Abstract
The cloning of a human G-protein-coupled receptor (GPCR) that specifically responds to UDP-glucose and related sugar-nucleotides has been reported recently. This receptor has important structural similarities to known members of the P2Y receptor family but also shows a distinctly different pharmacological response profile. Here, the IUPHAR Subcommittee for P2Y receptor nomenclature and classification review the current knowledge of this receptor and present their reasons for including this receptor in the P2Y receptor family as the P2Y14 receptor.
UDP-glucose serves well-established biochemical roles in the synthesis of carbohydrates and protein glycosylation. However, it is far less appreciated that this nucleotide and other related sugar-nucleotides can exert pharmacological activity and thus receptor(s) for these molecules might exist. The recent cloning of a human GPCR (also known as GPR105 or KIAA0001) that specifically responds to UDP-glucose and related sugar-nucleotides [1] has important structural similarities to known members of the P2Y receptor family (which use simpler nucleotides as their agonists), but also shows a distinctly different pharmacological response profile. We discuss the pharmacological and signalling properties of this novel receptor and its potential role(s) in intercellular communication.
Subclassification of P2 nucleotide receptors
The role of extracellular adenine (ATP and ADP) and uracil (UTP and UDP) nucleotides as signalling molecules is phylogenetically ancient and universal in plants and animals. Receptors for these compounds – the P2 receptor family – are found on the surface of all animal tissues [2,3]. The existence of P2X and P2Y receptor subtypes was first suggested, on the basis of pharmacology, by Burnstock and Kennedy [4] in 1985. Following later studies of transduction mechanisms and cloning, in 1994 Abbracchio and Burnstock [5] proposed that P2 receptors should be subdivided into two families: (1) P2X ionotropic ligand-gated ion channel receptors; and (2) P2Y metabotropic G-protein-coupled receptors. This proposal has been adopted unanimously, with growing numbers of subtypes recognized since that time [3,6].
Current and putative P2Y receptors
The recognized members of the P2Y receptor family are the mammalian P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12 and P2Y13 receptors [7–10]. The missing numbers in the P2Y1–n sequence represent receptors cloned from non-mammalian vertebrates (whose mammalian orthologues have not yet been identified), or receptors that are currently under functional characterization. These and other potential members of the P2Y receptor family will be the subjects of future publications from the IUPHAR Subcommittee for P2Y receptor nomenclature and classification.
Alignment of the deduced amino acid sequences of the cloned P2Y receptors shows that human members of this family are 21–57% identical. When conservative substitutions are taken into consideration (i.e. where amino acid residues have been exchanged by other residues of similar structure or charge), the degree of relatedness among these receptors is much higher (36–69%). Pharmacologically, P2Y receptors can be subdivided into the adenine-nucleotide-preferring receptors mainly responding to ADP and ATP (human and rodent P2Y1, P2Y12 and P2Y13, and human P2Y11), the uracil-nucleotide-preferring receptors (human P2Y4 and P2Y6) responding to either UTP or UDP, and receptors of mixed selectivity (human and rodent P2Y2 and rodent P2Y4) [7–12].
Structural motifs involved in P2Y receptor activity
From a phylogenetic and structural point of view, two distinct P2Y receptor subgroups with a relatively high level of structural divergence have been identified [10,13]: the first subgroup encompasses P2Y1, P2Y2, P2Y4, P2Y6 and P2Y11 receptors and the second subgroup includes P2Y12 and P2Y13 receptors (Fig. 1). Site-directed mutagenesis of P2Y receptors, to probe for regions of agonist–receptor interactions, has suggested that four amino acid residues of the transmembrane (TM) regions TM6 and TM7 might be important for agonist potency and specificity [12,14,15]. In particular, all cloned P2Y receptors share the TM6 H-X-X-R/K motif, which is crucial for agonist activity (Fig. 2). For P2Y1, P2Y2, P2Y4, P2Y6 and P2Y11 receptors, a Y-Q/K-X-X-R motif in TM7 is also considered to participate in ligand binding. In the long-sought platelet P2Y12 receptor [9] and the recently identified P2Y13 receptor [10,11], this defining motif is substituted with K-E-X-X-L (Fig. 2), which suggests that these receptors possess a different mode of agonist binding.
Fig. 1.
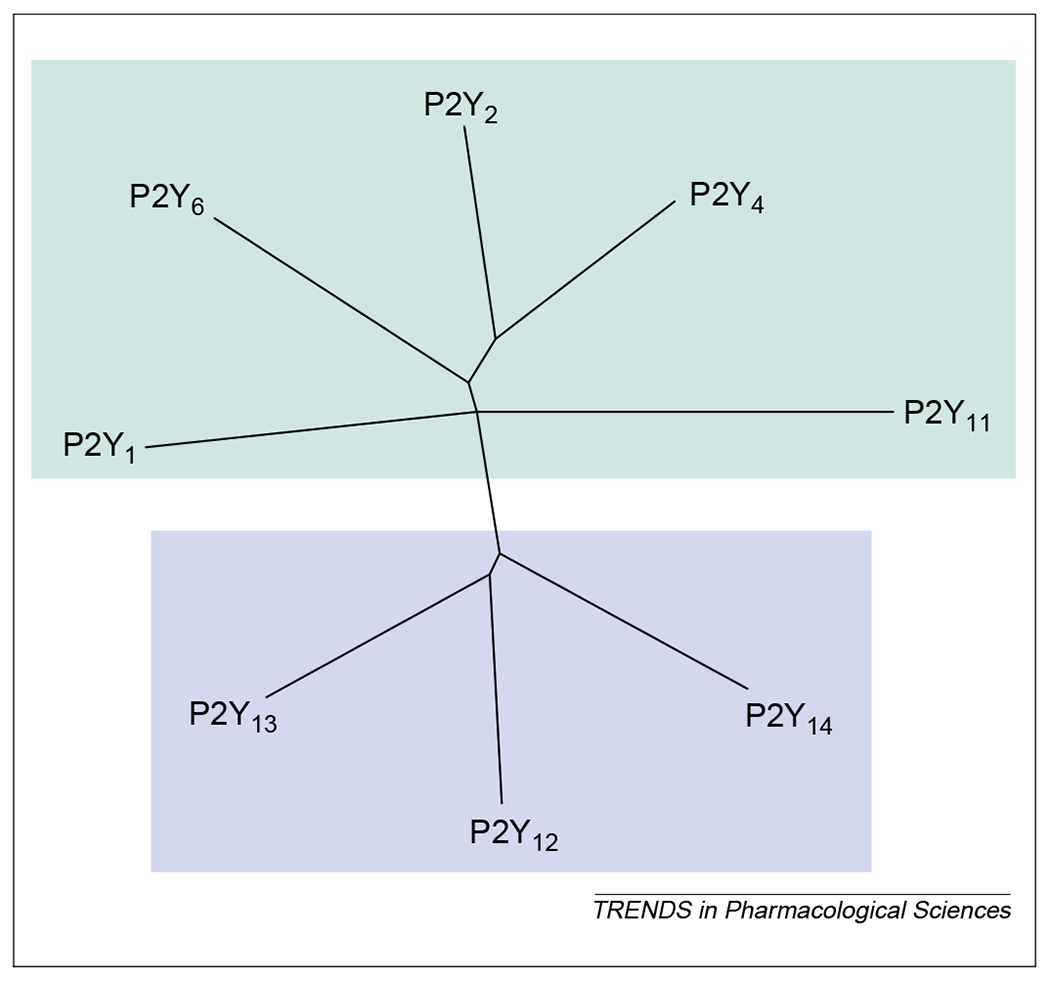
A phylogenetic tree (dendrogram) showing the relationships among the current members of the P2Y receptor family (human P2Y1 P2Y2, P2Y4, P2Y6, P2Y11, P2Y12 and P2Y13 receptors) and the human UDP-glucose receptor (here indicated as the P2Y14 receptor). The P2Y receptors can be divided into two subgroups shown with green and blue backgrounds. Sequences were aligned using clustalx and the tree was built using the treeview software.
Fig. 2.
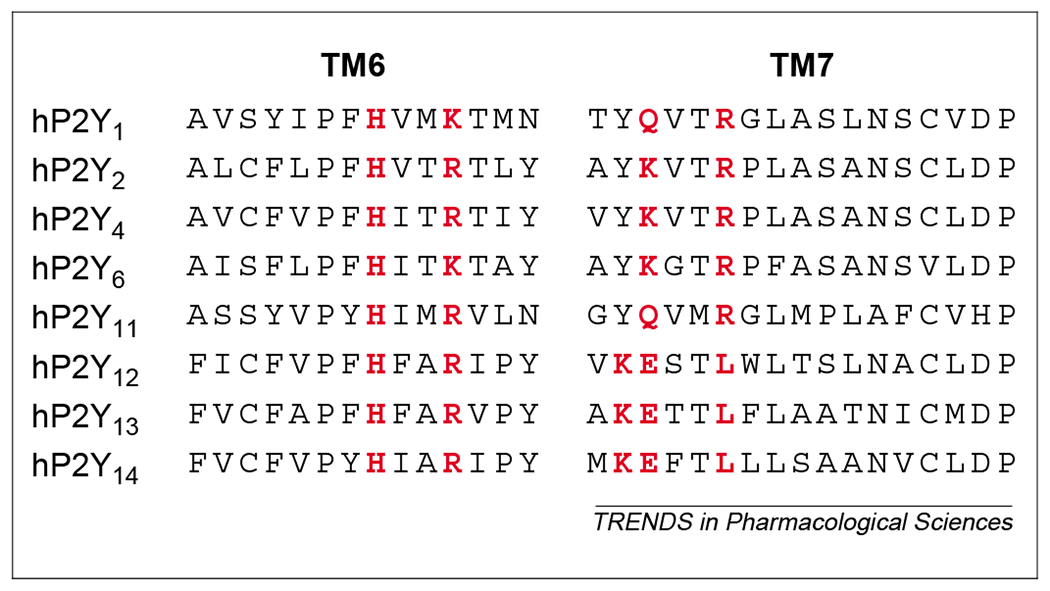
Alignment of putative nucleotide binding motifs in transmembrane domain 6 (TM6) and TM7 of human (h) P2Y receptors. All receptor subtypes share in TM6 the presence of the H and R/K amino acid residues proposed to be crucial for receptor activity. In P2Y1 P2Y2, P2Y4, P2Y6 and P2Y11 receptors, a Y-Q/K-X-X-R motif in TM7 has also been proposed to participate in ligand binding. In P2Y12 and P2Y13 receptors and in the UDP-glucose receptor (here indicated as the P2Y14 receptor subtype), this motif is substituted with K-E-X-X-L. Crucial amino acids for nucleotide binding are highlighted in red. Sequences were aligned using clustalx.
The final number of P2Y receptors is likely to exceed the cloned members listed above. This is based on the following considerations: (1) pathophysiological responses have been reported to be mediated by P2Y-like receptors characterized by pharmacological profiles that do not readily correspond with the known recombinant P2Y receptors [16,17]; and (2) a series of ’orphan’ GPCRs (i.e. cloned receptors available in the public database for which a natural ligand has not been identified) share significant sequence identity with some P2Y receptors. Importantly, these orphan receptors also retain the amino acid motifs in TM6 and TM7 that are considered to be essential for P2Y receptor activation. In line with this hypothesis, one of these orphan receptors (formerly known as GPR86, GPR94 or SP174) is the P2Y13 receptor [10,11]. Moreover, some of the cloned P2Y receptors cluster in the same regions of human chromosomes where several orphan GPCRs are also present. A cluster of seven related GPCRs, consisting of the P2Y1, P2Y12 and P2Y13 receptors and four other receptors, has been found in human chromosome 3q24–3q25 [18]. Three of these genes (GPR87, GPR91 and H963) still await identification; however, the fourth gene (GPR105) has been identified recently as the UDP-glucose receptor [1].
The UDP-glucose receptor
Similarities with known P2Y receptors
The UDP-glucose receptor was originally cloned from human myeloid cells (KIAA0001) and designated an orphan receptor (GPR105) [19]. This human receptor is most closely related in structure to the orphan GPCRs H963, GPR34 and EBI2; however, the thrombin and platelet-activating factor receptors represent the closest relatives with known functions. Sequence comparisons reveal that P2Y1,2,4,6,11 receptors are also structurally related to the UDP-glucose receptor, and that they might share a recent common ancestor [1]. Alignment of the sequences of the UDP-glucose receptor and human P2Y receptors reveals an overall identity of 18–45% (with highest identities in the regions corresponding to the TM1–TM7 domains) and further reveals the retention of the typical H-X-X-R and K-E-X-X-L motifs found in P2Y12 and P2Y13 receptors (Fig. 2). In the dendrogram of P2Y receptors shown in Fig. 1, the UDP-glucose receptor lies with the P2Y12 and P2Y13 receptors in the second main branch of the P2Y family.
The characteristic TM6 and TM7 amino acid motifs indicated above are also fully conserved in a previously cloned rat receptor (initially named VTR15–20 because it was isolated from the ventral tegmentum of rat) [20], whose complete sequence has been reported recently [21], as well as in the sequence of mouse GPR105 [21]. These receptors showing 80% and 83% amino acid identity, respectively, to the human UDP-glucose receptor [21], and indeed represent its rodent orthologues.
Pharmacological profile and coupling to G proteins
In recombinant systems that express human GPR105 or its rodent orthologues, the sugar-nucleotides UDP-glucose, UDP-galactose, UDP-glucuronic acid and UDP-N-acetyl-glucosamine exhibited agonist activity, with EC50 values in the 100–500 nM range [1,21]. No significant responses were detected with ATP, ADP, UTP, UDP, other nucleotides, dinucleotides, nucleosides, and other sugar-nucleotides, demonstrating a distinctly different pharmacological profile from that of the currently known P2Y receptors. Cell membranes expressing human GPR105 responded to UDP-glucose with increased binding of radiolabelled GTPγS – a characteristic indicator of G-protein activation [1]. Pertussis toxin completely abolished agonist responses, confirming the receptor to be coupled to G proteins of the Gi/o class [18]. The transduction pathway(s) used by this receptor in native systems still remains to be defined.
Tissue distribution and potential functional roles
Widespread distribution of GPR105 has been observed in humans, with highest expression in placenta, adipose tissue, stomach and intestine, and moderate levels in the brain, spleen, lung and heart [1]. This expression pattern differs slightly from that reported in the rat for VTR15–20, with higher levels of expression in cells of haemopoietic origin (including kidney, liver, lung and spleen) and lower, yet significant, expression in discrete brain regions [20]. The recently reported tissue distribution of the mouse UDP-glucose receptor [21] is in broad agreement with the results obtained with the human receptor. Rat VTR15–20 has also been detected in several cell lines (including astrocytoma, neuronal and promyelocytic cells), and in rat primary microglia and astrocytes [20]. To confirm the potential importance of this receptor in glial cells, rat primary astrocytes have been shown recently to respond to UDP-glucose with increases of intracellular Ca2+ concentration [M. Fumagalli et al., unpublished]. Interestingly,VTR15–20 has been reported to be regulated by immunological challenge. Following challenge with zymosan, a stimulator of macrophage phagocytosis, expression of VTR15–20 in rat primary microglia and astrocytes was significantly upregulated [20]. Acute in vivo lipopolysaccharide challenges in the rat also resulted in upregulation of VTR15–20 mRNA in discrete brain regions [20]. These data suggest that, at least in the rat, the UDP-glucose receptor might link the humoral and nervous systems to infection and inflammation.
One of the key elements in the definition of a membrane receptor-mediated signalling system is the demonstration of the release of the putative natural agonist into the extracellular space. Recently, using an enzymatic and high performance liquid chromatography (HPLC)-based methodology, the release of UDP-glucose in the lumen of primary cultures of bronchial epithelial cells from normal subjects and from patients with cystic fibrosis has been detected [22]. Under resting conditions, UDP-glucose levels were found in the 10–20 nM range. Similar levels have been detected in other cell lines including Calu-3 endothelial, 1321N1 astrocytoma, C6 glioma, Cos-7 and Chinese hamster ovary (CHO)-K1 cells.
Concluding remarks
UDP-glucose and UDP-galactose have long been thought to act exclusively as activated carriers of sugar moieties in the intermediary metabolism of carbohydrates. The data reviewed above showing the existence of a specific membrane receptor for UDP-glucose and its upregulation following immunological challenge requires a careful reappraisal of the physiological roles of these substances. Furthermore, based on: (1) sequence homology with cloned P2Y receptors; (2) the presence of key amino acids essential for receptor activation; (3) chromosomal colocalization with other P2Y receptors; (4) similarity of the cognate ligand to the natural ligands for P2Y receptors; and (5) identification of UDP-glucose in the extracellular space, the IUPHAR Subcommittee rename this receptor the P2Y14 receptor.
The limited evidence on the potential pathophysiological roles of the P2Y14 receptor needs to be confirmed by functional studies, preferably including the development of null alleles (i.e. knockout mice), anti-sense and RNA interference technology, and the development of small-molecule antagonists. However, the similarity of UDP-glucose and related molecules to the natural ligands for the P2Y receptors, which have established roles in intercellular communication, provides an exciting challenge for future research. It also encourages a search for new members of the P2Y12–14 branch of this ubiquitous receptor family because the divergence of the two branches is so great that new members could not have been detected by homology screening based on the previously known P2Y receptors.
Acknowledgements
We are grateful to Jon Chambers (Millennium Pharmaceutical Ltd, Cambridge, UK) for useful discussion.
References
- 1.Chambers JK et al. (2000) A G protein-coupled receptor for UDP-glucose. J. Biol. Chem 275, 10767–10771 [DOI] [PubMed] [Google Scholar]
- 2.Neary JT et al. (1996) Trophic actions of extracellular nucleotides and nucleosides on glial and neuronal cells. Trends Neurosci. 19, 13–18 [DOI] [PubMed] [Google Scholar]
- 3.Ralevic V and Burnstock G (1998) Receptors for purines and pyrimidines. Pharmacol. Rev 60, 413–492 [PubMed] [Google Scholar]
- 4.Burnstock G and Kennedy C (1985) Is there a basis for distinguishing two types of P2-purinoceptor? Gen. Pharmacol 16, 433–440 [DOI] [PubMed] [Google Scholar]
- 5.Abbracchio MP and Burnstock G (1994) Purinoceptors: are there families of P2X and P2Y purinoceptors? Pharmacol. Ther 64, 445–475 [DOI] [PubMed] [Google Scholar]
- 6.Fredholm BB et al. (1994) International union of pharmacology. VI. Nomenclature and classification of purinoceptors. Pharmacol. Rev 46, 143–156 [PMC free article] [PubMed] [Google Scholar]
- 7.King BF et al. (2000) P2Y receptors. IUPHAR Compendium of Receptor Characterization and Classification, pp. 307–320, IUPHAR Media [Google Scholar]
- 8.Hollopeter G et al. (2001) Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature 409, 202–207 [DOI] [PubMed] [Google Scholar]
- 9.Communi D et al. (2001) Identification of a novel human ADP receptor coupled to Gi. J. Biol. Chem 276, 41479–41485 [DOI] [PubMed] [Google Scholar]
- 10.Zhang FL et al. (2002) P213: identification and characterization of a novel Gαi- coupled ADP receptor from human and mouse. J. Pharm. Exp. Ther 301, 705–713 [DOI] [PubMed] [Google Scholar]
- 11.Boarder M and Webb TE (2001) P2Y receptors structure and function. Purinergic and Pyrimidinergic Signalling Handbook of Experimental Pharmacology (Vol. 151/I) (Abbracchio MP, Williams M eds), pp. 65–88, Springer-Verlag [Google Scholar]
- 12.Barnard EA and Simon J (2001) An elusive receptor is finally caught: P2Y12, an important drug target in platelets. Trends Pharmacol. Sci 22, 388–391 [DOI] [PubMed] [Google Scholar]
- 13.Jacobson KA et al. (2002) Purine and pyrimidine (P2) receptors as drug targets. J. Med. Chem 45, 4057–4093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erb L et al. (1995) Site-directed mutagenesis of P2U receptors: positively charged amino acids in transmembrane helix-6 and helix-7 affect agonist potency and specificity. J. Biol. Chem 270, 4185–4188 [DOI] [PubMed] [Google Scholar]
- 15.Jiang Q et al. (1997) A mutational analysis of residues essential for ligand recognition at the human P2Y1 receptor. Mol. Pharmacol 52, 499–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brambilla R et al. (1999) Cyclo-oxygenase 2 mediates P2Y-receptor-induced reactive astrogliosis. Br. J. Pharmacol 126, 563–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Windscheif U et al. (1995) Inhibitory action of PPADS on the relaxant responses to adenine nucleotides or electrical field stimulation in guinea-pig taenia-coli and rat duodenum. Br. J. Pharmacol 115, 1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wittenberger T et al. (2001) An expressed sequence tag (EST) data mining strategy succeeding in the discovery of new G-protein coupled receptors. J. Mol. Biol 307, 799–813 [DOI] [PubMed] [Google Scholar]
- 19.Nomura N et al. (1994) Prediction of the coding sequences of unidentified human genes. I. The coding sequences of 40 new genes (KIAA0001–KIAA0040) deduced by analysis of randomly sampled cDNA clones from human immature myeloid. DNA Res. 1, 27–35 [DOI] [PubMed] [Google Scholar]
- 20.Charlton ME et al. (1997) The isolation and characterization of a novel G protein-coupled receptor regulated by immunologic challenge. Brain Res. 764, 141–148 [DOI] [PubMed] [Google Scholar]
- 21.Freeman K et al. (2001) Cloning, pharmacology, and tissue distribution of G-protein-coupled receptor GPR105 (KIAA0001) rodent orthologs. Genomics 78, 124–128 [DOI] [PubMed] [Google Scholar]
- 22.Lazarowski E and Boucher R (2002) Luminal accumulation of UDP-glucose reveals a novel pathway for nucleotide release by airway epithelial cells. Ped. Pulmon. Suppl 24, 235 [Google Scholar]


