Abstract
Liver transplant recipients are at an increased risk of opportunistic infections due to the use of immunosuppression. Coronavirus disease of 2019 (COVID-19) increases the risk of these infections further due to associated immune dysfunction and the use of high-dose steroids. We present a case of a liver transplant recipient who developed disseminated tuberculosis and invasive pulmonary aspergillosis complicated by acquired hemophagocytic lymphohistiocytosis after recovering from severe COVID-19.
Keywords: COVID-19, invasive aspergillosis, hemophagocytic lymphohistiocytosis, disseminated tuberculosis, liver transplantation
Abbreviations: AFB, Acid-fast bacilli; AKI, Acute kidney Injury; ATT, Antitubercular therapy; BDG, Beta-D Glucan; COVID-19, Coronavirus disease of 2019; DEB-TACE, Drug eluting bead transarterial chemoembolization; GM, Galactomannan; HCC, Hepatocellular Carcinoma; HLH, Hemophagocytic Lymphohistiocytosis; HRCT, High-resolution computed tomography; IDSA, Infectious Diseases Society of America; IPA, Invasive pulmonary aspergillosis; IVIg, Intravenous immunoglobulin; mRECIST, modified response evaluation criteria in solid tumors; NODAT, New onset diabetes after transplant; PAS, Periodic acid Schiff; RT-PCR, Reverse transcriptase-polymerase chain reaction; SARS-CoV-2, Severe acute respiratory syndrome corona virus 2; sHLH, Secondary hemophagocytic lymphohistiocytosis
Liver transplant recipients are associated with adverse outcomes after infection with coronavirus disease of 2019 (COVID-19).1 Use of high dose immunosuppression has also led to a rise in cases of invasive aspergillosis worldwide.2 We describe a case highlighting the role of post-transplant immunosuppression and COVID-19 in causing reactivation of tuberculosis and invasive pulmonary aspergillosis, complicated by acquired Hemophagocytic Lymphohistiocytosis (HLH).
Case report
We report the case of a 47-year-old gentleman who underwent a living donor liver transplant in 2018 for chronic hepatitis B and hepatitis C co-infection related decompensated cirrhosis and hepatocellular carcinoma (HCC) within Milan criteria. Post-transplant, his liver functions were stable on maintenance immunosuppression with Sirolimus 2 mg twice a day. He had an early recurrence of HCC three months post-transplant, which was managed with a combination of lenvatinib and locoregional therapy in the form of two sessions of microwave ablation (MWA) and two sessions of doxorubicin drug-eluting bead transarterial chemoembolization (DEB-TACE) and was in remission since the last 18 months as determined by serial imaging as per mRECIST (modified response evaluation criteria in solid tumors) criteria. The patient developed new-onset diabetes after transplant (NODAT) a year ago and was on metformin therapy. The patient tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the month of March 2021 after complaining of two weeks of fever and dry cough.
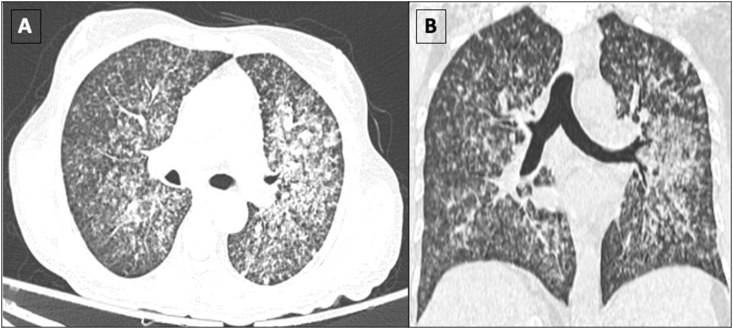
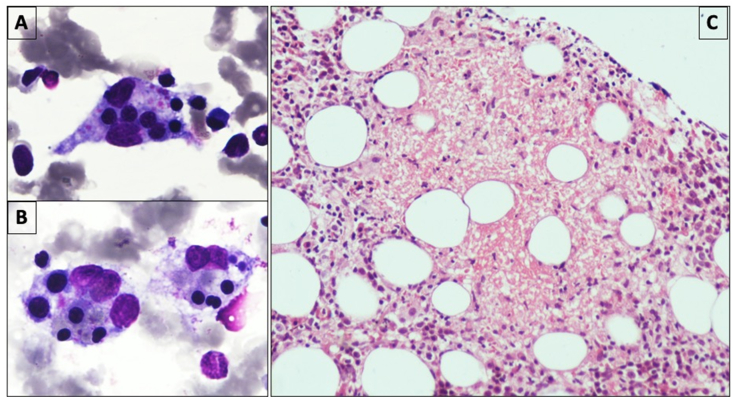
He had not been vaccinated for coronavirus disease of 2019 (COVID-19) at the time of infection. He was found to have moderate COVID-19 due to the presence of respiratory distress, for which he required admission in outside hospital for 3 days where his immunosuppression was switched from sirolimus (4 mg per day) to oral prednisolone (40 mg per day for a week, followed by 10 mg daily). He also received low molecular heparin for 7 days. Ten days later, he complained of progressive shortness of breath along with high-grade fever and fatigue. At this admission, he tested negative for COVID-19 on the SARS-CoV-2 reverse transcriptase-polymerase chain reaction (RT-PCR) report. On examination, he had tachycardia, tachypnea, and hypoxemia, with chest crepitations, and a palpable spleen. He required high flow oxygen by Venturi-mask with an FiO2 of 60%. Laboratory investigations were suggestive of pancytopenia, acute kidney injury (AKI), elevated liver enzymes, and elevated serum procalcitonin (Table 1). Ultrasound of the abdomen revealed splenomegaly with a spleen size of 17 cm. A high-resolution computed tomography (HRCT) scan of the chest revealed bilateral multiple diffuse centrilobular and acinar coalescing nodular opacities suggestive of an infective etiology (Figure 1). In view of fever, pancytopenia, splenomegaly, and calculated H-score being 306 (Table 2), secondary hemophagocytic lymphocytic histiocytosis (HLH) was suspected. He had serum ferritin of 27717 μg/L and fibrinogen levels of 0.8 mg/dL. Bone marrow examination revealed increased histiocytes, with many showing hemophagocytosis (Figure 2A and 2B), thus confirming the diagnosis of secondary HLH. Bone marrow biopsy additionally showed multiple necrotizing granulomas (Figure 2C); however, stains for acid-fast bacilli (AFB) and periodic acid-Schiff (PAS) did not reveal any organisms. Sputum microscopy was positive for acid-fast bacilli on Ziehl–Neelson stain, and sputum fungal culture grew Aspergillus flavus.
Table 1.
Laboratory Parameters of the Patient.
| Parameters | At Admission | On Follow-up |
|---|---|---|
| Hemoglobin (g/dL) | 8.1 | 10.1 |
| Total Leukocyte Count (per cu. mm.) | 1600 | 9700 |
| Platelet count (× 103 per cu. mm) | 11 | 362 |
| Serum Sodium (mEq/L) | 128 | 136 |
| Serum Potassium (mEq/L) | 5.38 | 3.8 |
| Blood Urea (mg/dL) | 133 | 97 |
| Serum Creatinine (mg/dL) | 2.39 | 1.09 |
| Serum Bilirubin (mg/dL) | 1.87 | 1.6 |
| Aspartate Aminotransferase (Units/L) | 404 | 98 |
| Alanine Aminotransferase (Units/L) | 195 | 36 |
| Alkaline Phosphatase (Units/L) | 636 | 720 |
| Prothrombin Index (PTI; %) | 63 | 92 |
| International Normalized Ratio (INR) | 1.55 | 1.02 |
| Serum Ferritin (μg/L) | 27717 | 245 |
| Lactate Dehydrogenase (Units/L) | 457 | 231 |
| Procalcitonin (ng/mL) | 20 | 0.34 |
| Fibrinogen (mg/dL) | 0.8 | 2.6 |
| Triglycerides (mg/dL) | 417 | 132 |
Figure 1.
Axial (A) and Coronal (B) reformatted sections in lung window settings showing multiple centrilobular and coalescing acinar nodules in both lungs, predominantly in the left upper and middle lobe. Features are consistent with infective aetiology.
Table 2.
H-score Criteria (2014) for Diagnosis of HLH and H-score of the Index Patient.
| Parameters | Number of points (criteria for scoring) | Index case |
|---|---|---|
| Known underlying immunosuppression | No (0), yes (18) | 18 |
| Temperature (°C) | <38.4 (0), 38.4–39.4 (33), >39.4 (49) | 33 |
| Organomegaly | No (0), hepatomegaly or splenomegaly (23), hepatomegaly and splenomegaly (38) | 23 |
| No. of cytopenias | 1 lineage (0), 2 lineages (24), 3 lineages (34) | 34 |
| Ferritin (ng/ml) | >2000 (0), 2000–6000 (35), >6000 (50) | 50 |
| Triglyceride (mg/dl) | >132.7 (0), 132.7–354 (44), >354 (64) | 64 |
| Fibrinogen (g/l) | >2.5 (0), ≤2.5 (30) | 30 |
| AST (U/L) | <30 (0), ≥30 (19) | 19 |
| Hemophagocytosis features on bone marrow aspirate | No (0), yes (35) | 35 |
| Total H-score | 306 | |
| Probability of sHLH | >99% |
Figure 2.
Bone marrow aspirate showing many histiocytes with ingested erythroid precursors and platelets (A & B: May-Grunwald-Giemsa, × 1000). Bone marrow biopsy showing necrotizing granulomatous inflammation (C: Hematoxylin & Eosin, × 200).
He was managed with intravenous immune globulin for HLH, liposomal Amphotericin B, and antitubercular therapy (ATT). In view of elevated procalcitonin, AKI, and multiorgan involvement, he received intensive fluid management along with broad-spectrum antibiotic coverage with intravenous meropenem. Liposomal amphotericin B was switched to oral Posaconazole after 3 weeks. AKI was likely prerenal as there was no proteinuria, and it improved with fluid resuscitation. He improved clinically with the resolution of fever, hypoxia, and pancytopenia. He was switched again to sirolimus-based immunosuppression, with complete resolution of cytopenia after 7 days of therapy (Table 1). A repeat positron emission tomographic scan revealed resolution of chest nodules and old neoplastic lesions with post TACE/ablation changes in the graft liver. The patient has completely recovered and is on modified antitubercular therapy and tapering steroid/sirolimus immunosuppression.
Discussion
Infectious complications remain important preventable causes of morbidity and mortality among liver transplant patients. Opportunistic infections, including mycobacterium tuberculosis infection and invasive fungal infections, occur most commonly 1–6 months post-transplant when immunosuppression is at its peak.3 A systematic review showed that liver transplant recipients have an 18-fold increased risk of active tuberculosis infection than the general population and four times increased mortality.4 A systematic review and meta-analysis demonstrated dyspnoea on presentation, hypertension, diabetes mellitus, use of corticosteroids and age 60 years or older to be significantly associated with increased mortality.5 Initially liver transplant recipients were considered to be at increased risk of adverse outcomes due to COVID-19, however, a recent systematic review and meta-analysis demonstrated similar outcomes in liver transplant recipients as nontransplant recipients.6
This case shows the tricky management of post-COVID-19 sequelae in a liver transplant recipient who was on immunosuppression, multikinase inhibitor therapy, with background diabetes and HCC in remission. In the index case, sirolimus was switched to prednisolone at the time of diagnosis of COVID-19. Theoretically, Sirolimus is expected to reduce infection in high-risk populations and severity of COVID-19;7 however, the evidence for the same is lacking. The proven benefit of steroids in improving mortality in patients with severe COVID-198 could have led to the decision to switch to prednisolone for immunosuppression in the index case. However, the use of steroids for COVID pneumonia resulted in reactivation of tuberculosis, superadded invasive pulmonary aspergillosis, and granulomatous inflammation in the marrow leading to secondary HLH. Diagnosis of tuberculosis was based on the presence of acid-fast bacilli in the sputum; however, culture for mycobacteria tuberculosis or molecular assays was not done. Although alkaline phosphatase was markedly raised in the index case, a liver biopsy to demonstrate granulomatous involvement of the liver was not performed as the patient was started treatment for both disseminated TB and HLH, and no additional information was expected from a liver biopsy. A diagnosis of probable invasive pulmonary aspergillosis (IPA) was as per the Infectious Diseases Society of America (IDSA) guidelines with CT imaging, aspergillus culture positivity in a sputum sample, and raised BDG/GM as biomarkers.9 Broncho-alveolar lavage or lung biopsy was not done due to the poor general condition of the patient.
Secondary hemophagocytic lymphohistiocytosis (sHLH) is a life-threatening syndrome of excessive immune activation that results in a hyperinflammatory state and consequently tissue destruction. Acquired HLH after COVID-19 infection is seen in about 6.3% of cases and is associated with a high mortality.10 A majority of HLH cases occur during the active phase of the disease; however, recent reports have highlighted the development of HLH even after the patient has recovered from COVID-19,11,12 as was seen in our index case. Cardinal features of sHLH include unremitting fever, cytopenias, and hyperferritinemia; pulmonary involvement (including ARDS) occurs in approximately 50% of patients.13 Treatment options include steroids, immunoglobulins, and interleukin inhibitors.14 High suspicion is needed to diagnose sHLH. Our patient was successfully managed by timely intravenous immunoglobulin (IVIg) with biopsy confirming the presence of both hemophagocytosis and granulomatous inflammation.
Disseminated tuberculosis was the third infection picked up during this admission. Many cases of reactivation of disease have been reported in the light of the COVID-19 pandemic.15,16 Worsening of glycemic control after reintroduction of steroids and COVID-19-related immune dysfunction could have all contributed to this presentation.
Management of late post-transplant infections remains a challenge in the COVID-19 era. Our unique case indicates the timely detection and treatment of IPA and disseminated tuberculosis post-COVID-19 infection, complicated by sHLH, in a liver transplant patient with a successful outcome.
Credit authorship contribution statement
Akash Gandotra – Writing – original draft; Rohit Mehtani – Writing – review & editing; Madhumita Premkumar – Conceptualization, writing – review and editing, data curation; Ajay Duseja – Writing – review and editing; Arka De – Writing – review and editing; Nabhajit Mallik – Investigation, Writing – review and editing; Durgadevi S - Investigation, Writing – review and editing; Ashim Das - Investigation, Writing – review and editing; Naveen Kalra – Investigation, Writing – review and editing.
Conflicts of interest
The authors have none to declare.
Acknowledgments
The authors have no funding sources or conflicts of interests to declare.
Source of funding
None.
Article guarantor
The first author, Dr Akash Gandotra, is a Fellow in the Department of Hepatology, PGIMER, Chandigarh. The attending physician, Dr. Madhumita Premkumar is the article guarantor.
Consent
Informed consent was obtained from the patient regarding publishing this case report.
References
- 1.Fraser J., Mousley J., Testro A., Smibert O.C., Koshy A.N. Clinical presentation, treatment, and mortality rate in liver transplant recipients with coronavirus disease 2019: a systematic review and quantitative analysis. Transplant Proc. 2020;52:2676–2683. doi: 10.1016/j.transproceed.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh S., Verma N., Kanaujia R., Chakrabarti A., Rudramurthy S.M. Mortality in critically ill patients with coronavirus disease 2019-associated pulmonary aspergillosis: a systematic review and meta-analysis. Mycoses. 2021:1–13. doi: 10.1111/myc.13328. 00. [DOI] [PubMed] [Google Scholar]
- 3.Romero F.A., Razonable R.R. Infections in liver transplant recipients. World J Hepatol. 2011 Apr 27;3:83–92. doi: 10.4254/wjh.v3.i4.83. PMID: 21603030; PMCID: PMC3098392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holty J.E., Gould M.K., Meinke L., Keeffe E.B., Ruoss S.J. Tuberculosis in liver transplant recipients: a systematic review and meta-analysis of individual patient data. Liver Transpl. 2009;15:894–906. doi: 10.1002/lt.21709. [DOI] [PubMed] [Google Scholar]
- 5.Kulkarni A.V., Kumar P., Tevethia H.V., et al. Systematic review with meta-analysis: liver manifestations and outcomes in COVID-19. Aliment Pharmacol Ther. 2020 Aug;52:584–599. doi: 10.1111/apt.15916. Epub 2020 Jul 8. PMID: 32638436; PMCID: PMC7361465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kulkarni A.V., Tevethia H.V., Premkumar M., et al. Impact of COVID-19 on liver transplant recipients-A systematic review and meta-analysis. EClinicalMedicine. 2021;38:101025. doi: 10.1016/j.eclinm.2021.101025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bischof E., Siow R.C., Zhavoronkov A., Kaeberlein M. The potential of rapalogs to enhance resilience against SARS-CoV-2 infection and reduce the severity of COVID-19. Lancet Healthy Longev. 2021;2:e105–e111. doi: 10.1016/S2666-7568(20)30068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.RECOVERY Collaborative Group. Horby P., Lim W.S., Emberson J.R., et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patterson T.F., Thompson G.R., Denning D.W., et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases Society of America. Clin Infect Dis. 2016;63:e1–e60. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang K., My Xing, Ly Jiang, et al. Infection-associated hemophagocytic syndrome in critically ill patients with COVID-19. Curr Med Sci. 2021;41:39–45. doi: 10.1007/s11596-021-2315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalita P., Laishram D., Dey B., Mishra J., Barman B., Barman H. Secondary hemophagocytic lymphohistiocytosis in post-COVID-19 patients: a report of two cases. Cureus. 2021 Aug;13 doi: 10.7759/cureus.17328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naous E., Nassani B.M., Yaghi C., Nasr F., Medlej R. Hemophagocytic lymphohistiocytosis, a new cause of death during 'post-acute COVID-19 syndrome?' A case report. J Hematop. 2021 Apr 20:1–5. doi: 10.1007/s12308-021-00452-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henter J.I., Horne A., Aricó M., et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124–131. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 14.Madkaikar M., Shabrish S., Desai M. Current updates on classification, diagnosis and treatment of hemophagocytic lymphohistiocytosis (HLH) Indian J Pediatr. 2016;83:434–443. doi: 10.1007/s12098-016-2037-y. [DOI] [PubMed] [Google Scholar]
- 15.Garg N., Lee Y.I. Reactivation TB with severe COVID-19. Chest. 2020;158:A777. doi: 10.1016/j.chest.2020.08.724. [DOI] [Google Scholar]
- 16.Pozdnyakov A., Andrew J., Mazen B. Reactivation of pulmonary tuberculosis in a patient with COVID-19. Infect Dis Clin Pract. April 26, 2021 doi: 10.1097/IPC.0000000000001032. (Published ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]