Abstract
Introduction:
Diabetes mellitus is the most common endocrinological disease associated with different life-threatening infections. Urinary Tract Infection is one of them which may lead to Intensive care Unit admission and even death. In this study, we would like to find out the spectrum of uropathogen and its antibiotic sensitivity, so that we can choose empirical antibiotics early to save lives.
Aim and Objectives:
To find out spectrum of uropathogens in Diabetic patients attending to Diabetes Clinic of a tertiary hospital and to find out the Antibiotic sensitivity pattern in isolated bacteria.
Material and Methods:
In this cross-sectional observational hospital-based study, consecutive patients of any age and gender having Diabetes mellitus and symptoms of Urinary Tract Infection, who attended Out-patient Department and Diabetes Clinic of General Medicine Department from August 2020 to October 2020, were included. After taking consent and brief history, mid-stream clean catch urine sample was collected in sterile container and sent to a Microbiology laboratory for culture of micro-organism and sensitivity to antibiotics.
Results:
Among 202 diabetic patients recruited in our study, 138 (68.31%) were female and 64 (31.69%) were male. The mean age of all participants was 50.23 ± 11.45 years. Culture confirmed UTI was 24.3% and patients showing classic UTI symptoms were 32.7%. Compared to male, most of the culture-positive and symptomatic patients were female (26.0% and 37% respectively). Culture-positive patients who showed classic UTI symptoms were 42.42%; however, we found 15.44% asymptomatic patients who showed culture positivity. The mean HbA1c level was 7.5 ± 1.6%. Subgroup analysis revealed that patients with HbA1c >7.5% were at a significantly higher risk of developing culture-positive UTI (P < 0.00001, Odds ratio 21.71). Prevalence of gram-negative and gram-positive bacteria were 65.3% (32 out of 49) and 28.57% (14 out of 49), respectively. The major organism isolated were Escherichia coli (39%), Klebsiella spp. (19%), Enterococcus spp. (12%), Staphylococcus aureus (12%), and Candida spp. (6%). The sensitivity pattern of the gram-negative bacilli showed the presence of Extended-spectrum betalactamases (ESBLs) in 36.84% (11 out of 32 isolates). The bacteria grown were most sensitive to Piperacillin-tazobactam (100%), Cefoperazone-sulbactam (100%) and Meropenem (100%) whereas Fluoroquinolone and Co-Amoxyclav showed least sensitivity (43.8% and 37.5% respectively). The Staphylococcus spp. showed 100% sensitivity to Vancomycin, Teicoplanin, Linezolid whereas Penicillin-G and Ampicillin showed 12.5% sensitivity. The Enterococcus spp. revealed 100% sensitivity to Vancomycin, Teicoplanin, Linezolid, and Fosfomycin. Drug resistance is emerging in clinical isolates. Prevalence of ESBL in Enterobacteriacea was found to be 34%.
Conclusion:
All patients with diabetes must be searched for urinary tract bacterial colonization by simple routine urinary culture even though they are asymptomatic. Resistance to common antibiotics, particularly to oral formulations (especially Fluoroquinolones and Ampicillin) is increasing day by day due to indiscriminate use of antibiotics. This study highlighted that the policy makers should formulate antibiotic policy for rational use of antibiotics, which could help clinicians to prescribe proper antibiotics. However, regular monitoring of susceptibility pattern of urinary pathogens is essential.
Keywords: Antibiotic sensitivity, antibiotic-resistance, diabetes, urinary tract infection, uropathogen
Introduction
Diabetes mellitus (DM) is the most common endocrinological disorder in the world having high morbidity and complications and ultimately death. Changing lifestyle and rapid urbanization has caused an increased incidence of DM in the developing countries like India. In 2020, according to the International Diabetes Federation (IDF), world diabetes population is approximately 463 million. Indian diabetes population is approximately 77 million. The Indian Council of Medical Research-INdiaDIABetes (ICMR-INDIAB) study which was conducted in 15 states showed the overall prevalence of diabetes was 7.3%.[1] Another study, The Delhi Urban Diabetes Survey (DUDS) revealed a strikingly high prevalence of diabetes among residents of urban areas of east Delhi (18.3%: known, 10.8%; newly detected 7.5%)).[2,3]
Urinary tract infection (UTI) is one of the most common infections seen in all age groups with DM. Susceptibility to UTI among diabetic patients is very high compared to non-diabetics.[4] In various literatures, it is found that E. coli is the most common isolate followed by Klebsiella pneumoniae, and Enterococci.[5]
The emergence of drug-resistant UTIs is increasing both in community and healthcare setups. This situation is posing a challenge in country like India due to irrational use of antibiotics.[6] Hence, calculating the prevalence of UTI among diabetic patients and determining the pattern of bacterial strains, sensitivity of bacterial isolates to antimicrobial agents is of utmost important for the epidemiologist, scientist, health planner, and clinician. So, in this study, we would like to find out the causative uropathogens and their antibiotic susceptibility pattern among patients with DM.
Material and Methods
A cross-sectional observational hospital-based study was performed at Out-patient Department and Diabetes Clinic of General Medicine Department, College of Medicine and JNM Hospital, WBUHS, Kalyani, West Bengal, India from August 2020 to October 2020. After getting permission from the Institutional Scientific Research Committee and Ethics Committee, we started recruiting cases. Consecutive male and female patients of any age with DM who attended the above-said clinic were approached to participate in the study, irrespective of the presence or absence of UTI symptoms. DM patients were selected according to World Health Organization (WHO) criteria. Pregnant women, patients with own underlying renal pathology or chronic renal disease, patients who were using Sodium-glucose co-transporter-2 (SGLT-2) and patients with history of use of antimicrobial therapy during the previous month were excluded from the study. After getting written informed consent, relevant clinical and socio-demographic characteristics were collected using pre-tested questionnaires. Consecutive patients were questioned regarding classic symptoms suggestive of UTI (e.g., urgency, dysuria, urinary frequency, loin pain, and nausea) and history of co-morbid conditions, such as hypertension and, for males, prostate enlargements were asked. We used HbA1c as a glycemic control index. HbA1c levels more than 6.5% were considered suboptimal controlled diabetes and HbA1c levels more than 7.5 were considered as poor control of diabetes. The anticipated sample size for the study was determined using the formula: n = z2 pq/d2. After thoroughly applying inclusion and exclusion criteria, 202 participants got included in our study.
We asked the participants to provide a midstream urine sample according to the clean-catch procedure. Sample was then sent to Microbiology Department of our Hospital where it was inoculated on Cysteine lactose electrolyte deficient (CLED) agar (Oxoid, Basingstoke, UK), MacConkey, 5% Sheep Blood agar, and chromogenic UTI (Oxoid) agar plates and incubated at 37° for 24–48 h. Organisms grown on culture discs were classified by standard protocols using various identification and biochemical tests, that is, colony morphology, gram staining, positive oxidase reaction, production of pyocyanin on Mueller-Hinton agar (Oxide, Ltd, UK), citrate utilization and growth at 42°. Significant bacteriuria was defined as urine culture plates showing ≥105 colony-forming units (CFU)/mL of single bacterial species.
Kirby–Bauer's disc diffusion method was used to test Antibiotics susceptibility. Bacterial colonies were suspended in normal saline to 0.5 McFarland standard. Then, with the help of disposable sterile swabs, the suspensions were inoculated on Muller-Hinton agar (Oxoid) and incubated for 18–24 h, according to Clinical and Laboratory Standards Institute (CLSI) guidelines. Antimicrobial susceptibility and resistance was determined by isolate growth zone diameter according to CLSI guidelines. All antibiotic discs were from Oxoid. The antibiotics disc that were used to classify the susceptibility pattern of bacterial pathogen include ampicillin (10 mcg), amoxiclav/Clavulanate (30 mcg), sulfamethoxazole/Trimethoprim (TMP/SMX) (25 mcg), fosfomycin (50 mcg), ciprofloxacin (5 mcg), piperacillin/tazobactam (110 μg), cefotaxime (30 mcg), amikacin (30 mcg) meropenem (10 mcg), ceftazidime (30 mcg), imipenem (10 mcg), cefoperazone-sulbactam (30 mcg), nitrofurantoin (300 mcg), aztreonam (30 mcg), vancomycin (30 mcg), cefalexin (30 mcg) and cefipime (30 mcg). In this study, intermediate susceptibility was labeled as susceptible.
Data analysis was done using Statistical Package for the Social Sciences software for Windows version 20.0 (SPSS, Chicago, Illinois). Number and proportions were analyzed for categorical data. Odds ratio was determined using Chi-square statistics for categorical data. Statistical significance was set at <0.05.
Result
Demographic data analysis
Among 202 diabetic patients recruited in our study, 138 (68.31%) were female and 64 (31.69%) were male. The mean age of all participants was 50.23 ± 11.45 years. Culture confirmed UTI was 24.3% and patients showing classic UTI symptoms were 32.7%. Compared to male, most of the culture-positive and symptomatic patients were female (26.0% and 37% respectively). Culture-positive patients who showed classic UTI symptoms were 42.42%; however, we found 15.44% asymptomatic patients who showed culture positivity [Table 1]. Among all culture-positive patients, most participants were housewives (71.42%). Most of the patients were hypertensive (61.88%).
Table 1.
Association of culture positive UTI and symptoms of UTI
| Patient attributes | Culture positive | Culture Negative | Total |
|---|---|---|---|
| Symptomatic | 28 | 38 | 66 |
| Asymptomatic | 21 | 115 | 136 |
| Total | 49 | 153 | 202 |
In our study, we found patients with Type 2 DM were 193 (95.5%) and the rests were Type 1 (4.5%). The mean HbA1c level was 7.5 ± 1.6%. Subgroup analysis revealed that patients with HbA1c >7.5% were at significantly higher risk of developing culture-positive UTI (P < 0.00001, Odds ratio 21.71). Most of the patients were undergoing Oral hypoglycemic drugs (76.23%) alone, 81.68% patients on injection Insulin and oral drugs, and 18.31% were on injection insulin therapy.
Spectrum of bacteria in isolates and their antibiotic sensitivity pattern
In the current study, among all culture-positive isolates in this study, prevalence of gram-negative and gram-positive bacteria were 65.3% (32 out of 49) and 28.57% (14 out of 49), respectively. The major organisms isolated [Picture 1] were Escherichia coli (38.77%), Klebsiella spp. (18.36%), Enterococcus spp. (12.24%), Staphylococcus aureus (12.24%), and Candida spp. (6.12%). Other than these isolates, Pseudomonas aeruginosa, Proteus spp., coagulase-negative Staphylococcus aureus (CONS) were also isolated in this study; however, they were in less numbers. Distribution of culture-positive isolates among symptomatic and asymptomatic patient was summarized in Table 2. Escherichia coli were the prominent organism in both symptomatic and asymptomatic group. Among the Candida spp. isolates, the predominant organism was Candida albicans (60%). Among all 32 gram-negative isolates, 11 isolates (34.37%) were the Extended-Spectrum Betalactamases (ESBLs) producers and 22 (68.75%) were non-ESBL producers. Out of 6 Staphylococcus isolates, 2 (33.3%) were methicillin-resistant (MRSA) and 4 (66.6%) were methicillin sensitive (MSSA).
Picture 1.
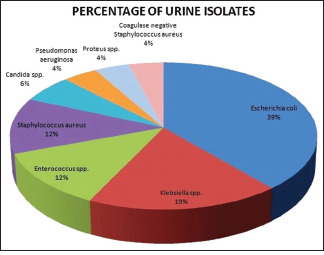
Pie diagram for Percentages of Urine isolates
Table 2.
Distribution of culture positive isolates among symptomatic and asymptomatic patient
| ISOLATES | SYMPTOMATIC PATIENT | ASYMPTOMATIC PATIENT | TOTAL |
|---|---|---|---|
| Escherichia coli | 8 | 11 | 19 |
| Klebsiella spp. | 4 | 5 | 9 |
| Enterococcus spp. | 4 | 2 | 6 |
| Staphylococcus aureus | 4 | 2 | 6 |
| Candida spp. | 3 | 0 | 3 |
| Pseudomonas aeruginosa | 1 | 1 | 2 |
| Proteus spp. | 2 | 0 | 2 |
| Coagulase negative Staphylococcus aureus | 2 | 0 | 2 |
| Total | 28 | 21 | 49 |
In our study, the gram-negative bacteria grown were most sensitive to Piperacillin-tazobactam (100%), Cefoperazone-sulbactam (100%), and Meropenem (100%), whereas Fluoroquinolone and Co-Amoxyclav showed least sensitivity (43.8% and 37.5% respectively). Detail of antibiotic sensitivity pattern among gram-negative organisms was depicted in Picture 2.
Picture 2.
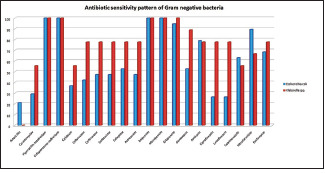
Detail of antibiotic sensitivity pattern among gram negative organisms
In this study, among the gram-positive organisms isolated, Staphylococcus spp. showed 100% sensitivity to vancomycin, teicoplanin, and linezolid. Amikacin (87.5%), gentamicin, ceftriaxone, tetracycline, and cefoxitin (75%) showed moderate sensitivity. But, ciprofloxacin, levofloxacin, ampicillin and penicillin-G again revealed poor sensitivity. Among the six Enterococcus spp., all 6 were found to be sensitive to vancomycin, teicoplanin, linezolid, and fosfomycin. We found moderate sensitivity to ampicillin, imipenem, gentamicin, nitrofurantoin, but the fluoroquinolones again showed least sensitivity (16.7%). These patterns were depicted in Picture 3.
Picture 3.
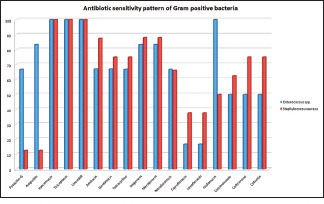
Detail of antibiotic sensitivity pattern among gram positive organisms
Discussion
In general, diabetic patients pose increased risk of infection, in particular, to UTI.[4] Severe UTI and associated complications can lead to significant morbidity and mortality. Patients with DM develop neuropathy resulting in neurogenic bladder and urinary stasis which promote the probability of infection.[7] Patients with diabetes also show decreased neutrophil activity, decrease urinary cytokines, and leukocyte concentrations, which could promote the adhesion of microorganisms to uroepithelial cells. Moreover, hyperglycemia is itself a promoter of the colonization and growth of a range of organism.[8]
In our current study, we tried to determine the prevalence of UTI among DM patients and explore the spectrum of uropathogens along with their antibiotic sensitivity pattern without comparing the data with non-diabetic individuals. In this study, the prevalence of culture-confirmed UTI was 24.3% and symptomatic UTI was 32.7%. This finding was almost similar to the data published in a study in Nepal[9] and other studies in and around our country.[10,11] Interestingly, in our study, asymptomatic culture-positive UTI was 15.44%. This finding indicates a serious concern regarding DM patient because it may lead to fatal complications if not treated early and properly. Hence, all DM patients should be screened for UTI at regular interval. A limitation of our study was that we didn't compare the data with non-diabetic subjects.
It is stated that UTI is predominantly a disease of the female due to short urethra and proximity to the anal opening. The majority of the study all over the world has concluded female preponderance to UTI over male.[12] In a related study in a tertiary Indian hospital revealed that UTI occurred more frequently in females (70.5%) as compared to males (29.5%).[13] In our study, we also noticed higher prevalence of UTI in female, especially in housewives (71.42% culture-positive and 66.01 culture-negative UTI). In the current study, majority of the culture-positive patients were observed in 51-60 years age group (42.85%) which was similar to a study by R. Simkhada[14] in Nepal and in Pakistan[6] but on the contrary to the work done by May Sewify et al.[4] in Kuwait and also in Nepal by Jha PK, et al.[15]
In a study in Sudan, diabetic patients having poor glycemic control had a high prevalence of UTI.[16] Poor control of DM increases the risk of UTI by 24%.[17] In our study, we also noticed that uncontrolled glycemia (HbA1c >7.5%) was also related to higher risk of culture-positive UTI (P < 0.00001, Odds ratio 21.71).
There are several studied all over the world including India which revealed the most common pathogen causing UTI in DM is a Gram-negative bacteria and Escherichia coli is the major isolate in culture in both symptomatic and asymptomatic patients.[16,18,19] We also observed similar isolates in this study – Gram-negative bacteria (65.3%) vs. Gram-positive bacteria (28.57%). In our study too, E. coli was the most prevalent pathogen.
In our study, gram-negative isolates were nearly 100% susceptible to antibiotics such as Piperacillin-tazobactam, cefoperazone-sulbactam, Meropenem, Imipenem, etc., possibly because of the fact that the patients were all from the community and therefore not exposed to unnecessary antibiotics or not exposed to highly resistant bacteria. But the resistant pattern revealed in this study was comparable to study in Ethiopia.[20] High resistance to fluoroquinolones and ampicillin observed in our study was probably due to erroneous use in empirical treatment in our country. Easy availability and indiscriminate use of commonly prescribed antibiotic such as cotrimoxazole, ciprofloxacin, levofloxacin, and ampicillin may explain the observed resistance. ESBLs producers were found in moderate numbers (34%) with good sensitivity to carbapenems. These findings were similar to many studies abroad but dissimilar to a study from Kerala.[21,22] However, Gram-negative isolates were sensitive to carbapenems and antipseudomonal penicillins, these drugs cannot be recommended in outpatient settings due to their supervised drug administration protocols. Fosfomycin and nitrofurantoin have shown intermediate to full susceptibility pattern (68.8% and 71.9% respectively). This observation questions the use of fluoroquinolone as the empiric treatment.[22] This is consistent with earlier studies.[5] Therefore, nitrofurantoin and fosfomycin may be used as the empirical drug of choice in the study area as a potential treatment of UTI.
Among the gram-positive isolates, Staphylococcus aureus was the most common isolates followed by Enterococcus spp. Antibiotic susceptibility patterns were comparable to the study done in Kerala.[21]
Knowledge regarding the local sensitivity pattern of the organisms is cornerstone to the selection of antibiotics for proper management. Indiscriminate use of antibiotics and especially the use of less sensitive antibiotics as empirical therapy make more complications. This study will help the policy makers to formulate the antibiotic policy for rational use of antibiotics, which could help primary care physician to prescribe proper empirical antibiotics. This will reduce the probability of local antibiotics resistance pattern at community level. The limitations of our study are many including small sample size, sampling technique, non-inclusion of control group, not taking into account the history of nephrolithiasis or any bladder disorders, and sexual history.
Conclusion
High prevalence of UTI in diabetic patients was observed. Female (housewives) showed high frequency of UTI compared to male. Poor glycemic control was highly associated with culture positive UTI. All patients with diabetes must be searched for urinary tract bacterial colonization by simple routine urinary culture even though they are asymptomatic. E. coli species and Klebsiella species were the most common isolates. Imipenem, meropenem, Anti-Pseudomonal Penicillin, fosfomycin, and nitrofurantoin had high susceptibility profile against the isolated pathogens. Resistance to common antibiotics, particularly to oral formulations (especially Fluoroquinolones and Ampicillin) is increasing day by day due to indiscriminate use of antibiotics. This study highlighted that the policy makers should formulate antibiotic policy for rational use of antibiotics, which could help clinicians to prescribe proper antibiotics after getting culture sensitivity report. However, regular monitoring of susceptibility pattern of urinary pathogens is essential to establish reliable information for optimal empirical therapy of diabetic patients with urinary tract infection.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Anjana RM, Deepa M, Pradeepa R, Mahanta J, Narain K, Das HK, et al. Prevalence of diabetes and prediabetes in 15 states of India: Results from the ICMR-INDIAB population-based cross-sectional study. Lancet Diabetes Endocrinol. 2017;5:585–96. doi: 10.1016/S2213-8587(17)30174-2. [DOI] [PubMed] [Google Scholar]
- 2.Chawla R, Madhu SV, Makkar BM, Ghosh S, Saboo B, Kalra S. RSSDI-ESI clinical practice recommendations for the management of type 2 diabetes mellitus 2020. Indian J Endocr Metab. 2020;24:1–122. doi: 10.4103/ijem.IJEM_225_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madhu SV, Sandeep G, Mishra BK, Aslam M. High prevalence of diabetes, prediabetes and obesity among residents of East Delhi – The Delhi urban diabetes survey (DUDS) Diabetes Metab Syndr. 2018;12:923–7. doi: 10.1016/j.dsx.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 4.Sewify M, Nair S, Warsame S, Murad M, Alhubail A, Behbehani K, et al. Prevalence of urinary tract infection and antimicrobial susceptibility among diabetic patients with controlled and uncontrolled glycemia in Kuwait. J Diabetes Res. 2016. 2016:6573215. doi: 10.1155/2016/6573215. DOI:10.1155/2016/6573215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmad S, Hussain A, Khan MSA, Shakireen N, Ali I. Diabetes mellitus and urinary tract infection: Causative uropathogens, their antibiotic susceptibility pattern and the effects of glycemic status. Pak J Med Sci. 2020;36:1550–7. doi: 10.12669/pjms.36.7.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zubair KU, Shah AH, Fawwad A, Sabir R, Butt A. Frequency of urinary tract infection and antibiotic sensitivity of uropathogens in patients with diabetes. Pak J Med Sci. 2019;35:1664–8. doi: 10.12669/pjms.35.6.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alemu M, Belete MA, Gebreselassie S, Belay A, Gebretsadik D. Bacterial profiles and their associated factors of urinary tract infection and detection of extended spectrum beta-lactamase producing gram-negative uropathogens among patients with diabetes mellitus at Dessie Referral Hospital, Northeastern Ethiopia. Diabetes Metab Syndr Obes. 2020;13:2935–48. doi: 10.2147/DMSO.S262760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scholes D, Hooton TM, Roberts PL, Gupta K, Stapleton AE, Stamm WE. Risk factors associated with acute pyelonephritis in healthy women. Ann Intern Med. 2005;142:20–7. doi: 10.7326/0003-4819-142-1-200501040-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Acharya D, Bogati B, Shrestha G, Gyawali P. Diabetes mellitus and urinary tract infection: Spectrum of uropathogens and their antibiotic sensitivity. J Manmohan Mem Inst Health Sci. 2015;1:24–8. [Google Scholar]
- 10.Sarvepalli VK, Patak NP, Kandati J, Pathapati RM, Buchineni M. Prevalence of uropathogens and their antibiogram in diabetic patients a cross sectional study. Int J Curr Microbiol App Sci. 2015;4:226–35. [Google Scholar]
- 11.Sharma V, Gupta V, Mittal M. Prevalence of uropathogens in diabetic patients and their antimicrobial susceptibility pattern. Natl J lab Med. 2012;1:26–8. [Google Scholar]
- 12.Akash MSH, Rehman K, Fiayyaz F, Sabir S, Khurshid M. Diabetes-associated infections: Development of antimicrobial resistance and possible treatment strategies. Arch Microbiol. 2020;202:953–65. doi: 10.1007/s00203-020-01818-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janifer J, Geethalakshmi S, Satyavani K, Viswanathan V. Prevalence of lower urinary tract infection in South Indian type 2 diabetic subjects. Indian J Nephrol. 2009;19:107–11. doi: 10.4103/0971-4065.57107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simkhada R. Urinary tract infection and antibiotic sensitivity pattern among diabetics. Nepal Med Coll J. 2013;15:1–4. [PubMed] [Google Scholar]
- 15.Jha PK, Baral R, Khanal B. Prevalence of uropathogens in diabetic patients and their susceptibility pattern at a Tertiary care center in Nepal-A retrospective study. Int J Biomed Lab Sci. 2014;3:29–34. [Google Scholar]
- 16.Hamdan HZ, Kubbara E, Adam AM, Hassan OS, Suliman SO, Adam I. Urinary tract infections and antimicrobial sensitivity among diabetic patients at Khartoum, Sudan. Ann Clin Microbiol Antimicrob. 2015;14:26. doi: 10.1186/s12941-015-0082-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirji I, Guo Z, Andersson SW, Hammar N, Gomez-Caminero A. Incidence of urinary tract infection among patients with type 2 diabetes in the UK General practice research database (GPRD) J Diabetes Complications. 2012;26:513–6. doi: 10.1016/j.jdiacomp.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Aswani SM, Chandrashekar U, Shivashankara K, Pruthvi B. Clinical profile of urinary tract infections in diabetics and non-diabetics. Australas Med J. 2014;7:29–34. doi: 10.4066/AMJ.2014.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kakde P, Redkar NN, Yelale A. Urinary tract infection in elderly: Clinical profile and outcome. J Assoc Physicians India. 2018;66:14–7. [PubMed] [Google Scholar]
- 20.Yenehun Worku G, Belete Alamneh Y, Erku Abegaz W. Prevalence of bacterial urinary tract infection and antimicrobial susceptibility patterns among diabetes mellitus patients attending Zewditu memorial hospital, Addis Ababa, Ethiopia. Infect Drug Resist. 2021;14:1441–54. doi: 10.2147/IDR.S298176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prabhu H, Oommen AT, Henry R. Clinico-microbiological profile of urinary tract infections in diabetic patients in Tertiary centre in Kerala. Kerala Med J. 2016;9:97–104. [Google Scholar]
- 22.Kumar N, Chatterjee K, Deka S, Shankar R, Kalita D. Increased isolation of extended-spectrum beta-lactamase-producing Escherichia coli from community-onset urinary tract infection cases in Uttarakhand, India. Cureus. 2021;13:e13837. doi: 10.7759/cureus.13837. [DOI] [PMC free article] [PubMed] [Google Scholar]


