Abstract
Background.
Incidence rates of testicular cancer (TC) have been increasing in many countries since at least the mid-20th century without clear explanation. Examining the varying trends across countries and time provides clues to understanding the causes of TC.
Objective.
We have presented incidence data from 41 countries and evaluated incidence trends for the 35-year period from 1978 to 2012.
Design, Setting and Participants.
Cancer registry data from Cancer Incidence in Five Continents (CI5) Volumes V-XI, CI5plus, and the NORDCAN database were analyzed.
Outcome Measurements and Statistical Analysis.
Age-standardised rates of testicular cancer overall and by histologic type were calculated. A joinpoint regression model of the natural log-transformed rates was used to calculate the average annual percent change (AAPC) in incidence. Age-period-cohort modeling was used to examine the effect of birth cohort on rates.
Results.
While the highest incidence of testicular cancer remains in Northern Europe, the gap is closing between higher- and lower-incidence regions. Age-period-cohort modeling found a flattening of risk among recent cohorts in Denmark and the United Kingdom, a steady increase in risk in the United States (particularly for seminomas), and an increase in risk among more recent cohorts in Costa Rica, Croatia and Slovakia.
Conclusions.
The gap between low and high incidence countries is closing due to increases in the former and stabilisation in the latter. Understanding the causes of these and other differences in incidence rates between, and within, countries may help to further our understanding of the aetiology of this cancer.
Keywords: testicular cancer, testicular germ cell tumours, incidence, global trends
Patient Summary.
We examined the rates of testicular cancer in different countries over time. Rates have been increasing, although rates in high incidence countries seem to be slowing down, while rates in low incidence countries are catching up. These trends might help us understand what is causing testicular cancer in general.
Introduction
Testicular cancer is the most common cancer among young men aged 15–40 years.1 While the prognosis is very favourable, with more than 90% of men surviving beyond 5 years, survivors are at increased risk of infertility, sexual dysfunction and other side-effects of treatment.2–5 Around 98% of all testicular cancers are testicular germ cell tumours (TGCT), which are histologically divided into seminomas (50–60% of tumours), nonseminomas (40–50%) and spermatocytic tumours (<1%).6,7 Seminomas are typically diagnosed at around 35 years of age and nonseminomas at around 25,6 with both forms arising from germ cell neoplasia in-situ (GCNIS).6,7
Incidence rates of testicular cancer have been increasing in many countries since at least the mid-20th century without clear explanation. Incidence tends to be greatest in Northern European countries, while the lowest rates occurring in Eastern European, Asian, African and South American countries.8 However, even within high-incidence regions (including Northern Europe) there are examples of stark divergence in incidence between (and to an extent within) countries. In recent decades, there has been an increase in rates of testicular cancer in countries which had previously had, in relative terms, low incidence,9 predicted to continue in the coming decades.10 These phenomena are not well explained, and require careful monitoring and consideration given the public health importance of testicular cancer among young men, and the need for high-quality cancer service delivery to maximise survival prospects and quality of life.
In this manuscript, we have updated and extended our previous assessment of international temporal trends in testicular cancer incidence,9 with the addition of new registries, examination of within-country differences, and an assessment of temporal changes in incidence rate by histological sub-type. We have presented incidence data from 41 separate countries and evaluated incidence trends for the 35 year period from 1978 to 2012. We have also conducted age-period-cohort modeling to distinguish the effects of birth cohort from those of age and period on rates.
Methods
Data sources
Cancer registry data from Cancer Incidence in Five Continents (CI5) Volumes V-XI, CI5plus, and the NORDCAN database were analyzed to examine temporal trends in testicular cancer incidence over the past 35 years. The quality of the data sources used in this manuscript have been evaluated elsewhere.11–14 The International Agency for Research on Cancer (IARC) has high data quality standards for registries to be included in CI5, based on completeness, comparability, validity/accuracy, and timeliness. The CI5plus database contains annual incidence data reported from regional and national cancer registries and includes data on cancer site, sex, age, age-specific populations, age-standardised incidence, and histology, where available. Similarly, a new CI5 volume is published every 5 years with the same variables as CI5plus, but with the data aggregated over the 5-year interval rather than the 1-year interval. In the current analysis, the CI5plus data were used where available from 1978 to 2012. Data from countries not included in the latest CI5plus database release were obtained from previous CI5 volumes including Volume V (1978–1982), Volume VI (1983–1987), Volume VII (1988–1992), Volume VIII (1993–1997), Volume IX (1998–2002), Volume X (2003–2007), and Volume XI (2008–2012). Lastly, two countries (Finland and Sweden) that were not able to submit data to CI5 for Volume XI were included by appending data from NORDCAN, a database of cancer statistics from the Nordic countries to the data previously submitted to CI5 (1978–2007).15–17
As a measure of each registry’s data quality over time, registries that had been included in the most recent CI5 volume (Volume XI) or had CI5plus data from 2008–2012 and that had 15 consecutive years of data within either CI5plus or previous CI5 volumes were eligible for inclusion in the current analysis.15,16 Forty-one countries from Africa, Asia, Europe, the Americas, and Oceania were included (Supplementary Table 1).
Statistical analysis
Age-standardised rates of testicular cancer overall and by histologic type, where available, were calculated using the World Standard Population 18,19 and plotted by 5-years intervals from 1978–1982 through 2008–2012. To examine trends in rates over time, a joinpoint regression model of the natural log-transformed rates was used to calculate the estimated annual percent change (APC) and to determine whether a second joinpoint was appropriate to characterize the change in rates within each country.20 A joinpoint model allows for non-linear trends in incidence over time, by indicating at which time points the trend significantly changes. If one or more joinpoints were incorporated into the model, an average annual percent change (AAPC) was calculated to more precisely describe the changes in rates over the 35-year period. If no joinpoints were incorporated into the model, then the AAPC exactly reflected the APC. For Volume XI or CI5plus years 2008–2012 included plotting the age-standardised rates against AAPC on an arithmetic scale, plotting the overall age-standardised rate by country.
Histology was categorized as seminoma, nonseminoma, or other histology. Volumes V through VIII classified spermatocytic seminomas (recently renamed to spermatocytic tumours, ICD-O morphology code 9063) with classic seminomas, thus these volumes include spermatocytic seminomas in the overall estimate of seminomas (ICD-O morphology codes 9060–9064). In Volumes IX-XI and CI5plus, spermatocytic tumours were not included with seminomas, thus seminomas were identified by ICD-O morphology codes 9060–9062 and 9064. Embryonal carcinoma, malignant teratoma, yolk sac tumour, choriocarcinoma, and mixed germ cell tumours were categorized as nonseminomas and were identified by ICD-O morphology codes 9065–9102 for all included CI5 volumes. Histologies categorized as ‘Other/Unspecified’ in the current analysis include sex cord-stromal tumours and any tumour with ICD-O morphology codes 8000–8005. Age-standardised rates by histologic type were calculated and plotted by calendar time for the period 1978–2012 for selected high-quality registries representing various regions of the world: Chile, Colombia, Croatia, Denmark, France, India, Israel, Italy, Japan, New Zealand, Norway, Philippines, Slovakia, Slovenia, United Kingdom, and United States.
Age-period-cohort modeling was used to examine the effect of birth cohort on testicular cancer rates. Birth cohort trends were calculated for Costa Rica, Croatia, Denmark, Slovakia, United States, and United Kingdom by subtracting the midpoints of 5-year age groups from the corresponding 5-year calendar periods. Due to the identification problem inherent in allaAge-period-cohort analyses, wherein age, period and cohort are linearly dependent on each other, the linear component of the period effect was assumed to have zero slope when presenting the cohort effects.9 The cohort effects were estimated using the full age-period-cohort model with incidence rate ratios presented relative to the reference cohort, with midpoint 1965. More detailed methods used to perform these calculations have been previously reported.9
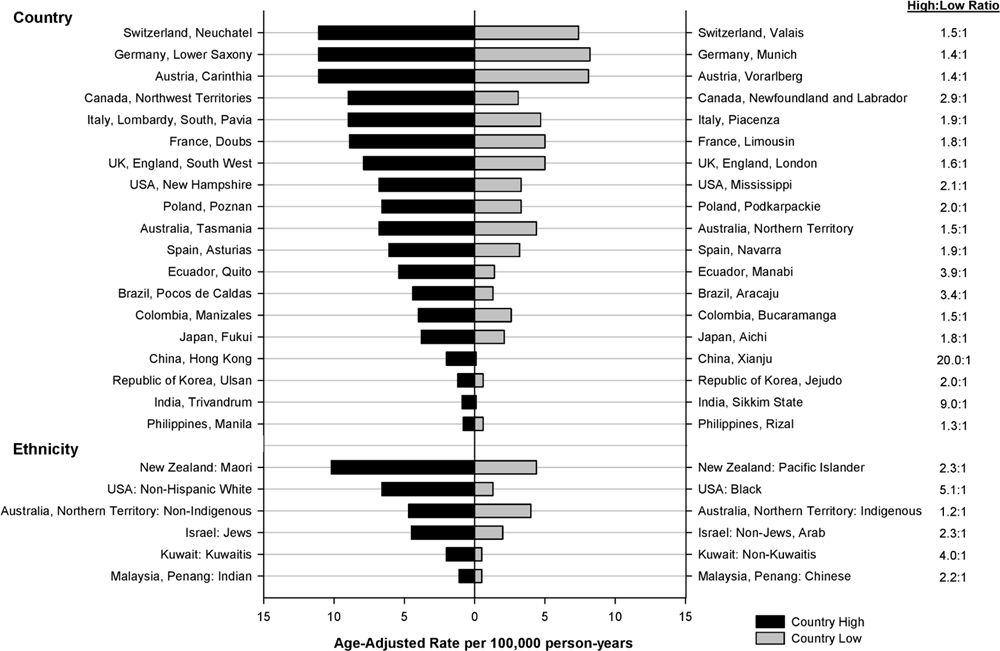
As a supplementary analysis, the highest and lowest incidence rates were plotted by registry/ ethnic group within each country where applicable (Box 1). We also plotted seminoma rates against nonseminoma rates to visually represent the magnitude of the burden according to major histological subtypes (Supplementary Figure 1).
Box 1: Inter- and intra-region disparities in TGCT incidence.
There is substantial international variation in the incidence of TGCT, with strong patterning of disease incidence towards those countries with a majority population of European ethnicity, with disease incidence greatest amongst Northern European countries. However, even within these regions there are several examples of puzzling epidemiological patterns within regions: for example, despite geographic proximity and very similar population demography, rates of disease among Norwegian males are more than two-fold higher than those observed across the border in Finland (Figure 2).1,2
There are also noteworthy patterns within countries, particularly between ethnic groups: in New Zealand, incidence rates of TGCT are 80% higher among indigenous Māori men compared to European/Other men (age-standardised relative risk: 1.80, 95% CI 1.58–2.05).3 This is in stark contrast to other countries, where men of European ethnicity have by far the highest rates of disease compared to other ethnic groups.4,5 An additional curiosity is the fact that Pacific New Zealanders have very low rates of TGCT compared to Māori and European/Other men, a rare example where disease incidence does not move in parallel between Māori and Pacific men (see below).3 Understanding the drivers of these and other unusual inter- and intra-region disparities may provide important clues regarding the currently-obscure aetiology of testicular cancer.
SAS statistical software was used for data management and rate calculations (v9.4, SAS Institute Inc., Cary, NC, USA). The highest and lowest age-standardised rates within each country were obtained from the International Agency for Research on Cancer’s online analysis tool.21 Sigmaplot was used to create figures (v12.5, SY Software Inc., San Jose, CA, USA). The Joinpoint Regression Program was used to calculate joinpoints, APC, and AAPC (v4.6.0.0, IMS, Inc., Calverton, MD, USA). All age-period-cohort modeling was performed using apc.fit 22 in R (v3.5.3) and graphics were performed using Stata (v13, StataCorp, LP, College Station, TX, USA).
Results
The highest incidence rates of testicular cancer circa 2008–2012 occurred in Europe, with all of the top-12 highest-incidence countries being from either Northern, Central, Southern or Eastern Europe (Figure 1). The highest incidence was observed in the Northern European countries of Norway (age-standardised rate: 11.5 cases/100,000 person-years) and Denmark (10.2/100,000). These very high rate countries were followed by Switzerland (8.9/100,000) and Slovenia (8.8/100,000). In contrast, the lowest incidence of TGCT occurred in Africa (Uganda 0.3/100,000) and Asia (Thailand 0.5/100,000; India 0.6/100,000; the Philippines 0.8/100,000; China 1.5/100,000).
Figure 1:
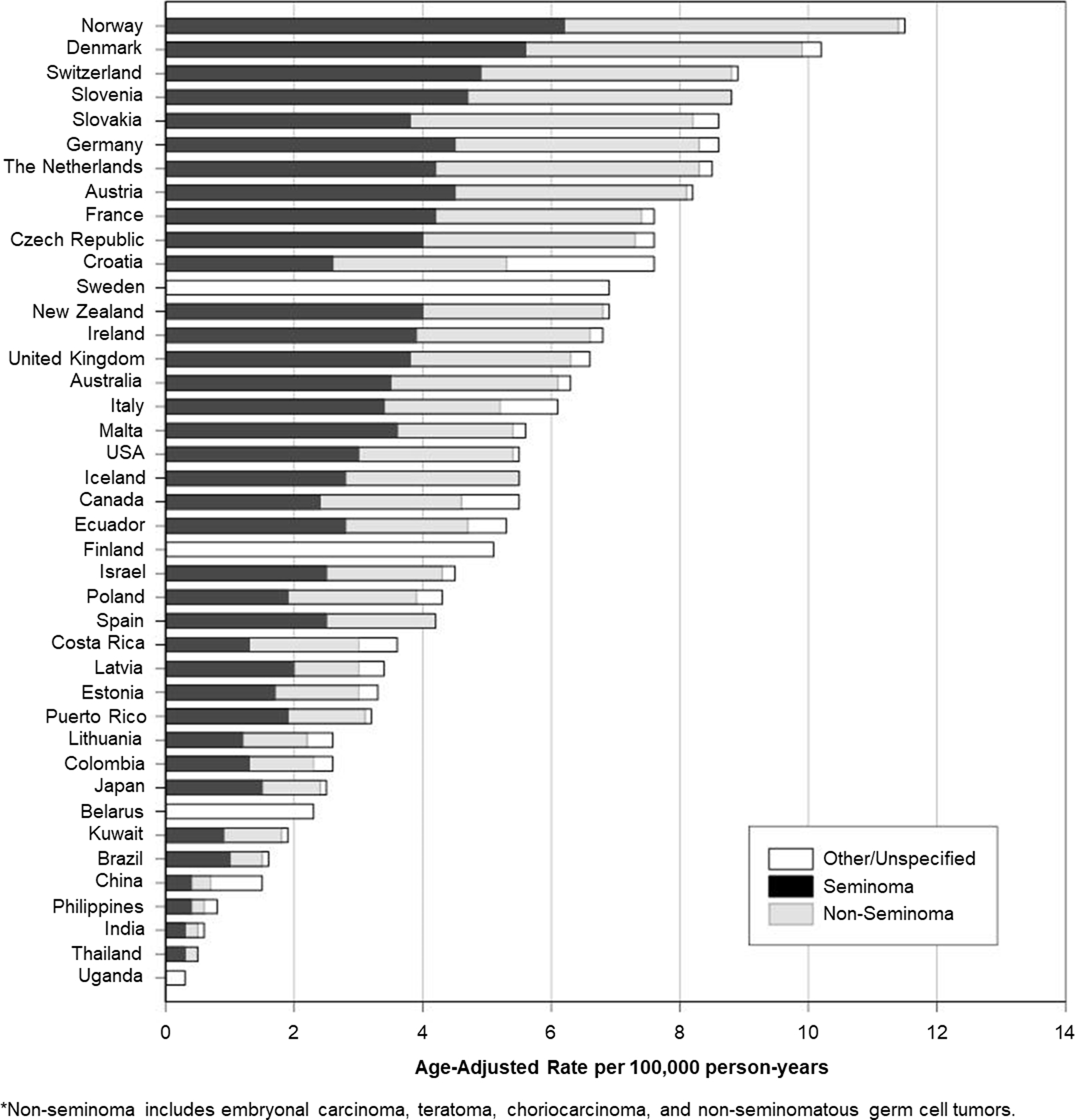
Age-standardised (World Standard Population) rates of testicular cancer incidence by histologic type, 2008–2012.
The trends in rates among the 41 countries examined are displayed in Table 1. According to the average annual percent change (AAPC), the greatest rise in TGCT incidence rates over the 35 year study period occurred in Croatia, with an average of 6% growth in incidence per year (95% CI 2.5–9.5%) and a current rate of 7.6/100,000 (Table 1). Other high-incidence countries including Slovakia (3.9%, 95% CI 3.6–4.2), Slovenia (3.7%, 95% CI 2.3–5.2) and The Netherlands (4.0%, 95% CI 3.5–4.5) also exhibited strong annual growth in rates. The rate of change in the highest incidence countries of Norway (2.4%, 95% CI 2.0–2.8) and Denmark (0.8%, 95% CI 0.4–1.3%) were more modest over the follow-up period, with APCs nearing 0% in more recent decades (Supplementary Material 2). The majority of countries from regions with relatively low testicular cancer incidence experienced 3%−5% average annual growth in incidence rates, including all Central/Eastern European (ranging from 2.2%−4.1%) and Latin American and Caribbean countries (2.1%−4.1%; Figure 2 and Table 1).
Table 1:
Age-standardised (World Standard Population) testicular cancer incidence rates per 100,000 person-years (2008–2012).
| Population | Number of Registries Included | 1978–1982 |
2008–2012 |
AAPC | AAPC 95% CI | ||
|---|---|---|---|---|---|---|---|
| Rate | 95% CI | Rate | 95% CI | (%) | |||
|
| |||||||
| Africa | |||||||
| Uganda | 1 | 0.3 | (0.1, 0.4) | −3.9 | (−10.5, 3.3) | ||
| Asia | |||||||
| China | 2 | 1.5 | (1.3, 1.6) | 1.5 | (1.3, 1.6) | 0.6 | (−1.3, 2.5) |
| India | 2 | 0.6 | (0.5, 0.6) | −1.1* | (−1.7, −0.4) | ||
| Japan | 3 | 1.3 | (1.2, 1.5) | 2.5 | (2.3, 2.7) | 2.1* | (1.5, 2.6) |
| Philippines | 1 | 0.8 | (0.6, 0.9) | −0.3 | (−2.4, 1.8) | ||
| Thailand | 1 | 0.5 | (0.3, 0.7) | −0.2 | (−2.9, 2.6) | ||
| Middle East | |||||||
| Israel | 1 | 2.1 | (1.8, 2.5) | 4.5 | (4.1, 4.8) | 2.7* | (1.6, 3.9) |
| Kuwait | 1 | 1.1 | (0.2, 1.9) | 1.9 | (1.4, 2.5) | 3.0* | (1.2, 4.8) |
| Central/Eastern Europe | |||||||
| Belarus | 1N | 2.3 | (2.2, 2.5) | 3.4* | (2.5, 4.4) | ||
| Czech Republic | 1N | 7.6 | (7.3, 7.9) | 2.2* | (1.3, 3.1) | ||
| Poland | 1 | 4.3 | (3.6, 5.0) | 4.1* | (3.3, 4.9) | ||
| Slovakia | 1N | 2.6 | (2.4, 2.9) | 8.6 | (8.0, 9.2) | 3.9* | (3.6, 4.2) |
| Northern Europe | |||||||
| Denmark | 1N | 7.8 | (7.3, 8.3) | 10.2 | (9.7, 10.8) | 0.8* | (0.4, 1.3) |
| Iceland | 1N | 3.0 | (1.6, 4.3) | 5.5 | (3.9, 7.0) | 1.9 | (−0.5, 4.3) |
| Finland1 | 1N | 1.5 | (1.3, 1.7) | 5.1 | (4.7, 5.5) | 4.2* | (3.6, 4.8) |
| Norway | 1N | 5.8 | (5.4, 6.3) | 11.5 | (10.9, 12.1) | 2.4* | (2.0, 2.8) |
| Sweden1 | 1N | 3.3 | (3.1, 3.6) | 6.9 | (6.5, 7.2) | 2.2* | (1.8, 2.7) |
| Estonia | 1N | 3.3 | (2.7, 3.9) | 3.1* | (2.1, 4.1) | ||
| Latvia | 1N | 3.4 | (2.8, 4.0) | 4.5* | (3.8, 5.3) | ||
| Lithuania | 1N | 2.6 | (2.2, 2.9) | 3.6* | (1.0, 6.2) | ||
| Western Europe | |||||||
| France | 4 | 3.5 | (3.0, 3.9) | 7.6 | (7.0, 8.2) | 2.5* | (2.1, 2.9) |
| Ireland | 1N | 6.8 | (6.4, 7.3) | 2.6 | (−0.6, 6.0) | ||
| The Netherlands | 1N | 8.5 | (8.2, 8.8) | 4.0* | (3.5, 4.5) | ||
| United Kingdom | 11 | 6.6 | (6.5, 6.8) | 1.0 | (−0.3, 2.3) | ||
| Southern Europe | |||||||
| Croatia | 1N | 7.6 | (7.1, 8.2) | 6.0* | (2.5, 9.5) | ||
| Italy | 2 | 2.7 | (1.8, 3.6) | 6.1 | (4.9, 7.3) | 3.2* | (2.1, 4.4) |
| Malta | 1N | 5.6 | (4.2, 6.9) | 3.6 | (−1.5, 9.1) | ||
| Slovenia | 1N | 8.8 | (8.0, 9.6) | 3.7* | (2.3, 5.2) | ||
| Spain | 2 | 1.3 | (0.7, 1.8) | 4.2 | (3.4, 5.1) | 4.3* | (2.9, 5.8) |
| Austria | 1N | 8.2 | (7.0, 9.5) | 1.2* | (0.1, 2.3) | ||
| Germany | 1 | 5.3 | (4.4, 6.2) | 8.6 | (7.4, 9.8) | 1.5* | (0.9, 2.0) |
| Switzerland | 3 | 6.8 | (5.8, 7.8) | 8.9 | (7.9, 9.9) | 0.5 | (−0.3, 1.3) |
| North America | |||||||
| Canada | 8 | 3.3 | (3.2, 3.5) | 5.5 | (5.3, 5.7) | 1.5* | (1.1, 1.9) |
| Costa Rica | 1N | 1.6 | (0.8, 2.4) | 3.6 | (3.2, 4.0) | 3.4* | (2.4, 4.5) |
| Puerto Rico | 1N | 1.0 | (0.8, 1.2) | 3.2 | (2.9, 3.6) | 4.1* | (3.5, 4.8) |
| United States2 | 1 | 3.8 | (3.7, 4.0) | 5.5 | (5.3, 5.7) | 1.1* | (0.9, 1.4) |
| Latin America and Caribbean | |||||||
| Brazil | 1 | 1.6 | (1.1, 2.0) | 4.0* | (0.2, 7.9) | ||
| Colombia | 1 | 2.6 | (2.1, 3.0) | 2.1* | (0.6, 3.5) | ||
| Ecuador | 1 | 5.3 | (4.6, 6.0) | 2.5* | (1.3, 3.8) | ||
| Oceania | |||||||
| Australia | 5 | 6.3 | (6.1, 6.5) | 1.9* | (1.2, 2.6) | ||
| New Zealand | 1N | 6.9 | (6.4, 7.4) | 1.0* | (0.5, 1.6) | ||
Statistically significant at the 0.05 level.
Registry contributed national level data.
Data provided by the NORDCAN database for years 2008–2012.
Includes SEER 9 registries (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Fransisco -Oakland, Seattle-Puget Sound, and Utah).
Figure 2:
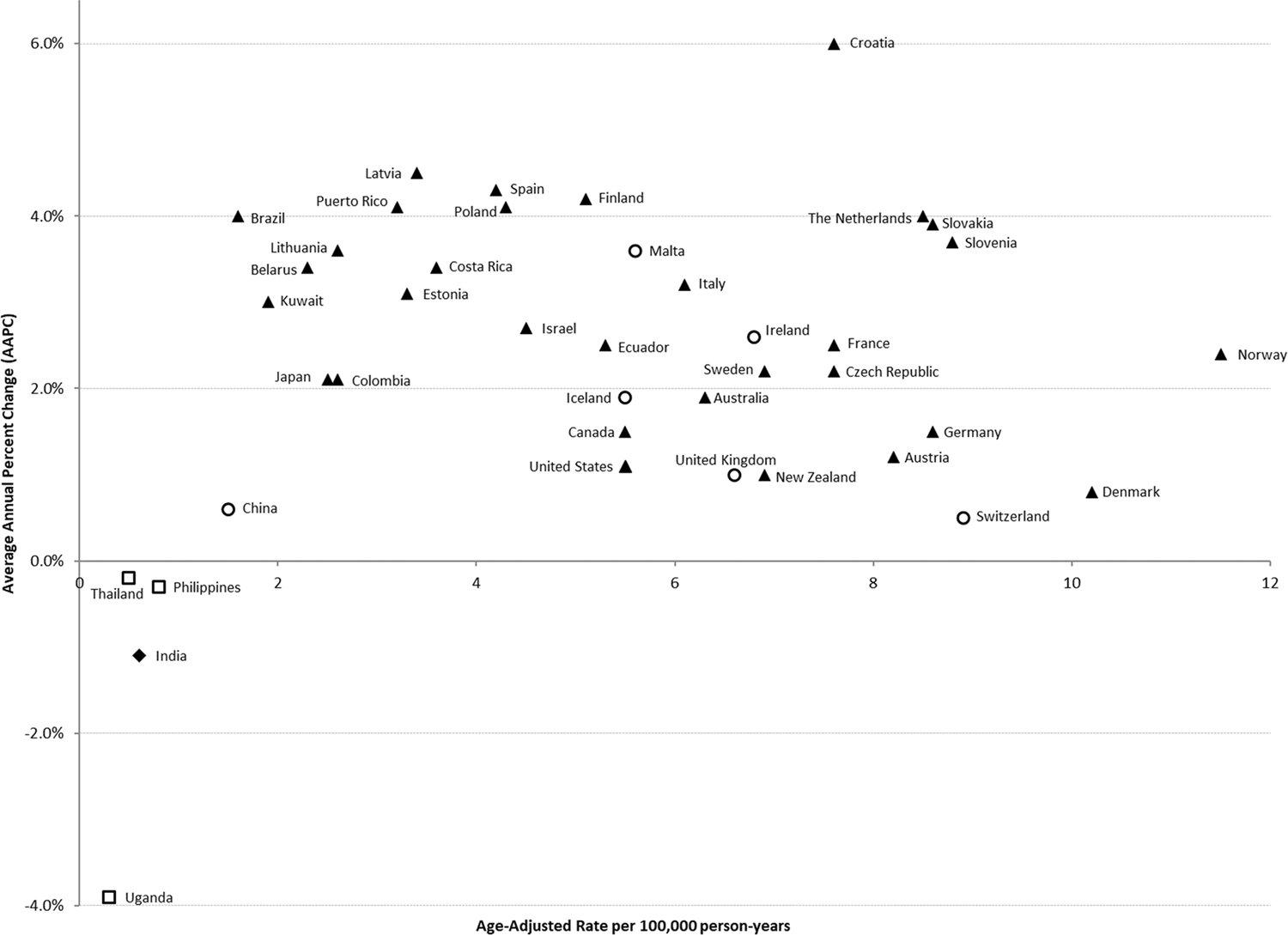
Average annual percent change 1978–2012 and age-standardized rates of testicular cancer incidence by country, 2008–2012. Triangle icons refer to a significant increase over time (p < 0.05); circles refer to a non-significant increase; squares refer to a non-significant decrease; and diamonds refer to a significant decrease.
In most countries where histology data were available, the incidence of seminoma tended to be greater than the incidence of nonseminoma, with both histologic types increasing over time (Figure 3). In terms of temporal trends, rates of seminomas and nonseminomas tended to move in parallel with each other over time; however, there was some evidence of divergence over the last decade, wherein rates of seminoma appear to be increasing at a somewhat greater rate relative to nonseminoma in some higher incidence countries including Denmark, the United States, Italy and the United Kingdom. A sensitivity analysis was conducted to examine the difference between rates including, and excluding, spermatocytic seminoma among selected CI5plus countries and negligible changes in rates were found (Supplemental Table 3).
Figure 3:

Trends in testicular cancer incidence rates for seminoma and nonseminoma for selected countries, 1978–2012.
The age-period-cohort models (Figure 4) demonstrated that the peak age at diagnosis remains approximately 10 years later for seminoma (~35 years) than for nonseminoma (~25 years). This was true across all investigated regions, with the unusual exception of Croatia in which the peak age of seminoma diagnosis occurred between the ages of 40–45. Regarding a birth cohort effect, we observed a flattening of risk among recent cohorts in Denmark and the United Kingdom, a steady increase in risk in the United States (particularly for seminomas), and an increase in risk among more recent cohorts in Costa Rica, Croatia and Slovakia. There was heterogeneity between regions in terms of birth cohort trends by histological sub-type, with some regions experiencing uniformly greater generational increases in the risk of seminoma (Croatia, Denmark, and the United States), while this pattern was unclear for other regions.
Figure 4:
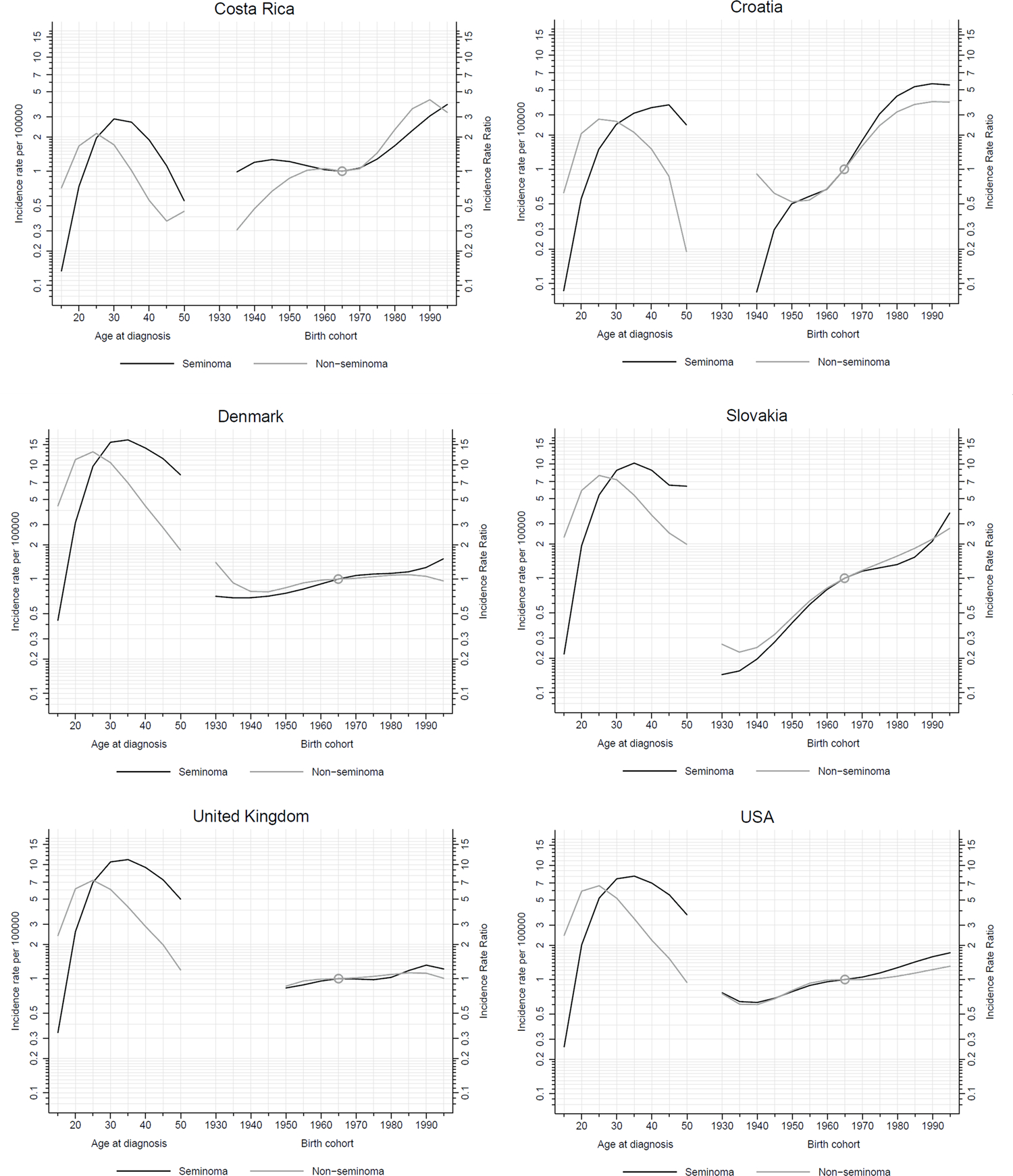
Age-period-cohort models of testicular cancer incidence: age-specific rates per 100 000 man-years (left) and incidence rate ratios by birth cohort (right) for Costa Rica, Croatia, Denmark, Slovakia, the United Kingdom and the United States.
Discussion
The peak incidence of testicular cancer remains centred in Europe, with the highest rates of disease remaining in Norway and Denmark. This observation is in-keeping with previous evidence.8,9 However, given the increasing average annual percent increase in testicular cancer incidence rates continue are showing signs of stabilisation or decline in most of the highest-incidence countries, there is a suggestion that countries are nearing (or may have already hit) an incidence ‘ceiling’. The stabilisation of testicular cancer incidence among high-incidence countries was noted more than a decade ago as possibly representing “a mature phase in the epidemic”.23 Just as the reasons for the abrupt increases in incidence in these countries in the latter half of the 20th century remain largely unexplained, so too does the stabilisation in the early part of the 21st Century.
By contrast, the rising incidence rates in previously lower incidence countries (such as Croatia and Slovakia) appears to have accelerated in recent decades, leading to a narrowing of the incidence gap among European countries. As previously noted by Znaor et al.,8 the steepest increases in incidence appear to be occurring in Southern European states. Our age-period-cohort models suggest that recent birth cohorts (from the 1960’s onwards) have experienced an increase in the relative risk of testicular cancer (Figure 4). However what exposure(s) could be driving the risk in the more recent birth cohorts is unclear, as risk factors for TGCT remain poorly understood in all countries.
The quickening of the rise in incidence among previously low incidence regions has not been limited to Europe. In Latin America and the Caribbean, both Costa Rica and Puerto Rico continue to experience average annual increases of 3–4%, while the Middle East (2–4%) is also experiencing notable increases in incidence. The acceleration of rates in these regions, and in Europe, could be related to a) an actual increase in the prevalence of aetiological risk factors within those populations, b) changes in population screening for testicular cancer, c) an increase in diagnosis of testicular cancer (ascertainment bias), in which the detection of disease has increased (as opposed to an actual increase in disease development), d) changes in competing morbidity risks, and e) migration of peoples from high incidence populations toward previously-low incidence populations. Among these possibilities, a) is the most likely – but it is not clear which factors could be driving the increased risk, as there are very few identified risk factors for testicular cancer. Changes in population screening practices cannot explain changes in incidence, because there are no testicular cancer screening programs in any country. Increased detection of disease due to better access to medical care is also an unlikely explanation, as men with undiagnosed testicular cancer are still likely to die from it (and thus have the cancer detected at a later stage). In terms of competing morbidity, this is an unlikely explanation as there are few competing risks among young men and the presentation of testicular cancer is fairly specific. In terms of migration, this is also unlikely as most migration is from low-incidence areas to high-incidence areas, rather than from high-incidence to low-incidence areas. In summary, there is little evidence to suggest that the increases in incidence rates are due to anything other than actual increases in incidence.
While in many regions the gap between high and low incidence countries appears to be closing (e.g. Northern Europe and Central/Eastern Europe), surprising differences in incidence between apparently similar countries remain. Key examples are found between border-sharing countries with similar demography – for example, the difference observed between Finland and Norway, or between Poland and Slovakia. In addition, as discussed in Box 1 incidence rates for different ethnic groups can differ markedly within the same country, even when those ethnic groups share many demographic and environmental characteristics (for example Māori and Pacific men in New Zealand). The reasons for these disparities remain obscure, but likely include a combination of genetic and environmental drivers. Furthering our understanding of the root causes of these disparities could lead to new clues in understanding the aetiology of testicular cancer in general.
Rates of seminoma and nonseminoma tended to move in parallel over time, although as previously noted by Speaks et al.24 there is evidence that rates of seminoma appear to be increasing at a greater rate relative to nonseminoma in some higher incidence countries including Denmark and the United States. However, these observations were somewhat heterogeneous across regions. Seminomas are diagnosed, on average, 10 years later than nonseminomas, and nonseminomas tend to be more aggressive.9,25,26 Because of these differences in tumour presentation, it has been suggested that the most important exposures which lead to seminoma may differ to those that are most important in the development of nonseminoma.27 However, as previously noted by Bray et al.,28 the lack of consistent and substantial differences over time suggests that the exposures causing the general increase in rates of testicular cancer are relevant to the aetiology of both histologic types.28
Key new information
While the current manuscript provides a comprehensive update on previous descriptions of global trends in testicular cancer incidence, it has also revealed and/or confirmed several key pieces of information about the incidence of this disease. Firstly, our broadened perspective using data from 41 countries has allowed us to clearly convey evidence of a ‘gap-closing’ between high incidence and previously-low incidence countries. We have updated the evidence that shows this gap is closing due to a combination of increasing rates among low incidence countries, along with a recent stabilisation of rates among high incidence countries. Secondly, using these combined registries, it has shown the extent to which rates of testicular cancer can vary within countries (Box 1), which may offer clues regarding the aetiology of this complex disease.
Strengths and Limitations
A strength of this study is the inclusion of data from the Cancer in Five Continents (CI5C) and NORDCAN datasets,15–17 both of which have strict quality control rules for data collection and management. A limitation of this study is that not all countries included in this study have national cancer registries, which required us to combine regional registry data from those countries to infer national incidence rates. Another limitation is the lack of data from several regions, particularly Africa, Asia and the Pacific Islands – regions that are less likely to have cancer registries. As noted previously,8 the International Agency for Research on Cancer is currently development Regional Hubs for Cancer Registration, and so future studies may be able to incorporate more data from these regions.29
Conclusion
While the majority of the testicular cancer burden remains in European countries, the gap is closing between higher-incidence and lower-incidence regions – driven by a combination of increasing rates in lower-incidence countries and rate stabilisation in higher-incidence countries. While this stabilisation follows decades of an epidemic rise in incidence, and thus offers some reassurance that we are observing an incidence ‘ceiling’, the absence of clear evidence for the cause(s) underlying these trends is notable, and is thus an emerging priority. Unusual disparities within geographically-similar regions – and between ethnic groups living within a country – persist, and may offer clues to the currently-obscure aetiology of this disease.
Supplementary Material
Supplementary Table 1: Registries included in testicular cancer trends analysis.
Supplementary Table 2: Age-standardised testicular cancer incidence rates per 100,000 person-years (1978–1982 and 2008–2012), including numbers of cases, annual percent change and numbers of joinpoints.
Supplemental Table 3. Comparison of age-standardised rates for seminomas with and without spermatocytic seminoma included, 2008–2012.
Supplementary Figure 1. Age-standardised rates of testicular cancer by histologic type, 2008–2012.
Acknowledgements
We would like to thank the main organiser of the 9th Copenhagen Workshop on Testicular Germ Cell Tumours, Dr Ewa Rajpert-De Meyts for facilitating a presentation and discussion of some of the preliminary data from this manuscript during the 2018 meeting. JG would like to acknowledge the funding support of the Health Research Council of New Zealand (HRC #14/052).
References
- 1.Winter C, Albers P. Testicular germ cell tumors: Pathogenesis, diagnosis and treatment. Nature Reviews Endocrinology. 2011;7(1):43–53. [DOI] [PubMed] [Google Scholar]
- 2.Brydøy M, Fosså SD, Klepp O, et al. Paternity following treatment for testicular cancer. Journal of the National Cancer Institute. 2005;97(21):1580–1588. [DOI] [PubMed] [Google Scholar]
- 3.Pühse G, Wachsmuth JU, Kemper S, Husstedt IW, Evers S, Kliesch S. Chronic pain has a negative impact on sexuality in testis cancer survivors. Journal of Andrology. 2012;33(5):886–893. [DOI] [PubMed] [Google Scholar]
- 4.Kim C, McGlynn KA, McCorkle R, et al. Sexual functioning among testicular cancer survivors: A case-control study in the U.S. Journal of Psychosomatic Research. 2012;73(1):68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fosså SD. Long-term sequelae after cancer therapy: Survivorship after treatment for testicular cancer. Acta Oncologica. 2004;43(2):134–141. [DOI] [PubMed] [Google Scholar]
- 6.Rajpert-De Meyts E, McGlynn KA, Okamoto K, Jewett MAS, Bokemeyer C. Testicular germ cell tumours. The Lancet. 2016;387(10029):1762–1774. [DOI] [PubMed] [Google Scholar]
- 7.Cheng L, Albers P, Berney DM, et al. Testicular cancer. Nature Reviews Disease Primers. 2018;4(1). [DOI] [PubMed] [Google Scholar]
- 8.Znaor A, Lortet-Tieulent J, Jemal A, Bray F. International Variations and Trends in Testicular Cancer Incidence and Mortality. European Urology. 2014;65(6):1095–1106. [DOI] [PubMed] [Google Scholar]
- 9.Trabert B, Chen J, Devesa SS, Bray F, McGlynn KA. International patterns and trends in testicular cancer incidence, overall and by histologic subtype, 1973–2007. Andrology. 2015;3(1):4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le Cornet C, Lortet-Tieulent J, Forman D, et al. Testicular cancer incidence to rise by 25% by 2025 in Europe? Model-based predictions in 40 countries using population-based registry data. European Journal of Cancer. 2014;50(4):831–839. [DOI] [PubMed] [Google Scholar]
- 11.Bray F, Ferlay J, Laversanne M, et al. Cancer Incidence in Five Continents: Inclusion criteria, highlights from Volume X and the global status of cancer registration. Int J Cancer. 2015;137(9):2060–2071. [DOI] [PubMed] [Google Scholar]
- 12.Bray F, Parkin DM. Evaluation of data quality in the cancer registry: principles and methods. Part I: comparability, validity and timeliness. European journal of cancer (Oxford, England : 1990). 2009;45(5):747–755. [DOI] [PubMed] [Google Scholar]
- 13.Parkin DM, Bray F. Evaluation of data quality in the cancer registry: principles and methods Part II. Completeness. European journal of cancer (Oxford, England : 1990). 2009;45(5):756–764. [DOI] [PubMed] [Google Scholar]
- 14.Bray F, Ferlay J. Chapter 5: Data comparability and quality. In: Bray F, Colombet M, Mery L, et al. , eds. Cancer Incidence in Five Continents, Vol. XI (electronic version). Lyon: International Agency for Research on Cancer. Lyon, France: International Agency for Research on Cancer; 2017. [Google Scholar]
- 15.Bray F, Colombet M, Mery L, et al. Cancer Incidence in Five Continents, Vol. XI (electronic version). Lyon: International Agency for Research on Cancer;2017. [Google Scholar]
- 16.Ferlay J, Colombet M, Bray F. Cancer Incidence in Five Continents, CI5plus: IARC CancerBase No. 9 [Internet]. Lyon, France: International Agency for Research on Cancer;2018. [Google Scholar]
- 17.Danckert B, Ferlay J, Engholm G, et al. NORDCAN: Cancer Incidence, Mortality, Prevalence and Survival in the Nordic Countries. Association of the Nordic Cancer Registries; Danish Cancer Society.;2019. [Google Scholar]
- 18.Segi M Cancer mortality for selected sites in 24 countries (1950–57). Sendai, Japan: Department of Public Health, Tohoku University School of Medicine;1960. [Google Scholar]
- 19.Doll R, Payne P, Waterhouse J. Cancer incidence in five continents: a technical report. New York: 1966. [Google Scholar]
- 20.Kim HJ, Fay MP, Feuer EJ, Midthune D. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351 (correction: 2001;2020:2655). [DOI] [PubMed] [Google Scholar]
- 21.International Agency for Research on Cancer. Global Cancer Observatory. https://gco.iarc.fr/. Accessed 31/01/2019. [Google Scholar]
- 22.Epi: A Package for Statistical Analysis in Epidemiology. [computer program]. 2018.
- 23.Bray F, Richiardi L, Ekbom A, Pukkala E, Cuninkova M, Møller H. Trends in testicular cancer incidence and mortality in 22 European countries: Continuing increases in incidence and declines in mortality. International Journal of Cancer. 2006;118(12):3099–3111. [DOI] [PubMed] [Google Scholar]
- 24.Speaks C, McGlynn KA, Cook MB. Significant calendar period deviations in testicular germ cell tumors indicate that postnatal exposures are etiologically relevant. Cancer causes & control : CCC. 2012;23(10):1593–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horwich A, Shipley J, Huddart R. Testicular germ-cell cancer. Lancet (London, England). 2006;367(9512):754–765. [DOI] [PubMed] [Google Scholar]
- 26.Shah MN, Devesa SS, Zhu K, McGlynn KA. Trends in testicular germ cell tumours by ethnic group in the United States. International journal of andrology. 2007;30(4):206–213; discussion 213–204. [DOI] [PubMed] [Google Scholar]
- 27.Coupland CA, Chilvers CE, Davey G, Pike MC, Oliver RT, Forman D. Risk factors for testicular germ cell tumours by histological tumour type. United Kingdom Testicular Cancer Study Group. British journal of cancer. 1999;80(11):1859–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bray F, Richiardi L, Ekbom A, et al. Do Testicular Seminoma and Nonseminoma Share the Same Etiology? Evidence from an Age-Period-Cohort Analysis of Incidence Trends in Eight European Countries. Cancer Epidemiology Biomarkers & Prevention. 2006;15(4):652–658. [DOI] [PubMed] [Google Scholar]
- 29.International Agency for Research on Cancer. IARC Regional Hubs. 2019; https://gicr.iarc.fr/iarc-regional-hubs-for-cancer-registration/. Accessed 16th May 2019.
- 1.Chia VM, Quraishi SM, Devesa SS, et al. International trends in the incidence of testicular cancer, 1973-2002. Cancer Epidemiology Biomarkers and Prevention 2010;19(5):1151–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray F, Richiardi L, Ekbom A, et al. Trends in testicular cancer incidence and mortality in 22 European countries: Continuing increases in incidence and declines in mortality. International Journal of Cancer 2006;118(12):3099–111. [DOI] [PubMed] [Google Scholar]
- 3.Gurney J, Sarfati D, Stanley J. Obscure etiology, unusual disparity: the epidemiology of testicular cancer in New Zealand. Cancer Causes and Control 2015;26:561–69. [DOI] [PubMed] [Google Scholar]
- 4.Shah MN, Devesa SS, Zhu K, et al. Trends in testicular germ cell tumours by ethnic group in the United States. International journal of andrology 2007;30:206–13. [DOI] [PubMed] [Google Scholar]
- 5.McGlynn KA, Devesa SS, Sigurdson AJ, et al. Trends in the incidence of testicular germ cell tumors in the United States. Cancer 2003;97:63–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Registries included in testicular cancer trends analysis.
Supplementary Table 2: Age-standardised testicular cancer incidence rates per 100,000 person-years (1978–1982 and 2008–2012), including numbers of cases, annual percent change and numbers of joinpoints.
Supplemental Table 3. Comparison of age-standardised rates for seminomas with and without spermatocytic seminoma included, 2008–2012.
Supplementary Figure 1. Age-standardised rates of testicular cancer by histologic type, 2008–2012.


