics | |||||
| Gender n(%) | Male | 137 (49.8) | 91 (46) | 46 (59.7) | 0.04* |
| Female | 138 (50.2) | 107 (54) | 31 (40.3) | ||
| Age: mean (SD) years | 37.7 (23.3) | 40.4(23.4) | 30.7(21.7) | 0.002* | |
| Weight: mean (SD) kg | 68.4(28.5) | 71.02(27.9) | 61.7 (29.1) | 0.015* | |
| Height; mean (SD) cm | 151.9(21.5) | 152.9(20.1) | 149.3(24.9) | 0.26 | |
| BMI category | Underweight | 13 (4.7) | 6 (3) | 7 (9) | 0.053 |
| Normal weight | 74 (26.9) | 51 (25.8) | 23 (29.9) | 0.05 | |
| Overweight | 52 (18.9) | 32 (16.2) | 20 (26) | 0.062 | |
| Obese | 136 (49.5) | 109 (55) | 27 (35.1) | 0.003* | |
| Smoking history n(%) | 8 (2.9) | 7 (3.5) | 1 (1.3) | 0.45 | |
| Comorbidities n(%) | Non-asthmatic pulmonary disease | 20 (7.3) | 17 (8.6) | 3 (3.9) | 0.179 |
| Renal failure | 11 (4) | 11 (5.6) | 0 | 0.038* | |
| Cardiovascular disease | 27 (9.8) | 24 (12.2) | 3 (3.9) | 0.04* | |
| HTN | 64 (23.3) | 54 (27.3) | 10 (13) | 0.012* | |
| Diabetes | 72 (26.2) | 57 (28.8) | 15 (19.5) | 0.11 | |
| Malignancy | 8 (2.9) | 6 (3) | 2 (2.6) | 0.603 | |
| Liver disease | 5 (1.8) | 3 (1.5) | 2 (2.6) | 0.62 | |
| Obesity | 62 (22.5) | 47 (23.7) | 15 (19.5) | 0.44 | |
| Stroke | 5 (1.8) | 5 (2.5) | 0 | 0.326 | |
| On immunosuppression | 1 (0.4) | 1 (0.5) | 0 | 0.72 | |
| Dyslipidemia | 26 (9.5) | 14 (7.1) | 12 (15.6) | 0.02* | |
| Hypothyroidism | 7 (2.5) | 5 (2.5) | 2 (2.6) | 0.63 | |
| Concurrent Medications n(%) | Beta-blockers | 9 (3.3) | 9 (4.5) | 0 | 0.066 |
| ACEI | 27 (9.8) | 20 (10.1) | 7 (9.1) | 0.8 | |
| Asthma history | |||||
| Severity of Asthma n(%) | Intermittent | 40 (14.5) | 3 (1.5) | 37(48.1) | <0.001 |
| Mild persistent | 117(42.5) | 79 (39.9) | 38 (49.4) | 0.15 | |
| Moderate persistent | 85 (30.9) | 83 (41.9) | 2 (2.6) | <0.001 | |
| Severe persistent | 33 (12) | 33 (16.7) | 0 | <0.001 | |
| Other asthma medications n(%) | Salbutamol | 215 (78.2) | 169 (85.4) | 46 (59.7) | <0.001 |
| Ipratropium | 65 (23.6) | 58 (29.3) | 7 (9.1) | <0.001 | |
| Tiotropium | 3 (10.9) | 2 (1) | 1 (1.3) | 0.63 | |
| Racepinephrine | 1 (0.4) | 1 (0.5) | 0 | 0.72 | |
| Montelukast | 14 (5.1) | 9 (4.5) | 5 (6.5) | 0.545 | |
Significant.
Prescribed medication
A small proportion of the sample was using other medications, 9 (3.3%) were using beta-blockers, 27 (9.8%) ACE inhibitors, and 1 immunosuppression. The majority were using other asthma medication than ICS, including salbutamol (n = 215, 78.2%) and ipratropium (n = 65, 23.6%). These medications were also used with ICS, 169 (85.4%) were using salbutamol and 58 (29.3%) ipratropium (P < 0.001). The majority of the ICS users were using the ICS/LABA combination, 90 (45.5%) fluticasone/salmeterol, and 45 (22.7%) budesonide/formoterol. The most frequently used ICS monotherapy was fluticasone (n = 47, 23.7%) (Fig. 2 ).
Fig. 2.
Types of inhaled corticosteroids used by patients.
Primary outcomes
The variability of COVID-19 severity was assessed in terms of the variables related to the patient, disease, and medication (Table 2 ). The disease severity was similar in the ICS and non-ICS user groups (P = 0.12). However, the use of the combination ICS/LABA therapy resulted in more mild to moderate (n = 89, 55.6%), and severe-critical cases (n = 12, 85.7%), compared to the asymptomatic cases in the ICS monotherapy users (P < 0.001). No significant difference in disease severity was noted with salbutamol (P = 0.75), Ipratropium/tiotropium (P = 0.53), or gender (P = 0.24). The COVID-19 severity was significantly different between smokers and non-smokers (P = 0.015), with 2 (14.3%) of the severe-critical patients currently smoking compared to 4 (4%) in the asymptomatic group and only 2 (1.3%) in the mild to moderate cases. COVID-19 disease severity was significantly increased in patients presenting with non-asthmatic pulmonary disease (P = 0.006), renal failure (P = 0.03), cardiovascular disease (P = 0.003), hypertension (P < 0.001), diabetes (P < 0.001), and malignancy (P = 0.008). There was no significant difference in the disease severity in patients suffering from liver disease (P = 0.064), stroke (P = 0.056), dyslipidemia (P = 0.48), or hypothyroidism (P = 0.31). COVID-19 severity was also not significantly different in patients taking beta-blockers (P = 0.18), ACE inhibitors (P = 0.15), and immunosuppressing therapy (P = 0.58). Lastly, the BMI category and asthma severity were positively correlated with COVID-19 severity (Spearman: 0.12; P = 0.042) and (Spearman: 0.165; P = 0.006) respectively. Increased age was also significantly correlated with a more severe presentation of the disease (Spearman: 0.39; P < 0.001).
Table 2.
Association of COVID-19 disease severity with ICS use and other patient characteristics; N(%).
| Nominal or ordinal variables | Disease severity |
||||||
|---|---|---|---|---|---|---|---|
| All (n = 275) n (%) | Asymptomatic (n = 101) n (%) | Mild- moderate (n = 160) n (%) | Severe- critical (n = 14) n (%) | P-value | |||
| ICS users | 198 (72) | 66 (65.3) | 120 (75) | 12 (85.7) | 0.12 | ||
| Non-ICS user | 77 (28) | 35 (34.7) | 40 (25) | 2 (14.3) | |||
| ICS + LABA combinations (Budesonide + Formoterol OR Fluticasone + salmeterol) | 135 (49.1) | 34 (33.7) | 89 (55.6) | 12 (85.7) | <0.001* | ||
| ICS only | 140(50.9) | 67 (66.3) | 71 (44.4) | 2 (14.3) | |||
| Salbutamol users | 215(78.2) | 78 (77.2) | 127 (79.4) | 10 (71.4) | 0.75 | ||
| Ipratropium/tiotropium users | 67 (24.4) | 18 (17.8) | 45 (28.1) | 4 (28.6) | 0.53 | ||
| Gender | Male | 137 (49.8) | 57 (77.2) | 74 (46.3) | 6 (42.9) | 0.24 | |
| Female | 138 (50.2) | 44 (43.6) | 86 (53.8) | 8 (57.1) | |||
| Current smokers | 8 (2.9) | 4 (4) | 2 (1.3) | 2 (14.3) | 0.015* | ||
| Comorbidities | Non-asthmatic pulmonary disease | 20 (7.3) | 7 (6.9) | 9 (5.6) | 4 (28.6) | 0.006* | |
| Renal failure | 11 (4) | 2 (1.9) | 6 (3.8) | 3 (21.4) | 0.03* | ||
| Cardiovascular disease | 27 (9.8) | 7 (6.9) | 15 (9.4) | 5 (35.7) | 0.003* | ||
| Hypertension | 64 (23.3) | 12 (11.8) | 39 (24.4) | 13 (92.9) | <0.001* | ||
| Diabetes | 72 (27.3) | 14 (13.9) | 49 (30.6) | 9 (64.3) | <0.001* | ||
| Malignancy | 8 (2.9) | 0 | 6 (3.8) | 2 (14.3) | 0.008* | ||
| Liver disease | 5 (1.8) | 0 | 5 (3.1) | 0 | 0.064 | ||
| Stroke | 5 (1.8) | 1 (0.9) | 2 (1.3) | 2 (14.3) | 0.056 | ||
| Dyslipidemia | 26 (9.5) | 7 (6.9) | 18 (11.3) | 1 (7.1) | 0.48 | ||
| Hypothyroidism | 7 (2.5) | 1 (0.9) | 5 (3.1) | 1 (7.1) | 0.31 | ||
| Concurrent Medications | Beta-blockers | 9 (3.3) | 1 (0.9) | 7 (4.4) | 1 (7.1) | 0.18 | |
| ACE inhibitors | 27 | 7 (6.9) | 20 (12.5) | 0 | 0.15 | ||
| Immunosuppressants | 1 (0.4) | 0 | 1 (0.6) | 0 | 0.58 | ||
| Asthma Severity | Intermittent | 40 (14.5) | 19 (18.8) | 19 (11.9) | 2 (14.3) | 0.302 | |
| Mild persistent | 117 (42.5) | 48 (47.5) | 67 (41.9) | 2 (14.3) | 0.06 | ||
| Moderate persistent | 85 (30.9) | 25 (24.8) | 53 (33.1) | 7 (50) | 0.103 | ||
| Severe persistent | 33 (12) | 9 (8.9) | 21 (13.1) | 3 (21.4) | 0.32 | ||
| BMI Category | Underweight | 13 (4.7) | 6 (5.9) | 7 (4.4) | 0 | 0.57 | |
| Normal weight | 74 (26.9) | 36 (35.6) | 34 (21.3) | 4 (28.5) | 0.038* | ||
| Overweight | 52 (18.9) | 14 (13.9) | 36 (22.5) | 2 (14.3) | 0.2 | ||
| Obese | 136 (49.5) | 45 (44.6) | 83 (51.9) | 8 (57.1) | 0.96 | ||
| Scale/ordinal variables | COVID-19 severity | ||||||
| Pearson/Spearman correlation | P-value | ||||||
| BMI category | 0.12 | 0.042 | |||||
| Severity of Asthma | 0.165 | 0.006 | |||||
| Age | 0.39 | <0.001 | |||||
| Weight | 0.209 | <0.001 | |||||
| Height | 0.156 | 0.009 | |||||
Significant.
Ordinal logistic regression
After adjusting for asthma severity in the ordinal logistic regression (Table 3 ), none of the variables were significantly associated with disease severity. The use of the ICS/LABA combination (adj OR: 0.72 [0.15,1.2]; P = 0.021), being hypertensive (adj OR: 0.98 [0.28,1.6]; P = 0.006), having cancer (adj OR: 1.49 [0.12, 2.8]; P = 0.033), or having diabetes (adj OR: 0.75 [0.09, 1.4]; P = 0.024) could not increase the risk for more severe disease.
Table 3.
Ordinal logistic regression modeling of COVID-19 severity versus significantly associated variables.
| Patient variables |
Disease severity |
|||
|---|---|---|---|---|
| All (n = 275) n (%) | Hazard ratio (CI) | P-value | ||
| ICS use | Reference: ICS users | 0.37 (−0.36,1.12) | 0.317 | |
| Combo (ICS + LABA) vs. Mono (ICS only) | Budesonide/Formoterol | 135 (49.1) | 3.9 (2.3, 5.4) | <0.001* |
| OR | ||||
| Fluticasone/salmeterol combinations (reference: no ICS/LABA) | ||||
| Comorbidities | Hypertension (reference: no hypertension) | 64 (23.3) | 0.72 (0.15, 1.2) | 0.021* |
| Malignancy (reference: no malignancy) | 8 (2.9) | 0.98 (0.28, 1.6) | 0.006* | |
| Diabetes (reference: no diabetes) | 72 (26.2) | 1.49 (0.12, 2.8) | 0.033* | |
Significant.
Stratified analysis of secondary outcomes by ICS use status
The COVID-19 related clinical outcomes of the sample were compared between ICS and non-ICS users (Table 4 ). The majority of the sample (n = 227, 82.5%) were home-isolated, compared to 33 (12%) admitted to the ward, and 14 (5.1%) admitted to the ICU. There was no significant difference in the care setting, based on ICS use. In total, 19 (6.9%) of the admitted group were ventilated, 9 used ICS with 10 non-ICS users (P = 0.56). No significant difference was observed in the type of ventilation support (i.e., invasive versus non-invasive) between the groups (P = 0.082). However, the mean days of ventilation were lower in the non-ICS users 2.25 (0.95) days compared with the ICS users 16.07 (18.74) (P = 0.006). The mode of ventilation at baseline and after three days were either PRVC/VC+, BiLevel, and BiPAP, with PRVC/VC+ mostly used at baseline, 8 (2.9%), and after 3 days, 10 (3.6%), which was not statistically significant for the two groups. The change in the type of ventilation mode between days 0 and 3 was different between ICS and non-ICS users. No change in the three ventilation modes was observed in the ICS users, but subsequently a significant change to BIPAP occurred after 3 days of ventilation (P < 0.0001) (Table 5 ). Oxygen use was similar between the two groups (P = 0.74). The oxygen devices used included NC (n = 7, 2.5%), and HFNC (n = 7, 2.5%) (P = 0.704), also similar in the two groups. The majority of the hospitalized group were ICS users, yet this finding was not statistically significant (P = 0.65). The mean LOS was comparable between the two groups (P = 0.43). Of the 19 (6.9%) patients admitted to the ICU, 14 (73.7%) were ICS users compared to 5 (6.5%) non-ICS users (P = 0.78). The mean ICU LOS was slightly higher in the ICS users (n = 11.9, 9.4), compared to the non-ICS users (n = 7.8, 5.3) (P = 0.43). ARDS (n = 6, 2.2%), and AKI (n = 6, 2.2%) were the most frequent complications, followed by septic shock (n = 5, 1.8%); however, no statistically significant difference in complications between ICS and non-ICS users was noted. The mortality rate was 9 (3.3%) and the majority were ICS users (n = 8, 4%) (P = 0.45).
Table 4.
Clinical outcomes of the sample differentiated by the ICS use status (N,%) unless otherwise stated.
| Clinical outcomes | All patients (n = 275) | ICS users (n = 198) | Non-ICS users (n = 77) | P-value | |
|---|---|---|---|---|---|
| Care setting | Home Isolation | 227 (82.5) | 165 (83.3) | 62 (80.5) | 0.58 |
| Ward-Based | 33 (12) | 20 (10.1) | 13 (16.9) | 0.12 | |
| High dependency unit | 0 | 0 | 0 | – | |
| Intensive care unit | 14 (5.1) | 13 (6.6) | 1 (1.3) | 0.12 | |
| Ventilated Patients | 19 (6.9) | 15 (7.6) | 4 (5.2) | 0.56 | |
| Type of ventilatory support | Invasive | 9 (3.3) | 9 (4.5) | 0 | 0.082 |
| Non-invasive | 9 (3.3) | 5 (2.5) | 4 (5.2) | ||
| Days of ventilation: mean (SD) | 18 (6.5) | 16.07 (18.74) | 2.25 (0.95) | 0.006* | |
| Oxygenated Patients | 14 (5.1) | 8 (4) | 6 (7.8) | 0.74 | |
| Oxygen Device | NC | 7 (2.5) | 4 (2) | 3 (3.9) | 0.704 |
| HFNC | 7 (2.5) | 4 (2) | 3 (3.9) | ||
| Other oxygen devices | 0 | 0 | 0 | ||
| Hospital Course | Hospital admitted patients | 49 (17.8) | 34 (17.2) | 15 (19.5) | 0.65 |
| Hospital length of stay | 49 (17.8) | 9.97 (8.2) | 10.5 (15.8) | 0.43 | |
| ICU admitted patients | 19 (6.9) | 14 (7.1) | 5 (6.5) | 0.78 | |
| ICU length of stay | 19 (6.9) | 11.9 (9.4) | 7.8 (5.3) | 0.43 | |
| COVID-19 Severity | Asymptomatic | 101 (36.7) | 66 (33.3) | 35 (45.5) | 0.061 |
| Mild-moderate | 160 (58.2) | 120 (60.6) | 40 (52) | 0.19 | |
| Severe- critical | 14 (5.1) | 12 (6.1) | 2 (2.6) | 0.36 | |
| COVID-19 Complications | ARDS | 6 (2.2) | 6 (3) | 0 | 0.19 |
| AKI | 6 (2.2) | 5 (2.5) | 1 (1.3) | 0.46 | |
| Septic Shock | 5 (1.8) | 4 (2) | 1 (1.3) | 0.56 | |
| Case fatality rate | 9 (3.3) | 8 (4) | 1 (1.3) | 0.45 | |
Table 5.
The change in the type of ventilation device for ICS and non-ICS users on days 0 and 3.
| Mode of ventilation | ICS users |
Non-ICS users |
||||
|---|---|---|---|---|---|---|
| Day 0 | Day 3 | P-value | Day 0 | Day 3 | P-value | |
| PRVC/VC+ | 8 (4%) | 10 (5.1%) | 0.5 | 0 | 0 | – |
| Bilevel | 1 (0.5%) | 1 (0.5%) | 0.99 | 0 | 0 | – |
| BiPAP | 5 (2.5%) | 3 (1.5%) | 0.5 | 3 (3.9%) | 0 | <0.0001 |
| Other | 0 | 0 | – | 0 | 0 | – |
Discussion
The prevalence of asthma in patients with COVID-19 is 5.6% in Italy [23], 5.2% in Spain [24], 14% in the UK [25], and 17% in the USA [26]. Similar findings were reported for the general population in these countries [27,28]. In an analysis of 641 COVID-19-positive patients, Zhu et al. reported an increased disease severity in asthmatic patients (OR = 1.39; 95% CI 1.13–1.71) [29], and similar findings were reported by Mendy et al. [30]. The current study aimed to assess the variation in the COVID-19 disease severity based on the baseline clinical features of asthmatic patients and medication use, including ICS. The clinical course of COVID-19 was also compared between ICS and non-ICS users in asthma patients in Saudi Arabia.
Most of the sample had mild-moderate disease (n = 160, 58.2%), followed by 101 (36.7%) symptomatic cases, and 14 (5.1%) severe-critical cases. The majority (n = 198, 72%) were prescribed ICS therapy. No significant difference in COVID-19 severity was observed between the ICS and non-ICS users (P = 0.12). Nonetheless, the use of inhaled budesonide for non-asthmatic out-patients enrolled in the PRINCIPLE study, who were at higher risk of complications, improved the time to recovery with a concurrent reduction of hospital admissions and mortality [31]. Similarly, the use of ICS was markedly lower among patients requiring hospitalization due to COVID-19 in Izquierdo et al.'s study; that is, suggesting a possible protective role [32]. On the flip side, according to Sen et al. [20], the use of ICS in COPD patients did not significantly affect COVID-19 related clinical outcomes (i.e., hospitalization, ICU admission, need for mechanical ventilation, and mortality).
Likewise, the use of other asthma medications, such as β2 adrenergic agonists (salbutamol), and anticholinergics (ipratropium and tiotropium) in the current study was not associated with COVID-19 severity (P = 0.75 and P = 0.53, respectively). Mahdanivia et al. also reported that bronchodilators did not alter the intubation-time, suggesting a lack of benefit [33]. The use of bronchodilators, such as nebulized albuterol, as instructed by some ICU protocols should be questioned, and their use in the targeted intubated COVID-19 patients should be assessed.
However, the use of such bronchodilators shall be continued in case of exacerbations triggered by COVID-19. In fact, there is no current evidence as to where the continued ICS administration results in adverse or beneficial outcomes in COVID-19. Furthermore, no clear data are currently available to recommend a change in ICS dosing in case of exacerbation [32].
On the other hand, the use of the ICS/LABA combination was significantly associated with a more severe-critical disease presentation but not after adjustment for asthma severity (adj OR: 0.72 [0.15, 1.2], P = 0.021). This may be in part related to the fact that patients prescribed combination therapy, tend to have more severe asthma and they are susceptible to more severe COVID-19 (Spearman: 0.165, P = 0.006).
It is worth mentioning that more severe disease was experienced by smokers in this study (P = 0.015). Similarly, in Lohia et al.’s cohort study, a higher ICU admission rate was observed in COVID-19 patients with preexisting respiratory diseases and a history of smoking (adj OR: 1.25 [1.01–1.55]; P = 0.03) [34]. Literature demonstrated a crude association between smoking and developing worse clinical outcomes when infected with COVID-19 [[35], [36], [37]].
Patients presenting with comorbidities had a worse disease prognosis, especially renal failure (P = 0.03), non-asthmatic pulmonary diseases (P = 0.006), cardiovascular diseases (P = 0.003), hypertension (P < 0.001), diabetes (P < 0.001), and malignancy (P = 0.008). Nevertheless, after adjusting for asthma severity none of them were found to impact COVID-19 severity. Feng et al. also reported more comorbidities in severe-critical cases, especially diabetes (35.7% vs. 20.7%; P = 0.05) and hypertension [6]. Hypertension, diabetes, and coronary artery disease are the most frequently identified comorbidities in COVID-19 patients, and are associated with a more complicated and severe disease prognosis [3,[38], [39], [40], [41], [42]]. In contrast, having a normal weight is associated with an asymptomatic disease manifestation as supported by the current study (P = 0.038). Remarkably, immunocompromised patients did not have a significantly different disease severity (P = 0.58), contrary to our expectations.
Although only asymptomatic and mild to moderate cases were using ACE inhibitors, the severity of the COVID-19 variability was similar in patients taking ACE inhibitors compared to other patients (P = 0.15), which rules out any protective or predisposing role of ICS. However, Feng et al. reported that more patients were on ACE inhibitors in the moderate group compared to the severe group [6]. This suggests that ICS may protect asthmatic patients from developing more severe disease.
There was no difference in the hospital admission rate based on ICS-use status (P = 0.65) and the hospital LOS (P = 0.43). Similarly, the risk of hospitalization was not altered with the ongoing use of ICS (RR: 1.39; [0.90–2.15]) as reported by Chhiba et al. [43]. Three other studies [22,33,44] investigated the LOS in asthmatic patients, and none found a prolonged hospital LOS in this group of patients. However, Feng et al. found a longer LOS in severe-critical COVID-19 patients presenting with respiratory problems. In the current study, there was no difference in ICU admission between the two groups (P = 0.78), and the ICU LOS was comparable between the groups (P = 0.43). Two additional studies reported that the risk of ICU transfer were similar between the two groups [22,[43], [44], [45]]. In the current study, there was no significant difference in the rate of complications (i.e., ARDS, AKI, and septic shock), oxygenation, ventilation, type of oxygen device mode, and type of ventilation when ICS was used. However, the days of ventilation were significantly increased in ICS users (P = 0.006) and the improvement in ventilation mode after three days from baseline was evident in the non-ICS users (P < 0.0001). According to literature, asthma was significantly associated with a longer intubation time, with or without ICS therapy (P = 0.002) [33]. We posit that the duration of ventilation is more indicative of the disease prognosis than the hospital or ICU LOS.
According to Williamson et al. most comorbidities (i.e., cardiovascular diseases, diabetes, severe asthma, obesity, malignancy, kidney and liver diseases, and autoimmune conditions) were associated with an increased risk of COVID-19 related mortality. In the current study, the COVID-19 case fatality rate (CFR) was 3.3% in asthmatic patients. This rate is slightly higher than the CFR reported in the general population (2%) [2], however, it is lower than the mortality rate reported by Lohia et al. in patients with antecedent respiratory diseases (adj OR: 1.36 [1.08–1.72], P = 0.01) [35]. Notably, no difference was observed between ICS and non-ICS users (P = 0.45). According to Feng et al., critical patients had a higher mortality rate compared to severe or moderate cases [6].
Limitations
Several limitations of this study need to be addressed in further research. Firstly, although the baseline characteristics were varied, a limited number of patients from a single center was recruited which may affect the generalizability of the study. Another key limitation is that most patients had mild persistent asthma; and ICS and non-ICS users were not perfectly matched. Such variations may ultimately affect the representativeness of the sample. Additionally, the severity of the disease was not investigated in terms of the ICS dose and frequency. The data collected were based on previously written clinical notes which may be subject to information bias. For more accurate results, these findings should be validated with larger sample size in a prospective study setting, targeting post-COVID-19 respiratory sequelae and the effect of the ICS dose changes, in addition to evaluating other asthma medications which were minimally used in the current study.
Conclusions
Overall, more severe or critical cases occurred in patients using the ICS/LABA combination, as well as hypertensive and cancer patients. However, the ICS therapy had no effect on the severity of the COVID-19, disease complications, and mortality in asthmatic patients. The continued use of ICS during this pandemic should be encouraged to prevent asthma exacerbations.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interests
The authors have no conflicts of interest to declare concerning the research, authorship, and/or publication of this article.
Ethical approval
The study was approved by the Institutional Review Board of the King Abdullah International Medical Research Center (KAIMRC) (RC 20/504/R).
Appendix I
| Severity components | Intermittent | Mild persistent asthma | Moderate persistent asthma | Severe persistent asthma |
| Symptoms | Less than once a week | More than twice per week but not daily | Daily | Throughout the day |
| Nocturnal symptoms | Less than twice a day per month | Three-four times per month | More than once a week but not every night | Often every night per week |
| Interference with activity | Brief exacerbations | Exacerbations may cause minor limitation of activity and sleep | Exacerbations more than twice a week and may cause some limitation of activity and sleep | Frequent exacerbations with marked limitation of physical activity |
| SABA use | </=2 days per week | >2 days per week but not daily and not more than once on any day | Daily | Several times per day |
| Pulmonary function test | Normal FEV1 between exacerbations | FEV1 > 80% predicted | FEV1 > 60% but < 80% predicted | FEV1 < 60% predicted |
| FEV1 > 80% predicted | FEV1/PVC: normal | FEV1/PVC: reduced 5% | FEV1/PVC: reduced 5% | |
| FEV1/PVC: normal |
Adapted from the national heart, blood, and lung institute expert panel report 3 (EPR 3): guidelines for the diagnosis and management of asthma. NIH publications no.09-4051, 2007.
References
- 1.Mason R.J. Pathogenesis of COVID-19 from a cell biology perspective. Eur Respir J [Internet] 2020;55(4) doi: 10.1183/13993003.00607-2020. http://erj.ersjournals.com/lookup/doi/10.1183/13993003.00607-2020 Apr [cited 2021 Jul 3]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA [Internet] 2020;323(13):1239. doi: 10.1001/jama.2020.2648. https://jamanetwork.com/journals/jama/fullarticle/2762130 Apr 7 [cited 2021 Jul 3]. Available from: [DOI] [PubMed] [Google Scholar]
- 3.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet [Internet] 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. https://linkinghub.elsevier.com/retrieve/pii/S0140673620301835 Feb [cited 2021 Jul 4]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Archer S.L., Sharp W.W., Weir E.K. Differentiating COVID-19 pneumonia from acute respiratory distress syndrome and high altitude pulmonary edema: therapeutic implications. Circulation [Internet] 2020;142(2):101–104. doi: 10.1161/CIRCULATIONAHA.120.047915. https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.120.047915 Jul 14 [cited 2021 Jul 3]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X., Ma X. Acute respiratory failure in COVID-19: is it “typical” ARDS? Crit Care [Internet] 2020;24(1):198. doi: 10.1186/s13054-020-02911-9. https://ccforum.biomedcentral.com/articles/10.1186/s13054-020-02911-9 Dec [cited 2021 Jul 3]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng Y., Ling Y., Bai T., Xie Y., Huang J., Li J., et al. COVID-19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med [Internet] 2020;201(11):1380–1388. doi: 10.1164/rccm.202002-0445OC. https://www.atsjournals.org/doi/10.1164/rccm.202002-0445OC Jun 1 [cited 2021 Jul 3]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnston S.L. Overview of virus-induced airway disease. Proc Am Thorac Soc [Internet] 2005;2(2):150–156. doi: 10.1513/pats.200502-018AW. http://pats.atsjournals.org/cgi/doi/10.1513/pats.200502-018AW Aug 1 [cited 2021 Jul 3]. Available from: [DOI] [PubMed] [Google Scholar]
- 8.Akenroye A.T., Wood R., Keet C. Asthma, biologics, corticosteroids, and coronavirus disease 2019. Ann Allergy Asthma Immunol [Internet] 2020;125(1):12–13. doi: 10.1016/j.anai.2020.05.001. https://linkinghub.elsevier.com/retrieve/pii/S1081120620303173 Jul [cited 2021 Jul 4]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Youssef J., Novosad S.A., Winthrop K.L. Infection risk and safety of corticosteroid use. Rheum Dis Clin N Am [Internet] 2016;42(1):157–176. doi: 10.1016/j.rdc.2015.08.004. https://linkinghub.elsevier.com/retrieve/pii/S0889857X1500068X Feb [cited 2021 Jul 3]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ni W., Yang X., Yang D., Bao J., Li R., Xiao Y., et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care [Internet] 2020;24(1):422. doi: 10.1186/s13054-020-03120-0. https://ccforum.biomedcentral.com/articles/10.1186/s13054-020-03120-0 Dec [cited 2021 Jul 10]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horby P., Lim W.S., Emberson J., Mafham M., Bell J., Linsell L., et al. Effect of dexamethasone in hospitalized patients with COVID-19 – preliminary report [Internet] Infect Dis (except HIV/AIDS) 2020 http://medrxiv.org/lookup/doi/10.1101/2020.06.22.20137273 Jun [cited 2021 Jul 3]. Available from: [Google Scholar]
- 12.Ahmed M.H., Hassan A. Dexamethasone for the treatment of coronavirus disease (COVID-19): a review. SN Compr Clin Med [Internet] 2020;2(12):2637–2646. doi: 10.1007/s42399-020-00610-8. http://link.springer.com/10.1007/s42399-020-00610-8 Dec [cited 2021 Jul 10]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasan S.S., Capstick T., Zaidi S.T.R., Kow C.S., Merchant H.A. Use of corticosteroids in asthma and COPD patients with or without COVID-19. Respir Med [Internet] 2020;170 doi: 10.1016/j.rmed.2020.106045. https://linkinghub.elsevier.com/retrieve/pii/S0954611120301852 Aug [cited 2021 Jul 10]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morais-Almeida M., Pité H., Aguiar R., Ansotegui I., Bousquet J. Asthma and the coronavirus disease 2019 pandemic: a literature review. Int Arch Allergy Immunol [Internet] 2020;181(9):680–688. doi: 10.1159/000509057. https://www.karger.com/Article/FullText/509057 [cited 2021 Jul 10]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halpin D.M.G., Singh D., Hadfield R.M. Inhaled corticosteroids and COVID-19: a systematic review and clinical perspective. Eur Respir J [Internet] 2020;55(5) doi: 10.1183/13993003.01009-2020. http://erj.ersjournals.com/lookup/doi/10.1183/13993003.01009-2020 May [cited 2021 Jul 3]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wenzel S.E. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med [Internet] 2012;18(5):716–725. doi: 10.1038/nm.2678. http://www.nature.com/articles/nm.2678 May [cited 2021 Jul 3]. Available from: [DOI] [PubMed] [Google Scholar]
- 17.Al Ghobain M.O., Algazlan S.S., Oreibi T.M. Asthma prevalence among adults in Saudi Arabia. Saudi Med J [Internet] 2018;39(2):179–184. doi: 10.15537/smj.2018.2.20974. https://smj.org.sa/lookup/doi/10.15537/smj.2018.2.20974 Feb [cited 2021 Jul 5]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Y., Liu Y. Recent trends in current asthma prevalence among US adults, 2009-2018. J Allergy Clin Immunol Pract [Internet] 2020;8(8):2814–2816. doi: 10.1016/j.jaip.2020.04.041. https://linkinghub.elsevier.com/retrieve/pii/S2213219820303986 Sep [cited 2021 Jul 5]. Available from: [DOI] [PubMed] [Google Scholar]
- 19.Juniper E.F., Kline P.A., Vanzieleghem M.A., Ramsdale E.H., O’Byrne P.M., Hargreave F.E. Long-term effects of budesonide on airway responsiveness and clinical asthma severity in inhaled steroid-dependent asthmatics. Eur Respir J. 1990;3(Nov (10)):1122–1127. [PubMed] [Google Scholar]
- 20.Sen P., Majumdar U., Zein J., Hatipoğlu U., Attaway A.H. Inhaled corticosteroids do not adversely impact outcomes in COVID-19 positive patients with COPD: an analysis of Cleveland Clinic’s COVID-19 registry. Loukides S, editor. PLOS ONE [Internet] 2021;16(6) doi: 10.1371/journal.pone.0252576. https://dx.plos.org/10.1371/journal.pone.0252576 Jun 3 [cited 2021 Jul 5]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schultze A., Walker A.J., MacKenna B., Morton C.E., Bhaskaran K., Brown J.P., et al. Risk of COVID-19-related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: an observational cohort study using the OpenSAFELY platform. Lancet Respir Med [Internet] 2020;8(11):1106–1120. doi: 10.1016/S2213-2600(20)30415-X. https://linkinghub.elsevier.com/retrieve/pii/S221326002030415X Nov [cited 2021 Jul 6]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grandbastien M., Piotin A., Godet J., Abessolo-Amougou I., Ederlé C., Enache I., et al. SARS-CoV-2 pneumonia in hospitalized asthmatic patients did not induce severe exacerbation. J Allergy Clin Immunol Pract [Internet] 2020;8(8):2600–2607. doi: 10.1016/j.jaip.2020.06.032. https://linkinghub.elsevier.com/retrieve/pii/S221321982030667X Sep [cited 2021 Jul 5]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guerriero M., Caminati M., Viegi G., Senna G., Pomari C. Prevalence and features of asthma–chronic obstructive pulmonary disease overlap in Northern Italy general population. J Asthma [Internet] 2019;56(1):27–33. doi: 10.1080/02770903.2018.1424190. https://www.tandfonline.com/doi/full/10.1080/02770903.2018.1424190 Jan 2 [cited 2021 Jul 6]. Available from: [DOI] [PubMed] [Google Scholar]
- 24.Borobia A., Carcas A., Arnalich F., Álvarez-Sala R., Monserrat-Villatoro J., Quintana M., et al. A cohort of patients with COVID-19 in a major teaching hospital in Europe. J Clin Med [Internet] 2020;9(6):1733. doi: 10.3390/jcm9061733. https://www.mdpi.com/2077-0383/9/6/1733 Jun 4 [cited 2021 Jul 6]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Docherty A., Harrison E., Green C., Hardwick H., Pius R., Norman L., et al. Features of 16,749 hospitalised UK patients with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol [Internet] Infect Dis (except HIV/AIDS) 2020 doi: 10.1136/bmj.m1985. http://medrxiv.org/lookup/doi/10.1101/2020.04.23.20076042 Apr [cited 2021 Jul 6]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.COVID-FJD TEAM, Barroso B., Valverde-Monge M., Cañas Jose A., Rodrigo-Muñoz J., Gonzalez-Cano B., et al. Prevalence, characteristics, and outcome of asthmatic patients with type 2 diseases in hospitalized patients with COVID-19 in Madrid, Spain. J Investig Allergol Clin Immunol [Internet] 2020;30(5):382–384. doi: 10.18176/jiaci.0627. http://www.jiaci.org/summary/vol30-issue5-num2113 Oct 2 [cited 2021 Jul 6]. Available from: [DOI] [PubMed] [Google Scholar]
- 27.Jarvis D., Newson R., Janson C., Corsico A., Heinrich J., Anto J.M., et al. Prevalence of asthma-like symptoms with ageing. Thorax [Internet] 2018;73(1):37–48. doi: 10.1136/thoraxjnl-2016-209596. https://thorax.bmj.com/lookup/doi/10.1136/thoraxjnl-2016-209596 Jan [cited 2021 Jul 6]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urrutia I., Aguirre U., Sunyer J., Plana E., Muniozguren N., Martínez-Moratalla J., et al. Changes in the prevalence of asthma in the Spanish Cohort of the European Community Respiratory Health Survey (ECRHS-II) Arch Bronconeumol Engl Ed [Internet] 2007;43(8):425–430. doi: 10.1016/s1579-2129(07)60098-6. https://linkinghub.elsevier.com/retrieve/pii/S1579212907600986 Jan [cited 2021 Jul 6]. Available from: [DOI] [PubMed] [Google Scholar]
- 29.Zhu Z., Hasegawa K., Ma B., Fujiogi M., Camargo C.A., Liang L. Association of asthma and its genetic predisposition with the risk of severe COVID-19. J Allergy Clin Immunol [Internet] 2020;146(2):327–329.e4. doi: 10.1016/j.jaci.2020.06.001. https://linkinghub.elsevier.com/retrieve/pii/S009167492030806X Aug [cited 2021 Jul 6]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mendy A., Apewokin S., Wells A.A., Morrow A.L. Factors associated with hospitalization and disease severity in a racially and ethnically diverse population of COVID-19 patients [Internet] Epidemiology. 2020 http://medrxiv.org/lookup/doi/10.1101/2020.06.25.20137323 Jun [cited 2021 Jul 6]. Available from: [Google Scholar]
- 31.Dphil L., Bafadhel M., Dorward J., Hayward G., Saville B., Gbinigie O., et al. Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet. 2021;398(10303):843–855. doi: 10.1016/S0140-6736(21)01744-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Izquierdo J., Almonacid C., González Y., Del Rio-Bermudez C., Ancochea J., Cárdenas R., et al. The impact of COVID-19 on patients with asthma. Eur Respir J. 2021;57(3) doi: 10.1183/13993003.03142-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahdavinia M., Foster K.J., Jauregui E., Moore D., Adnan D., Andy-Nweye A.B., et al. Asthma prolongs intubation in COVID-19. J Allergy Clin Immunol Pract [Internet] 2020;8(7):2388–2391. doi: 10.1016/j.jaip.2020.05.006. https://linkinghub.elsevier.com/retrieve/pii/S2213219820304761 Jul [cited 2021 Jul 5]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lohia P., Sreeram K., Nguyen P., Choudhary A., Khicher S., Yarandi H., et al. Preexisting respiratory diseases and clinical outcomes in COVID-19: a multihospital cohort study on predominantly African American population. Respir Res [Internet] 2021;22(1):37. doi: 10.1186/s12931-021-01647-6. https://respiratory-research.biomedcentral.com/articles/10.1186/s12931-021-01647-6 Dec [cited 2021 Jul 5]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet [Internet] 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. https://linkinghub.elsevier.com/retrieve/pii/S0140673620305663 Mar [cited 2021 Jul 5]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med [Internet] 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. http://www.nejm.org/doi/10.1056/NEJMoa2002032 Apr 30 [cited 2021 Jul 5]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karanasos A., Aznaouridis K., Latsios G., Synetos A., Plitaria S., Tousoulis D., et al. Impact of smoking status on disease severity and mortality of hospitalized patients with COVID-19 infection: a systematic review and meta-analysis. Nicotine Tob Res [Internet] 2020;22(9):1657–1659. doi: 10.1093/ntr/ntaa107. https://academic.oup.com/ntr/article/22/9/1657/5860451 Aug 24 [cited 2021 Jul 5]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA [Internet] 2020;323(11):1061. doi: 10.1001/jama.2020.1585. https://jamanetwork.com/journals/jama/fullarticle/2761044 Mar 17 [cited 2021 Jul 5]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet [Internet] 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. https://linkinghub.elsevier.com/retrieve/pii/S0140673620302117 Feb [cited 2021 Jul 5]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu K., Fang Y.-Y., Deng Y., Liu W., Wang M.-F., Ma J.-P., et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) [Internet] 2020;133(9):1025–1031. doi: 10.1097/CM9.0000000000000744. https://journals.lww.com/10.1097/CM9.0000000000000744 May 5 [cited 2021 Jul 5]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu X.-W., Wu X.-X., Jiang X.-G., Xu K.-J., Ying L.-J., Ma C.-L., et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ [Internet] 2020 doi: 10.1136/bmj.m606. https://www.bmj.com/lookup/doi/10.1136/bmj.m606 Feb 19 [cited 2021 Jul 5]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guan W., Liang W., He J., Zhong N. Cardiovascular comorbidity and its impact on patients with COVID-19. Eur Respir J [Internet] 2020;55(6) doi: 10.1183/13993003.01227-2020. http://erj.ersjournals.com/lookup/doi/10.1183/13993003.01227-2020 Jun [cited 2021 Jul 5]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chhiba K.D., Patel G.B., Vu T.H.T., Chen M.M., Guo A., Kudlaty E., et al. Prevalence and characterization of asthma in hospitalized and nonhospitalized patients with COVID-19. J Allergy Clin Immunol [Internet] 2020;146(2):307–314.e4. doi: 10.1016/j.jaci.2020.06.010. https://linkinghub.elsevier.com/retrieve/pii/S009167492030840X Aug [cited 2021 Jul 5]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.COVID-FJD TEAM, Barroso B., Valverde-Monge M., Cañas Jose A., Rodrigo- Muñoz J., Gonzalez-Cano B., et al. Prevalence, characteristics, and outcome of asthmatic patients with type 2 diseases in hospitalized patients with COVID-19 in Madrid, Spain. J Investig Allergol Clin Immunol [Internet] 2020;30(5):382–384. doi: 10.18176/jiaci.0627. http://www.jiaci.org/summary/vol30-issue5-num2113 Oct 2 [cited 2021 Jul 6]. Available from: [DOI] [PubMed] [Google Scholar]
- 45.Lieberman-Cribbin W., Rapp J., Alpert N., Tuminello S., Taioli E. The impact of asthma on mortality in patients with COVID-19. Chest [Internet] 2020;158(6):2290–2291. doi: 10.1016/j.chest.2020.05.575. https://linkinghub.elsevier.com/retrieve/pii/S0012369220316457 Dec [cited 2021 Jul 6]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]