Abstract
Background:
The spectrum of Diabetes Mellitus and various complications associated with it have been regarded as major global health challenges. Raised TG/HDL has been regarded as one of the valid markers for Insulin resistance. It leads to increased risk of CVD by causing Insulin resistance and also by its own effect on the vessel wall. Detection of raised TG/HDL ratio and early intervention before the patients develop clinical disease can help in mitigation of future consequences of CVD.
Aims:
The aim of our study was to compare TG/HDL ratio between prediabetics and controls and further to look for any correlation between the TG/HDL ratio value with HOMA-IR and Carotid Intima Media Thickness (CIMT) in prediabetics.
Settings and Designs:
A cross sectional study
Methods and Material:
Study was done at ABVIMS and Dr RML Hospital, New Delhi. 60 prediabetics and 60 age, sex, BMI matched controls were employed. In both cases and controls fasting and postprandial blood glucose, glycated Hemoglobin (HbA1C) and fasting Insulin levels were measured. HOMA-IR values in both the groups were calculated using fasting glucose and Insulin levels. Serum lipid profile was obtained and TG/HDL ratio was analysed in two groups. Values obtained were compared between the two groups. CIMT was only measured in cases using B mode ultrasonography.
Statistical Analysis and Results:
Median (IQR) of fasting plasma Insulin (μIU/ml) in cases was 11.3 (10.175-13.505) versus that in controls being 5.73 (4.3-7.1). HOMA-IR (IQR) values in cases and controls were 3.12 (2.73 - 3.595) and 1.21 (0.918 – 1.505) respectively. Median (IQR) for TG/HDL ratio was 3.26 (2.712 – 4) for cases and 2.05 (1.755- 2.502) for controls. However no correlation was observed between either the mean CIMT (mm) or HOMA-IR with TG/HDL ratio.
Conclusions:
Diabetes Mellitus and its various complications are of a great burden to society. Diagnosing the risk factors early before the onset of these manifestations can help us in combating these major issues. One of the risk factors among them is raised TG/HDL ratio. Early detection of elevated TG/HDL in prediabetics may serve in early detection of atherosclerotic complications and help physicians in framing primary preventive strategies for tackling ASCVD in patients with prediabetes and full-blown Diabetes.
Keywords: CIMT, HOMA IR, prediabetes, TG/HDL ratio
Introduction
Prediabetes is a condition with elevated plasma glucose levels above normal but below that of Diabetes Mellitus. It is defined as fasting plasma glucose (FPG) of 100–125 mg/dL, 2-h postprandial blood glucose (2 h PG) of 140–199 mg/dL or HbA1c of 5.7%–6.4% (ADA guidelines).[1] Chronic hyperglycemia in diabetes has long been known to cause long-term microvascular and macrovascular complications, cardiovascular diseases (CVD) being the most common. Now even prediabetics have been found to have 20% more increased risk of developing CVD than euglycemic individuals.[2] This increased risk for CVD in prediabetes is multifactorial, with etiologies including insulin resistance, hyperglycemia, dyslipidemia, hypertension, systemic inflammation, and oxidative stress.[3] In individuals with dyslipidemia, it has been found that higher triglyceride/high-density lipoprotein (TG/HDL) increases the risk of CVD by its own effects on vessel wall and also causing insulin resistance.[4]
CVDs being subtle and silent in their progression make it worthwhile to identify them as early as we can so that we can course correct and halt atherosclerosis progression and cardiovascular morbidity and mortality as well.
Aim
The aim of the study was to assess the serum levels of TG/HDL in prediabetes, compare the same in normoglycemic individuals and to correlate the obtained value with HOMA-IR and carotid artery intima-media thickness (CIMT) in prediabetics.
Material and Methods
Study Place: Departments of Medicine, Biochemistry, Radiology at ABVIMS and Dr RML Hospital, New Delhi.
Study Design: Cross-sectional observational study.
Study Period: November 1, 2018 to March 31, 2020.
Sample Size: A convenient sample size of 60 consecutive patients of prediabetes and 60 matched control subjects were taken from medicine OPD, wards and emergencies of ABVIMS and Dr RML Hospital, New Delhi after fulfilling all inclusion and exclusion criteria.
Inclusion criteria
60 cases of prediabetes of age 30–60 years as defined by fasting plasma glucose between 100 and 125 mg/dL OR 2-h postprandial glucose/2 h OGTT (after 75 g of glucose solution ingestion) between 140 and 199 mg/dL OR HbA1c = 5.7–6.4% (ADA 2016).
60 control subjects matched for age, gender, ethnicity and BMI and with fasting plasma glucose of less than 100 mg/dL AND 2 h postprandial glucose of less than 140 mg/dL and HbA1c less than 5.7 with no known co-morbidities as per exclusion criteria.
{An informed bilingual written consent was taken from each of the patient/relatives for inclusion}.
Exclusion criteria
Known hypertensive
Known diabetics
Known cases of chronic liver disease
Known cases of non-alcoholic fatty liver disease
Known cases of coronary heart disease
Known case of cerebrovascular accidents (CVA) or transient ischemic attacks (TIA)
Pregnant females
Known smokers and alcoholics.
Methodology
Clearance from ethical committee was sought [(MD/MS) (139/2018)/IEC/PGIMER/RMLH].
All the cases and controls underwent the following examinations and tests:
Clinical examination
After a baseline data about age, sex, race, ethnicity and family history of diabetes or hypertension all the cases and controls underwent a detailed clinical examination including measurement of height (in centimeter using a wall-mounted stadiometer), weight (using weight measurement scale) and waist circumference (using a standard measuring tape). Body mass index was calculated as weight in kilograms divided by height in square meters. Resting systolic and diastolic blood pressures were recorded twice using an automated sphygmomanometer after a 5-min rest and mean obtained was considered.
Laboratory investigations
Around 10 mL of fasting blood sample was collected after venepuncture. Samples were taken in EDTA vial for HbA1c measurements and in red (Plain) vials for biochemical profile and lipid profile.
Investigations done in patients were:
Fasting and 2-h postprandial plasma glucose.
HbA1c (measured by Immunoturbidimetry method on Vitros dry chemistry analyser by NSGP guidelines)
Serum Lipid profile including Triglycerides, Total Cholesterol, HDL-cholesterol, LDL- cholesterol, VLDL-cholesterol were estimated on fully automated clinical chemistry analyser.
Fasting Plasma insulin levels (measured by Chemiluminescence Immuno Assay (CLIA) on Vitros ECiQ by Orthoclinical Diagnostics).
All the samples were analyzed in the Department of Biochemistry, ABVIMS and Dr. RML Hospital, New Delhi.
Basal insulin resistance of the individual was calculated using HOMA-IR (Homeostatic model assessment of insulin resistance) using the formula:
HOMA – IR = (FPI × FPG)/405 (where fasting plasma glucose was in mg/dL).
Ultrasonographic examination: (Only performed in cases)
All cases underwent high-resolution B-mode ultrasonography with a 7.5 MHz linear probe. CIMT was measured as distance between two echogenic lines (representing intima and media). All scans and image measurements were carried out by the same investigator, blinded to the risk factor status of the participants.
Statistical analysis
Categorical variables were presented in number and percentage (%) and continuous variables were presented as mean ± SD and median. Normality of data was tested by Kolmogorov-Smirnov test. If the normality was rejected then non-parametric test was used.
Statistical tests were applied as follows-
Quantitative variables were compared using the Independent t test/Mann–Whitney test (when the data sets were not normally distributed) between the two groups.
Qualitative variables were compared using Chi-square test.
A value of P < 0.05 was considered statistically significant.
The data was entered in MS EXCEL spreadsheet and analysis was done using Statistical Package for Social Sciences (SPSS) version 21.0.
Results
The following observation was made Tables 1, 2 and 3.
Table 1.
Demographic and anthropometric characteristics of cases and controls
| Parameters | Cases (n=60) | Controls (n=60) | P |
|---|---|---|---|
| Age (mean±SD) | 45.68±8.78 | 44.48±7.44 | 0.439 |
| Sex (%) | 0.855 | ||
| Males | 46.67% (n=28) | 48.33% (n=29) | |
| Females | 53.33% (n=32) | 51.67% (n=31) | |
| BMI (mean±SD) | 25.33±2.65 | 24.93±2.22 | 0.387 |
| Waist circumference (mean±SD) | 84.57±7.3 | 82.62±8.7 | 0.287 |
| Systolic blood pressure (mean±SD) | 116.23±6.66 | 116.93±8.13 | 0.541 |
| Diastolic blood pressure (mean±SD) | 74.97±5.17 | 73.43±4.97 | 0.079 |
Table 2.
Biochemical parameters among cases and controls
| Parameters (Median (IQR)) | Cases (n=60) | Controls (n=60) | P |
|---|---|---|---|
| Fasting Blood Sugar | 110 (106-115.25) | 86 (79-91.25) | <0.0001 |
| Postprandial Blood Sugar | 168 (156-184.25) | 125 (117-130) | <0.0001 |
| HbA1c | 6 (5.9-6.2) | 4.9 (4.6-5.125) | <0.0001 |
| Fasting Insulin Levels | 11.3 (10.175-13.505) | 5.73 (4.3-7.1) | <0.0001 |
| HOMA-IR Index | 3.12 (2.73-3.595) | 1.21 (0.918-1.505) | <0.0001 |
Table 3.
Descriptive statistics of mean carotid intima media thickness (mm) of study subjects
| Variable | Mean±Stdev | Median (IQR) | Range |
|---|---|---|---|
| Mean carotid intima media thickness (mm) | 0.61±0.1 | 0.6 (0.5-0.7) | 0.4-0.8 |
Significant difference was seen in levels of FPI (uIU/mL) between cases and controls (P < 0.05). Median (IQR) of FPI level (uIU/mL) in cases was 11.3 (10.175–13.505) which was significantly higher as compared to controls (5.73 (4.3–7.1)) [Table 2]. FPI levels (uIU/mL) were >=9 in 83.33% of as compared to controls (1.67%). HOMA-IR Index showed median (IQR) values of 3.12 (2.73 ± 3.595) in cases and 1.21 (0.918 ± 1.505) in controls with a statistically significant difference (P < 0.0001) [Table 2]. HOMA-IR was > or equal to 2 91.67% of cases and 1.67% of controls. [Table 4]
Table 4.
HOMA-IR distribution among cases and controls with a cut-off of 2
| HOMA-IR | Case (n=60) | Control (n=60) | Total | P | Test performed |
|---|---|---|---|---|---|
| <2 | 5 (8.33%) | 59 (98.33%) | 64 (53.33%) | <.0001 | Fisher Exact test |
| >=2 | 55 (91.67%) | 1 (1.67%) | 56 (46.67%) |
Lipid profiles of both the groups were assessed and compared and a significant difference in serum levels of HDL Cholesterol (gm/dL), LDL- cholesterol (gm/dL) and total Cholesterol (gm/dL) levels between cases and controls (P < 0.05) was obtained. [Table 5]
Table 5.
Comparison of HDL Cholesterol, LDL Cholesterol level, total Cholesterol, Triglyceride, VLDL Cholesterol and TG/HDL ratio between cases and controls
| Lipid profile | Case (n=60) | Control (n=60) | Total | P | Test performed |
|---|---|---|---|---|---|
| HDL Cholesterol (gm/dL) | |||||
| Mean±SD | 43.42±11.73 | 47.88±8.7 | 45.65±10.52 | 0.019 | t test; 2.369 |
| Median (IQR) | 44 (34.75-52.25) | 49 (41-54) | 47 (39-54) | ||
| Range | 23-69 | 17-64 | 17-69 | ||
| LDL- cholesterol (gm/dL) | |||||
| Mean±SD | 109.41±20.92 | 92.42±21.16 | 100.91±22.62 | <.0001 | t test; 4.425 |
| Median (IQR) | 109 (98.85-129.1) | 92 (84-101) | 100 (87.875-113.8) | ||
| Range | 56-161 | 22-134 | 22-161 | ||
| Total Cholesterol (gm/dL) | |||||
| Mean±Stdev | 165.02±35.44 | 133.93±34.87 | 149.48±38.33 | <.0001 | Mann Whitney test; 948 |
| Median (IQR) | 170.5 (134.75-188.25) | 130.5 (103.75-154) | 151 (120-185) | ||
| Range | 79-219 | 52-200 | 52-219 | ||
| Triglyceride (gm/dL) | |||||
| Mean±SD | 135.75±30.01 | 102.38±29.12 | 119.07±33.87 | <.0001 | Mann Whitney test; 720 |
| Median (IQR) | 142 (114.75-155) | 95.5 (84-106.75) | 113.5 (92-149.25) | ||
| Range | 70-213 | 40-197 | 40-213 | ||
| TG/HDL ratio | |||||
| Mean±SD | 3.3±0.98 | 2.17±0.54 | 2.73±0.97 | <.0001 | Mann Whitney test; 579 |
| Median (IQR) | 3.26 (2.712-4) | 2.05 (1.755-2.502) | 2.56 (1.948-3.3) | ||
| Range | 1.56-5.4 | 1.25-4.02 | 1.25-5.4 | ||
| VLDL Cholesterol (gm/dL) | |||||
| Mean±SD | 26.18±6.81 | 24.36±7.8 | 25.27±7.34 | 0.179 | Mann Whitney test; 1544.5 |
| Median (IQR) | 25.5 (22-32.25) | 24 (18-30.25) | 24.5 (19-31.25) | ||
| Range | 15-40 | 10-40 | 10-40 |
However, no significant difference was seen in VLDL Cholesterol (gm/dL) between cases and controls (P = 0.179). Median (IQR) of VLDL Cholesterol (gm/dL) in cases was 25.5 (22-32.25) compared to controls where median was 24 (18–30.25). [Table 5]
For TG/HDL ratio a significant difference was found between cases and controls [(P < .0001); Median (IQR) of TG/HDL in cases 3.26 (2.712-4) as compared to controls 2.05 (1.755–2.502)]. TG/HDL ratio was >=2 in 88.33%, >=3 in 63.33% and >=4 in 26.67% in cases which was significantly higher as compared to controls 58.33%, 6.67% and 1.67% respectively. [Table 5 and Figures 1, 2 and 3].
Figure 1.
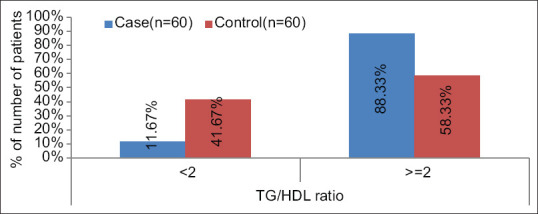
Showing serum TG: HDL ratio distribution among cases and controls with a cut-off of 2
Figure 2.
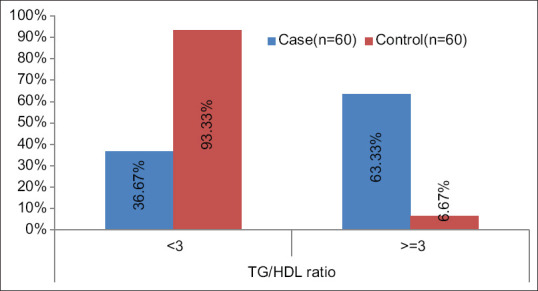
Showing serum TG: HDL ratio distribution among cases and controls with a cut-off of 3
Figure 3.
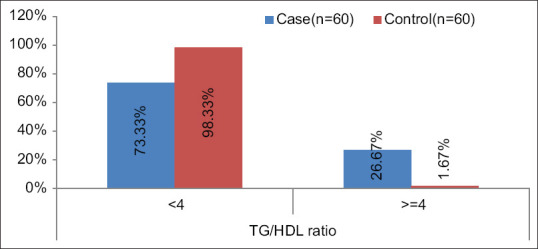
Showing serum TG: HDL ratio distribution among cases and controls with a cut-off of 4
But, when it came to finding its utility as an early marker of atherosclerosis or insulin resistance there was no correlation seen between either the mean carotid intima-media thickness (mm) and HOMA-IR with TG/HDL ratio. [Tables 6 and 7].
Table 6.
Correlation of mean carotid intima media thickness (mm) with TG/HDL ratio
| Variables | TG/HDL ratio |
|---|---|
| Mean carotid intima media thickness (mm) | |
| Correlation coefficient | 0.017 |
| P | 0.896 |
Spearman rank correlation coefficient
Table 7.
Correlation of TG/HDL ratio with HOMA IR
| Variables | Homeostasis model assessment of insulin resistance (HOMA-IR) |
|---|---|
| TG/HDL ratio | |
| Correlation coefficient | -0.063 |
| P | 0.631 |
Spearman rank correlation coefficient
Discussion
The aim of our study was to primarily compare the TG/HDL in prediabetics versus normoglycemic individuals and further to see for any correlation between TG/HDL ratio value with HOMA-IR and carotid intima-media thickness (CIMT) in prediabetics.
Our study showed a conclusive increased TG/HDL in prediabetics as compared to euglycemic individuals; we were however not able to find any correlation of TG/HDL with either the HOMA IR or mean CIMT (TG/HDL with HOMA IR, Correlation coefficient = -0.063 and P = 0.631; TG/HDL with mean CIMT, Correlation coefficient = 0.017 and P = 0.896). In a study of China which was done in diagnosed cases of T2DM, significantly higher HOMA-IR values were found in patients with higher tertiles of TG/HDL-C than patients in the lower tertiles [T1: 2.68 (1.74–3.70); T2: 2.96 (2.29–4.56); T3: 3.09 (2.30–4.99)].[5] Ray S and team[6] also observed association of lipoprotein ratios with HOMA-IR. 100 patients with IFG (Impaired Fasting Glucose) admitted with ACS (Acute Coronary Syndrome) were evaluated for fasting glucose, insulin concentrations and HOMA-IR and significantly elevated levels lipoproteins in patients with HOMA- IR >2 was found compared to patients with values <2.
Kansal S et al.[7] in their study observed significantly raised TC, LDL, TG, VLDL TG/HDL ratio and LDL/HDL ratio in prediabetic individuals as compared to normal healthy controls (P < 0.05) and significantly lower HDL levels in prediabetics compared to controls (P < 0.05). A positive correlation between TG/HDL ratio and metabolic syndrome (MS) was observed by Spanish MESYAS (Metabolic Syndrome in Active Subjects) group in a similar study.[8] They observed prevalence of MS of 18.8% in men and 6.1% in women and it was observed that mean value of the TG/HDL ratio of 2.50 ± 2.2 increases with each component of MS. Subjects with MS had a 2 times higher ratio of TG/HDL than those without (P < 0.001). It was concluded that TG/HDL ratio >2.75 in men and >1.65 in woman were highly predictive of MS with a sensitivity of 80% and a specificity of 78%. Thus we see that TG/HDL has also been regarded as one of the most useful marker in identification of insulin-resistant states. It also ultimately risk of various adverse outcomes along with other parameters like plasma TG concentration and insulin concentration.[4] Kimm H et al.[9] reported that lipid ratios of TG/HDL-C, LDL-C/HDL-C and TG/HDL-C, as well as TG and HDL were consistently associated with MetS and insulin resistance in subjects without MS. An Indian study was done by Kohli A and her colleagues[10] to study the pattern of TG/HDL-C ratio among healthy, young, and middle-aged 121 Indian men of age 25–44 years and its relationship with other lipid and nonlipid factors. It showed a significant association of TG/HDL-C ratio with other lipid parameters and measures of adiposity, such as BMI, body fat etc.
In a South Indian study, TG/HDL-C ratio has also been considered to be a predictor of insulin resistance in non-diabetic ACS. It was concluded in the study that the plasma TG/HDL-C ratio provides simple means to identify insulin resistance and can be used as a marker of the same and also for other CVD risks in adult non-diabetic patients.[11]
We noticed a significant difference in the distribution of TG/HDL ratio between cases and controls (P < 0.05). With a cut-off of 2 about 88.33% cases and 58.33% controls had a value of more than or equal to 2; with a cut-off of 3 around 63.33% cases and 6.67% controls had values more than or equal to 3, whereas, 26.67% of cases and only 1.67% controls were found to have a value of greater than or equal to 4 when 4 was used the cut-off value. So it can be proposed that while a cut-off of 2 or 3 might not differentiate all controls from the prediabetics, a cut-off value of 4 is better in determining the true negatives (normoglycemics) as only 1.67% of the controls have values over 4, thus increasing the specificity.
HOMA-IR and TG: HDL ratios, studied as markers of states of insulin resistance and metabolic syndrome can be employed routinely to identify high-risk individuals among the general population. It is a very useful tool in primary care settings as only a lipid profile test (which usually consists of both triglyceride and HDL) is required. If the primary care physician finds ratio to be high, he can identify patients for which an aggressive lifestyle modification approach and dietary control can be advised
Conclusion
Diabetes mellitus and the various complications associated with it are of great concern. Identifying the disease process or its complications in early course can help in minimizing further consequences. Among the various markers of insulin resistance, TG/HDL ratio is the simplest to assess. We were able to assess raised TG/HDL in prediabetics as compared to controls but could not get any significant correlation between HOMA-IR or CIMT with TG/HDL. This may perhaps be due to including prediabetic patients into the study, which is a stage quite early in the spectrum of dysglycemias. It requires significant time to develop CIMT thickening and the early stage of atherosclerotic process might not get picked up radiologically. Further prospective studies with a larger sample size might perhaps be helpful in defining the timeline of the development of TG/HDL correlation with CIMT as well as HOMA IR. However, even in prediabetics elevated TG/HDL may serve in the early detection of atherosclerotic complications. Being a very easily accessible and an inexpensive investigation, this can help primary care physicians in framing primary preventive strategies for ASCVD in patients with prediabetes and diabetes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.American Diabetes Association. 2.Classification and diagnosis of diabetes: Standards of medical care in diabetes-2020. Diabetes Care. 2020;43((Suppl 1)):S14–31. doi: 10.2337/dc20-S002. [DOI] [PubMed] [Google Scholar]
- 2.Hsueh WA, Orloski L, Wyne K. Prediabetes: The importance of early identification and intervention. Postgrad Med. 2010;122:129–43. doi: 10.3810/pgm.2010.07.2180. [DOI] [PubMed] [Google Scholar]
- 3.Brannick B, Dagogo-Jack S. Prediabetes and cardiovascular disease: Pathophysiology and interventions for prevention and risk reduction. Endocrinol Metab Clin North Am. 2018;47:33–50. doi: 10.1016/j.ecl.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hegele RA. The pathogenesis of atherosclerosis. Clin Chim Acta. 1996;246:21–38. doi: 10.1016/0009-8981(96)06224-9. [DOI] [PubMed] [Google Scholar]
- 5.Ren X, Chen ZA, Zheng S, Han T, Li Y, Liu W, et al. Association between triglyceride to HDL-C ratio (TG/HDL-C) and insulin resistance in Chinese patients with newly diagnosed type 2 diabetes mellitus. PloS One. 2016;11:e0154345. doi: 10.1371/journal.pone.0154345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ray S, Bairagi AK, Guha S, Ganguly S, Ray D, Basu AK, et al. A simple way to identify insulin resistance in non-diabetic acute coronary syndrome patients with impaired fasting glucose. Indian J Endocrinol Metab. 2012;16((Suppl 2)):S460–4. doi: 10.4103/2230-8210.104132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kansal S, Kamble TK. Lipid profile in prediabetes. J Assoc Physicians India. 2016;64:18–21. [PubMed] [Google Scholar]
- 8.Laclaustra M, Ordoñez B, Leon M, Andres EM, Cordero A, Pascual-Calleja I, et al. Metabolic syndrome and coronary heart disease among Spanish male workers: A case-control study of MESYAS. Nutr Metab Cardiovasc Dis. 2012;22:510–6. doi: 10.1016/j.numecd.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Kimm H, Lee SW, Lee HS, Shim KW, Cho CY, Yun JE, et al. Associations between lipid measures and metabolic syndrome, insulin resistance and adiponectin. Circ J. 2010;74:931–7. doi: 10.1253/circj.cj-09-0571. [DOI] [PubMed] [Google Scholar]
- 10.Kohli A, Siddhu A, Pandey RM, Reddy KS. Relevance of the triglyceride-to-high-density lipoprotein cholesterol ratio as an important lipid fraction in apparently healthy, young, and middle-aged Indian men. Indian J Endocrinol Metab. 2017;21:113–8. doi: 10.4103/2230-8210.196020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajappa M, Sridhar MG, Balachander J, Sethuraman KR, Rajendiran KS. Lipoprotein ratios as surrogate markers for insulin resistance in South Indians with normoglycemic nondiabetic acute coronary syndrome. ISRN Endocrinol. 2014;2014:1–6. doi: 10.1155/2014/981524. [DOI] [PMC free article] [PubMed] [Google Scholar]


