Abstract
Background and objectives:
Neoadjuvant chemotherapy (NAT) for pancreatic adenocarcinoma (PDAC) is increasingly being utilized. However, a significant number of patients will experience early recurrence, possibly negating the benefit of surgery. We aimed to identify factors implicated in early disease recurrence.
Methods:
A retrospective review of pancreaticoduodenectomies performed between 2005–2017 at our institution for PDAC following NAT was performed. A 6-month cut-off was used to stratify patients into early/late recurrence groups. Multivariate analysis was performed to identify predictors of recurrence.
Results:
Of 273 patients, 64 (23%) developed early recurrence or died within 90-days of surgery. The median time to recurrence was 4 months (95% CI 2.2–4.3) in the early group vs 16 months (95% CI 13.7–19.9) in the late group. The former had higher baseline and post-NAT Ca19–9 levels than the latter (472 vs 153 IU/ml, p=0.001 and 71 vs 39 IU/ml, p=0.005, respectively). A higher positive lymph node ratio significantly increased the risk of early recurrence (hazard ratio (HR):15.9, p<0.001) while adjuvant chemotherapy was protective (HR:0.4, p<0.001).
Conclusions:
Our findings acknowledge the limitations of clinically measured factors used to ascertain response to NAT and underline the need for individualized molecular markers that take into consideration the specific tumor biology.
Keywords: Pancreatic cancer, neoadjuvant chemotherapy, early recurrence, pancreaticoduodenectomy
INTRODUCTION
Despite advances in multimodal therapy, pancreatic ductal adenocarcinoma (PDAC) remains an aggressive disease with a 5-year overall survival (OS) rate approaching 10% [1,2]. Upfront surgical resection followed by adjuvant chemotherapy has long been the standard of care for patients with localized PDAC; recent clinical trials have demonstrated significant improvement in OS rates with adjuvant treatment following curative-intent surgical resection [3–5]. However, a significant proportion of patients are unable to initiate/complete adjuvant therapy either due to a decline in performance status, postoperative complications, or evidence of early disease recurrence [6,7].
In contrast to adjuvant chemotherapy, neoadjuvant chemotherapy (NAT) not only guarantees delivery of systemic therapy, but also increases the probability of R0 resection and decreases the incidence of lymph node metastatic disease [8–11]. Similarly, NAT offers several key theoretical advantages, including early treatment of occult micro-metastatic disease, in vivo assessment of tumor response, and proper patient selection based on appropriate tumor biology [12,13]. Currently, both the American Society of Clinical Oncology (ASCO) [14] and the National Comprehensive Cancer Network (NCCN) guidelines [15] endorse NAT for borderline resectable (BR) disease and support the use of either upfront resection or NAT for resectable (R) PDAC.
In a significant proportion of patients with R and BR PDAC following NAT and curative-intent resection, early recurrence is often encountered, possibly obviating the benefit of surgical therapy. The primary aim of this work was to determine the factors associated with an increased risk of early recurrence for this subcategory of patients. The secondary aim was to discern whether a difference in the patterns of recurrence or complications exist between patients who experience early recurrence following surgical therapy as opposed to the group who experience a delayed recurrence.
MATERIALS AND METHODS
Patient population and data collection
A retrospective review of a prospectively maintained database was performed following approval from the Institutional Review Board at the University of Pittsburgh (STUDY #19020338). Included patients had R and BR PDAC, as per NCCN guidelines, and were treated with NAT followed by curative-intent surgical resection at the University of Pittsburgh Medical Center between 2005–2017 using selection criteria described previously [16]. Patients with metastatic disease and non-pancreaticoduodenectomy resections were excluded. Stratification was then performed based on the timing of recurrence into the early (within 6 months following surgery [17]) and late (>6 months) recurrence groups. It is our practice to obtain surveillance imaging within 6-months from the day of surgery, therefore the choice of this temporal cut-off ensured that nearly all patients included in this series had at least one postoperative surveillance imaging within the first postoperative semester, making it a reliable time point.
Demographics, pathologic variables, and clinical outcomes for the study cohort were obtained through review of the institutional electronic medical record system. The performance status of the study cohort was determined by the Charlson Comorbidity Index (CCI) [18] and the American Society of Anesthesiology (ASA) classification. The stage at diagnosis and resectability status were determined utilizing both computed tomography (CT) scans and endoscopic ultrasound (EUS). Values for carcinoembryonic antigen 19–9 (Ca19–9) levels that were associated with a normal total bilirubin level (<2mg/dl) were collected at the time of diagnosis and post-completion of NAT. Pathologic variables retrieved included treatment response (stratified into none/poor, mild-to-moderate and near-complete/complete response), grade of differentiation, lymphovascular invasion, perineural invasion, and AJCC 8th edition pathologic stage [19] margins ≤1 mm were classified as positive residual margins. The positive lymph node ratio-which corresponds to the number of positive lymph nodes divided by the total number of lymph nodes harvested-was calculated and compared between the two study groups.
Postoperative complications were assessed utilizing the Clavien-Dindo score for surgical complications [20] and tabulated up to one year after the index operation, with the exception of pancreatic leak, bile leak, and surgical site infections, which were defined per the timelines set by the International Study Group of Pancreatic Fistula [21], International Study Group of Liver Surgery [22], and Centers for Disease Control and Prevention [23], respectively. Recurrence was diagnosed on routine surveillance cross-sectional imaging and classified as local, single distant-organ, multiple distant organs, or frank carcinomatosis and only occasionally was recurrence confirmed by histopathology. The last recorded visit at our institution was used as the date of last follow-up.
Study endpoints
The primary endpoint was to identify predictors of early disease recurrence following NAT and pancreaticoduodenectomy. Secondary aims were to delineate the patterns of recurrence, complications, overall survival (OS, defined from the time of surgery to the date of death or last recorded follow-up), and recurrence-free survival (RFS; defined from the time of surgery to the time of first identified recurrence) between the two study groups.
Statistical analysis
Descriptive statistics were used to summarize baseline patient characteristics and clinico-pathologic variables; continuous variables are presented using median and interquartile range (IQR), while categorical variables are presented as raw numbers with corresponding percentages. Differences between categorical variables were analyzed using either Chi-square or Fisher’s exact test. Differences between continuous variables that were normally distributed were analyzed using two-tailed Student’s t-test, while the Wilcoxon rank-sum test was used for continuous variables that were not normally distributed. To accurately discerns differences between the study groups, a time to “event” analysis was used where event was defined as either recurrence, death, or last follow-up (censored observations).
Survival was characterized using Kaplan-Meier estimates and log-rank tests. A multivariable Cox-proportional hazard regression model was used to identify independent predictors of early recurrence/death following NAT and curative-intent surgical resection. All clinico-pathological factors that were examined in univariate analysis with p≤0.2 were considered for entry into multivariate modeling and were selected for the final multivariate model based on a backward stepwise selection method. Variables included in these models were ASA class, size of tumor at diagnosis on CT scan, vascular involvement, pathologic treatment response, adjuvant treatment receipt, positive lymph node to total lymph nodes harvested ratio, 90-day readmission, and complications. All inferential testing was conducted using the entire cohort except when building models that involved Ca19–9 as an independent variable. Cases without a validated Ca19–9 value-due to non-secretor status (<37U/ml)-were excluded. The time to “event” in the early cohort included either death or documented recurrence within 6 months of resection. Sensitivity analysis was performed after excluding 90-day mortality (patients who died without definitive evidence of recurrence) to accurately discern significant predictors for early recurrence and compare the RFS and OS across the two groups. All statistical tests were two-sided with an α (type I) error of 0.05 and performed using STATA 13 (StataCorp LP, College Station, TX, USA).
RESULTS
Study population and preoperative variables
A total of 273 patients with R and BR PDAC who received NAT followed by surgical resection were included, of which 64 (23%) patients experienced recurrence or death within 6 months of surgery. The median age at diagnosis was 65 years and 137 (50%) were females (Table 1). There were no significant differences in the baseline demographic and preoperative variables between the two groups. BR lesions were predominant in both cohorts at the time of diagnosis as indicated by a variable degree of abutment/involvement of the portal venous-superior mesenteric vein confluence in 155 (57%) patients. However, the early recurrence cohort had a significantly larger median tumor size on the pre-NAT CT scan than the late recurrence group (3.1 vs 2.8 cm, p=0.002). Similarly, the median CA19–9 levels both pre-and post-completion of NAT were significantly higher in the early recurrence group (472 vs 153 IU/ml, p=0.001 and 71 vs 39, p=0.005) (Table 1). Gemcitabine-based therapy was the predominant systemic therapy utilized in the neoadjuvant setting for both cohorts (73% vs 79%, p=0.531). There were no significant differences in the number of NAT cycles administered and receipt of neoadjuvant radiation therapy (p=0.719 and p=0.201, respectively).
Table 1.
Baseline demographic and preoperative variables in R and BR PDAC patients who received NAT and underwent curative-intent pancreaticoduodenectomy.
| Variable | Overall cohort (n = 273) |
Event ≤6 months (n = 64) |
Event >6 month/no recurrence (n = 209) |
P-value |
|---|---|---|---|---|
| Age, yrs | 65 (58–72) | 65 (55–73) | 66 (59–71) | 0.259 |
| Male gender | 136 (50) | 35(55) | 101 (48) | 0.373 |
| White race | 263 (96) | 63 (98) | 200 (96) | 0.461 |
| BMI, kg/m2 | 26 (23–30) | 26 (23–30) | 27 (23–30) | 0.339 |
| ASA class | ||||
| 1 | 1 (0.4) | 0 (0) | 1 (0.5) | |
| 2 | 33 (13) | 2 (3) | 31 (15) | 0.080 |
| 3 | 214 (81) | 56 (90) | 158 (78) | |
| 4 | 16 (6) | 4 (7) | 12 (6) | |
| CCI (age-adjusted) | 5(4–5) | 4(4–5) | 5(4–6) | 0.347 |
| Radiologic stage at diagnosis | ||||
| Resectable | 78 (29) | 62 (30) | 16 (25) | 0.470 |
| Borderline resectable | 195 (71) | 147 (70) | 48 (75) | |
| EUS size, cm | 2.8 (2.3–3.4) | 3 (2.5–3.5) | 2.8 (2.3–3.4) | 0.135 |
| CT size, cm | 2.9 (2.2–3.5) | 3.2 (2.7–3.8) | 2.8 (2.1–3.3) | 0.002 |
| Vessel involvement | 201 (73.9) | 49 (76.6) | 152 (73.1) | 0.579 |
| None | 71 (26.1) | 15 (23.4) | 56 (26.9) | |
| Venous | 155 (57) | 33 (51.6) | 122 (58.7) | 0.157 |
| Arterial | 10 (3.7) | 2 (3.1) | 8 (3.9) | |
| Venous and arterial | 36 (13.2) | 14 (21.9) | 22 (10.6) | |
| Type of NAT | ||||
| Gemcitabine based | 211 (77) | 47 (73) | 164 (79) | |
| 5-FU based | 45 (17) | 11 (17) | 34 (16) | 0.531 |
| Crossover | 13 (4.8) | 5 (7.8) | 8 (3.8) | |
| Other | 4 (1.5) | 1 (1.6) | 3 (1.4) | |
| Number of NAT cycles | 3 (2–4) | 3 (2–5) | 3 (2–4) | 0.719 |
| Neoadjuvant radiotherapy | 101 (37) | 28 (44) | 73 (35) | 0.201 |
| CA19–9 level, U/ml | ||||
| Pre-NAT | 201 (54–626) | 472 (75–1469) | 153 (49–413) | 0.001 |
| Post-NAT | 43 (15–136) | 71 (20–320) | 39 (14–100) | 0.005 |
Variables are presented as medians (interquartile range) and raw numbers (percentage). BMI: body mass index, ASA: American Society of Anesthesiology score, CCI: Charlson-comorbidity index, EUS: endoscopic ultrasound, CT: computed tomography, NAT: neoadjuvant treatment
Surgical variables and histopathology
Open pancreaticoduodenectomy was the predominant surgical approach performed in 168 (62%) patients, while robotic pancreaticoduodenectomy was performed in 105 (39%) patients (Table 2). Patients in the early recurrence group were found to have a significantly higher median intraoperative blood loss than the late recurrence group (400 ml vs 300 ml, p=0.007). On histopathology, there were no significant difference in the T stage, N stage, lymph node ratio (LNR), residual positive margins, or pathologic response to NAT. However, the early recurrence group was characterized by a significantly higher incidence of lymphovascular invasion than patients with late recurrence (81% vs 67%, p=0.032).
Table 2.
Operative, pathologic, and outcome variables in R and BR PDAC patients who received NAT and underwent curative-intent pancreaticoduodenectomy.
| Variable | Overall cohort (n = 273) |
Recurrence ≤6 months (n = 64) |
Recurrence >6 month/no recurrence (n = 209) |
P-value |
|---|---|---|---|---|
| Robotic approach | 105 (39) | 27 (42) | 78 (37) | 0.484 |
| EBL, mL | 300 (175–300) | 400 (300–750) | 300 (150–550) | 0.007 |
| T stage | ||||
| 0 | 4 (1.5) | 0 (0) | 4 (1.9) | |
| 1 | 94 (34) | 21 (33) | 73 (35) | |
| 2 | 150 (55) | 33 (52) | 117 (60) | 0.217 |
| 3 | 24 (9) | 10 (16) | 14 (7) | |
| 4 | 1 (0.4) | 0 (0) | 1 (0.5) | |
| Pathologic tumor size, cm | 2.5 (2–3.3) | 3 (2–3.5) | 2.5 (2–3) | 0.106 |
| N stage | ||||
| 0 | 96 (35) | 19 (30) | 77 (37) | |
| 1 | 101 (37) | 25 (39) | 76 (36) | 0.544 |
| 2 | 76 (28) | 20 (31) | 56 (27) | |
| Total number of LN harvested | 27 (20–36) | 26 (18–35) | 28 (20–37) | 0.307 |
| Number of positive LN | 1 (0–4) | 2 (0–5) | 1 (0–4) | 0.177 |
| Ratio of positive LN | 0.05 (0–0.14) | 0.08 (0–0.21) | 0.04 (0–0.14) | 0.058 |
| LVI | 186 (70) | 51 (81) | 135 (67) | 0.032 |
| PNI | 222 (82) | 56 (90) | 166 (80) | 0.057 |
| Positive margins | 130 (48) | 35 (55) | 95 (46) | 0.196 |
| Response to NAT | ||||
| None/absent | 50 (22) | 14 (28) | 36 (20) | |
| Partial | 167 (73) | 36 (22) | 131 (74) | 0.495 |
| Near complete | 7 (3) | 1 (2.0) | 6 (3) | |
| Complete | 4 (1.8) | 0 (0) | 4 (2.3) | |
| 30-day mortality | 4 (1.5) | 4 (6) | 0 (0) | 0.003 |
| 90-day mortality | 10 (4) | 10 (16) | 0 (0) | <0.001 |
| Clavien-Dindo score ≥3 | 73 (26.7) | 27 (42) | 46 (22) | 0.001 |
| Adjuvant chemotherapy | 201 (75.6) | 31 (51) | 170 (83) | <0.001 |
| Gemcitabine based | 166 (83) | 23 (74) | 143 (85) | |
| 5-fluorouracil based | 27 (13.5) | 6 (19) | 21 (12) | 0.239 |
| Crossover | 7 (3.5) | 2 (6.5) | 5 (3.0) | |
| Number of Adjuvant cycles | 4 (3–6) | 3 (2–4) | 5 (4–6) | <0.001 |
| Total number of cycles (NAT + adjuvant) |
7 (4–9) | 4 (3–6) | 8 (5–9) | <0.001 |
| Recurrence | ||||
| Local recurrence | 65 (24) | 8 (16) | 57 (39) | |
| Carcinomatosis | 8 (3) | 3 (6) | 5 (34) | 0.004 |
| Single distant organ | 80 (30) | 22 (43) | 58 (40) | |
| Multiple sites | 43 (16) | 18 (35) | 25 (17) | |
Variables are presented as medians (interquartile range), raw numbers (percentage). EBL: estimated blood loss, LN: lymph nodes, LVI: lympho-vascular invasion, PNI: peri-neural invasion, NAT: neoadjuvant chemotherapy
Postoperative outcomes and recurrence patterns
Since 90-day mortality was included within the early recurrence group, it is not surprising that we found a significantly higher incidence of major complications as defined by a Clavien-Dindo score of >2 in the early recurrence group (42% vs 22%, p=0.001) (Table 2). However, direct comparison of all recorded complications between the two study arms (Supplementary Table 1) yielded no differences except in the incidence of anastomotic strictures, which were significantly more frequent in the early recurrence cohort (8% vs 1%, p=0.009). A total of seven patients developed benign anastomotic strictures: three at the gastrojejunostomy, three at the hepaticojejunostomy, and one at both anastomoses; no strictures developed at the pancreaticojejunostomy.
A total of 201 (76%) patients received adjuvant chemotherapy within a median of 64 days from surgery. Patients in the early recurrence groups were less likely to receive adjuvant chemotherapy than the late recurrence group (51% vs 83%, p<0.001); in patients who did receive adjuvant chemotherapy, the median number of cycles was significantly lower in the early recurrence compared to the late recurrence group (3 vs 5, p<0.001). The predominant regimen in patients who received adjuvant treatment was gemcitabine-based in both study groups (74% vs 85%, p=0.239).
The median duration of follow-up for the overall study cohort was 69 months (95% confidence interval (CI) 54.8–75.9). Of note, after excluding patients who died within 90-days from surgery and for whom no definitive evidence of recurrence was identified (n=10), a total of 189 out of 263 patients (72%) experienced a recurrence during this time. Among this cohort, the overall early-recurrence rate was 20% (54 of 263); while the remaining 145 patients (55%) recurred greater than six months from surgery. Early recurrence most commonly developed simultaneously at multiple distant sites when compared to late recurrence (35 vs 17%, p=0.004). On the other hand, patients in the late recurrence group were more likely to develop local recurrence (39 vs 16%, p=0.004) and carcinomatosis (34 vs 6%, p=0.004) compared to the early recurrence group. When we categorized recurrence patterns by site of recurrence (Figure 1), both lung and local recurrence developed predominately in the late recurrence group, while liver recurrence developed predominately in the early recurrence group.
Figure 1.
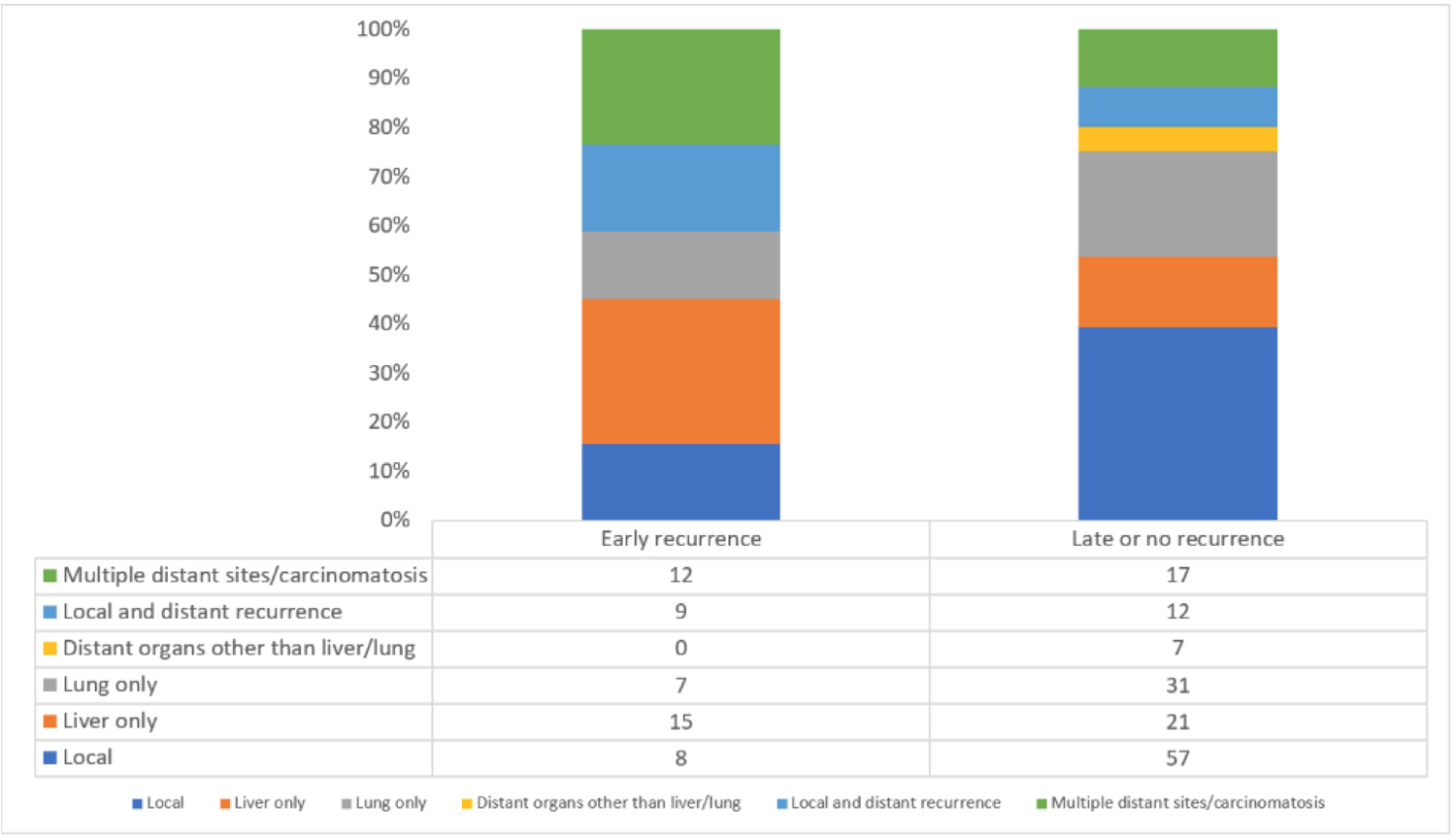
Patterns of early (≤6 months after resection) vs. late (>6 months after resection) recurrence in R and BR PDAC patients who received NAT followed by pancreaticoduodenectomy.
Survival outcomes and predictors of recurrence
To accurately assess survival outcomes and identify predictors of recurrence, sensitivity analysis was performed following exclusion of 90-day mortality (n=10). The median OS and RFS for the whole study cohort from the time of diagnosis was 29.0 months (95% CI 25.7–34.6) and 17.1 months (95% CI 15.4–19.5), respectively. As illustrated in Figure 2A, the median OS for the late recurrence group was significantly higher than the early recurrence group (38.1 months (95% CI 31.1–44.6) vs 14.2 months (95% CI 10.9–15.4), p<0.0001).
Figure 2.
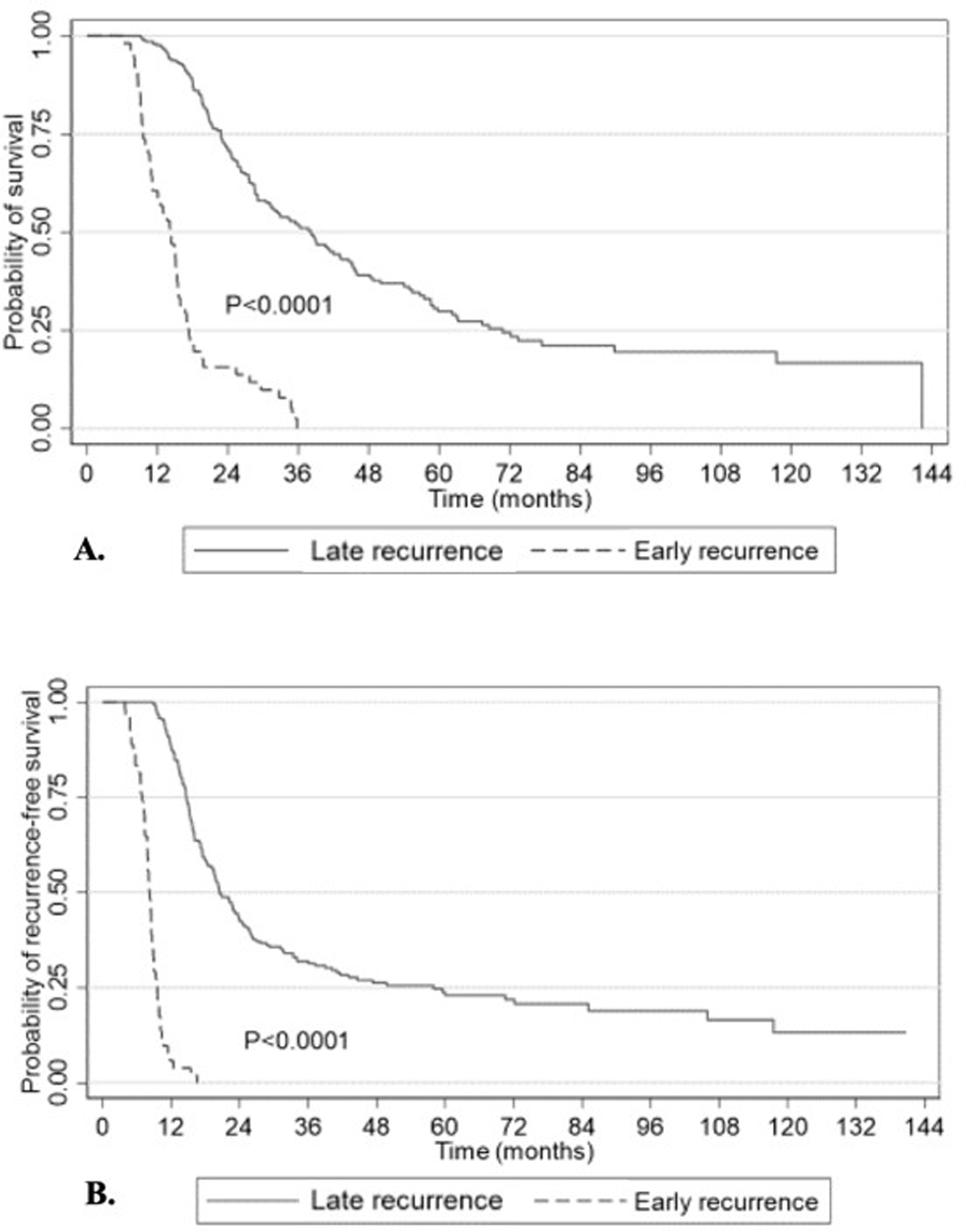
Overall (a) and progression-free (b) survival of R and BR PDAC patients after NAT and surgical resection.
The median time to recurrence for the early recurrence group was 3.7 months (95% CI 3.2–4.3) while it was 16.3 months (95% CI 13.7–19.9) for the late recurrence group. Expectedly, the median RFS from the time of diagnosis for the early recurrence group was significantly lower than the late recurrence group (8.2 months (95% CI 7.8–8.7) vs 20.4 months (95% CI 18.5–23.9), p<0.0001) (Figure 2B).
On Cox-proportional hazard regression, designed to identify predictors of early recurrence (Table 3), indicated that an increased ratio of positive lymph nodes to total harvested lymph nodes conferred a 16-fold increase in the risk of early recurrence (Hazard ratio (HR): 15.9, 95% CI 3.8–66.2, p<0.001) which represented the strongest overall predictor of early recurrence. Furthermore, readmission within 90 days (HR: 1.9, 95% CI 1.1–3.3, p=0.030) and anastomotic stricture (HR:6.0, 95% CI 1.8–20.3, p=.004) were also found to increase the risk of early recurrence, while adjuvant chemotherapy was protective (HR: 0.4, 95% CI .2–0.7, p=0.001). Tumor size measured by pre-NAT CT scan and ASA had no influence on the timing of recurrence.
Table 3.
Cox regression model for independent predictors of early recurrence in PDAC patients who received neoadjuvant therapy.
| Variable | Hazard ratio | 95% confidence interval | P-value |
|---|---|---|---|
| ASA | 1.8 | 0.8–3.9 | 0.127 |
| CT size | 1.0 | 1.0–1.1 | 0.024 |
| Arterial and venous involvement | 1.9 | 0.9–3.7 | 0.086 |
| Ratio of positive LN | 15.9 | 3.8–66.2 | <0.001 |
| Anastomotic stricture | 6.0 | 1.8–20.3 | 0.004 |
| Readmission within 90 days | 1.9 | 1.1–3.3 | 0.030 |
| Adjuvant chemotherapy | 0.4 | 0.2–0.7 | 0.001 |
DISCUSSION
In this retrospective review of patients with R and BR PDAC who received NAT followed by pancreaticoduodenectomy at a high-volume pancreaticobiliary center, we demonstrate that approximately 23% will experience rapid disease progression or succumb to early postoperative complications despite receipt of NAT. The median time to recurrence for the early recurrence group following pancreaticoduodenectomy was 3.7 months. The strongest predictor of early disease recurrence was an increase in the positive lymph node ratio; in contrast, administration of adjuvant chemotherapy was protective against early recurrence.
In a meta-analysis of prospective randomized controlled trials comparing NAT to surgery-first approach for R and BR PDAC by Cloyd et al [8], the authors demonstrated approximately a 30% improvement in the OS for the NAT group as opposed to the surgery-first group on an intent-to-treat basis. This effect was independent of the NAT protocol or the resectability status of PDAC (R vs BR). Furthermore, NAT increased the likelihood of margin-negative resection (Risk Ratio (RR):1.51, 95% CI 1.18–1.93) and lymph node negative disease (RR:1.51, 95% CI 1.18–1.93). In congruence to the aforementioned findings, the benefit of NAT over a surgery-first approach for potentially resectable PDAC has also been demonstrated in meta-analyses and systematic reviews of retrospective and non-randomized prospective studies [24–27], propensity-matched analysis from a national cancer database [28] and Markov decision models [29]. Yet, the utilization of NAT for the treatment of potentially resectable PDAC has not gained widespread popularity across the United States, potentially owing to the lack of prospective level I evidence [30]. Nevertheless, and due to mounting evidence supporting the use of NAT over a surgery-first approach, we aimed to analyze whether NAT followed by curative-intent pancreaticoduodenectomy influences the timing and/or patterns of disease recurrence in patients with R and BR PDAC.
In a secondary analysis of the European Study Group for Pancreatic Cancer (ESPAC)-4 randomized clinical trial [5], the patterns of recurrence in patients who received adjuvant gemcitabine or combination gemcitabine and capecitabine were evaluated [30]. The authors found that recurrence developed in 479 (66%) of 730 patients over a median follow-up of 43 months. The median time to recurrence was 12.7 months (95% CI 11.9–13.5); local recurrence occurred in a median of 13.6 months (95% CI 12.6–14.1) while distant-organ metastasis-predominantly diagnosed in the liver-developed after a median of 11.3 months (95% CI 10.4–12.6). In our study, the median RFS was 12.8 months (95% CI 10.6–14.0), similar to the results from ESPAC-4 [5,32]. Given that the median time to recurrence was comparable between our cohort and that of ESPAC-4, it appears that NAT affects neither the patterns nor the timing of recurrence. Furthermore, we found that the predominant site of early recurrence was the liver (Figure 1), while local recurrence and lung metastases developed after an extended median RFS. These findings corroborate with the findings of the ESPAC-4 secondary analysis [30] as well as previous retrospective studies from our [32] and other institutions [33,34].
The patterns of recurrence in our study cohort are also comparable to another single-institution retrospective study by Groot et al [34], in which the authors analyzed the patterns and predictors of recurrence following curative-intent resection of PDAC in patients who had not received NAT. Recurrence developed in the majority of their cohort (78%) over a median follow-up of 25.3 months. Again, the liver was the predominant site of first recurrence developing within 6.9 months (95% CI 4.9–8.9) of resection. In contrast, patients with lung and local recurrence had an extended RFS (18.6 and 14.6 months, respectively). Additionally, Groot et al. reported that a lymph node ratio >0.2 was associated with distant-organ recurrence (HR:1.93, 95% CI 1.6–2.3, p<0.001) while adjuvant chemotherapy was protective (HR:0.75, 95% CI 0.57–0.97, p=0.027). These steadfast patterns of recurrence are replicated in patient populations across multiple institutions and suggest a biology intrinsic to PDAC that would be intriguing to explore further.
Lymph node enlargement detected on either radiologic evaluation (CT or MRI) or on EUS has been suggested to be a potential marker of lymph node metastasis in PDAC patients with a 68% positive predictive value (PPV) and 43.1% negative predictive value (NPV). When limited to patients with biliary obstruction, the PPV of lymph node enlargement was found to be even higher at 84.2% [35]. Recent studies using nomograms based on radiographic features of contrast-enhanced CT scans demonstrate promising rates of positive lymph node identification in both the test and validation cohorts [36,37]. With similar advances in progress using abdominal MRIs [38] and based on the aforementioned implications of an elevated lymph node positive ratio on early disease recurrence demonstrated in our analysis, improving imaging modalities may facilitate more judicious patient selection for surgery following NAT.
Previous systematic reviews and meta-analyses of mainly observational studies that examined the impact of NAT on recurrence reported that NAT provides more effective local tumor control as opposed to upfront surgical resection [39,40]. However, NAT did not appear to influence the overall rate of distant or peritoneal metastasis. These studies included a heterogenous patient population and treatment modalities and therefore their results should be interpreted with caution. Nonetheless, in our study, local recurrence was seen predominantly in the late recurrence cohort, lending further evidence that NAT has some efficacy in controlling the local tumor burden for at least several months following surgery.
Our study has several limitations that are mainly inherent to its retrospective design. Although NAT is routinely administered for BR PDAC at our institution, 78 (29%) patients had R PDAC; as a result, there is a selection bias as the indications for NAT in the R PDAC group varied. Secondly, our results only represent a cross-section of the natural history of recurrent PDAC as only the first site of recurrence was documented while further disease progression was not accounted for in this analysis. Moreover, overestimation of the timing/location of recurrence should be assumed as the diagnoses of recurrence was mainly based on findings on surveillance cross-sectional imaging and histopathologic confirmation was seldom established. Despite these limitations however, our study adds significant support to the growing body of evidence on the effects of NAT on potentially-resectable PDAC.
CONCLUSIONS
We provide a large and homogeneous patient population from a major tertiary care referral center and describe select predictors of early disease recurrence and progression, including positive lymph node ratios and receipt of adjuvant chemotherapy. Although these are factors that are not always modifiable, future investigations can be built on our findings to improve existing surveillance and treatment strategies to identify and treat patients at risk for early recurrence.
Supplementary Material
Supplementary Table 1. Postoperative complications in R and BR PDAC patients who received NAT and underwent pancreaticoduodenectomy. CR-POPF: clinically relevant-postoperative pancreatic fistula, GJ: gastrojejunostomy, GIB: gastrointestinal bleed, DVT/PE: deep vein thrombosis/pulmonary embolism.
Synopsis:
Through a retrospective review of a large patient population from a high-volume tertiary care center, we identified select predictors of early disease recurrence following neoadjuvant therapy and pancreaticoduodenectomy for pancreatic adenocarcinoma.
Footnotes
Disclosures: none, financial or otherwise
Data Availability Statement:
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1).McGuigan A, Kelly P, Turkington R, et al. : Pancreatic cancer: a review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol 2018;24:4846–4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Siegel RL, Miller KD, Jemal A.: Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 3).Conroy T, Hammel P, Hebbar M, et al. : FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med 2018;379:2395–2406. [DOI] [PubMed] [Google Scholar]
- 4).Merkow RP, Bilimoria KY, Tomlinson JS, et al. : Postoperative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer. Ann Surg 2014;260:372–377. [DOI] [PubMed] [Google Scholar]
- 5).Neoptolemos JP, Palmer DH, Ghaneh P, et al. : Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): A multicentre, open-label, randomised, phase 3 trial. Lancet 2017;389:1011–1024. [DOI] [PubMed] [Google Scholar]
- 6).Mayo SC, Gilson MM, Herman JM, et al. : Management of patients with pancreatic adenocarcinoma: national trends in patient selection, operative management, and use of adjuvant therapy. J Am Coll Surg 2012;214:33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Bilimoria KY, Bentrem DJ, Ko CY, et al. : Multimodality therapy for pancreatic cancer in the U.S.: Utilization, outcomes, and the effect of hospital volume. Cancer 2007;110:1227–1234. [DOI] [PubMed] [Google Scholar]
- 8).Cloyd J, Heh V, Pawlik T, et al. : Neoadjuvant therapy for resectable and borderline resectable pancreatic cancer: a meta-analysis of randomized controlled trials. J Clin Med 2020; 9:1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Janssen Q, Buettner S, Suker M, et al. : Neoadjuvant FOLFIRINOX in patients with (borderline) resectable pancreatic cancer: A systematic review and patient-level meta-analysis. J Natl Cancer Inst 2019;111:782–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Sugimoto M, Takahashi N, Farnell MB, et al. : Survival benefit of neoadjuvant therapy in patients with non-metastatic pancreatic ductal adenocarcinoma: A propensity matching and intention-to-treat analysis. J Surg Oncol 2019;120:976–984. [DOI] [PubMed] [Google Scholar]
- 11).Motoi F, Kosuge T, Ueno H, et al. : Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP-05). Jpn J Clin Oncol 2019;49:190–194. [DOI] [PubMed] [Google Scholar]
- 12).Cloyd JM, Katz MHG, Prakash L, et al. : Preoperative therapy and pancreatoduodenectomy for pancreatic ductal adenocarcinoma: a 25-year single-institution experience. J Gastrointest Surg 2017;21:164–174. [DOI] [PubMed] [Google Scholar]
- 13).Cloyd JM, Wang H, Egger ME, et al. : Association of clinical factors with a major pathologic response following preoperative therapy for pancreatic ductal adenocarcinoma. JAMA Surg 2017;152:1048–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Khorana AA, Mangu PB, Berlin J, et al. : Potentially curable pancreatic cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2016;34:2541–2556. [DOI] [PubMed] [Google Scholar]
- 15).Tempero MA, Malafa MP, Chiorean EG, et al. : Pancreatic adenocarcinoma, Version 1.2019. J Natl Compr Cancer Netw 2019;17:202–210. [DOI] [PubMed] [Google Scholar]
- 16).Paniccia A, Gleisner AL, Zenati MS, et al. : Predictors of disease progression or performance status decline in patients undergoing neoadjuvant therapy for localized pancreatic head adenocarcinoma. Ann Surg Oncol 2020; 27:2961–2971. [DOI] [PubMed] [Google Scholar]
- 17).Suto H, Okano K, Oshima M, et al. : The predictors and patterns of the early recurrence of pancreatic ductal adenocarcinoma after pancreatectomy: the influence of pre- and post- operative adjuvant therapy. BMC Surgery 2019;19:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Charlson M, Szatrowski TP, Peterson J, Gold J.: Validation of a combined comorbidity index. J Clin Epidemiol 1994;47:1245–1251. [DOI] [PubMed] [Google Scholar]
- 19).Amin MB, Edge SB, Greene FL, et al. , (eds). AJCC Cancer Staging Manual. 8th ed. New York: Springer; 2017, 303–309. [Google Scholar]
- 20).Clavien PA, Barkun J, de Oliveira ML, et al. : The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187–196. [DOI] [PubMed] [Google Scholar]
- 21).Bassi C, Marchegiani G, Dervenis C.: The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery 2017;161:584–591. [DOI] [PubMed] [Google Scholar]
- 22).Koch M, Garden OJ, Padbury R, et al. : Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery (ISGLS). Surgery 2011;149:680–688. [DOI] [PubMed] [Google Scholar]
- 23).Berríos-Torres SI, Umscheid CA, Bratzler DW, et al. : Centers for disease control and prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg 2017;152:784–791. [DOI] [PubMed] [Google Scholar]
- 24).Versteijne E, Vogel JA, Besselink MG, et al. : Meta-analysis comparing upfront surgery with neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer. Br J Surg 2018;105:946–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Bradley A, Van Der Meer R.: Upfront surgery versus neoadjuvant therapy for resectable pancreatic cancer: systematic review and Bayesian network meta-analysis. Sci Rep 2019; 9:4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Unno M, Hata T, Motoi F.: Long-term outcome following neoadjuvant therapy for resectable and borderline resectable pancreatic cancer compared to upfront surgery: A meta-analysis of comparative studies by intention-to-treat analysis. Surg Today 2019;49:295–299. [DOI] [PubMed] [Google Scholar]
- 27).Ye M, Zhang Q, Chen Y, et al. : Neoadjuvant chemotherapy for primary resectable pancreatic cancer: A systematic review and meta-analysis. HPB (Oxford) 2020; 22:831–832. [DOI] [PubMed] [Google Scholar]
- 28).Mokdad AA, Minter RM, Zhu H, et al. : Neoadjuvant therapy followed by resection versus upfront resection for resectable pancreatic cancer: a propensity score matched analysis. J Clin Oncol 2016;35:515–522. [DOI] [PubMed] [Google Scholar]
- 29).De Geus SWL, Evans DB, Bliss LA, et al. : Neoadjuvant therapy versus upfront surgical strategies in resectable pancreatic cancer: A Markov decision analysis. Eur J Surg Oncol 2016;42:1552–1560. [DOI] [PubMed] [Google Scholar]
- 30).Hashmi A, Kozick Z, Fluck M et al. : Neoadjuvant versus adjuvant chemotherapy for resectable pancreatic adenocarcinoma: a national cancer database analysis. Am Surg 2018;84:1439–1445. [PubMed] [Google Scholar]
- 31).Jones R, Psarelli E, Jackson R, et al. : Patterns of recurrence after resection of pancreatic ductal adenocarcinoma: a secondary analysis of the ESPAC-4 randomized adjuvant chemotherapy trial. JAMA Surgery 2019;154:1038–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Downs-Canner S, Zenati M, Boone B, et al. : The indolent nature of pulmonary metastases from ductal adenocarcinoma of the pancreas. J Surg Oncol 2015;112(1):80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Van den Broeck A, Sergeant G, Ectors N, et al. : Patterns of recurrence after curative resection of pancreatic ductal adenocarcinoma. Eur J Surg Oncol 2009;35:600–604. [DOI] [PubMed] [Google Scholar]
- 34).Groot VP, Rezaee N, Wu W, et al. Patterns, timing, and predictors of recurrence following pancreatectomy for pancreatic ductal adenocarcinoma. Ann Surg 2018;267:936–945. [DOI] [PubMed] [Google Scholar]
- 35).Masuda T, Dann AM, Elliott IA, et al. A comprehensive assessment of accurate lymph node staging and preoperative detection in resected pancreatic cancer. J Gastrointest Surg. 2018;22:295–302. [DOI] [PubMed] [Google Scholar]
- 36).Gao J, Han F, Jin Y, Wang X, et al. : A radiomics nomogram for the preoperative prediction of lymph node metastasis in pancreatic ductal adenocarcinoma. Front Oncol. 2020;10:1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Li K, Yao Q, Xiao J, et al. : Contrast-enhanced CT radiomics for predicting lymph node metastasis in pancreatic ductal adenocarcinoma: a pilot study. Cancer Imaging 2020;20:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Rong D, Mao Y, Hu W, et al. : Intravoxel incoherent motion magnetic resonance imaging for differentiating metastatic and non-metastatic lymph nodes in pancreatic ductal adenocarcinoma. Eur Radiol 2018;28:2781–2789. [DOI] [PubMed] [Google Scholar]
- 39).Schorn S, Demir I, Samm N, et al. : Meta-analysis of the impact of neoadjuvant therapy on patterns of recurrence in pancreatic ductal adenocarcinoma. BJS Open 2018;2(2):52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Ratnayake B, Savastyuk A, Nayar M, et al. : Recurrence patterns for pancreatic ductal adenocarcinoma after upfront resection versus resection following neoadjuvant therapy: a comprehensive meta-analysis. J Clin Med 2020;9(7):2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Postoperative complications in R and BR PDAC patients who received NAT and underwent pancreaticoduodenectomy. CR-POPF: clinically relevant-postoperative pancreatic fistula, GJ: gastrojejunostomy, GIB: gastrointestinal bleed, DVT/PE: deep vein thrombosis/pulmonary embolism.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


