Abstract
Therapeutic drug monitoring (TDM) uses drug concentrations, primarily from plasma, to optimize drug dosing. Optimisation of drug dosing may improve treatment outcomes, reduce toxicity and reduce the risk of acquired drug resistance. The aim of this narrative review is to outline and discuss the challenges of developing multi-analyte assays for anti-tuberculosis (TB) drugs using liquid chromatography-tandem mass spectrometry (LC-MS/MS) by reviewing the existing literature in the field. Compared to other analytical methods, LC-MS/MS offers higher sensitivity and selectivity while requiring relatively low sample volumes. Additionally, multi-analyte assays are easier to perform since adequate separation and short run times are possible even when non-selective sample preparation techniques are used. However, challenges still exist, especially when optimizing LC separation techniques for assays that include analytes with differing chemical properties. Here, we have identified seven multi-analyte assays for first-line anti-TB drugs that use various solvents for sample preparation and mobile phase separation. Only two multi-analyte assays for second-line anti-TB drugs were identified (including either nine or 20 analytes), with each using different protein precipitation methods, mobile phases and columns. The 20 analyte assay did not include bedaquiline, delamanid, meropenem or imipenem. For these drugs, other assays with similar methodologies were identified that could be incorporated in the development of a future comprehensive multi-analyte assay.
TDM is a powerful methodology for monitoring patient’s individual treatments in TB programmes, but its implementation will require different approaches depending on available resources. Since TB is most-prevalent in low- and middle-income countries where resources are scarce, a patient-centred approach using sampling methods other than large volume blood draws, such as dried blood spots or saliva collection, could facilitate its adoption and use. Regardless of the methodology of collection and analysis, it will be critical that laboratory proficiency programmes are in place to ensure adequate quality control.
It is our intent that the information contained in this review will contribute to the process of assembling comprehensive multiplexed assays for the dynamic monitoring of anti-TB drug treatment in affected individuals.
Abbreviations: ADR, acquired drug resistance; DBS, dried blood spots; DS-TB, drug-susceptible tuberculosis; EMA, European Medicines Agency; FDA, Food and Drug Administration; HILIC, hydrophilic interaction liquid chromatography; HPLC, high performance liquid chromatography; LC-MS/MS, liquid chromatography-tandem mass spectrometry; MDR-TB, multidrug-resistant tuberculosis; MTB, Mycobacterium tuberculosis; SIL-IS, stable isotopically labelled-internal standards; TDM, therapeutic drug monitoring; TB, tuberculosis; UHPLC-UV, ultra-high performance liquid chromatography-ultra violet detection; WHO, World Health Organisation
Keywords: Tuberculosis, Liquid chromatography, LC-MS/MS, TDM, Multi-analyte assays
1. Introduction
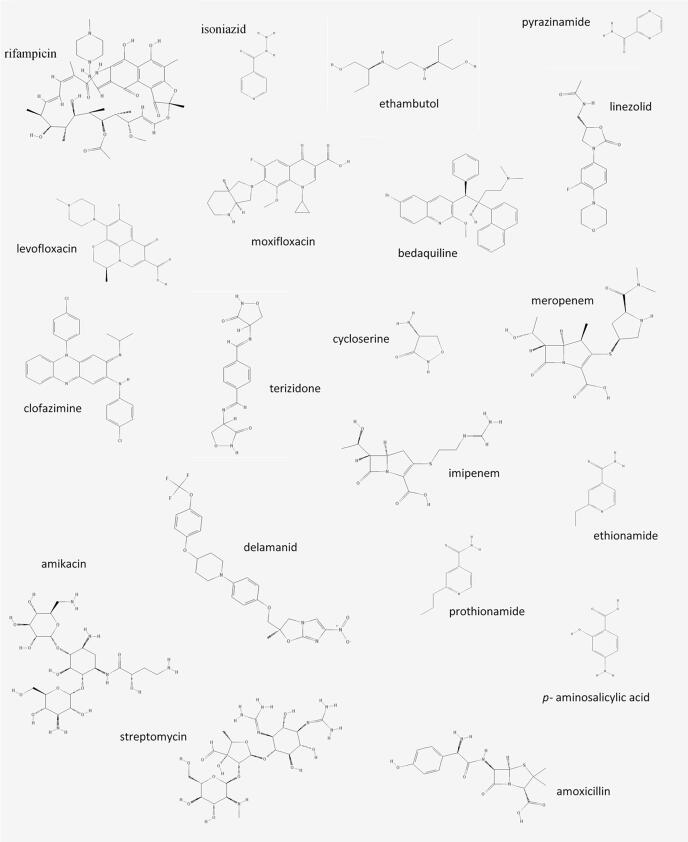
Tuberculosis (TB), caused by Mycobacterium tuberculosis (MTB), is a worldwide infectious disease feared for its high morbidity and mortality. TB is treated with a cocktail of antimicrobial drugs to prevent acquired drug resistance (ADR) and treatment failure. Drug-susceptible TB (DS-TB) is currently treated with the first-line drugs isoniazid, rifampicin, ethambutol and pyrazinamide for two months, followed by isoniazid and rifampicin for an additional four months [1]. Multidrug-resistant TB (MDR-TB), defined by resistance to at least isoniazid and rifampicin, requires simultaneous treatment with second-line anti-TB drugs, which are generally less efficient and more toxic [2]. For the short course MDR-TB regimen, a cocktail of seven anti-TB drugs is prescribed for nine to twelve months, while for the longer standard treatment a cocktail of at least five anti-TB drugs is prescribed for at least 20 months [2], [3]. The World Health Organisation's (WHO) newly released rapid communication divides the second-line drugs for the treatment of MDR-TB into groups A, B and C based on their effectiveness and safety [3]. Group A consists of the fluoroquinolones, levofloxacin and moxifloxacin, as well as the novel MDR-TB drug bedaquiline and linezolid. Group B consists of clofazimine, cycloserine and terizidone. Group C is a mixed group consisting of ethambutol, the novel drug delamanid, pyrazinamide, imipenem-cilastin, meropenem, the two injectable drugs amikacin and streptomycin, ethionamide, prothionamide and p-aminosalicylic acid. Amoxicillin-clavulanate is not included in any group and not counted as a separate drug, but should be included together with the carbapenems, imipenem-cilastin and meropenem. In the longer standard treatment, WHO recommends including three Group A drugs (using one of the fluoroquinolones), two Group B drugs (using one of either cycloserine or terizidone), and then adding Group C drugs to complete the regimen, up to at least five drugs if Group A and B drugs cannot be used [3]. Gatifloxacin, thioacetazone and the injectables capreomycin and kanamycin are no longer recommended anti-TB drugs. The newly excluded drugs will be mentioned briefly since the changes were recently made. The chemical structures of the recommended drugs are depicted in Fig. 1 [2].
Fig. 1.
Chemical structure of first- and second-line anti-tuberculosis drugs [89] (imipenem is depicted without cilastin component and amoxicillin without clavulanate component).
As current treatment strategies under programmatic conditions cure only 50% of MDR-TB patients, healthcare professionals face numerous challenges concerning treatment improvement [4]. A tool that is increasingly being recommended to improve outcome is therapeutic drug monitoring (TDM). TDM is focused on the measurement of drug concentrations in body fluids and aims to improve patient treatment outcome by adjusting the dose of drugs to increase efficacy of treatment, thereby reducing the risk of ADR, while minimizing toxicity.
TDM is valuable when it is difficult to otherwise directly measure the pharmacological effect of a drug. To effectively perform TDM, a relationship between drug concentration (pharmacokinetics) and pharmacological effect (pharmacodynamics), which can be efficacy and/or toxicity, needs to have been established [5]. The correlation between anti-TB drug exposure and mycobactericidal activity has been demonstrated using data from hollow fibre infection models, which mimic human pharmacokinetics [6]. Key drugs such as isoniazid, rifampicin, pyrazinamide and the fluoroquinolones show a clinically relevant pharmacokinetic variability between and within patients [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17]. Additionally, a randomized controlled trial demonstrated that isoniazid dosing based on an N-acetyltransferase polymorphism resulted in fewer treatment failures for rapid acetylators and fewer adverse drug events in slow acetylators [18]. Ideally, detection, within the first two weeks of treatment, of patients likely to fail to respond to treatment (e.g., determined by sputum culture conversion), due to insufficient drug exposure is desired.
To adequately implement TDM, accurate and precise analytical methods are required. This narrative review will focus on challenges we have identified in the development of multi-analyte assays for anti-TB drugs using liquid chromatography-tandem mass spectrometry (LC-MS/MS).
2. Application and methods of therapeutic drug monitoring
2.1. Therapeutic drug monitoring in a programmatic setting
TDM is a component of personalized medicine and may appear contradictory to a programmatic treatment plan. However, TDM can be part of a programmatic setting to optimize individualized treatment plans, especially in the treatment of TB which requires long term treatment with high toxicity and the risk of developing ADR.
For DS-TB, the most recent joint American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America guideline suggests that TDM can be helpful when either drug malabsorption or drug underdosing is suspected [19]. Medical conditions such as malnutrition, HIV infection, diabetes mellitus or gastrointestinal abnormalities may cause inadequate drug exposure [19], [20]. TDM can also be used to minimize toxicity, e.g. in patients with renal impairment. However, the guideline does not advise on how to practice or implement TDM during treatment [19].
For MDR-TB, the WHO recommends that aminoglycoside concentrations should be monitored in patients with renal impairment, and cycloserine concentrations should be monitored to avoid central nervous system toxicity [21]. Other local and national guidelines also specifically recommend dose monitoring for aminoglycosides, cycloserine and ethambutol [22], [23], [24], [25], [26]. For the first-line drug ethambutol, which is often included in MDR-TB treatment, toxicity studies suggest that higher and/or prolonged doses of ethambutol are associated with optic neuritis, although no data on TDM is available [27], [28]. Higher cycloserine serum concentrations have been related to increased neuropsychiatric toxicity [29], [30]. TDM using defined pharmacokinetic/pharmacodynamic targets can decrease the incidence of hearing loss due to aminoglycoside toxicity, while maintaining high treatment success rates [31]. TDM has been used to lower the dose of linezolid and, thus, reduce toxicity considerably while retaining efficacy [32], [33], [34]. In the Netherlands, an 86% (89/104) treatment success in MDR-TB patients has been reported using dose adjustments with TDM for several drugs while reducing toxicity [35], [36]. Using TDM for aminoglycosides only, a Swedish study reported a 77% (132/158) relapse-free successful outcome for MDR-TB patients [37]. While high success rates can be achieved using the standard MDR-TB regimen, although severe side-effects are often present, these preliminary studies have shown that TDM may be beneficial in reducing toxicity, increasing efficacy and preventing ADR.
Before TDM can be implemented in a programmatic setting in resource constrained countries, several hurdles need to be addressed. Ghimire et al. have proposed an infrastructure for TDM according to the WHO hierarchy of TB diagnostics [38]. At the peripheral level (e.g., subdistrict or community level), dried blood spots (DBS) could be sampled and, subsequently, analysed at the intermediate level (e.g., regional or district laboratory) using a semi-quantitative thin-layer chromatography. At the central level (e.g., reference laboratory), DBS-samples could be analysed using LC-MS/MS, providing country-wide access to TDM. For resource-rich areas, DBS sampling could also be beneficial at peripheral levels, or for certain groups, such as children. In intermediate and central level hospitals, venous blood could also be used, as adequate transportation and storage of samples are usually available. The desired total time from patient sampling to result output, in our opinion, is no more than one week for out-patients. Lengthier times could make results more difficult to interpret as the patient’s medications or condition may change (e.g., due to unbearable side effects). For severely ill patients in the intensive care unit, we recommend results be provided within one day.
2.2. Multi-analyte assays and their pitfalls
When performing TDM for TB regimens, the laboratory needs to be able to quantify a wide range of analytes within a short time frame. While a multiplexed assay, which analyses multiple drugs simultaneously, may provide a shorter turnaround time for a panel analysis when compared to single-plex analysis, the development of such a method is not without pitfalls. The use of stand-alone photometric detection in multi-analyte assays can be cumbersome for complex biological matrices, like plasma. The chance of multiple components absorbing light in the same spectral region is high. If there is a significant spectral difference, it is possible to correct for interference by mathematically processing the absorption spectra [39]. However, with each analyte added, the chance of spectral similarities increases and the correction methods become less successful. Using chromatographic separation reduces the likelihood for interference. High performance liquid chromatography/ultra-high performance liquid chromatography-ultra violet detection (HPLC/UHPLC-UV) can be used to separate analytes from interfering compounds and increase selectivity.
Over the past 15 years LC-MS/MS has become an increasingly preferred method for molecular analysis. In particular, the introduction of the relatively small and robust triple quadrupole has boosted the use of LC-MS/MS [40]. LC-MS/MS combines the advantages of HPLC/UHPLC with a selective detection technique, known as Selected Reaction Monitoring (SRM). Subsequently, chromatographic separation of all compounds or interfering peaks is less of an issue, thereby enabling short run times. Co-eluting endogenous and exogenous compounds can, however, result in ionization suppression or enhancement (i.e., matrix effects), which can adversely affect quantification. Ionization effects can be minimized by optimizing chromatography and corrected for using stable isotopically labelled-internal standards (SIL-IS) for each analyte.
A timed gradient mobile phase can be used to optimize the balance between analyte peak resolution and run time for LC-methods. When including many analytes, relatively long run times remain a drawback. Dedicated assays for smaller clusters of analytes (e.g., all first-line anti-TB drugs) may result in shorter turnaround times. Although a single multi-analyte assay encompassing all desired analytes may be unpractical or practically impossible due to differences in analyte characteristics (Table 1) that are likely to require different separation conditions and detection settings (e.g., solvents, gradient of the mobile phase, analytical column and ionization technique).
Table 1.
Chemical properties of anti-tuberculosis drugs.
| Molecular mass (g/mol) | pKa (strongest acidic/basic) | LogP | Solubility* (mg/ml) | Stability** |
|||
|---|---|---|---|---|---|---|---|
| Room temp. (hours) | Freeze/thaw cycles | Freezer (weeks) | |||||
| Rifampicin | 823.0 | +6.80/+7.37 | +2.95 | 2.70 × 10−3 | 4–12 [45], [47], [48] | yes [45], [47] | 4 [45], [47], [48] |
| Isoniazid | 137.1 | +13.6/+3.35 | −0.690 | 5.34 × 101 | 4–12 [45], [47], [48] | yes [45], [47] | 4 [45], [47], [48] |
| Ethambutol | 204.3 | +14.8/+9.55 | −0.0600 | 1.26 × 102 | 4–12 [45], [47], [48] | yes [45], [47] | 4 [45], [47], [48] |
| Pyrazinamide | 123.1 | +13.1/−0.550 | −1.23 | 4.49 × 102 | 4–12 [45], [47], [48] | yes [45], [47] | 4–12 [45], [47], [48] |
| Levofloxacin | 361.4 | +5.29/+6.16 | +0.510 | 2.01 × 100 | 6 [48] | NA | 4–12 [48] |
| Moxifloxacin | 401.4 | +5.49/+9.42 | −0.500 | 3.20 × 10−1 | 6 [48] | NA | 4–12 [48] |
| Bedaquiline | 555.5 | +13.6/+8.91 | +7.13 | 1.01 x10−5 | 72 [55] | yes [55] | >1 year [55] |
| Linezolid | 337.4 | +14.9/−1.18 | +0.64 | 2.29 × 10−1 | 6 [48] | NA | 4 [48] |
| Clofazimine | 473.4 | NA/+6.63 | +7.30 | 2.64 × 10−5 | 6 [48] | NA | 4 [48] |
| Cycloserine | 102.1 | +4.21/+8.36 | −2.42 | 1.06 × 102 | 6 [48] | NA | 4–12 [48] |
| Terizidone | 302.3 | −0.65/+4.46 | −0.56 | 9.57 × 10−2 | 6 [48]*** | NA | 4–12 [48]*** |
| Delamanid | 534.5 | NA/+5.51 | +6.14 | 6.86 x10−6 | NA | yes [62] | 780 days [62] |
| Imipenem | 317.4 | +3.44/+11.8 | −3.80 | 1.96 × 100 | 4 [64] | no [64] | 10 days [64] |
| Meropenem | 383.5 | +3.28/+9.39 | −4.35 | 1.76 × 100 | 24 [64] | yes [64] | 20 days to 6 months [64], [65] |
| Amikacin | 585.6 | +12.2/+9.61 | −8.58 | 1.49 × 103 | 6 [48] | NA | 4–12 [48] |
| Streptomycin | 581.6 | +10.9/+11.5 | −7.49 | 2.44x102 | 4–12 [45], [47], [48] | yes [45], [47] | 4–12 [45], [47], [48] |
| Ethionamide | 166.2 | +11.89/+5.00 | +1.33 | 1.81 × 100 | no [48] | NA | no [48] |
| Prothionamide | 180.3 | +8.19/+7.25 | +1.94 | 6.18 × 10−1 | no [48] | NA | 4 [48] |
| PAS | 153.1 | +3.68/+2.19 | +0.83 | 1.85 × 101 | 6 [48] | NA | 4 [48] |
| Amoxicillin | 365.4 | +3.23/+7.22 | −2.31 | 1.63 × 10−2 | 6 [48] | NA | 4–12 [48] |
The chemical properties molecular mass, pKa, logP and solubility are calculated [88]. References in the table are only for stability assessments. *In water, **within 15% of nominal concentrations [41], [42], autosampler stability is not included as it is based on the properties of the prepared sample (e.g. acidic, methanolic, aqueous) and for details we refer to the original publication, ***tested as cycloserine. PAS = p-aminosalicylic acid, NA = not available.
2.3. LC-MS/MS versus HPLC-UV
If HPLC-UV and LC-MS/MS methods are validated according to Food and Drug Administration/European Medicines Agency (FDA/EMA) guidelines, they should produce comparable results with similar quality [41], [42]. Due to the relatively low sensitivity and selectivity of HPLC-UV systems, an optimized liquid–liquid extraction or solid phase extraction is often required in conjunction with a concentration step that requires a relatively high sample volume (e.g., 1000 µl). These optimised sample treatments aim to uniquely extract the analyte of interest from the sample matrix. With multiple analytes, the extraction targets become more heterogeneous with potentially broad disparities in chemical properties, such as polarity, molecular weight and pKa (Table 1), making it extremely complex or impossible to achieve high extraction recoveries for all compounds without compromising the sample clean up itself. Selective sample treatment methods targeting different analyte groups are only feasible if there is adequate sample volume.
Compared to UV detection, triple quadrupole MS offers improved sensitivity, selectivity and enables a wider linear detection range. The increased sensitivity and selectivity of the detector reduces the demand for selective sample treatment and sample concentration during extraction and chromatography. Non-selective sample treatments, such as protein precipitation, become feasible and also bring the advantage of high yield recoveries for a wide range of compounds. Non-selective sample treatments are, thus, ideal for multi-analyte assays and permit the sensitive analysis of multiple drugs and their metabolites simultaneously, while requiring relatively low sample volumes (e.g., 5–100 µl). These advantages result in LC-MS/MS-based methods having a higher throughput capacity in comparison to HPLC-UV [43]. The operation of an MS, however, requires a skilled analyst with specific training and knowledge. Furthermore, MS detectors are more expensive than UV-detectors, both for acquisition and maintenance.
3. Multi-analyte assays for anti-tuberculosis drugs
3.1. Multi-analyte assays for first-line anti-tuberculosis drugs
Seven different multi-analyte methods for first-line anti-TB drugs have been published (Table 2) [44], [45], [46], [47], [48], [49], [50]. A paper from Song et al. [44] describes a two-step deproteinization extraction method using methanol. The chromatographic run time was 4 min with adequate calibration ranges for all four first-line drugs [20]. Unfortunately, the polar compounds isoniazid, pyrazinamide and ethambutol eluted near the void volume of the system, co-eluting with endogenous compounds resulting in substantial ion suppression. As matrix effects may vary within and between patients, ion suppression at the time of elution of the compounds of interest should be avoided or overcome using a SIL-IS [51].
Table 2.
Multi-analyte assays for first- and second-line anti-tuberculosis drugs and single -analyte assays for second-line drugs using LC-MS/MS.
| Drug | Internal standard | Prep. time* (min) | Sample volume (µl), matrix | Protein precipitation solvent | Chromatographic column | Range (µg/ml) | Mobile phase Solvent A, B and C, pH | Run time (min) | Retention time (min) | Mass transitions |
|---|---|---|---|---|---|---|---|---|---|---|
| R, H, E, Z [44] | Rifabutin (R), 6-aminonicotinic acid (H, E, Z) | >10 | 50, serum | methanol | YMC Co, Hydrosphere C18 (50 × 2.0 mm, 3 μm) | R 1.1–320, H 0.07–19, E 0.07–18, Z 0.4–290 | A: 0.3% formic acid in 100% methanol B: 0.3% formic acid in water. pH: NA | 4 | R 3.43, H 1.27, E 0.89, Z 134 | R 824–792, H 138–121, E 205–116, Z 124–81 |
| R, H, E, Z, S [45] | Rifaxim (R), Phenformin hydrochloride (other) | >12 | 100, plasma | methanol, formic acid, ammonium acetate | GL Sciences, Interstil HILIC (75 × 2.1 mm, 3 μm) | R, H, Z 0.004–4, E 0.005–0.5, S 0.01–16 | A: methanol B: water with 0.1% formic acid and 5 mM ammonium acetate** pH 3.0. | 2 | R 1.42, H 1.41, E 1.22, Z 1.64, S 1.11 | R 823.7–791.6, H 138.1–121.1, E 205.3–116.2 Z 124.1–81.1, S 582.5–263.3 |
| R, H, E, Z [46], [43] | 6,7-dimethyl-2,3-di(2-pyridyl)quioxaline (R), Thymidine (H, E, Z) | >10 | 200, plasma | acetonitrile | Waters, Acquity HSS T3 (150 × 2.1 mm, 1.8 μm) | R 0.47–60, H, E 0.12–15 Z 0.55–70 | A: water with 0.05% formic acid B: acetonitrile with 0.05% formic acid pH: NA | 6 | R ca 4.4, H ca 1.6, E ca 0.8, Z ca 3.1 | R 824–792, H 138–79, E 205–116, Z 124–81 |
| R, H, E, Z, S [47] | Phenacetin | >18 | 100, plasma | methanol, formic acid | Agilent, Zorbax SB-C18 (100 × 2.1 mm, 3.5 μm) | R, Z 0.2–4, H 0.08–2, E 0.0002–1, S 0.002–0.2 | A: 0.02% heptaflourobutyric acid, 0.2% formic acid in methanol B: 0.02% heptaflourobutyric acid, 0.2% formic acid in water pH: NA | 8.5 | R ca 7.2, H ca 1.3, E ca 1.6, Z ca 1.7, S ca 1.5 | R 823.4–791.2, H 138.1–120.9, E 205.3–116.2 Z 124.1–79.1, S 582.3–262.6 |
| R, H, E, Z [50] | D3-Rifampicin (R), D4-isoniazid (H) 15 N,D3-pyrazinamide (Z), D4-ethambutol dihydrochloride (E) | >82 | 50, plasma | methanol, acetonitrile | Merck, Chromolith Flash RP-18e (25 × 4.6 mm, 2 μm) | R 0.75–30, H 0.5–10, E 0.25–10, Z 4–80 | A: 5 mM ammonium acetate, 5% methanol and 0.45% formic acid in water. B: 5 mM ammonium acetate, 95% methanol and 0.45% formic acid in water C: 5 mM ammonium acetate in water. pH: NA | 10.2 | R ca 5.5, H ca 1.1, E ca 0.9, S ca 1.5 | R 824.0–792.5, H 138.1–121.1, E 205.1–116.1, Z 124.1–81.1 |
| R, H, E, Z, Lfx, Mfx, Am, Km, S, Eto, Pto, Cs, Lzd, Cfz, PAS, Amx [48] | Apramycin (Z, Am, Km, S), D4-Moxifloxacin (other) | >10 | 100 (Z, Am, Km, S) 50 (other), plasma | acetonitrile | Waters, Atlantis HILIC (150 × 2.1 mm, 3 μm) (Z, Am, Km, S) Waters, Atlantis dC18 (150 × 2.0 mm, 3 μm) (other) | R, Lfx, Mfx, Pto 0.2–10, H, E, Eto 0.1–5.0, Z 2.0–100, Am, Km, S, PAS 1.0–50, Cs 0.8–40, Cfz 0.04–2.0, Lzd, Amx 0.4–20 | A: 0.1% formic acid solution B: 0.1% formic acid in acetonitrile pH: NA | Z, Am, Km, S 9 Other 13 | R ca 7.3, H ca 2.1, E ca 1.8, Z ca 2.8, Lfx ca 6, Mfx ca 6.5, Am ca 2.6, Km ca 2.6, S ca 2.6, Eto ca 3.3, Pto ca 4.7, Cs ca 2, Lzd ca 6.7, Cfz ca 7.5, PAS ca 6.3, Amx ca 2.8 | R 823.4–791.1, H 138.0–121.0, E 167.1–107.0, Z 124.0–81.0, Lfx 362.0–318.0, Mfx 402.0–384.0, Am 586.2–425.3, Km 485.5–163.3, S 582.2–263.3, Eto 167.1–107.0, Pto 181.0–154.3, Cs 103.0–58.0, Lzd 338.0–296.0, Cfz 473.2–431.2, PAS 154.0–119.0, Amx 366.0–114.0 |
| Lfx, Mfx, Km, S, Pto, Cs, Lzd, PAS [52] | Gm (Km), Dihydrostreptomycin (S), Lomefloxacin (Lfx, Mfx, Lzd), Ethionamide (Pto), Muscimol (Cs), 4-aminobenzoic acid (PAS) | >8 | 50 (Lfx, Mfx, PAS), 10 (other), serum | acidified methanol | Waters, Acquity HSS T3 (50 × 2.1 mm, 1.8 µm) | Lfx, Mfx, Lzd 1.0–20, Km, S, Cs, 5–100, Pto 0.5–10, PAS 0–100 | A: 10 mM ammonium formate in 0.1% formic acid B: acetonitrile in 0.1% formic acid pH: NA | 3 | Lfx 1.37, Mfx 1.90, Km 0.51, S 0.56, Pto 1.88, Cs 0.63, Lzd 1.96, PAS 1.42 | Lfx 362.3–318.2, Mfx 402.4–384.3, Km 485.5–163.1, S 582.6–263.2, Pto 181.0–154.1, Cs 103.0–75.1, Lzd 338.3–296.2, PAS 154.1–119.0 |
| Bdq [55] | D6-bedaquiline | >6 | 10, serum | acetonitrile, methanol | Thermo Scientific, Hypurity C18 (50 × 2.1 mm, 3 μm) | 0.05–6 | A: 5 g/l ammonium acetate in 100% acetic acid 25 ml/L and 100% triflouroacetic acid 2 ml/L B: water C: acetonitrile pH: NA | 2.6 | 1.9 | 555.1–58.4 |
| Dlm [62] | OPC-14714 | >7 | 50, plasma | acetonitrile | Waters, UPLC BEH C18 (50 × 2.1 mm, 1.7 μm) | 0.001–0.5 | A: 5 mM ammonium bicarbonate and 0.2% ammonium hydroxide in water B: 0.2% ammonium hydroxide in methanol pH: NA | 4 | 1.9 | 535.1–352.2 |
| Mpm, Ipm [64] | D6-Meropenem | >10 | 100, plasma | methanol | Thermo Fischer Scientific, Accucore C18 (100 × 2.1 mm, 2.6 μm) | 0.5–32 | A: 2 mM ammonium formate and 0.1% formic acid in water B: acetonitrile with 0.1% formic acid | 9 | Mpm ca 3.4, Ipm 3.04 + 3.09 | Mpm 384.2, Ipm 300.1 |
| Mpm [65] | D6-Meropenem | >12 | 50, plasma | acetonitrile | Waters, Acquity UPLC BEH C18 (100 × 2.1 mm, 1.7 μm) | 0.27–150 | A: 0.1% formic acid in water B: 0.1% formic acid in acetonitrile pH: NA | 3 | 1.05 | 384.2–141.0 |
| Clv**** [66] | NA | >5 | 1000, serum | acetonitrile | Waters, Acquity UPLC BEH C18 (100 × 2.1 mm, 1.7 μm) | 0.1–5 | A: methanol B: 0.01% formic acid in water pH: NA | 5.8 | 1.05–1.35 | 198.1–136.2 |
All studies used protein precipitation as extraction method, gradient elution for the mobile phase and positive electrospray ionization mode unless specified. *Times specified in each manuscript for different sample preparation steps (e.g. vortexing and centrifugation), **isocratic elution, ***solid phase extraction, ****negative electrospray ionization mode. LC-MS/MS = liquid chromatography-tandem mass spectrometry, Prep. time = sample preparation time, R = rifampicin, H = isoniazid, E = ethambutol, Z = pyrazinamide, Lfx = levofloxacin, Mfx = moxifloxacin, Am = amikacin, Km = kanamycin, S = streptomycin, Eto = ethionamide, Pto = prothionamide, Cs = cycloserine, Lzd = linezolid, Cfz = clofazimine, Bdq = bedaquiline, Dlm = delamanid, PAS = p-aminosalicylic acid, Ipm = imipenem, Mpm = meropenem, Amx = amoxicillin, Clv = clavulanate, Gm = Gentamycin, NA = not available.
Zhou et al. [45] developed a rapid and simple method for the first-line drugs together with streptomycin. To overcome the matrix problems described by Song et al., they used a hydrophilic interaction liquid chromatography (HILIC) column. Sample preparation was a single-step deproteinization using methanol, formic acid and ammonium acetate with an analytical run time of two minutes. However, Zhou et al. [45] also used structural analogues instead of isotopes as internal standards and calibration ranges were insufficient for all drugs [20]. Two other methods describe LC-MS/MS-based analysis of the first-line drugs with simple sample pre-treatment and reduced matrix effects, however, neither used SIL-IS and one suffered from low recoveries (i.e., mean recovery 10–29%) for all drugs [46], [47].
Kim et al. [48] developed an LC-MS/MS method including all first-line and numerous second-line drugs, although analysis of the full complement of first-line drugs required two separate sample preparation protocols, which compromises the methods applicability. A further drawback was higher than expected lower limits of quantification for the first-line drugs. This study is discussed in more detail in Section 3.2.
For the simultaneous analysis of rifampicin, ethambutol, pyrazinamide and streptomycin, but not isoniazid, a matrix assisted laser desorption/ionisation time of flight (MALDI-TOF/TOF) method has been described [49], although it involves a laborious sample clean up, involving extraction and evaporation, and the initial costs of the platform are high. Quantification was performed by standard addition (i.e., spiking with known concentrations) of the analytes. Although analytically interesting, this method is not useful as an affordable high-throughput assay for analysis of the first-line drugs.
The first report of using SIL-IS to drastically reduce matrix effects for all first-line drugs was Prahl et al. [50]. Sample preparation comprised two serial deproteinization steps between which the sample was frozen for 60 min, prolonging turn-around time by one hour. Chromatographic separation was performed on a C18 column with a run time of 10.2 min. Validation was performed according to FDA guidelines [41], not including a full stability validation, and calibration ranges were sufficient for clinically relevant concentrations [20].
While there has been progress in the development of a simple assay for the first-line anti-TB drugs, comprising of a single step sample clean-up followed by LC-MS/MS separation and analysis with short run times and use of SIL-IS, additional work is needed.
3.2. Multi-analyte assays for second-line anti-tuberculosis drugs
Three studies were identified where three or more second-line anti-TB drugs were analysed simultaneously in serum or plasma [48], [52], [53]. The study by Kim et al., introduced in 3.1, measured 20 anti-TB drugs in plasma [48], while Han et al. detected nine second-line drugs in serum [52] and also modified their assay demonstrating analysis of the same drugs using DBS [53], which will be discussed in Section 4.1.
In the assay by Kim et al. [48], anti-TB drugs were divided into two groups based on chemical properties. The samples were extracted and analysed differently for the two groups, resulting in effectively two separate assays. The Group One compounds included amikacin, kanamycin, streptomycin and pyrazinamide, whereas the Group Two compounds included levofloxacin, moxifloxacin, ciprofloxacin, ethionamide, prothionamide, cycloserine, linezolid, clofazimine, ethambutol, isoniazid, rifabutin, rifampicin, p-aminosalicylic acid, amoxicillin, clarithromycin and roxithromycin. Since clarithromycin and other macrolides are no longer recommended anti-TB drugs, as noted in the last two WHO guidelines [2], [3], they will not be discussed further. SIL-IS was used for Group Two, but not for Group One. Positive aspects of this methodology are a single-step sample preparation involving protein precipitation with methanol for Group One and acetonitrile for Group Two [48]. Separation was performed using a HILIC column and a C18 column for Group One and Two, respectively. Formic acid and acetonitrile were used for the mobile phase with different gradient compositions for both Groups and the run time was 9 min and 13 min for Group One and Group Two, respectively (Table 2). Both assays were validated according to the FDA guidelines, except for full stability validation [41]. Calibration ranges of all drugs, except p-aminosalicylic acid, were within range of the clinically relevant maximum concentration [20], [54]. Stability was acceptable for all drugs, except prothionamide and ethionamide.
The study by Han et al. included nine second-line drugs (i.e., kanamycin, streptomycin, levofloxacin, moxifloxacin, linezolid, prothionamide, cycloserine and p-aminosalicylic acid) [52] and followed a two-step deproteinization step with methanol, but did not use SIL-IS. The separation column was a High Strength Silica Technology 3 (HSS T3) with a run time of 3 min (Table 2) and the mobile phase used ammonium formate, formic acid and acetonitrile. All drugs were validated within clinically relevant concentration ranges [20], [54], but there was no evaluation on the stability of the drugs in serum.
Regarding the published multi-analyte assays of second-line anti-TB drugs, much work has already been done, although there is a need to further optimize the assays with regard to run time, the inclusion of other relevant anti-TB drugs (e.g., bedaquiline and delamanid) and internal standards, addressing matrix effect and analyte stability, and establishing clinically relevant calibration ranges. Details of these assays are described in Table 2, while individual drug assays are discussed below in 3.2.1, Sections 3.2.1–3.2.3, 3.2.3.
3.2.1. Group A: Levofloxacin, moxifloxacin, bedaquiline and linezolid
The two fluoroquinolones levofloxacin and moxifloxacin, as well as linezolid, were included in both multi-analyte assays by Kim et al. and Han et al. (Table 2) [48], [52]. Bedaquiline was not included in the multi-analyte assays and one study quantifying bedaquiline in serum was found that was fully validated and had enough descriptions that makes it replicative [55]. D6-bedaquiline was used as the internal standard in a single-step extraction method using acetonitrile and methanol for protein precipitation (Table 2). Separation was performed on a C18 column via a mobile phase consisting of acetonitrile, ammonium acetate, acetic acid and triflouroacetic anhydride. The linear calibration range was adequate for clinically relevant dosing [56]. Before bedaquiline can be included in a multi-analyte assay, the method will need further development, most specifically regarding adapting the extraction method to only using methanol or acetonitrile and simplifying the mobile phase using only formic acid and acetonitrile with the possible addition of ammonium formate [48], [52].
The fluoroquinolone, gatifloxacin is no longer a recommended anti-TB drug according to the recent WHO rapid communication [3]. We only found one article by Vishwanathan et al. that quantified gatifloxacin in human plasma using solid phase extraction with a mobile phase using isocratic elution [57]. If WHO recommendations for MDR-TB treatment regarding gatifloxacin change, additional work will be needed to include it as part of a multi-analyte assay panel.
3.2.2Group B: Clofazimine, cycloserine and terizidone
Cycloserine was included in both multi-component assays by Kim et al. and Han et al. [48], [52] and showed low recovery in one study [48], while clofazimine was only included in the study by Kim et al. (Table 2) [48]. Since terizidone hydrolyses to its active metabolite cycloserine, WHO recommends pharmacokinetic analysis via measurement of cycloserine [58], which was recently described by Court et al. [59].
3.2.3. Group C: Ethambutol, delamanid, pyrazinamide, imipenem-cilastin, meropenem, amikacin, streptomycin, ethionamide, prothionamide, p-aminosalicylic acid and the companion drug amoxicillin-clavulanate
Group C is a highly heterogeneous group of anti-TB drugs that have been subject to quantitative analysis by many different methods and techniques. Ethambutol, pyrazinamide, ethionamide, prothionamide, amikacin, streptomycin and p-aminosalicylic acid have been incorporated in one or both multi-analyte assays by Kim et al. and Han et al. (Table 2) [48], [52]. Ethionamide and prothionamide were subject to stability issues in the study by Kim et al. [48] and clinically relevant calibration ranges were too high for p-aminosalicylic acid in both studies [48], [52], [54]. Delamanid was not included in the assays and only one study was identified that reported on the measurement of delamanid in plasma using LC-MS/MS [62]. A structural analogue (OPC-14714) was used as an internal standard and the extraction was a one-step centrifugation method using acetonitrile for protein precipitation (Table 2). The mobile phase consisted of methanol, ammonium hydroxide and ammonium bicarbonate with separation on a C18 column. The study was fully validated and calibration ranges were adequate for concentrations achieved with recommended dosing [63]. The method shows similarities with the methods developed for the multi-analyte assays [48], [52], however, the mobile phases need to be adapted using formic acid, acetonitrile and, possibly, ammonium formate.
Since carbapenems are often used for treatment of bacterial infections, there were multiple methods identified for the analysis of the carbapenem antibiotics and the manuscripts often included simultaneous analysis of other drugs. Lefeuvre et al. [64] describe a validated method for the analysis of meropenem and imipenem that could be adapted to the multi-analyte assays (Table 2) [48], [52]. A one-step method for protein precipitation with methanol was used and the mobile phase consisted of formic acid, acetonitrile and ammonium formate on a C18 column. However, the linear calibration range was at the lower end of the maximum concentration for imipenem (i.e., 30–40 µg/ml) [54]. Meropenem has also been validated in a method using acetonitrile for a one-step protein precipitation assay with a mobile phase consisting of formic acid and acetonitrile (Table 2) [65].
There is low priority to include streptomycin in a multi-analyte assay since it is no longer recommended by WHO to be used in retreatment regimens together with first-line drugs [1] and it is rarely used in MDR-TB due to high rates of resistance [21].
Amoxicillin was included in the study by Kim et al. [48] and it was adequately evaluated (Table 2). For the analysis of the clavulanate compound, a similar method was identified (Table 2) [66]. Detection was performed in negative ionization mode and the method suffered from low recoveries and matrix effects indicating further improvement is needed.
The two injectables, kanamycin and capreomycin, as well as thioacetazone, have recently been removed as recommended anti-TB drugs by WHO [3]. Kanamycin was included in both multi-analyte assays [48], [52]. However, capreomycin was not included in the multi-analyte assays and we did not find any study for capreomycin, except for a WHO Drug Information Report [60]. No studies quantifying thioacetazone using LC-MS/MS were found, although a study from 1983 using HPLC-UV was found [67].
4. Alternative matrices
4.1. DBS for therapeutic drug monitoring in a programmatic setting
Analysis of DBS on specially designed filter paper has been used for screening new-born babies for decades and is a well-established and validated method for detection of anti-epileptic drugs, immunosuppressants, antiretrovirals and some antibiotics [72], [73]. Benefits of DBS are the low blood volume that is required, often higher analyte stability in the dried matrix and reduced biohazard risk [74], [75]. Possible benefits, at a programmatic level, include increased availability of TDM in rural areas and low- to middle-income countries, and reduction of costs for storage and transportation. In TB care, DBS, in relation to blood drug concentrations, has been evaluated for rifampicin, moxifloxacin and linezolid [76], [77], [78]. Lee et al. recently reported a method using LC-MS/MS with good correlation between venous and capillary concentrations of isoniazid [79]. However, the assay lacked important parameters, such as evaluation of long term stability and extraction time, required for analytical validation of a DBS method. Therefore, the results of this assay are considered preliminary.
A multi-analyte assay using DBS was established for simultaneous analysis of nine second-line anti-TB drugs (i.e., kanamycin, streptomycin, levofloxacin, moxifloxacin, linezolid, prothionamide, cycloserine, and p-aminosalicylic acid) using UPLC-MS/MS as mentioned in Section 3.1 [53]. A similar method was used for sample preparation, chromatography and detection, as in the study by Han et al. [52]. However, for the DBS, 100 µl of whole blood was added to filter paper and a partial spot was punched out and extracted with sonification using either of two elutions, methanol or acidified methanol, depending on the drugs being analysed. The study had a number of limitations, including (i) DBS drug concentrations were mostly lower compared to plasma samples, (ii) haematocrit was not measured, (iii) extended sample preparation time, and (iv) the method was not fully validated. Despite its limitations, this is the first study analysing multiple anti-TB drugs using DBS and could be a basis for further development. As such, a limited number of methods are available for certain anti-TB drugs for programmatic TDM using DBS [76], [77], [78], although for multi-analyte assays further improvement is needed.
4.2. Saliva
As an alternative to a blood draw for TDM analysis, saliva offers a compelling alternative. With its primary advantage being the ease of sample acquisition, home collection, following adequate patient instruction, is an achievable reality. Such a collection paradigm would be especially beneficial for patients living in remote areas as well as those for whom blood-based TDM is not preferred (e.g., children) [80]. One caveat of using saliva for TDM is that that the passive diffusion of anti-TB drugs into saliva is influenced by the salivary pH and salivary flow, as well as the physical characteristics of the analyte (e.g., the acid dissociation constant, protein binding capacity, molecular mass and lipophilicity). Stimulation of salivary flow increases its pH due to excretion of bicarbonate, hence, tracking sample pH as part of salivary TDM will be needed. Additionally, precautions should be taken to sterilize saliva via membrane filtration, since saliva from smear-positive patients could contain TB bacteria from coughed-up sputum [81].
In order to correlate the concentration of analysed drugs in the saliva with that in the blood, saliva-blood ratios for relevant drugs will need to be determined. For some drugs such as linezolid, the saliva-blood ratio is within a narrow range [80]. However, since other anti-TB drugs show low (e.g., amoxicillin and amikacin) [82] or variable (e.g., isoniazid, rifampicin and moxifloxacin) [80] penetration into saliva, the low detection levels attainable with LC-MS/MS assays will be useful in assessment of salivary drug concentrations.
While this avenue holds promise, further evaluation of the sample stability and recovery of drugs from saliva will be needed. Currently, quantitative analysis of saliva samples using LC-MS/MS has only been described for linezolid and doripenem [83], [84].
5. Proficiency testing programme
A variety of analytical methods have been published addressing the quantification of anti-TB drugs in human serum or plasma. Few commercial assays are available for TDM of anti-TB drugs; instead laboratories are required to develop and validate methods themselves. The reliability of these analytical methods is essential to providing accurate information on drug concentration [5]. During development, the intra-laboratory (internal) quality control procedures, such as validation of equipment and qualification of technicians, are essential. By combining these procedures with intra-laboratory method validation, a methodology with sufficient accuracy, precision and specificity at clinically relevant concentrations would be expected. An external inter-laboratory quality control, or proficiency testing, programme is imperative to objectively evaluate the accuracy of the results [85], [86]. Such a proficiency testing programme is an essential component of quality assurance, and also provides evidence of laboratory competence for clinicians, researchers, accrediting bodies and regulatory agencies [85], [86].
To date, only one proficiency testing programme exists in the field of anti-TB drug analysis [86]. Unfortunately, this programme only tests the first-line drugs, moxifloxacin and linezolid. In the first round of evaluations taken in 2015, participating laboratories performed generally well, with about 80% quantifying the results within 20% accuracy limits. Rifampicin proved to be the most difficult drug to analyse accurately [86]. In the more recent round of analysis evaluation levofloxacin has been added to the programme [87]. The importance of proficiency testing programmes cannot be under emphasized.
6. Suggestions for future multi-analyte assays
On an evaluation of the landscape, it appears as though it would be most beneficial to develop a number of multi-analyte assays that are each tailored to different settings. Countries that have a highly programmatic approach, those with a high burden of drug-resistant TB, or those that mostly use individually tailored regimens would benefit from using different multi-analyte assays. Apart from taking into account the chemical properties of the drugs, a multi-analyte assay would ideally be adapted to clinically relevant regimens, current clinical evidence of target values for TDM and available therapeutic windows.
For patients with DS-TB, assays including all first-line drugs have already been developed, but a more robust and simple assay would be useful. Although there is weak evidence indicating a need for TDM of ethambutol, it seems prudent to include it, especially since the quality of the assays are not likely to be affected by adding ethambutol to the panel.
To develop assays for MDR-TB drugs we propose one assay for the polar drug amikacin, and one multi-analyte assay for the less polar drugs isoniazid, ethambutol, pyrazinamide, levofloxacin, moxifloxacin, linezolid and cycloserine. Patients treated with either the standard MDR-TB regimen or the short-course regimen would benefit from these assays. Since only small blood volumes are needed for LC-MS/MS, both assays could be performed using a single blood sample. The assays could be based on the assays already developed by Kim et al. [48], but would need further testing and refinement to adjust to differing drug combinations.
In settings where individually tailored regimens are used and where extended TDM is used, more drugs would need to be included. Although evidence that TDM for the additional drugs is not currently compelling, we recommend including bedaquiline, clofazimine, ethionamide, prothionamide, p-aminosalicylic acid, meropenem, imipenem and delamanid in the less polar assay (for a total of 15 drugs). Streptomycin and amoxicillin have been omitted due to limited usage. We believe it is important not to neglect incorporation of the newer drugs, bedaquiline and delamanid, in any developed assays, and perhaps also pretonamid in future assays, since these drugs are often used in difficult to treat cases where only limited drug treatment options are left, which also further stresses the need for TDM in order to preserve the efficacy of the limited drugs remaining [68], [69], [70].
7. Summary
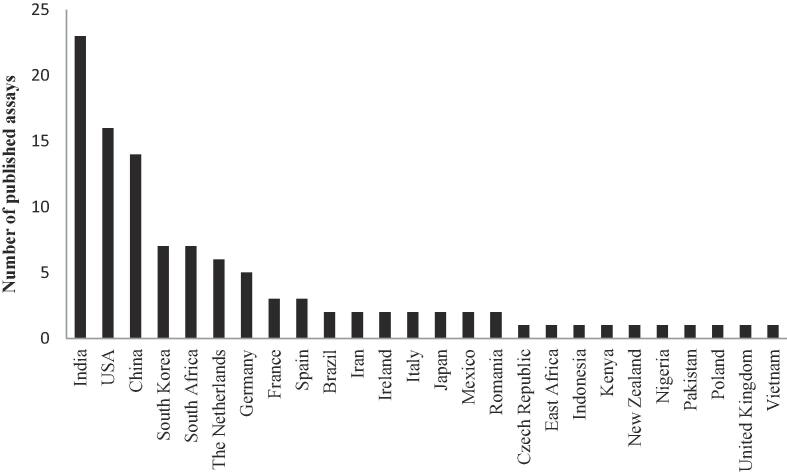
In providing an overview of the current state-of-the-art with regard to TDM-level analysis of the anti-TB drugs, we have highlighted the challenges of developing LC-MS/MS methods for multi-analyte assays. While there are a limited number of multi-analyte assays available for both first-line and second-line anti-TB drugs, many drugs are not covered; for these we have identified single-plex assays that could be incorporated into multi-analyte assay panels in the future. Suggestions have also been proposed for the development of custom multi-analyte assays. Already, TDM for anti-TB drugs is included in many TB guidelines and numerous assays have been published from multiple countries (Fig. 2). We believe that we have only seen the beginning of TDM for anti-TB drugs, although many technical and organisational challenges still exist. By developing patient-friendly sampling techniques and simpler methods for drug quantification with more robust and reliable assays, TDM could soon be available in all settings and countries. Lastly, but perhaps most crucially, a laboratory proficiency programme is key to assure adequate quality control of these assays.
Fig. 2.
Number of published anti-tuberculosis assays for therapeutic drug monitoring per country PubMed was searched on 27 March 2018 using the search terms (“levofloxacin” OR “moxifloxacin” OR “gatifloxacin”) AND (“tuberculosis” OR “TB” OR “Mtb”) AND (“pharmacokinetics” OR “concentration” OR “therapeutic drug monitoring” OR “TDM” OR “drug exposure” OR “drug monitoring” OR “pharmacology” OR “pharmacodynamics” OR “pharmacol*” OR “pharmacod*”) NOT (“review”[Publication Type] OR “review literature as topic” [MeSH Terms] OR “review” [All Fields]) NOT (“case reports” [Publication Type] OR “case report” [All Fields]).
Declarations of interest
None.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.World Health Organisation (WHO), Guidelines for treatment of drug-susceptible tuberculosis and patient care, 2017 update, Geneva, 2017.
- 2.World Health Organisation (WHO), WHO treatment guidelines for drug-resistant tuberculosis: 2016 update, Geneva, 2016. [PubMed]
- 3.World Health Organisation (WHO), Rapid Communication: Key changes to treatment of multidrug- and rifampicin-resistant tuberculosis (MDR/RR-TB) Geneva, 2018.
- 4.World Health Organisation (WHO), Global tuberculosis report, Geneva, 2017.
- 5.Veringa A., Sturkenboom M.G.G., Bart G.J., Dekkers B.G., Koster R.A., Roberts J.A., Peloquin C.A., Touw D., Alffenaar J.C. LC-MS/MS for Therapeutic Drug Monitoring of anti-infective drugs. Trends Anal. Chem. 2016;84:34–40. doi: 10.1016/j.trac.2015.11.026. [DOI] [Google Scholar]
- 6.Srivastava S., Pasipanodya J.G., Meek C., Leff R., Gumbo T. Multidrug-resistant tuberculosis not due to noncompliance but to between-patient pharmacokinetic variability. J. Infect. Dis. 2011;204:1951–1959. doi: 10.1093/infdis/jir658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiner M., Burman W., Vernon A., Benator D., Peloquin C.A., Khan A., Weis S., King B., Shah N., Hodge T. Low isoniazid concentrations and outcome of tuberculosis treatment with once-weekly isoniazid and rifapentine. Am. J. Respir. Crit. Care Med. 2003;167:1341–1347. doi: 10.1164/rccm.200208-951OC. [DOI] [PubMed] [Google Scholar]
- 8.Mehta J.B., Shantaveerapa H., Byrd R.P., Jr., Morton S.E., Fountain F., Roy T.M. Utility of rifampin blood levels in the treatment and follow-up of active pulmonary tuberculosis in patients who were slow to respond to routine directly observed therapy. Chest. 2001;120:1520–1524. doi: 10.1378/chest.120.5.1520. [DOI] [PubMed] [Google Scholar]
- 9.Chideya S., Winston C.A., Peloquin C.A., Bradford W.Z., Hopewell P.C., Wells C.D., Reingold A.L., Kenyon T.A., Moeti T.L., Tappero J.W. Isoniazid, rifampin, ethambutol, and pyrazinamide pharmacokinetics and treatment outcomes among a predominantly HIV-infected cohort of adults with tuberculosis from Botswana. Clin. Infect. Dis. 2009;48:1685–1694. doi: 10.1086/599040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van't Boveneind-Vrubleuskaya N., Seuruk T., van Hateren K., van der Laan T., Kosterink J.G.W., van der Werf T.S., van Soolingen D., van den Hof S., Skrahina A., Alffenaar J.C. Pharmacokinetics of levofloxacin in multidrug- and extensively drug-resistant tuberculosis patients. Antimicrob. Agents. Chemother. 2017;61 doi: 10.1128/aac.00343-17. e00343–00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pasipanodya J.G., McIlleron H., Burger A., Wash P.A., Smith P., Gumbo T. Serum drug concentrations predictive of pulmonary tuberculosis outcomes. J. Infect. Dis. 2013;208:1464–1473. doi: 10.1093/infdis/jit352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magis-Escurra C., van den Boogaard J., Ijdema D., Boeree M., Aarnoutse R. Therapeutic drug monitoring in the treatment of tuberculosis patients. Pulm. Pharmacol. Ther. 2012;25:83–86. doi: 10.1016/j.pupt.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Weiner M., Benator D., Burman W., Peloquin C.A., Khan A., Vernon A., Jones B., Silva-Trigo C., Zhao Z., Hodge T. Association between acquired rifamycin resistance and the pharmacokinetics of rifabutin and isoniazid among patients with HIV and tuberculosis. Clin. Infect. Dis. 2005;40:1481–1491. doi: 10.1086/429321. [DOI] [PubMed] [Google Scholar]
- 14.Te Brake L., Dian S., Ganiem A.R., Ruesen C., Burger D., Donders R., Ruslami R., van Crevel R., Aarnoutse R. Pharmacokinetic/pharmacodynamic analysis of an intensified regimen containing rifampicin and moxifloxacin for tuberculous meningitis. Int. J. Antimicrob. Agents. 2015;45:496–503. doi: 10.1016/j.ijantimicag.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 15.Prahl J.B., Johansen I.S., Cohen A.S., Frimodt-Moller N., Andersen A.B. Clinical significance of 2 h plasma concentrations of first-line anti-tuberculosis drugs: a prospective observational study. J. Antimicrob. Chemother. 2014;69:2841–2847. doi: 10.1093/jac/dku210. [DOI] [PubMed] [Google Scholar]
- 16.Swaminathan S., Pasipanodya J.G., Ramachandran G., Hemanth Kumar A.K., Srivastava S., Deshpande D., Nuermberger E., Gumbo T. Drug concentration thresholds predictive of therapy failure and death in children with tuberculosis: bread crumb trails in random forests. Clin. Infect. Dis. 2016;63:S63–S74. doi: 10.1093/cid/ciw471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ebers A., Stroup S., Mpagama S., Kisonga R., Lekule I., Liu J., Heysell S. Determination of plasma concentrations of levofloxacin by high performance liquid chromatography for use at a multidrug-resistant tuberculosis hospital in Tanzania. PLoS One. 2017;12 doi: 10.1371/journal.pone.0170663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azuma J., Ohno M., Kubota R., Yokota S., Nagai T., Tsuyuguchi K., Okuda Y., Takashima T., Kamimura S., Fujio Y., Kawase I. NAT2 genotype guided regimen reduces isoniazid-induced liver injury and early treatment failure in the 6-month four-drug standard treatment of tuberculosis: a randomized controlled trial for pharmacogenetics-based therapy. Eur. J. Clin. Pharmacol. 2013;69:1091–1101. doi: 10.1007/s00228-012-1429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nahid P., Dorman S.E., Alipanah N., Barry P.M., Brozek J.L., Cattamanchi A., Chaisson L.H., Chaisson R.E., Daley C.L., Grzemska M., Higashi J.M., Ho C.S., Hopewell P.C., Keshavjee S.A., Lienhardt C., Menzies R., Merrifield C., Narita M., O'Brien R., Peloquin C.A., Raftery A., Saukkonen J., Schaaf H.S., Sotgiu G., Starke J.R., Migliori G.B., Vernon A. Official american thoracic society/centers for disease control and prevention/infectious diseases society of america clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin. Infect. Dis. 2016;63:e147–e195. doi: 10.1093/cid/ciw376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alsultan A., Peloquin C.A. Therapeutic drug monitoring in the treatment of tuberculosis: an update. Drugs. 2014;74:839–854. doi: 10.1007/s40265-014-0222-8. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organisation, Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis, Geneva, 2014. [PubMed]
- 22.Curry International Tuberculosis Center, C.D.o.P. Health, Drug-Resistant Tuberculosis: A Survival Guide for Clinicians, 3rd, San Francisco, 2016.
- 23.Canadian Thoracic Society (CTS), Canadian Lung Association (CLA), Public Health Agency of Canada (PHAC), Canadian Tuberculosis Standards 7th Edition: 2014, Ottawa, 2014.
- 24.Ministry of Health & Family Welfare, India, Revised National Tuberculosis Control Programme: DOTS-Plus Guidelines, New Dehli, 2010.
- 25.Ministry of Health and Social Welfare, The Government of the Kingdom of Swaziland, Drug resistant tuberculosis management guidelines and manual, Mbabane, 2008.
- 26.Department of Health, Republic of South Africa, Management of drug-resistant tuberculosis: Policy Guidelines, Johannesburg, 2013.
- 27.Patel A.M., McKeon J. Avoidance and management of adverse reactions to antituberculosis drugs. Drug Saf. 1995;12:1–25. doi: 10.2165/00002018-199512010-00001. [DOI] [PubMed] [Google Scholar]
- 28.Talbert Estlin K.A., Sadun A.A. Risk factors for ethambutol optic toxicity. Int. Ophthalmol. 2010;30:63–72. doi: 10.1007/s10792-009-9293-z. [DOI] [PubMed] [Google Scholar]
- 29.Holmes C.X., Martin G.E., Fetterhoff K.I. The role of the cycloserine (seromycin) blood level in the treatment of pulmonary tuberculosis and the prevention and control of cycloserine (seromycin) toxicity. Dis. Chest. 1959;36:591–593. doi: 10.1378/chest.36.6.591. [DOI] [PubMed] [Google Scholar]
- 30.Deshpande D., Alffenaar J.W., Koser C.U., Dheda K., Chapagain M.L., Simbar N., Schon T., Sturkenboom M.G., McIlleron H., Lee P.S., Koeuth T., Mpagama S.G., Banu S., Foongladda S., Ogarkov O., Pholwat S., Houpt E.R., Heysell S.K., Gumbo T. D-cycloserine pharmacokinetics-pharmacodynamics, susceptibility, and dosing implications in multidrug-resistant tuberculosis: a Faustian deal. Clin. Infect. Dis. 2018 doi: 10.1093/cid/ciy624. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Altena R., Dijkstra J.A., van der Meer M.E., Borjas Howard J.F., Kosterink J.G., van Soolingen D., van der Werf T.S., Alffenaar J.W. Reduced chance of hearing loss associated with therapeutic drug monitoring of aminoglycosides in the treatment of multidrug-resistant tuberculosis. Antimicrob. Agents Chemother. 2017;61 doi: 10.1128/aac.01400-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bolhuis M.S., Tiberi S., Sotgiu G., De Lorenzo S., Kosterink J.G., van der Werf T.S., Migliori G.B., Alffenaar J.W. Linezolid tolerability in multidrug-resistant tuberculosis: a retrospective study. Eur. Respir. J. 2015;46:1205–1207. doi: 10.1183/13993003.00606-2015. [DOI] [PubMed] [Google Scholar]
- 33.Sotgiu G., Centis R., D'Ambrosio L., Alffenaar J.W., Anger H.A., Caminero J.A., Castiglia P., De Lorenzo S., Ferrara G., Koh W.J., Schecter G.F., Shim T.S., Singla R., Skrahina A., Spanevello A., Udwadia Z.F., Villar M., Zampogna E., Zellweger J.P., Zumla A., Migliori G.B. Efficacy, safety and tolerability of linezolid containing regimens in treating MDR-TB and XDR-TB: systematic review and meta-analysis. Eur. Respir. J. 2012;40:1430–1442. doi: 10.1183/09031936.00022912. [DOI] [PubMed] [Google Scholar]
- 34.Bolhuis M.S., Akkerman O.W., Sturkenboom M.G., Ghimire S., Srivastava S., Gumbo T., Alffenaar J.W. Linezolid based regimens for MDR-TB: a systematic review to establish or revise the current recommended dose for TB treatment. Clin. Infect. Dis. 2018 doi: 10.1093/cid/ciy625. In press. [DOI] [PubMed] [Google Scholar]
- 35.van Altena R., de Vries G., Haar C.H., de Lange W.C., Magis-Escurra C., van den Hof S., van Soolingen D., Boeree M.J., van der Werf T.S. Highly successful treatment outcome of multidrug-resistant tuberculosis in the Netherlands, 2000–2009. Int. J. Tuberc. Lung Dis. 2015;19:406–412. doi: 10.5588/ijtld.14.0838. [DOI] [PubMed] [Google Scholar]
- 36.van Altena R., Akkerman O.W., Alffenaar J.C., Kerstjens H.A., Magis-Escurra C., Boeree M.J., van Soolingen D., de Lange W.C., Bolhuis M.S., Hoefsloot W., de Vries G., van der Werf T.S. Shorter treatment for multidrug-resistant tuberculosis: the good, the bad and the ugly. Eur. Respir. J. 2016;48:1800–1802. doi: 10.1183/13993003.01208-2016. [DOI] [PubMed] [Google Scholar]
- 37.Davies Forsman L., Jonsson J., Wagrell C., Chryssanthou E., Giske C.G., Groenheit R., Hoffner S., Mansjö M., Wijkander M., Werngren J., Schön T., Bruchfeld J. European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), Vienna, Austria. 2017. Minimum inhibitory concentrations in MDR-TB patients from Sweden – a national cohort study. [Google Scholar]
- 38.Ghimire S., Bolhuis M.S., Sturkenboom M.G., Akkerman O.W., de Lange W.C., van der Werf T.S., Alffenaar J.W. Incorporating therapeutic drug monitoring into the World Health Organization hierarchy of tuberculosis diagnostics. Eur. Respir. J. 2016;47:1867–1869. doi: 10.1183/13993003.00040-2016. [DOI] [PubMed] [Google Scholar]
- 39.D. Skoog, D. West, F. Holler, S. Crouch, Skoog & and Wests' Fundamentals of Analytical Chemistry, 9th ed., Cengage Learning EMEA, 2013.
- 40.Annesley T.M., Cooks R.G., Herold D.A., Hoofnagle A.N. Clinical mass spectrometry-achieving prominence in laboratory medicine. Clin. Chem. 2016;62:1–3. doi: 10.1373/clinchem.2015.251272. [DOI] [PubMed] [Google Scholar]
- 41.United States Food and Drug Administration (US FDA), Guidance for industry: Bioanalytical method validation, 2013.
- 42.European Medicins Agency (EMA), Guideline on bioanalytical method validation, London, 2011.
- 43.Shipkova M., Svinarov D. LC-MS/MS as a tool for TDM services: where are we? Clin. Biochem. 2016;49:1009–1023. doi: 10.1016/j.clinbiochem.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Song S.H., Jun S.H., Park K.U., Yoon Y., Lee J.H., Kim J.Q., Song J. Simultaneous determination of first-line anti-tuberculosis drugs and their major metabolic ratios by liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007;21:1331–1338. doi: 10.1002/rcm.2961. [DOI] [PubMed] [Google Scholar]
- 45.Zhou Z., Wu X., Wei Q., Liu Y., Liu P., Ma A., Zou F. Development and validation of a hydrophilic interaction liquid chromatography-tandem mass spectrometry method for the simultaneous determination of five first-line antituberculosis drugs in plasma. Anal. Bioanal. Chem. 2013;405:6323–6335. doi: 10.1007/s00216-013-7049-0. [DOI] [PubMed] [Google Scholar]
- 46.Baietto L., Calcagno A., Motta I., Baruffi K., Poretti V., Di Perri G., Bonora S., D'Avolio A. A UPLC-MS-MS method for the simultaneous quantification of first-line antituberculars in plasma and in PBMCs. J. Antimicrob. Chemother. 2015;70:2572–2575. doi: 10.1093/jac/dkv148. [DOI] [PubMed] [Google Scholar]
- 47.Gao S., Wang Z., Xie X., You C., Yang Y., Xi Y., Chen W. Rapid and sensitive method for simultaneous determination of first-line anti-tuberculosis drugs in human plasma by HPLC-MS/MS: application to therapeutic drug monitoring. Tuberculosis (Edinburgh, Scotland) 2018;109:28–34. doi: 10.1016/j.tube.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 48.Kim H.J., Seo K.A., Kim H.M., Jeong E.S., Ghim J.L., Lee S.H., Lee Y.M., Kim D.H., Shin J.G. Simple and accurate quantitative analysis of 20 anti-tuberculosis drugs in human plasma using liquid chromatography-electrospray ionization-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2015;102:9–16. doi: 10.1016/j.jpba.2014.08.026. [DOI] [PubMed] [Google Scholar]
- 49.Notari S., Mancone C., Sergi M., Gullotta F., Bevilacqua N., Tempestilli M., Urso R., Lauria F.N., Pucillo L.P., Tripodi M., Ascenzi P. Determination of antituberculosis drug concentration in human plasma by MALDI-TOF/TOF. IUBMB Life. 2010;62:387–393. doi: 10.1002/iub.321. [DOI] [PubMed] [Google Scholar]
- 50.Prahl J.B., Lundqvist M., Bahl J.M., Johansen I.S., Andersen A.B., Frimodt-Moller N., Cohen A.S. Simultaneous quantification of isoniazid, rifampicin, ethambutol and pyrazinamide by liquid chromatography/tandem mass spectrometry. APMIS. 2016;124:1004–1015. doi: 10.1111/apm.12590. [DOI] [PubMed] [Google Scholar]
- 51.Sturkenboom M.G., van der Lijke H., Jongedijk E.M., Kok W.T., Greijdanus B., Uges D.R.A., Alffenaar J.W.C. Quantification of isoniazid, pyrazinamide and ethambutol in serum using liquid chromatography-tandem mass spectrometry. J. Applied Bioanalysis. 2015;1:89–98. doi: 10.17145/jab.15.015. [DOI] [Google Scholar]
- 52.Han M., Jun S.H., Lee J.H., Park K.U., Song J., Song S.H. Method for simultaneous analysis of nine second-line anti-tuberculosis drugs using UPLC-MS/MS. J. Antimicrob. Chemother. 2013;68:2066–2073. doi: 10.1093/jac/dkt154. [DOI] [PubMed] [Google Scholar]
- 53.Lee K., Jun S.H., Han M., Song S.H., Park J.S., Lee J.H., Park K.U., Song J. Multiplex assay of second-line anti-tuberculosis drugs in dried blood spots using ultra-performance liquid chromatography-tandem mass spectrometry. Ann. Lab. Med. 2016;36:489–493. doi: 10.3343/alm.2016.36.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lange C., Abubakar I., Alffenaar J.W., Bothamley G., Caminero J.A., Carvalho A.C., Chang K.C., Codecasa L., Correia A., Crudu V., Davies P., Dedicoat M., Drobniewski F., Duarte R., Ehlers C., Erkens C., Goletti D., Gunther G., Ibraim E., Kampmann B., Kuksa L., de Lange W., van Leth F., van Lunzen J., Matteelli A., Menzies D., Monedero I., Richter E., Rusch-Gerdes S., Sandgren A., Scardigli A., Skrahina A., Tortoli E., Volchenkov G., Wagner D., van der Werf M.J., Williams B., Yew W.W., Zellweger J.P., Cirillo D.M. Tbnet, Management of patients with multidrug-resistant/extensively drug-resistant tuberculosis in Europe: a TBNET consensus statement. Eur. Respir. J. 2014;44:23–63. doi: 10.1183/09031936.00188313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alffenaar J.W., Bolhuis M., van Hateren K., Sturkenboom M., Akkerman O., de Lange W., Greijdanus B., van der Werf T., Touw D. Determination of bedaquiline in human serum using liquid chromatography-tandem mass spectrometry. Antimicrob. Agents Chemother. 2015;59:5675–5680. doi: 10.1128/aac.00276-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diacon A.H., Pym A., Grobusch M., Patientia R., Rustomjee R., Page-Shipp L., Pistorius C., Krause R., Bogoshi M., Churchyard G., Venter A., Allen J., Palomino J.C., De Marez T., van Heeswijk R.P., Lounis N., Meyvisch P., Verbeeck J., Parys W., de Beule K., Andries K., Mc Neeley D.F. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N. Engl. J. Med. 2009;360:2397–2405. doi: 10.1056/NEJMoa0808427. [DOI] [PubMed] [Google Scholar]
- 57.Vishwanathan K., Bartlett M.G., Stewart J.T. Determination of gatifloxacin in human plasma by liquid chromatography/electrospray tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2001;15:915–919. doi: 10.1002/rcm.322. [DOI] [PubMed] [Google Scholar]
- 58.World Health Organisation (WHO), Notes on the Design of Bioequivalence Study: Terizidone https://extranet.who.int/prequal/sites/default/files/documents/29%20BE%20terizidone_Oct2015_0.pdf. WHO, 2015, (accessed 11 March 2018).
- 59.Court R., Wiesner L., Stewart A., de Vries N., Harding J., Maartens G., Gumbo T., McIlleron H. Steady state pharmacokinetics of cycloserine in patients on terizidone for multidrug-resistant tuberculosis. Int. J. Tuberc. Lung Dis. 2018;22:30–33. doi: 10.5588/ijtld.17.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.World Health Organisation (WHO), WHO Drug Information, Vol. 28, No. 4, Geneva, 2014.
- 62.Meng M., Smith B., Johnston B., Carter S., Brisson J., Roth S.E. Simultaneous quantitation of delamanid (OPC-67683) and its eight metabolites in human plasma using UHPLC-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015;1002:78–91. doi: 10.1016/j.jchromb.2015.07.058. [DOI] [PubMed] [Google Scholar]
- 63.Diacon A.H., Dawson R., Hanekom M., Narunsky K., Venter A., Hittel N., Geiter L.J., Wells C.D., Paccaly A.J., Donald P.R. Early bactericidal activity of delamanid (OPC-67683) in smear-positive pulmonary tuberculosis patients. Int. J. Tuberc. Lung Dis. 2011;15:949–954. doi: 10.5588/ijtld.10.0616. [DOI] [PubMed] [Google Scholar]
- 64.Lefeuvre S., Bois-Maublanc J., Hocqueloux L., Bret L., Francia T., Eleout-Da Violante C., Billaud E.M., Barbier F., Got L. A simple ultra-high-performance liquid chromatography-high resolution mass spectrometry assay for the simultaneous quantification of 15 antibiotics in plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017;1065-1066:50–58. doi: 10.1016/j.jchromb.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 65.Rigo-Bonnin R., Juvany-Roig R., Leiva-Badosa E., Sabater-Riera J., Perez-Fernandez X.L., Cardenas-Campos P., Sospedra-Martinez E., Colom H., Alia P. Measurement of meropenem concentration in different human biological fluids by ultra-performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2014;406:4997–5007. doi: 10.1007/s00216-014-7910-9. [DOI] [PubMed] [Google Scholar]
- 66.Cazorla-Reyes R., Romero-Gonzalez R., Frenich A.G., Rodriguez Maresca M.A., Martinez Vidal J.L. Simultaneous analysis of antibiotics in biological samples by ultra high performance liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2014;89:203–212. doi: 10.1016/j.jpba.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 67.Jenner P.J. High-performance liquid chromatographic determination of thiacetazone in body fluids. J. Chromatogr. 1983;276:463–470. doi: 10.1016/s0378-4347(00)85118-x. [DOI] [PubMed] [Google Scholar]
- 68.Pontali E., Sotgiu G., Tiberi S., Tadolini M., Visca D., D'Ambrosio L., Centis R., Spanevello A., Migliori G.B. Combined treatment of drug-resistant tuberculosis with bedaquiline and delamanid: a systematic review. Eur. Respir. J. 2018;52 doi: 10.1183/13993003.00934-2018. [DOI] [PubMed] [Google Scholar]
- 69.Borisov S.E., D'Ambrosio L., Centis R., Tiberi S., Dheda K., Alffenaar J.W., Amale R., Belilowski E., Bruchfeld J., Canneto B., Denholm J., Duarte R., Esmail A., Filippov A., Forsman L.D., Gaga M., Ganatra S., Igorevna G.A., Mastrapa B.L., Manfrin V., Manga S., Maryandyshev A., Massard G., Montaner P.G., Mullerpattan J., Palmero D.J., Pontarelli A., Papavasileiou A., Pontali E., Leyet R.R., Spanevello A., Udwadia Z.F., Viggiani P., Visca D., Sotgiu G., Migliori G.B. Outcomes of patients with drug-resistant-tuberculosis treated with bedaquiline -containing regimens and undergoing adjunctive surgery. J. Infect. 2018 doi: 10.1016/j.jinf.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 70.Schnippel K., Ndjeka N., Maartens G., Meintjes G., Master I., Ismail N., Hughes J., Ferreira H., Padanilam X., Romero R., Te Riele J., Conradie F. Effect of bedaquiline on mortality in South African patients with drug-resistant tuberculosis: a retrospective cohort study, The Lancet. Respiratory Med. 2018 doi: 10.1016/s2213-2600(18)30235-2. [DOI] [PubMed] [Google Scholar]
- 72.Parker S.P., Cubitt W.D. The use of the dried blood spot sample in epidemiological studies. J. Clin. Pathol. 1999;52:633–639. doi: 10.1136/jcp.52.9.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wilhelm A.J., den Burger J.C., Swart E.L. Therapeutic drug monitoring by dried blood spot: progress to date and future directions. Clin. Pharmacokinet. 2014;53:961–973. doi: 10.1007/s40262-014-0177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vu D.H., Alffenaar J.W., Edelbroek P.M., Brouwers J.R., Uges D.R. Dried blood spots: a new tool for tuberculosis treatment optimization. Curr. Pharm. Des. 2011;17:2931–2939. doi: 10.2174/138161211797470174. [DOI] [PubMed] [Google Scholar]
- 75.Zuur M.A., Bolhuis M.S., Anthony R., den Hertog A., van der Laan T., Wilffert B., de Lange W., van Soolingen D., Alffenaar J.W. Current status and opportunities for therapeutic drug monitoring in the treatment of tuberculosis. Expert Opin. Drug Metab. Toxicol. 2016;12:509–521. doi: 10.1517/17425255.2016.1162785. [DOI] [PubMed] [Google Scholar]
- 76.Allanson A.L., Cotton M.M., Tettey J.N., Boyter A.C. Determination of rifampicin in human plasma and blood spots by high performance liquid chromatography with UV detection: a potential method for therapeutic drug monitoring. J. Pharm. Biomed. Anal. 2007;44:963–969. doi: 10.1016/j.jpba.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 77.Vu D.H., Bolhuis M.S., Koster R.A., Greijdanus B., de Lange W.C., van Altena R., Brouwers J.R., Uges D.R., Alffenaar J.W. Dried blood spot analysis for therapeutic drug monitoring of linezolid in patients with multidrug-resistant tuberculosis. Antimicrob. Agents Chemother. 2012;56:5758–5763. doi: 10.1128/aac.01054-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vu D.H., Koster R.A., Alffenaar J.W., Brouwers J.R., Uges D.R. Determination of moxifloxacin in dried blood spots using LC-MS/MS and the impact of the hematocrit and blood volume. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011;879:1063–1070. doi: 10.1016/j.jchromb.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 79.Lee K., Jun S.H., Choi M.S., Song S.H., Park J.S., Lee J.H., Park K.U., Song J. Application of the isoniazid assay in dried blood spots using the ultra-performance liquid chromatography-tandem mass spectrometry. Clin. Biochem. 2017;50:882–885. doi: 10.1016/j.clinbiochem.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 80.van den Elsen S.H.J., Oostenbrink L.M., Heysell S.K., Hira D., Touw D.J., Akkerman O.W., Bolhuis M.S., Alffenaar J.C. Systematic review of salivary versus blood concentrations of antituberculosis drugs and their potential for salivary therapeutic drug monitoring. Ther. Drug Monit. 2018;40:17–37. doi: 10.1097/ftd.0000000000000462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van den Elsen S.H.J., van der Laan T., Akkerman O.W., van der Zanden A.G.M., Alffenaar J.C., van Soolingen D. Membrane filtration is suitable for reliable elimination of mycobacterium tuberculosis from saliva for therapeutic drug monitoring. J. Clin. Microbiol. 2017;55:3292–3293. doi: 10.1128/jcm.01248-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van den Elsen S.H.J., Akkerman O.W., Huisman J.R., Touw D.J., van der Werf T.S., Bolhuis M.S., Alffenaar J.C. Lack of penetration of amikacin into saliva of tuberculosis patients. Eur. Respir. J. 2018;51 doi: 10.1183/13993003.02024-2017. [DOI] [PubMed] [Google Scholar]
- 83.Burian B., Zeitlinger M., Donath O., Reznicek G., Sauermann R. Penetration of doripenem into skeletal muscle and subcutaneous adipose tissue in healthy volunteers. Antimicrob. Agents Chemother. 2012;56:532–535. doi: 10.1128/aac.05506-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bolhuis M.S., van Altena R., van Hateren K., de Lange W.C., Greijdanus B., Uges D.R., Kosterink J.G., van der Werf T.S., Alffenaar J.W. Clinical validation of the analysis of linezolid and clarithromycin in oral fluid of patients with multidrug-resistant tuberculosis. Antimicrob. Agents Chemother. 2013;57:3676–3680. doi: 10.1128/AAC.00558-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Burger D., Teulen M., Eerland J., Harteveld A., Aarnoutse R., Touw D. The international interlaboratory quality control program for measurement of antiretroviral drugs in plasma: a global proficiency testing program. Ther. Drug Monit. 2011;33:239–243. doi: 10.1097/FTD.0b013e31820fa528. [DOI] [PubMed] [Google Scholar]
- 86.Aarnoutse R.E., Sturkenboom M.G., Robijns K., Harteveld A.R., Greijdanus B., Uges D.R., Touw D.J., Alffenaar J.W. An interlaboratory quality control programme for the measurement of tuberculosis drugs. Eur. Respir. J. 2015;46:268–271. doi: 10.1183/09031936.001770144. [DOI] [PubMed] [Google Scholar]
- 87.Kwaliteitsbewaking Klinische Geneesmiddelanalyse en Toxicologie. KKGT. http://kkgt.nl/. KKGT, 2018, (accessed 11 October 2018).
- 88.ChemAxon, Chemicalize. https://chemicalize.com/. ChemAxon, 2018, (accessed 21 April 2018).
- 89.Kim S., Thiessen P., Bolton E., Chen J., Fu G., Gindulyte A., Han L., He J., He S., Shoemaker B., Wang J., Yu B., Zhang J., Bryant S. PubChem substance and compound databases. Nucleic Acids Res. 2016;44:D1202–1213. doi: 10.1093/nar/gkv951. [DOI] [PMC free article] [PubMed] [Google Scholar]