Abstract
Although periprosthetic osteolysis induced by wear debris particles is significantly elevated in senior (65+ years old) patients, most of the published pre-clinical studies were performed using young (less than three-month old) mice indicating the critical need to employ experimental models of particle-induced osteolysis involving mice with advanced age. Emerging evidence indicates that currently available antiresorptive bone therapies have serious age-dependent side effects. However, a resurgence of healthcare interest has occurred in glycyrrhizin (GLY), a natural extract from the licorice roots, as alternative sources of drugs for treating inflammatory bone lytic diseases and prevention of cellular senescence. This study investigated the effects of GLY on inflammatory bone loss as well as expression patterns of senescence-associated secretory phenotype and senescence-protective markers using an experimental calvarium osteolytic model induced in aged (twenty-four-month-old) mice by polymethylmethacrylate (PMMA) particles. Our results indicate that local treatment with GLY significantly diminished the size of inflammatory osteolytic lesions in aged mice via the number of CXCR4+OCPs and Tartrate-resistant acid phosphatase positive (TRAP+) osteoclasts. Furthermore, GLY dramatically decreased the amounts of senescence-associated secretory phenotype markers, including pro-inflammatory macrophage migration inhibitory factor (MIF) chemokine, and cathepsins B and K in the bone lesions of aged mice. By contrast, GLY significantly elevated expression patterns of senescence-protective markers, including homeostatic stromal derived factor-1 (SDF-1) chemokine, and sirtuin-1, and sirtuin-6, in the PMMA particle-induced calvarial lesions of aged mice. Collectively, these data suggest that GLY can be used for the development of novel therapies to control bone loss and tissue aging in senior patients with periprosthetic osteolysis.
Keywords: Aging, Glycyrrhizin, Particle-induced osteolysis, MIF, Cathepsins, Sirtuins
1. Introduction
Total joint arthroplasty (TJA) is one of the success stories of modern medicine because it is an effective and common procedure to treat the last stages of osteoarthritis in senior (>65 years old) patients [1–3]. However, approximately 70% of TJA cases require a replacement surgery within a decade as a result of the accumulation of wear debris particles from an implant device, leading to particle-induced periprosthetic osteolysis [4–7].
Although, particle-induced osteolysis is significantly elevated in patients of an advanced age, most of the published pre-clinical studies were performed using experimental models of periprosthetic bone loss induced in young mice (less than three-months old) [8–10]. By contrast, using CSF1r-eGFP knock in (KI) mice, whose monocyte-lineage cells predominantly express enhanced green fluorescent protein (eGFP), we recently demonstrated that the molecular mechanisms of innate responses and bone osteolysis are significantly different between young (two-month old) and aged (twenty-four-month old) mice indicating the critical need to employ experimental models of particle-induced periprosthetic osteolysis involving aged mice [11,12].
While our understanding of the molecular mechanisms underlying wear debris-induced inflammation has substantially improved in recent decades [13,14] there is still a need for the development of novel therapeutic regimens for elevated periprosthetic osteolysis in senior individuals. Furthermore, a growing body of evidence suggests that the currently available antiresorptive therapies have serious side effects in relation to aging, such as the development of medication-related osteonecrosis of the jaw and may not improve bone quality or bone union ratios as expected [15–17]. Thus, a resurgence of interest has occurred in natural plant extracts as alternative sources of drugs for treating various bone diseases, including wear debris-induced osteolysis [17–20].
A triterpenoid saponin glycoside glycyrrhizin (GLY), an active component of licorice plant roots (Glycyrrhiza glabra), possesses multiple pharmacological properties, including anti-inflammation, anti-tumor, anti-aging, and anti-oxidative activities [21–23] It was also suggested that GLY attenuates tissue aging and promotes cell rejuvenation via the suppression of senescence-associated secretory phenotype (SASP) markers including macrophage migration inhibitory factor (MIF) and lysosomal cathepsins [24–26] Besides the role of GLY in the downregulation of SASPs expression, several studies have reported that it promotes the expression of senescence-protective nicotinamide adenine dinucleotide (NAD+)-dependent deacetylases, known as sirtuins [27,28] It is true that the age-dependent accumulation of SASPs converts senescent cells into pro-inflammatory cells that alter osteoclastogenesis, leading to inflammatory osteolysis [11,29,30]. Furthermore, the incidence of senescent osteoclast precursors (OCPs) increases in the bone microenvironment of aging mice compared with young mice [31] and the inhibition of cellular senescence may prevent aging-related bone loss [32].
Although, GLY effectively inhibited osteoclast differentiation to prevent excessive bone resorption in vitro as well as in bone lesions induced in young mice [33–35], its therapeutic effects on the prevention of particle-induced inflammatory osteolysis in aged mice remain unclear. Thus, in this study we aimed to address whether GLY mitigates inflammatory osteolysis as well as SASPs and promotes expression of senescence-protective sirtuins in an experimental model of particle-induced calvarial osteolysis using aged mice.
2. Material and methods
2.1. Animals
Aged (twenty-four-month-old) male CSF1r-eGFP-KI mice and their wild type strain C57BL/6 were used in this study. Animals were kept in a conventional room with a 12-hour light-dark cycle at constant temperature. Aged C57BL/6 animals were obtained as a donation from the NIA Aging Rodent Colony. The experimental procedures were approved by the Institutional Animal Care and Use Committees (IACUC) at Nova Southeastern University.
2.2. RANKL-induced osteoclastogenesis and TRAP staining in vitro
Mononuclear bone marrow cells isolated from femur and tibia of aged wild type mice (twenty four month old) were seeded in 96-well plates at a density of 1 × 105 cells/well in α-MEM medium (Life Technologies) containing 10% FBS (Atlanta Biologicals) and 20 ng/ml of MCSF and 10 ng/ml of RANKL (BioLegend) recombinant proteins in the presence or absence of polymethylmethacrylate (PMMA) particles (mean diameter 6.5 μm; Bangs Laboratories), mouse MIF recombinant protein (R&D), and Glycyrrhizin (Tokyo Chemical Ind.) as described in earlier studies [12,33,36,37] Five days later, cells were stained for tartrate-resistant acid phosphatase (TRAP) using a leukocyte acid phosphatase kit (Sigma). TRAP positive (TRAP+) cells with more than three nuclei were considered mature osteoclasts and were microscopically counted. The results were expressed as numbers per well.
2.3. Particle-induced calvarial osteolysis model
An experimental PMMA particle-induced osteolytic mouse model was used in this study. A suspension of PMMA particles in phosphate buffered saline (PBS) was prepared as we described earlier [10]. Then, the suspension of particles (150 μL/site) was injected subcutaneously over the calvarial bone by using a Tuberculin syringe (Covidien, Inc). In addition, a group of animals was additionally injected subcutaneously over the calvaria bone with several concentrations of glycyrrhizin solution in PBS (20 or 200 mg/kg) once every other day for ten days. Control animals received sham injection of PBS. The injection volume was 100 μL/mouse and the concentrations of GLY were determined based on the previously published observations [38,39].
2.4. In situ imaging of cathepsin B and K activities at calvarial bone lesions
At day 11, wild type mice were administrated intravenously (i.v.) with CatB 680 FAST™ and CatK 680 FAST™ (Perkin Elmer) fluorescent imaging probes according to the manufacturer’s instructions. Then, the fluorescence activities were measured from the PMMA particle-induced calvarial lesions by In-Vivo Xtreme II imaging system (Brucker) in anesthetized mice. The total flux (measured in photons per second) in the ROI were quantified using In-Vivo Xtreme Image Software (Brucker).
2.5. Isolation of total proteins from mouse calvariae
Mouse calvariae were collected from euthanized wild type animals and weighed. Then bones were mechanically homogenized on ice with a mortar and pestle in PBS with 0.05% Tween 20 in the presence of 10 μg/ml of ethylenediaminetetraacetic acid (EDTA)-free protease inhibitor cocktail (Thermo Scientific). The suspension was centrifuged at 520 g and then collected proteins were isolated by ELISA.
2.6. ELISA
Concentrations of MIF and SDF-1 proteins from the collected cells supernatant and tissues suspension were measured using commercial sandwich ELISA kits (BioLegend).
2.7. Multicolor flow cytometry of OCPs
Mononuclear cells were isolated from calvarial tissues of CSF1r-eGFP-KI mice and then were incubated with anti-mouse CD16/32 (Fc-blocking antibody, Ab). Next, cells were stained with anti-CD11b conjugated to Pacific blue, anti-CD45 conjugated to Alexa 647, and anti-CXCR4 conjugated to PE Abs (BioLegend) to characterize OCPs as CD45+eGFP+CD11b+CXCR4+ cells. Cells were acquired on the BD FACSAria™ II Cell Sorter (BD Biosciences) and analyzed with FlowJo (ver. 10) software (Tree Star).
2.8. Histological analyses and immunohistochemistry
Dissected calvariae were decalcified in 10% EDTA (Thermo Fisher Scientific) and then were embedded in paraffin. Frontal calvarial Section 6 μm in thickness centered on the sagittal suture were obtained for histological analysis. To stain TRAP+ osteoclasts (OCs), sections were first incubated in 0.2 M acetate buffer containing 50 mM L-(+)-Tartaric acid (Sigma) at room temperature and then in TRAP staining solution (0.2 M acetate buffer, 50 mM L-(+)-Tartaric acid, 0.5 mg/ml Naphthol AS-MX phosphate, 1.1 mg/ml Fast Red ASTR salt; Sigma) at 37 °C. Finally, the sections were counterstained with hematoxylin solution (Sigma) at room temperature.
2.9. RNA Extraction and real-time PCR
Total RNA was isolated using PureLinkTM RNA Mini Kit (Ambion, Life Technologies), following the manufacturer’s instructions, and subjected to reverse transcription with the Verso cDNA Synthesis Kit (Thermo Fisher Scientific) in the presence of random primers and oligo-dT. Finally, sirtuin-1 (Mm01168521_m1), sirtuin-3 (Mm00452131_m1), and sirtuin-6 (Mm01149042_m1), cathepsin B (Mm00514443_g1), and cathepsin K (Mm00484039_m1) gene expressions were measured using TaqMan™ Fast Advanced PCR Master Mix (Applied Biosystems, Life Technologies). Data were analyzed using the 2−ΔΔCt method normalized to GAPDH.
2.10. Statistical analysis
Data are displayed as mean ± SEM. Statistical significance was evaluated using a one-way ANOVA with post hoc Tukey’s test. A p < 0.05 was considered statistically significant. Data were analyzed using PAST 2.1 statistical software.
3. Results and discussion
3.1. Glycyrrhizin (GLY) regulates the production of pro-inflammatory MIF and homeostatic SDF-1/CXCL12 chemokines for OCPs in the particle-induced calvarial lesions of aged mice
While stromal cell-derived factor-1 (SDF-1)/CXCL12 produced in normal bone tissue was accepted as a chemoattractant factor to recruit circulating OCPs to the homeostatic bone remodeling site [40], our group previously reported that macrophage migration inhibitory factor (MIF), but not SDF-1, is engaged in the recruitment of OCPs to particle-induced osteolysis lesion in young mice, and that MIF suppresses the local production of SDF-1 [10]. Therefore, we first tested the effects of locally administrated GLY solution (Fig. 1A) on the expression of SDF-1 and MIF chemokines in the particle-induced peripheral osteolytic lesions of aged wild type mice using an ELISA assay. The concentration of MIF was significantly elevated in the PMMA particle-induced osteolytic lesions compared to the control group of mice (Fig. 1B, C). In contrast, the local administration of GLY significantly reduced the MIF concentration in calvaria lesions compared to mice treated with a sham control (Fig. 1B). We also detected that SDF-1 levels in the PMMA-induced calvaria lesions of the GLY-treated mice were significantly elevated compared to the mice treated with PMMA alone (Fig. 1C); these findings correspond with the findings of our previous report, which showed that the downregulation of MIF has a positive effect on SDF-1 levels in PMMA-induced calvaria osteolysis lesions using CSF1r-eGFP-KI mice [10].
Fig. 1.
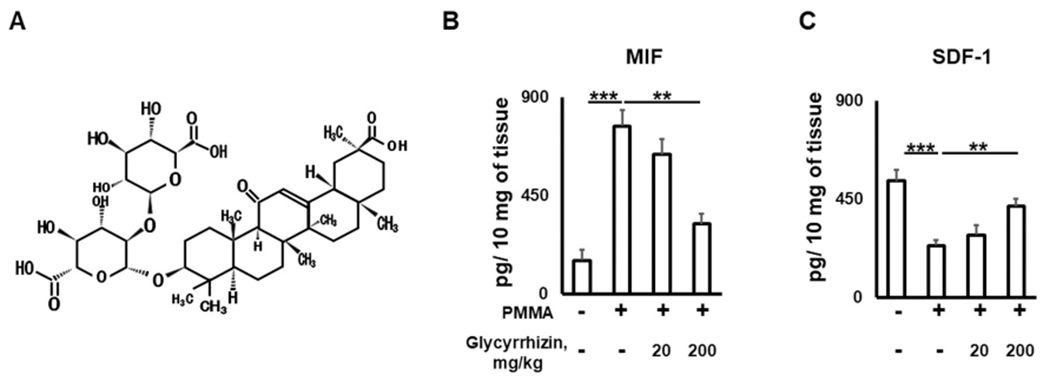
Glycyrrhizin (A; chemical structure) regulates the production of pro-inflammatory MIF (B) and homeostatic SDF-1/CXCL12 (C) chemokines for OCPs in the particle-induced calvarial lesions of aged mice. Calvarial tissues were sampled from wild type aged (twenty-four-month-old) mice and then were evaluated by an ELISA assay. *p < 0.05, **p < 0.01; ***p < 0.001. GLY – Glycyrrhizin.
In addition to MIF and SDF-1 chemokines, it was demonstrated that MCP-1 (CCL-2) chemokine promotes macrophage recruitment to particle-induced bone lesions [41–43]; however, the effects of MCP-1 on the macrophage chemotaxis were significantly mitigated in the MIF--knock out (KO) mice, indicating that MIF plays an essential role in the recruitment of OCPs to bone lesions [44,45]. Furthermore, a large number of studies demonstrated that MIF-KO mice have an extended lifespan and experience reduced osteoarthritis and particle-induced osteolysis severity compared to wild-type control mice, suggesting the important role of MIF as an SASP marker in the aging process and bone osteolysis [12,46–48]. Since aging decreases the level of homeostatic SDF-1 in peripheral blood while up-regulating the expression of pro-inflammatory MIF in various pathologies associated with aging, including peripheral osteolytic lesions [46,49–53], it is plausible that GLY could contribute to the prevention of osteolytic lesions in aged mice via the suppression of pro-inflammatory MIF production, restoring the concentration of homeostatic SDF-1.
3.2. GLY suppresses recruitment of CXCR4+OCPs to particle-induced osteolytic lesions in aged mice
Growing evidence suggests that bone inflammation promotes chemokine-induced recruitment of OCPs from the blood stream to bone lytic lesions, leading to elevated osteoclastogenesis at the site of inflammation [54,55]. Our previously published observations indicated that ligation of MIF and SDF-1 with CXCR4 receptor promotes OCPs chemotaxis and osteoclastogenesis in vitro as well as in the experimental model of particle-induced osteolysis using young CSF1r-eGFP-KI mice [10]. Because CSF1r-eGFP-KI mice express eGFP protein in osteoclast precursors, it was suggested that this transgenic strain could be used to elucidate the molecular mechanisms underlying chemotaxis of OCPs to osteolytic lesions in relation to aging [11,56]. Consequently, we examined next whether local administration of GLY could affect the recruitment of CXCR4+OCPs to PMMA particle-induced osteolysis in aged CSF1r-eGFP-KI mice by multicolor flow cytometry assay. It was clearly demonstrated that PMMA particles significantly increased the infiltration of CXCR4+OCPs in the calvarial lesions compared to the healthy group of aged mice. In contrast, a local injection of GLY significantly suppressed the infiltration of CXCR4+OCPs to the particle-induced bone lesion compared to the group of mice that received the control sham injection (Fig. 2A–C). These data concur with the findings of a previous study, which indicated that GLY suppresses the CXCR4-dependent migration of monocytes and fibroblasts in vitro and in vivo [57]. It is notable that a number of studies have detected an elevated expression of CXCR4 mRNA in senior patients when compared with younger individuals [58–61]. Altogether, our results strongly suggest that locally administered GLY diminished the migration of CXCR4+OCPs to particle-induced osteolytic lesions induced in aged mice.
Fig. 2.
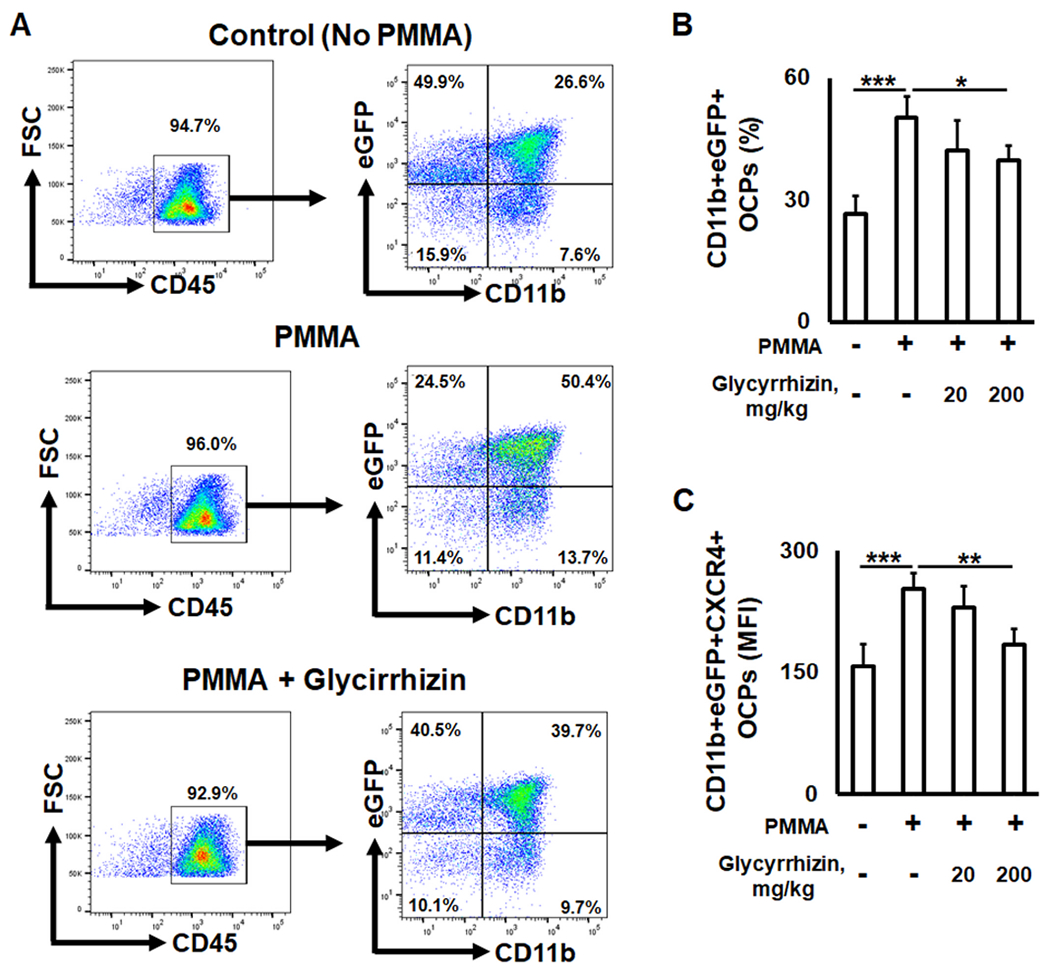
The impact of Glycyrrhizin (GLY) on the incidence of CXCR4+ osteoclast precursors (OCPs) in PMMA particle-induced calvarial lesions of aged (twenty-four-month-old) CSF1r-eGFRP-knock in mice. Representative contour plots (A) and percentage of eGFP+CD11b+ OCPs (B) identified in osteolytic lesions induced in CSF1r-eGFP-knock in mice that received subcutaneously over the calvarial bone: Control (no particles), PMMA particles alone, and PMMA particles + GLY solution. C: expression of CXCR4+ receptor on the surface of OCPs detected in the osteolytic lesions after treatment with GLY. *p < 0.05, **p < 0.01, ***p < 0.001.
3.3. GLY diminishes the effect of particles on the size of osteolytic calvanal lesions in aged mice
The elevated migration of CXCR4+OCPs to particle-induced osteolytic lesions was positively correlated with upregulated osteoclastogenesis and bone loss around the implant device [62–65]. Since GLY suppresses the recruitment of OCPs to the particle-induced calvaria osteolysis (Fig. 2), we evaluated the impact of GLY on PMMA particles- and recombinant MIF-elicited osteoclastogenesis in vitro using RANKL-primed bone marrow derived macrophages (BMDM) isolated from aged wild type mice. Addition of PMMA particles and recombinant MIF to RANKL-stimulated BMDM cells dramatically enhanced the development of TRAP+ multinucleated osteoclasts (OCs) (Fig. 3A, B). In contrast, GLY significantly reduced the number of OCs exposed to PMMA and MIF in vitro (Fig. 3A, B).
Fig. 3.
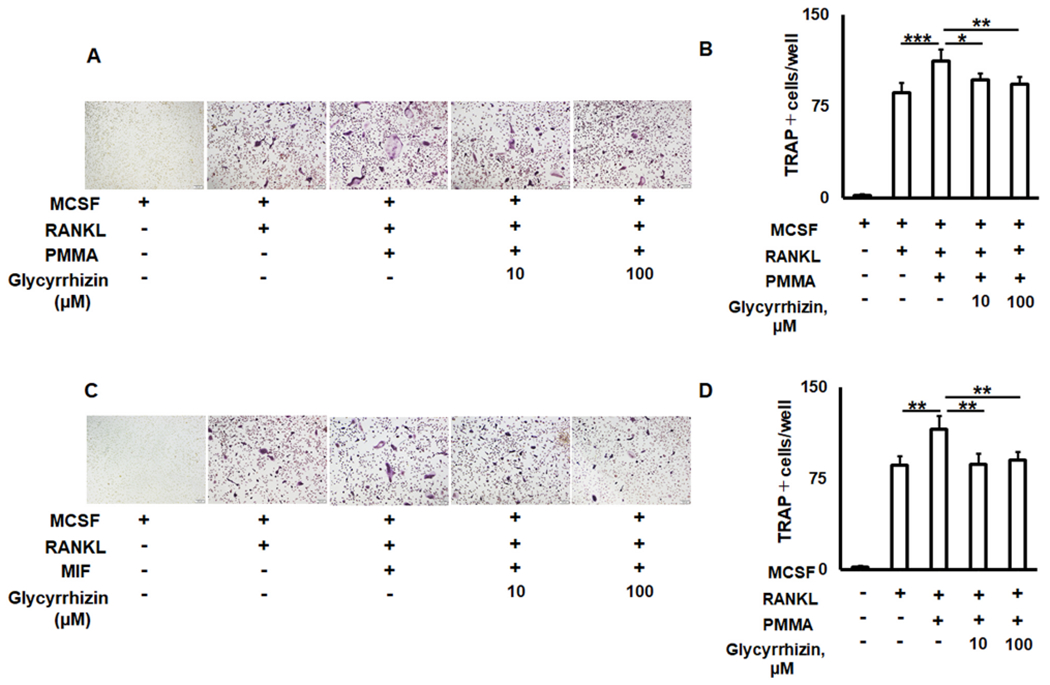
The effects of Glycyrrhizin (GLY) solution on aged RANKL-primed osteoclast precursors exposed to PMMA particles and recombinant MIF protein in vitro. Microscopic evaluation of the TRAP staining and number of TRAP multinucleated osteoclasts per well formed from the RANKL-stimulated bone marrow derived macrophages exposed to PMMA particles (A, B) and MIF (C, D) in the presence of GLY. Bone marrow derived macrophages were collected from twenty-four-month-old C57BL/6 mice. *p < 0.05, **p < 0.01, ***p < 0.001.
To further confirm our in vitro findings, we examined the effects of GLY on the expression of some important markers for osteoclastogenesis, including Dc-stamp, Oc-stamp, and Acp5/Trap mRNAs, and the development of osteolytic lesions along with numbers of TRAP+ cells in the PMMA particle injection site at the calvaria of aged mice. In response to a PMMA particles injection followed by GLY local treatment, the expression patterns of Dc-stamp, Oc-stamp, and Acp5 were significantly reduced (Fig. 4A). Furthermore, the size of osteolytic lesions and the number of TRAP+ osteoclasts in the mouse calvaria that received PMMA particles were significantly larger than those found in mice with particle-induced osteolytic lesions treated with GLY (Fig. 4B, C). These results accorded with those of previous reports, showing that GLY effectively inhibits RANKL-induced osteoclastogenesis in vitro and in an experimental mouse model of osteoporosis [33,37]. However, no effect of orally-administrated GLY on bone properties in ovariectomized rats was detected [66]. A previous study reported that the bioavailability of oral administering GLY is low because of its poor absorption in the intestine, indicating that an intraperitoneal administration of GLY significantly improves its beneficial effect on bone health in a model of osteoporosis using young mice [37]. These findings indicate that locally injected GLY attenuated the expression of pro-osteoclastogenic markers and reduced the amount of bone loss and the number of TRAP+ osteoclasts in vitro as well as in the osteolytic calvarial lesions induced in aged mice.
Fig. 4.
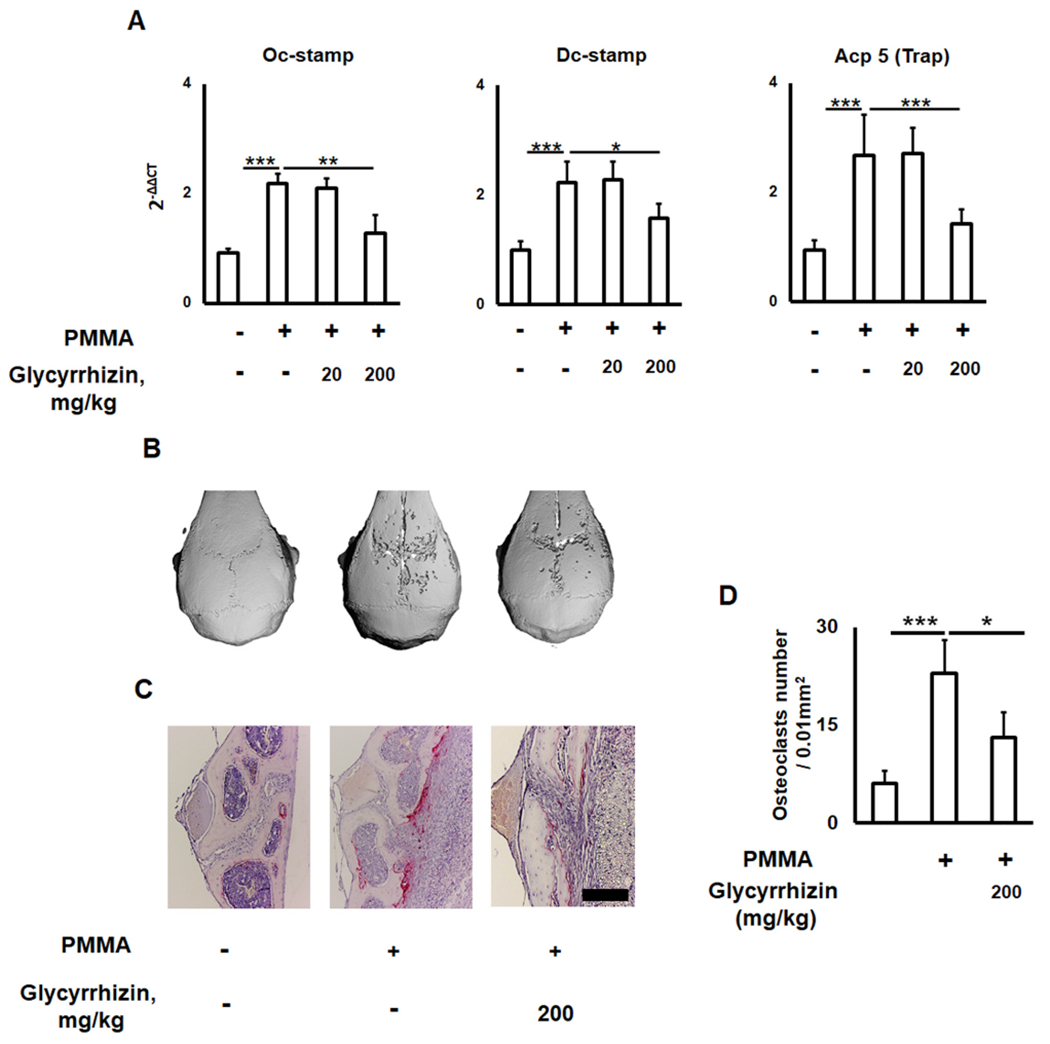
Glycyrrhizin (GLY) diminishes effects of PMMA particles on the size of osteolytic calvarial lesions in aged mice. (A) Expression of osteoclastogenesis-related Oc-stamp, Dc-stamp, and Acp 5/Trap genes in the dissected calvarial tissues of control (no particle placed) and PMMA particle-induced bone lesions of sham and GLY-treated aged wild type (twenty-four-month-old) mice. μ-CT images and histological evaluation of TRAP osteoclasts (B and C) in the PMMA particle-induced calvarial lesions of aged mice that received: Control (no particles), PMMA particles alone, and PMMA/GLY injection. D: The number of TRAP+ osteoclasts measured in a microscopic field (0.01 mm2) of TRAP-stained sections. Because no significant effect of GLY at 20 mg/kg concentration was observed, only a group of mice treated with 200 mg/kg of GLY solution was evaluated by μ-CT and immunohistochemistry. *p < 0.05, **p < 0.01, ***p < 0.001.
3.4. GLY reduces activities of bone resorptive cathepsins in the calvarium lesions of aged mice induced by PMMA particles
It is well-established that the activity of lysosomal cathepsin proteases is significantly elevated in senescent cells compared with young cells, indicating that cathepsins are a conserved component of SASPs [67–69]. Among the eleven known cathepsins, cathepsin B plays an essential role in the RANKL-mediated fusion of mononucleated OCPs, while cathepsin K is secreted by activated multinucleated osteoclasts to degrade various matrix proteins during bone resorption [70–72]. We examined whether GLY downregulates activities of cathepsins B and K in the particle-induced osteolytic lesions of aged wild type mice using real-time PCR and in situ intravital imaging assays. According to the real-time PCR evaluation, expression patterns of cathepsins B (catB) and K (catK) were significantly elevated in the PMMA-particle-induced osteolysis compared with control mice (Fig 5A, D), partially resembling findings of previously published reports, which found that patients with periprosthetic osteolysis have an elevated expression of cathepsin K [73,74]. In addition, some studies have reported that the levels of RANKL-induced osteoclastogenesis and pathogenic bone loss are positively correlated with elevated activities of cathepsins B and K in OCPs in relation to aging [68,75–77]. In this study, local injection of GLY significantly attenuated expression patterns of cathepsins B and K genes in the osteolytic lesions induced in aged mice (Fig. 5A, D). Furthermore, GLY significantly reduced the signal intensities for both cathepsins B (Fig. 5B, C) and cathepsin K (Fig. 5E, F) at the calvaria PMMA-induced inflammation site, indicating that GLY effectively downmodulated the enzymatic activities of cathepsins B and K in the bone lesions of aged mice. Since selective pharmaceutical inhibition of cathepsin B and K activities significantly reduces bone resorption in vitro as well as in vivo [78], these results strongly suggest that locally-administered GLY mitigates the activities of cathepsins B and K in the aging experimental model of particle-induced calvarial osteolysis.
Fig. 5.
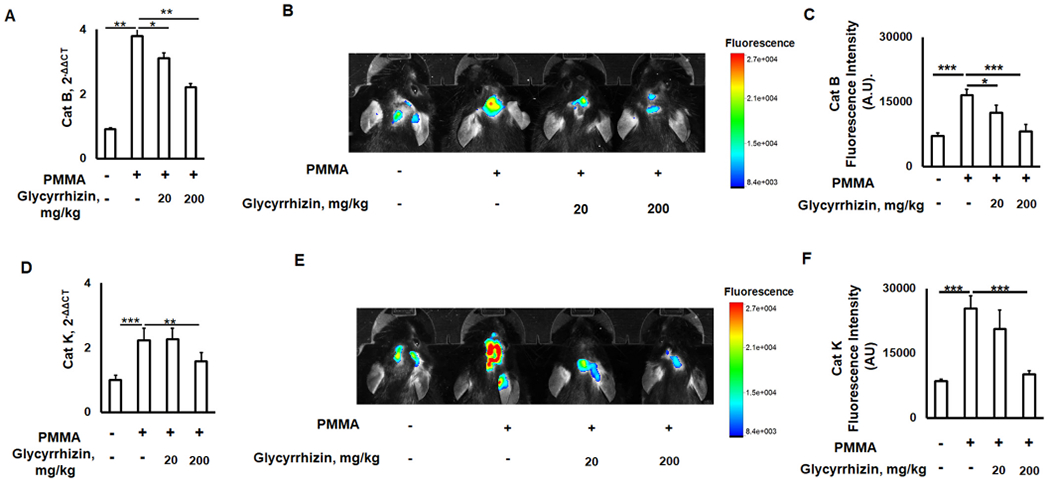
Glycyrrhizin (GLY) reduces activities of bone resorptive cathepsin B and K in the calvarium lesions of aged mice induced by PMMA particles. Expression patterns of cathepsin B and K mRNA (A, D) and in situ molecular imaging of cathepsin B and K activities (B, E) in the PMMA particle-induced calvarial lesions of wild type aged (twenty-four-month-old) mice that received: Control (no particles), PMMA particles alone, and PMMA/GLY injection. The levels of signal intensity of cathepsin B (C) and cathepsin K (F) with respect to total flux quantified from the molecular imaging assay. *p < 0.05, **p < 0.01, ***p < 0.001.
3.5. GLY diminishes the negative effect of PMMA particles on the expression of senescence-protective sirtuins in calvanal lesions of aged mice
Some studies reported that lysosomal cathepsins B and K promote the degradation of ubiquitously-expressed senescence-protecting sirtuins, which may also suppress particle-induced osteolysis [79,80]. Sirtuins are nicotinamide adenine dinucleotide (NAD)-dependent deacetylases that are implicated in a plethora of biological processes including metabolism, stress responses, and tumorigenesis [81]. Among the seven-known mammalian sirtuin families, growing evidence shows that sirtuin-1, sirtuin-3, and sirtuin-6 are essential factors in delaying cellular senescence and extending organismal lifespan by reversing some aspects of aging [82,83]. Sirtuin-1 and sirtuin-6 are predominately found in the nucleus, while sirtuin-3 resides in the mitochondria [83]. Nevertheless, the GLY-dependent effects on expression patterns of sirtuin-1, sirtuin-3, and sirtuin-6 in the particle-induced osteolytic lesions remains limited. As GLY suppressed the development of osteolytic lesion and reduced the activity of cathepsins B and K (Figs. 3–5), we next evaluated the effect of GLY on the expression patterns of sirtuin-1, sirtuin-3, and sirtuin-6 genes on osteolytic lesions of aged wild type mice. It was observed that the levels of sirtuin-1 and sirtuin-6 mRNAs in the group of aged mice locally injected with PMMA/GLY were significantly higher compared to the group treated with PMMA alone (Fig. 6A, C). It is important to note that, sirtuin-1 also enhances the expression of sirtuin-6 by interacting with FOXO3a and NRF1 at the sirtuin-6 promoter [84]. We also detected that GLY had either no or little significant effect on the expression of mitochondrial-localized sirtuin-3 in the peripheral osteolytic lesions induced in aged mice (Fig. 6B), indicating that GLY mainly promotes the expression of nuclear-localized sirtuin-1 and sirtuin-6. A recently published study demonstrated that sirtuin-3 elevated RANKL-mediated osteoclastogenesis in aged male mice, suggesting that sirtuin-3 promotes aging-related bone loss [85]. Our results support the previous finding by Deng et al. [86], that sirtuin-1 expression was significantly attenuated in the biopsies collected from patients with particle-induced osteolysis. Furthermore, it has been also demonstrated that the activation of sirtuin-1 and sirtuin-6 suppresses aging-dependent osteoclastogenesis [87,88]. Taken together, these results strongly suggest that GLY elevates the expression of senescence-protecting sirtuin-1 and sirtuin-6 in the PMMA particles-induced calvarial lesions, supporting our key finding that GLY promotes tissue rejuvenation and reduces bone osteolysis in aged mice.
Fig. 6.
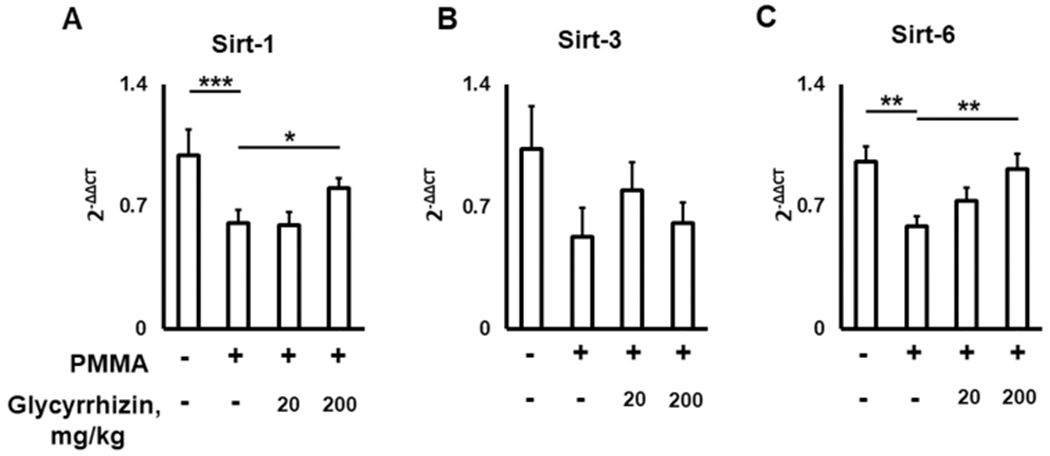
Glycyrrhizin (GLY) diminishes negative effects of PMMA particles on the expression of senescence-protective sirtuins (Sirt) in bone lesions of aged mice. Sirt-1 (A), sirt-3 (B), and sirt-6 (C) gene expression levels in the PMMA particle-induced calvarial lesions of aged mice that received: Control (no particles), PMMA particles alone, and PMMA/GLY injection. *p < 0.05, **p < 0.01.
4. Conclusion
In this study, we investigated the effects of locally injected glycyrrhizin (GLY) on experimental calvarial osteolysis induced by PMMA particles in aged (twenty-four-month-old) mice. We demonstrated that GLY significantly diminished the size of inflammatory osteolysis in aged mice via the number of CXCR4+OCPs and TRAP+ osteoclasts. Our results also indicated that GLY significantly downmodulates the amount of SASPs, including pro-inflammatory MIF chemokine and cathepsins B and K in the bone lesions of aged mice. We also demonstrated that GLY elevated the amount of homeostatic SDF-1 chemokine and the expression of senescence-protective factors, such as sirtuin-1 and sirtuin-6, in the calvarial lesions of aged mice. Therefore, GLY can be used for the development of novel therapies to control particle-induced osteolytic lesions and SASPs in senior patients after TJA.
Acknowledgements
This work was supported by a Nova Southeastern University President Faculty Research Development Grant and NIH Grants AG-053615, AG-064003, AG-068595, DE-028699, and DE-027153.
Footnotes
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- [1].Kurtz S, MOWAT F, ONG K, CHAN N, LAU E, HALPERN M, Kurtz S, Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002, J. Bone Jt. Surg. Am 87 (7) (2005) 1487–1497. [DOI] [PubMed] [Google Scholar]
- [2].Sculco TP, The role of constraint in total knee arthoplasty, J. Arthroplast 21 (4 Suppl 1) (2006) 54–56. [DOI] [PubMed] [Google Scholar]
- [3].Oussedik S, Abdel MP, Cross MB, Haddad FS, Alignment and fixation in total knee arthroplasty: changing paradigms, Bone Jt. J 97-B (10 Suppl A) (2015) 16–19. [DOI] [PubMed] [Google Scholar]
- [4].Steinbeck MJ, Jablonowski LJ, Parvizi J, Freeman TA, The role of oxidative stress in aseptic loosening of total hip arthroplasties, J. Arthroplast 29 (4) (2014) 843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lübbeke A, Garavaglia G, Barea C, Stern R, Peter R, Hoffmeyer P, Influence of patient activity on femoral osteolysis at five and ten years following hybrid total hip replacement, J. Bone Jt. Surg. Br 93 (4) (2011) 456–463. [DOI] [PubMed] [Google Scholar]
- [6].Gallo J, Goodman SB, Konttinen YT, Raska M, Particle disease: biologic mechanisms of periprosthetic osteolysis in total hip arthroplasty, Innate Immun. 19 (2) (2013) 213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kurtz SM, Ong KL, Schmier J, Mowat F, Saleh K, Dybvik E, Kŝrrholm J, Garellick G, Havelin LI, Furnes O, Malchau H, Lau E, Kurtz SM, Ong KL, Schmier J, Mowat F, Saleh K, Dybvik E, Kärrholm J, Garellick G, Havelin LI, Furnes O, Malchau H, Lau E, Future clinical and economic impact of revision total hip and knee arthroplasty, J. Bone Jt. Surg. Am 89 (Suppl 3) (2007) 144–151. [DOI] [PubMed] [Google Scholar]
- [8].Hiram-Bab S, Liron T, Deshet-Unger N, Mittelman M, Gassmann M, Rauner M, Franke K, Wielockx B, Neumann D, Gabet Y, Erythropoietin directly stimulates osteoclast precursors and induces bone loss, FASEB J. 29 (5) (2015) 1890–1900. [DOI] [PubMed] [Google Scholar]
- [9].Fujii J, Niida S, Yasunaga Y, Yamasaki A, Ochi M, Wear debris stimulates bone-resorbing factor expression in the fibroblasts and osteoblasts, Hip Int 21 (2) (2011) 231–237. [DOI] [PubMed] [Google Scholar]
- [10].Movila A, Ishii T, Albassam A, Wisitrasameewong W, Howait M, Yamaguchi T, Ruiz-Torruella M, Bahammam L, Nishimura K, Van Dyke T, Kawai T, Macrophage migration inhibitory factor (MIF) supports homing of osteoclast precursors to peripheral osteolytic lesions, J. Bone Min. Res 31 (9) (2016) 1688–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Akkaoui J, Yamada C, Duarte C, Ho A, Vardar-Sengul S, Kawai T, Movila A, Contribution of Porphyromonas gingivalis lipopolysaccharide to experimental periodontitis in relation to aging, Geroscience (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yamada C, Beron-Pelusso C, Algazzaz N, Heidari A, Luz D, Rawas-Qalaji M, Toderas I, Mascarenhas AK, Kawai T, Movila A, Age-dependent effect between MARCO and TLR4 on PMMA particle phagocytosis by macrophages, J. Cell Mol. Med 23 (8) (2019) 5827–5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Becerikli M, Jaurich H, Schira J, Schulte M, Dobele C, Wallner C, Abraham S, Wagner JM, Dadras M, Kneser U, Lehnhardt M, Behr B, Age-dependent alterations in osteoblast and osteoclast activity in human cancellous bone, J. Cell Mol. Med 21 (11) (2017) 2773–2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gibon E, Lu LY, Nathan K, Goodman SB, Inflammation, ageing, and bone regeneration, J. Orthop. Transl 10 (2017) 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Movila A, Mawardi H, Nishimura K, Kiyama T, Egashira K, Kim JY, Villa A, Sasaki H, Woo SB, Kawai T, Possible pathogenic engagement of soluble Semaphorin 4D produced by gammadeltaT cells in medication-related osteonecrosis of the jaw (MRONJ), Biochem. Biophys. Res. Commun 480 (1) (2016) 42–47. [DOI] [PubMed] [Google Scholar]
- [16].Lühe A, Künkele KP, Haiker M, Schad K, Zihlmann C, Bauss F, Suter L, Pfister T, Preclinical evidence for nitrogen-containing bisphosphonate inhibition of farnesyl diphosphate (FPP) synthase in the kidney: implications for renal safety, Toxicol. Vitr 22 (4) (2008) 899–909. [DOI] [PubMed] [Google Scholar]
- [17].Zhou X, Zhang P, Zhang C, Zhu Z, Promotion of bone formation by naringin in a titanium particle-induced diabetic murine calvarial osteolysis model, J. Orthop. Res 28 (4) (2010) 451–456. [DOI] [PubMed] [Google Scholar]
- [18].Liao JC, Wei ZX, Zhao C, Ma ZP, Cai DZ, Inhibition of osteoclastogenesis for periprosthetic osteolysis therapy through the suppression of p38 signaling by fraxetin, Int J. Mol. Med 42 (3) (2018) 1257–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yu L, Ao M, Wan J, Zhang Y, Anti-inflammatory effects of leaf and twig of Tripterygium wilfordii on paw edema in mice, Fitoterapia 79 (7–8) (2008) 529–532. [DOI] [PubMed] [Google Scholar]
- [20].Wong RW, Rabie B, Bendeus M, Hagg U, The effects of Rhizoma Curculiginis and Rhizoma Drynariae extracts on bones, Chin. Med 2 (2007) 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Abo El-Magd NF, El-Mesery M, El-Karef A, El-Shishtawy MM, Glycyrrhizin ameliorates high fat diet-induced obesity in rats by activating NrF2 pathway, Life Sci. 193 (2018) 159–170. [DOI] [PubMed] [Google Scholar]
- [22].Ekanayaka SA, McClellan SA, Barrett RP, Hazlett LD, Topical glycyrrhizin is therapeutic for Pseudomonas aeruginosa keratitis, J. Ocul. Pharm. Ther 34 (3) (2018) 239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Thakur V, Nargis S, Gonzalez M, Pradhan S, Terreros D, Chattopadhyay M, Role of glycyrrhizin in the reduction of inflammation in diabetic kidney disease, Nephron 137 (2) (2017) 137–147. [DOI] [PubMed] [Google Scholar]
- [24].Selyutina OY, Polyakov NE, Korneev DV, Zaitsev BN, Influence of glycyrrhizin on permeability and elasticity of cell membrane: perspectives for drugs delivery, Drug Deliv. 23 (3) (2016) 858–865. [DOI] [PubMed] [Google Scholar]
- [25].Madhavadas S, Subramanian S, Combination of Spirulina with glycyrrhizin prevents cognitive dysfunction in aged obese rats, Indian J. Pharm 47 (1) (2015) 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jiang R, Gao J, Shen J, Zhu X, Wang H, Feng S, Huang C, Shen H, Liu H, Glycyrrhizic acid improves cognitive levels of aging mice by regulating T/B cell proliferation, Front. Aging Neurosci 12 (2020), 570116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hayakawa T, Iwai M, Aoki S, Takimoto K, Maruyama M, Maruyama W, Motoyama N, SIRT1 suppresses the senescence-associated secretory phenotype through epigenetic gene regulation, PLoS One 10 (1) (2015), e0116480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mohammad G, Abdelaziz GM, Siddiquei MM, Ahmad A, De Hertogh G, Abu El-Asrar AM, Cross-talk between Sirtuin 1 and the proinflammatory mediator high-mobility group box-1 in the regulation of blood-retinal barrier breakdown in diabetic retinopathy, Curr. Eye Res 44 (10) (2019) 1133–1143. [DOI] [PubMed] [Google Scholar]
- [29].Coppé JP, Rodier F, Patil CK, Freund A, Desprez PY, Campisi J, Tumor suppressor and aging biomarker pl6(INK4a) induces cellular senescence without the associated inflammatory secretory phenotype, J. Biol. Chem 286 (42) (2011) 36396–36403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Aquino-Martinez R, Rowsey JL, Fraser DG, Eckhardt BA, Khosla S, Farr JN, Monroe DG, LPS-induced premature osteocyte senescence: implications in inflammatory alveolar bone loss and periodontal disease pathogenesis, Bone 132 (2020), 115220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Farr JN, Fraser DG, Wang H, Jaehn K, Ogrodnik MB, Weivoda MM, Drake MT, Tchkonia T, LeBrasseur NK, Kirkland JL, Bonewald LF, Pignolo RJ, Monroe DG, Khosla S, Identification of senescent cells in the bone microenvironment, J. Bone Min. Res 31 (11) (2016) 1920–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Farr JN, Xu M, Weivoda MM, Monroe DG, Fraser DG, Onken JL, Negley BA, Sfeir JG, Ogrodnik MB, Hachfeld CM, LeBrasseur NK, Drake MT, Pignolo RJ, Pirtskhalava T, Tchkonia T, Oursler MJ, Kirkland JL, Khosla S, Targeting cellular senescence prevents age-related bone loss in mice, Nat. Med 23 (9) (2017) 1072–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Li Z, Chen C, Zhu X, Li Y, Yu R, Xu W, Glycyrrhizin suppresses RANKL-induced osteoclastogenesis and oxidative stress through inhibiting NF-kappaB and MAPK and activating AMPK/Nrf2, Calcif. Tissue Int 103 (3) (2018) 324–337. [DOI] [PubMed] [Google Scholar]
- [34].Sun X, Zhang J, Wang Z, Liu B, Zhu S, Zhu L, Peng B, Licorice isoliquiritigenin-encapsulated mesoporous silica nanoparticles for osteoclast inhibition and bone loss prevention, Theranostics 9 (18) (2019) 5183–5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Pastorello EA, et al. , A double-blind study of hyposensitization with an alginate-conjugated extract of Dermatophagoides pteronyssinus (Conjuvac) in patients with perennial rhinitis. II, Immunol. Asp. Allergy 45 (7) (1990) 505–514. [DOI] [PubMed] [Google Scholar]
- [36].Sun Z, Zeng J, Wang W, Jia X, Wu Q, Yu D, Mao Y, Magnoflorine suppresses MAPK and NF-κB signaling to prevent inflammatory osteolysis induced by titanium particles in vivo and osteoclastogenesis via RANKL in vitro, Front. Pharm 11 (2020) 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yin Z, Zhu W, Wu Q, Zhang Q, Guo S, Liu T, Li S, Chen X, Peng D, Ouyang Z, Glycyrrhizic acid suppresses osteoclast differentiation and postmenopausal osteoporosis by modulating the NF-κB, ERK, and JNK signaling pathways, Eur. J. Pharmacol 859 (2019), 172550. [DOI] [PubMed] [Google Scholar]
- [38].Fei L, Jifeng F, Tiantian W, Yi H, Linghui P, Glycyrrhizin ameliorate ischemia reperfusion lung injury through downregulate TLR2 signaling cascade in alveolar macrophages, Front. Pharm 8 (2017) 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kim YM, Kim HJ, Chang KC, Glycyrrhizin reduces HMGB1 secretion in lipopolysaccharide-activated RAW 264.7 cells and endotoxemic mice by p38/Nrf2-dependent induction of HO-1, Int. Immunopharmacol 26 (1) (2015) 112–118. [DOI] [PubMed] [Google Scholar]
- [40].Yu X, Huang Y, Collin-Osdoby P, Osdoby P, Stromal cell-derived factor-1 (SDF-1) recruits osteoclast precursors by inducing chemotaxis, matrix metalloproteinase-9 (MMP-9) activity, and collagen transmigration, J. Bone Min. Res 18 (8) (2003) 1404–1418. [DOI] [PubMed] [Google Scholar]
- [41].Gibon E, Ma T, Ren PG, Fritton K, Biswal S, Yao Z, Smith L, Goodman SB, Selective inhibition of the MCP-1-CCR2 ligand-receptor axis decreases systemic trafficking of macrophages in the presence of UHMWPE particles, J. Orthop. Res 30 (4) (2012) 547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Huang Z, Ma T, Ren PG, Smith RL, Goodman SB, Effects of orthopedic polymer particles on chemotaxis of macrophages and mesenchymal stem cells, J. Biomed. Mater. Res. A 94 (4) (2010) 1264–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ren PG, Irani A, Huang Z, Ma T, Biswal S, Goodman SB, Continuous infusion of UHMWPE particles induces increased bone macrophages and osteolysis, Clin. Orthop. Relat. Res 469 (1) (2011) 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Fan H, Hall P, Santos LL, Gregory JL, Fingerle-Rowson G, Bucala R, Morand EF, Hickey MJ, Macrophage migration inhibitory factor and CD74 regulate macrophage chemotactic responses via MAPK and Rho GTPase, J. Immunol 186 (8) (2011) 4915–4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, Dewor M, Georgiev I, Schober A, Leng L, Kooistra T, Fingerle-Rowson G, Ghezzi P, Kleemann R, McColl SR, Bucala R, Hickey MJ, Weber C, MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment, Nat. Med 13 (5) (2007) 587–596. [DOI] [PubMed] [Google Scholar]
- [46].Rowe MA, Harper LR, McNulty MA, Lau AG, Carlson CS, Leng L, Bucala RJ, Miller RA, Loeser RF, Reduced osteoarthritis severity in aged mice with deletion of macrophage migration inhibitory factor, Arthritis Rheuma 69 (2) (2017) 352–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Harper JM, Wilkinson JE, Miller RA, Macrophage migration inhibitory factor-knockout mice are long lived and respond to caloric restriction, FASEB J. 24 (7) (2010) 2436–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Martínez de Toda I, Maté I, Vida C, Cruces J, De la Fuente M, Immune function parameters as markers of biological age and predictors of longevity, Aging (Albany NY) 8 (11) (2016) 3110–3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ichiyama H, Inhibition of joint inflammation and destruction induced by anti-type II collagen antibody/lipopolysaccharide (LPS)-induced arthritis in mice due to deletion of macrophage migration inhibitory factor (MIF), Cytokine 26 (5) (2004) 187–194. [DOI] [PubMed] [Google Scholar]
- [50].Singh A, Leng L, Fan J, Gajda M, Bräuer R, Fingerle-Rowson G, Bucala R, Illges H, Macrophage-derived, macrophage migration inhibitory factor (MIF) is necessary to induce disease in the K/BxN serum-induced model of arthritis, Rheuma Int. 33 (9) (2013) 2301–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Chen Z, Ma T, Huang C, Zhang L, Hu T, Li J, MIF, a potential therapeutic target for rheumatoid arthritis? Rheuma Int. 34 (10) (2014) 1481–1482. [DOI] [PubMed] [Google Scholar]
- [52].Suzuki K, Onodera S, Matsuno T, Kaneda K, Nishihira J, Identification of macrophage migration inhibitory factor in synovial membranes of loosened total joint replacement, Biochem. Mol. Biol. Int 39 (3) (1996) 529–537. [DOI] [PubMed] [Google Scholar]
- [53].Nishiguchi MA, Spencer CA, Leung DH, Leung TH, Aging suppresses skin-derived circulating SDF1 to promote full-thickness tissue regeneration, Cell Rep. 24 (13) (2018) 3383–3392 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Muto A, Mizoguchi T, Udagawa N, Ito S, Kawahara I, Abiko Y, Arai A, Harada S, Kobayashi Y, Nakamichi Y, Penninger JM, Noguchi T, Takahashi N, Lineage-committed osteoclast precursors circulate in blood and settle down into bone, J. Bone Min. Res 26 (12) (2011) 2978–2990. [DOI] [PubMed] [Google Scholar]
- [55].Kotani M, Kikuta J, Klauschen F, Chino T, Kobayashi Y, Yasuda H, Tamai K, Miyawaki A, Kanagawa O, Tomura M, Ishii M, Systemic circulation and bone recruitment of osteoclast precursors tracked by using fluorescent imaging techniques, J. Immunol 190 (2) (2013) 605–612. [DOI] [PubMed] [Google Scholar]
- [56].Ishii M, Kikuta J, Shimazu Y, Meier-Schellersheim M, Germain RN, Chemorepulsion by blood SIP regulates osteoclast precursor mobilization and bone remodeling in vivo, J. Exp. Med 207 (13) (2010) 2793–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Schiraldi M, Raucci A, Munoz LM, Livoti E, Celona B, Venereau E, Apuzzo T, De Marchis F, Pedotti M, Bachi A, Thelen M, Varani L, Mellado M, Proudfoot A, Bianchi ME, Uguccioni M, HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4, J. Exp. Med 209 (3) (2012) 551–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Shin HN, Moon HH, Ku JL, Stromal cell-derived factor-1 alpha and macrophage migration-inhibitory factor induce metastatic behavior in CXCR4-expressing colon cancer cells, Int J. Mol. Med 30 (6) (2012) 1537–1543. [DOI] [PubMed] [Google Scholar]
- [59].Drynda A, et al. , Metallic wear debris may regulate CXCR4 expression in vitro and in vivo, J. Biomed. Mater. Res. A (2014). [DOI] [PubMed] [Google Scholar]
- [60].Weisel KC, Bautz F, Seitz G, Yildirim S, Kanz L, Mohle R, Modulation of CXC chemokine receptor expression and function in human neutrophils during aging in vitro suggests a role in their clearance from circulation, Mediat. Inflamm 2009 (2009) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Cane S, Ponnappan S, Ponnappan U, Altered regulation of CXCR4 expression during aging contributes to increased CXCL12-dependent chemotactic migration of CD4(+) T cells, Aging Cell 11 (4) (2012) 651–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Zhang Z, Zhao S, Li X, Zhuo X, Zhang W, Nie Q, Wang S, Yan L, Sun Y, Amentoflavone inhibits osteoclastogenesis and wear debris-induced osteolysis via suppressing NF-kappaB and MAPKs signaling pathways, Planta Med. 84 (11) (2018) 759–767. [DOI] [PubMed] [Google Scholar]
- [63].Oparaugo PC, Clarke IC, Malchau H, Herberts P, Correlation of wear debris-induced osteolysis and revision with volumetric wear-rates of polyethylene: a survey of 8 reports in the literature, Acta Orthop. Scand 72 (1) (2001) 22–28. [DOI] [PubMed] [Google Scholar]
- [64].Chen D, Zhang X, Guo Y, Shi S, Mao X, Pan X, Cheng T, MMP-9 inhibition suppresses wear debris-induced inflammatory osteolysis through downregulation of RANK/RANKL in a murine osteolysis model, Int J. Mol. Med 30 (6) (2012) 1417–1423. [DOI] [PubMed] [Google Scholar]
- [65].Amstutz HC, Campbell P, Kossovsky N, Clarke IC, Mechanism and clinical significance of wear debris-induced osteolysis, Clin. Orthop. Relat. Res 276 (1992) 7–18. [PubMed] [Google Scholar]
- [66].Kaczmarczyk-Sedlak I, Klasik-Ciszewska S, Wojnar W, Glabridin and glycyrrhizic acid show no beneficial effect on the chemical composition and mechanical properties of bones in ovariectomized rats, when administered in moderate dose, Pharm. Rep 68 (5) (2016) 1036–1041. [DOI] [PubMed] [Google Scholar]
- [67].Keppler D, Walter R, Pérez C, Sierra F, Increased expression of mature cathepsin B in aging rat liver, Cell Tissue Res. 302 (2) (2000) 181–188. [DOI] [PubMed] [Google Scholar]
- [68].Stoka V, Turk V, Turk B, Lysosomal cathepsins and their regulation in aging and neurodegeneration, Ageing Res. Rev 32 (2016) 22–37. [DOI] [PubMed] [Google Scholar]
- [69].Valenzuela CA, Quintanilla R, Moore-Carrasco R, Brown NE, The potential role of senescence as a modulator of platelets and tumorigenesis, Front. Oncol 7 (2017) 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Cho E, Chen Z, Lee J, Lee S, Lee TH, PSTP-3,5-Me inhibits osteoclast differentiation and bone resorption, Molecules 24 (2019) 3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Yamada S, Ozaki N, Tsushima K, Yamaha S, Fujihara C, Awata T, Sakashita H, Kajikawa T, Kitagaki J, Yamashita M, Yanagita M, Murakami S, Transcriptome reveals cathepsin K in periodontal ligament differentiation, J. Dent. Res 95 (9) (2016) 1026–1033. [DOI] [PubMed] [Google Scholar]
- [72].Motyckova G, Fisher DE, Pycnodysostosis: role and regulation of cathepsin K in osteoclast function and human disease, Curr. Mol. Med 2 (5) (2002) 407–421. [DOI] [PubMed] [Google Scholar]
- [73].Koulouvaris P, Ly K, Ivashkiv LB, Bostrom MP, Nestor BJ, Sculco TP, Purdue PE, Expression profiling reveals alternative macrophage activation and impaired osteogenesis in periprosthetic osteolysis, J. Orthop. Res 26 (1) (2008) 106–116. [DOI] [PubMed] [Google Scholar]
- [74].Ross RD, Virdi AS, Liu S, Sena K, Sumner DR, Particle-induced osteolysis is not accompanied by systemic remodeling but is reflected by systemic bone biomarkers, J. Orthop. Res 32 (7) (2014) 967–973. [DOI] [PubMed] [Google Scholar]
- [75].Malavolta M, Giacconi R, Brunetti D, Provinciali M, Maggi F, Exploring the relevance of senotherapeutics for the current SARS-CoV-2 emergency and similar future global health threats, Cells 9 (4) (2020) 909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Yue X, Jiang H, Xu Y, Xia M, Cheng XW, Cathepsin K deficiency impaired ischemia-induced neovascularization in aged mice, Stem Cells Int. 2020 (2020) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Saftig P, et al. , Functions of cathepsin K in bone resorption. Lessons from cathepsin K deficient mice, Adv. Exp. Med Biol 477 (2000) 293–303. [DOI] [PubMed] [Google Scholar]
- [78].Stone JA, McCrea JB, Witter R, Zajic S, Stoch SA, Clinical and translational pharmacology of the cathepsin K inhibitor odanacatib studied for osteoporosis, Br. J. Clin. Pharm 85 (6) (2019) 1072–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Chen J, Xavier S, Moskowitz-Kassai E, Chen R, Lu CY, Sanduski K, Špes A, Turk B, Goligorsky MS, Cathepsin cleavage of sirtuin 1 in endothelial progenitor cells mediates stress-induced premature senescence, Am. J. Pathol 180 (3) (2012) 973–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Liu L, Zhou M, Zhu R, Zhou J, Ni L, Wang Z, Liu N, Zhu F, Shi T, Deng Z, Wang Y, Tian Y, Li R, Yang H, Wang Z, Jiang J, Xu Y, Hydrogen sulfide protects against particle-induced inflammatory response and osteolysis via SIRT1 pathway in prosthesis loosening, FASEB J. 34 (3) (2020) 3743–3754. [DOI] [PubMed] [Google Scholar]
- [81].Zhang B, Chen J, Cheng ASL, Ko BCB, Depletion of sirtuin 1 (SIRT1) leads to epigenetic modifications of telomerase (TERT) gene in hepatocellular carcinoma cells, PLoS One 9 (1) (2014), e84931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Grabowska W, Sikora E, Bielak-Zmijewska A, Sirtuins, a promising target in slowing down the ageing process, Biogerontology 18 (4) (2017) 447–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Michishita E, Park JY, Burneskis JM, Barrett JC, Horikawa I, Evolutionary conserved and nonconserved cellular localizations and functions of human SIRT proteins, Mol. Biol. Cell 16 (10) (2005) 4623–4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Kim HS, Xiao C, Wang RH, Lahusen T, Xu X, Vassilopoulos A, Vazquez-Ortiz G, Jeong WI, Park O, Ki SH, Gao B, Deng CX, Hepatic-specific disruption of SIRT6 in mice results in fatty liver formation due to enhanced glycolysis and triglyceride synthesis, Cell Metab. 12 (3) (2010) 224–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Ho L, Wang L, Roth TM, Pan Y, Verdin EM, Hsiao EC, Nissenson RA, Sirtuin-3 promotes adipogenesis, osteoclastogenesis, and bone loss in aging male mice, Endocrinology 158 (9) (2017) 2741–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Deng Z, Jin J, Wang Z, Wang Y, Gao Q, Zhao J, The metal nanopartide-induced inflammatory response is regulated by SIRT1 through NF-kappaB deacetylation in aseptic loosening, Int J. Nanomed 12 (2017) 3617–3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Kim HN, Han L, Iyer S, de Cabo R, Zhao H, O’Brien CA, Manolagas SC, Almeida M, Sirtuinl suppresses osteoclastogenesis by deacetylating FoxOs, Mol. Endocrinol 29 (10) (2015) 1498–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Moon YJ, Zhang Z, Bang IH, Kwon OK, Yoon SJ, Kim JR, Lee S, Bae EJ, Park BH, Sirtuin 6 in preosteodasts suppresses age- and estrogen deficiency-related bone loss by stabilizing estrogen receptor alpha, Cell Death Differ. 26 (11) (2019) 2358–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]


