Abstract
Chrysin is a promising naturally occurring flavonoid mainly found in honey and propolis. Although chrysin’s biological activities have been demonstrated and the mechanism of actions has been determined using in vitro and in vivo models, results from the current clinical studies were largely negative. A potential reason for chrysin’s low efficacy in humans is poor oral bioavailability. In this paper, we reviewed the preclinical and clinical pharmacokinetics studies of chrysin and analyzed the mechanism of poor in vivo efficacy with emphasis on its bioavailability and ADME mechanism. Low aqueous solubility, rapid metabolism mediated by UGTs and SULT, efficient excretion through efflux transporters including BCRP and MRP2 are the major reasons causing poor systemic bioavailability for chrysin. However, because of efficient enterohepatic recycling facilitated by phase II metabolism and efflux, chrysin’s bioavailability in the low GI tract is high. Thus, chrysin can be ideal for treating diseases in the terminal ileum and colon (e.g., carcinoma, local infection) since it is localized in the lower GI tract with limited delivery to other organs.
Keywords: Chrysin, Flavonoid, Bioavailability, Pharmacokinetic, ADME mechanism
1. Introduction
Chrysin (5,7-dihydroxy-flavone, Fig. 1), a natural occurring flavonoid found in diets (e.g., honey, propolis) possessing different pharmacological functions, has attracted increasing interest in the last few decades. The very first paper reporting chrysin in literature is back to 1893, where chrysin was isolated as a natural product and the structure was elucidated [1]. After that, many papers have been published on chrysin’s pharmacological efficacy, therapeutic properties, molecular mechanism, and dispositions. Chrysin’s pharmacological efficacy has been paid great attention to since the 1980s. A recent literature search using “Chrysin” as the keyword in PubMed resulted in 1242 hits in the last two decades (2000–2020), including 7 clinical studies papers, suggesting that chrysin is a promising compound in drug development.
Fig. 1.
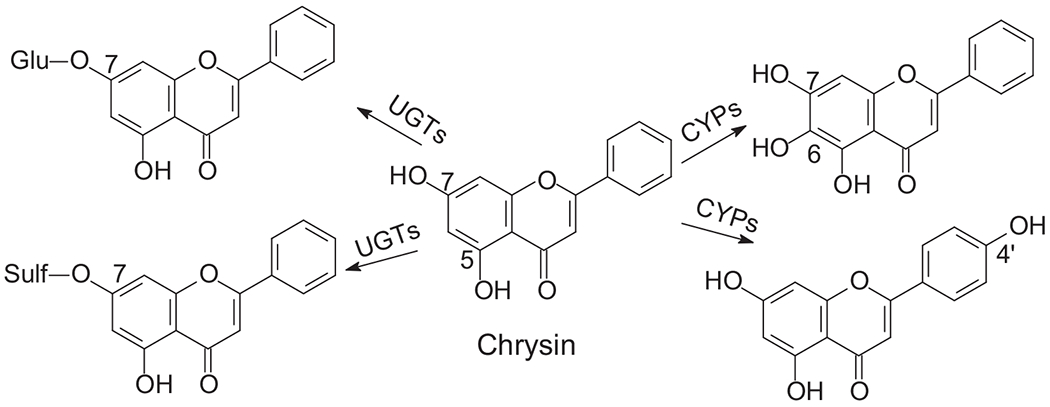
Chrysin metabolic pathways.
One of the major natural sources of chrysin is honey and propolis. The content of chrysin in honey could be up to 5.3 mg/kg [2]. Nowadays, extracts containing high concentration of chrysin such as propolis and honey, give rise to great interest to pharmacologists. Chrysin is also found in vegetables, fruits and mushrooms [3], passion flowers, and other herbal materials as one of the bioactive components [4]. In addition to the aglycone, chrysin also exists in nature as O-glycoside or C-glycoside forms. For example, chrysin-8-C-glucoside was identified in different types of cherries including Prunus cerasus and Prunus avium) [5]. Other examples include chrysin-7-O-glucoside from Moringa oleifera [6] and Podocytisus caramanicus [7], and chrysin-6-C-glucoside from passion flower Passiflora caerulea [8].
Chemically, chrysin belongs to the class of flavones containing two benzene rings (i.e., A and B ring) and a six-member heterocyclic ring (i. e., C ring). Unlike other flavonoids, chrysin only has hydroxyl groups on the A ring (5,7-dihydroxyl) with no substituents on the B ring. This unique substitutive feature makes it a suitable model compound in structure-activity relationship studies. It is generally believed that chrysin’s pharmacological efficacy is associated with moieties on the A ring and C rings, where the hydroxyl can exert antitoxic effects [2]. Theoretically, the carbonyl conjugated with the double bond at positions 2 and 3 could form a PAIN-(pan-assay interference compounds)-like moiety that may potentially cause certain toxicity [9]. However, chrysin is relatively safe as proven in preclinical models. A daily consumption of 0.5–3.0 g of chrysin is considered safe in humans [10].
In vitro and in vivo studies have demonstrated chrysin’s beneficial effect including neuroprotective [11], hepatoprotective [12], cardioprotective [13], nephroprotective [12], anti-inflammatory [14], antiasthmatic [15], antidepressant [16], and anticancer effects [17], etc. In addition, chrysin has also been tested as an adjuvant agent to improve other drugs efficacy [18,19] or to alleviate toxicity induced by other drugs [10,20–22]. Mechanism of action of these pharmacological effects has also been investigated [2]. However, pharmaceutical studies showed that oral bioavailability of chrysin is the biggest challenge in in vivo efficacy studies. It was reported that chrysin’s absolute oral bioavailability is ~1% [23]. Poor bioavailability is because poor aqueous solubility and rapid metabolism in the GI tract and the liver as chrysin is a good substrate of UGTs, a phase II metabolic enzymes catalyzing glucuronidation and SULTs, phase II metabolic enzymes mediating sulfonation. In addition, chrysin glucuronide and sulfate are good substrates of certain efflux transporters (e.g., MRP2, BCRP), which facilitate their elimination through urine and feces.
Several review papers have been published focusing on chrysin’s efficacy, molecular mechanism, clinical implications and figures/tables illustrating chrysin’s pharmacological efficacy and molecular mechanism were available in these review articles [2,4,13,17,24]. This paper reviews the pharmacokinetic and ADME studies of chrysin and discuss the challenges in developing chrysin as a therapeutic drug. We also provide several suggestions based on the biopharmaceutical consideration.
2. Clinical studies
Based on the results from PubMed query with “Chrysin” as the keyword and “clinical study” as the search criterion, seven clinical studies on chrysin have been conducted hereto as listed in Table 1 either using pure chrysin or using a dietary supplements or herbal extracts containing chrysin as the potential active ingredient. Among these clinical studies, four studies aimed to investigate the effect of chrysin on maintaining hormone homeostasis, one is for alleviating and preventing chemotherapeutic drug irinotecan-induced diarrhea, and one is for disposition study, and the other is for the treatment of ulcers using a buccal delivery system. The results of these studies revealed that chrysin’s efficacy as an aromatase inhibitor to prevent formation of estrogens and dihydrotestosterone is moderate. However, chrysin showed promising efficacy in preventing irinotecan-induced diarrhea without affecting irinotecan’s therapeutically efficacy in cancer patients. Chrysin prevents diarrhea through inducing intestinal UGT1A1, an enzyme that can metabolize the toxic compound SN-38 in the intestine.
Table 1.
Summary of chrysin clinical studies.
| # | Endpoints | Mechanism | Dose | Dosage form | Ref |
|---|---|---|---|---|---|
| 1 | Alleviating irinotecan-induced late onsite diarrhea | UGT1A1 induction | Twice daily for 1 week total 250 mg/day | Oral | [21] |
| 2 | Disposition | 400 mg, once | Oral capsules | [25] | |
| 3 | Prevent formation of estrogens and dihydrotestosterone | Aromatase inhibitor | Twice daily for 4 weeks, total dose 625 mg/day | Oral capsules | [26, 27] |
| 4 | Prevent formation of estrogens and dihydrotestosterone | Aromatase inhibitor | Three times per day for 4 weeks, total dose 300 mg/day | Oral capsules | [28] |
| 5 | Reduce conversion of androgens to estrogens | Aromatase inhibitor | Three times per day, Week 1–2, 4–5, 7–8, total dose 300 mg/day | Oral capsules | [29] |
| 6 | Effects of chrysin on Urinary testosterone levels | Aromatase inhibitor | 1280 mg of propolis, and 20 g of honey/day for 3 weeks. | Oral, in honey propolis | [30] |
| 7 | Oral recurrent aphthous ulcers | Antibacterial | Not provided | Buccal delivery film | [31] |
In these clinical studies, chrysin was administered through oral route either in capsules as a pure compound or in an herbal extract. The maximum dose was 625 mg/day for 4 weeks. Other than the disposition study, chrysin in vivo exposure has not been reported in these studies. Therefore, an intriguing and important question is whether the dosing regimen affects chrysin’s efficacy. Pharmaceutical studies showed that chrysin’s oral bioavailability is extremely poor (<1%) because of low aqueous solubility and extensive metabolism (e.g., glucuronidation and sulfonation), which will be discussed below. Therefore, in vivo efficacy studies without pharmaceutical analysis may result in inaccurate conclusion on chrysin. A suggestion for future clinical study is to determine the maximal absorption dose of chrysin and in vivo chrysin exposure and optimize the dosing regimen based on in vivo exposure. In addition, administering chrysin in certain formulations (e.g., nanoparticles) that can enhance its oral bioavailability should be considered to evaluate its in vivo efficacy more appropriately.
The study using chrysin to alleviate irinotecan-induced GI toxicity is fascinating. Although the systemic exposure is very poor, chrysin undergoes efficient enterohepatic recycling, resulting in repeated exposure in the GI tract. Low oral bioavailability but repeated exposure in the GI tract may be a benefit for chrysin when it is used to treat diseases or drug induced toxicity in the GI tract because low systemic exposure but high GI tract exposure could achieve local efficacy without or with limited off target side effects. In other words, chrysin could be considered as a drug that is formulated in a local delivery formulation for the lower GI tract.
3. Pharmacokinetics and oral bioavailability
3.1. Pharmacokinetic studies in animals and humans
A pharmacokinetic study in human volunteers has been conducted. The results showed that when chrysin was administered to heathy volunteers at 400 mg, chrysin-sulfate was the major form detected in the plasma with an AUC of 1490 ± 485 ng/mL·hr. Systemic exposure of chrysin was about 20-fold lower with an AUC of 64 ± 33 ng/mL·hr. The oral bioavailability of chrysin was estimated to be less than 1% by the author in this study, which could probably explain why chrysin is very potent in vitro but is not very promising in in vivo efficacy studies. This study also reported that more than 90% of the chrysin was eliminated as aglycone through the feces and the rest was eliminated as chrysin or chrysin-glucuronide through urine [25]. High fecal elimination could be because of either enterohepatic recycling or low aqueous solubility. Chrysin participates enterohepatic recycling, which will be discussed later, and the majority of chrysin could be excreted through the bile as conjugate forms (e.g., glucuronide, sulfate), which can be hydrolyzed back to chrysin in the terminal ileum or the colon by the intestine microbiome. If this process is the major reason for poor oral bioavailability, approaches focusing on enterohepatic recycling and first pass-metabolism (e.g., metabolic enzyme inhibitors) should be developed in order to enhance chrysin oral bioavailability. On the other hand, chrysin aqueous solubility is low, resulting in poor absorption in the intestine. In other words, a large proportion of chrysin dosed through the oral route is not absorbed due to high apparent fecal elimination. If low aqueous solubility is the major reason to cause poor oral bioavailability, approaches focusing on increasing aqueous solubility (e.g., nanoparticle formulation) should be developed to enhance chrysin’s oral bioavailability.
Chrysin PK studies in mice and rats have also been conducted using either pure chrysin or herbal extracts. Chrysin was administered orally or intravenously in these preclinical PK studies. The dose in animal studies ranged from 0.23 mg/kg to 100 mg/kg. The results showed that when chrysin was administered as a pure compound, the Tmax was 2–6.6 h, while the Tmax was < 1.0 h when chrysin was administered in herbal extracts. This is probably because the doses used as pure chrysin were higher than the Maximum Absorbable Dose (MAD) [32], resulting in chrysin being absorbed gradually, which delayed the Tmax. While being present at a lower dose in herbal extracts, chrysin was absorbed rapidly and completely in solution form in the intestine. This assumption is also supported by the fact that when chrysin was administered in formulations (e.g., folate-conjugated micelles) with better aqueous solubility, the Tmax was shortened significantly [33]. The results also revealed that the half-life of chrysin was relatively long at about 3–11 h no matter either given as a pure compound or herbal extracts, which is probably a result of enterohepatic recycling [34]. Double absorption peaks at 0.25 h and 8 h reported in one of the studies listed in Table 2 confirmed the existence of recycling [35]. In animal studies, exposure (i.e., Cmax, AUC) of chrysin conjugates was always higher than that of chrysin in both mice and rats, indicating extensive metabolism as one of the reasons for low systemic bioavailability.
Table 2.
Summary of chrysin pharmacokinetic studies in humans and animals.
| # | Species | Dose (mg/kg) | Route | Analytes | Tmax (hr) | Cmax | AUC | T1/2 (hr) | Ref |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Human | 400 mg | p.o. (capsule) | Chrysin | – | – | 0.06 ± 0.03 (h μg/mL) | 4.6 | [25] |
| Sulfate | – | – | 1.49 ± 0.49 (h μg/mL) | – | |||||
| 2 | FVB mice | 20 | p.o. (oral suspending vehicle) | Chrysin | 4.00 ± 1.36 | 0.01 ± 0.01 (μM) | 0.06 ± 0.03 (h μM) | – | [36] |
| Glucuronide | 7.00 ± 1.15 | 0.16 ± 0.04 (μM) | 2.00 ± 0.82 (h μM) | – | |||||
| Sulfate | 6.50 ± 1.00 | 0.13 ± 0.04 (μM) | 1.32 ± 0.42 (h μM) | – | |||||
| 3 | Bcrp(−/−) mice | 20 | p.o. (oral suspending vehicle) | Chrysin | 3.31 ± 3.12 | 0.02 ± 0.01(μM) | 0.11 ± 0.08 (h μM) | – | [36] |
| Glucuronide | 1.81 ± 1.63 | 0.02 ± 0.01(μM) | 2.83 ± 3.03 (h μM) | – | |||||
| Sulfate | 3.25 ± 3.57 | 0.43 ± 0.67(μM) | 2.62 ± 3.13 (h μM) | – | |||||
| 4 | Wistar rats | 10 | p.o., Folate-conjugated micelles | Chrysin | 1.00 | 0.99 ± 0.09 (μg/mL) | 10.42 ± 0.96(h μg/mL) | 8.42 ± 0.32 | [33] |
| 5 | Wistar rats | 10 | p.o. (formulation not mentioned) | Chrysin | 2.00 | 0.42 ± 0.05 (μg/mL) | 3.538 ± 0.78(h μg/mL) | 3.54 ± 0.18 | [33] |
| 6 | SD | 30 | p.o. suspension | Chrysin | 5.20 ± 1.11 | 0.02 ± 0.00 (μM) | 0.32 ± 0.05 (h μM) | 9.17 ± 3.16 | [37] |
| Glucuronide | 5.20 ± 1.09 | 0.76 ± 0.13 (μM) | 10.4 ± 1.01(h μM) | 8.24 ± 1.80 | |||||
| 7 | 30 | p.o. suspension in sodium oleate | Chrysin | 5.50 ± 1.00 | 0.02 ± 0.00 (μM) | 0.36 ± 0.06 (h μM) | 11.0 ± 1.53 | [37] | |
| Glucuronide | 6.25 ± 2.06 | 0.78 ± 0.13 (μM) | 10.3 ± 0.73 (h μM) | 8.53 ± 1.78 | |||||
| 8 | 30 | p.o. TW-80 nano-emulsion | Chrysin | 4.8 ± 1.09 | 0.02 ± 0.00 (μM) | 0.34 ± 0.08 (h μM) | 10.4 ± 2.67 | [37] | |
| Glucuronide | 6.20 ± 1.78 | 0.71 ± 0.13 (μM) | 10.7 ± 2.69(h μM) | 8.45 ± 2.62 | |||||
| 9 | 30 | sodium oleate nano-emulsion | Chrysin | 6.6 ± 1.37 | 0.05 ± 0.00 (μM) | 0.99 ± 0.25(h μM) | 11.6 ± 2.37 | [37] | |
| Glucuronide | 5.60 ± 0.89 | 0.34 ± 0.13 (μM) | 5.98 ± 2.47(h μM) | 9.11 ± 2.13 | |||||
| 10 | SD rats | 2 | i.v. (DMSO:PGE:ethanol:Saline) | Chrysin | – | – | 0.28 ± 0.05(h μg/mL) | 0.04 ± 0.01 | [23] |
| Glucuronide | – | 2.31 ± 1.07 (μg/mL) | 0.47 ± 0.18(h μg/mL) | 0.4 ± 0.6 | |||||
| Sulfate | – | 1.37 ± 0.47 (μg/mL) | 0.424 ± 0.19(h μg/mL) | 0.2 ± 0.1 | |||||
| 11 | SD rats | 100 | p.o. (corn oil) | Glucuronide | 3.6 ± 0.6 | 0.36 ± 0.12 (μg/mL) | 2.70 ± 0.96 (h μg/mL) | 3.0 ± 1.9 | [23] |
| 12 | Wistar Rats | 0.23 | p.o. in herbal extract | Chrysin | 0.40 ± 0.15 | 0.09 ± 0.02 (μg/mL) | 0.75 ± 0.24 (h μg/mL) | 9.72 ± 3.16 | [38] |
| 13 | Wistar rats | 1.03 | p.o. in herbal extract | Chrysin | 0.61 ± 0.25 | 0.09 ± 0.02 (μg/mL) | 0.39 ± 0.17 (h μg/mL) | 4.79 ± 2.81 | [39] |
| 14 | Wistar rats | 1.12 | p.o. in herbal extract | Chrysin | 0.30 ± 0.10 | 0.02 ± 0.01 (μg/mL) | 0.11 ± 0.08 (h μg/mL) | 6.6 ± 0.8 | [40] |
| 15 | SD | 0.25 | p.o. in herbal extract | Chrysin | 0.25, 8 | 0.02 ± 0.01 (μg/mL) | 0.08 ± 0.03 (h μg/mL) | 4.51 ± 1.02 | [35] |
| 16 | SD | 0.54 | p.o. in herbal extract | Chrysin | 0.58 ± 0.11 | 0.04 ± 0.01 (μg/mL) | 0.49 ± 0.07 (h μg/mL) | 11.78 ± 1.38 | [41] |
In mouse PK study, both sulfate and glucuronide were detected, and systemic exposure of chrysin-7-O-sulfate was higher than that of chrysin, which is similar to the scenario in humans. In rats, glucuronide is the major metabolite detected in the systemic circulation, probably because the relative expression of UGTs is higher than those of SULTs in rats. More pharmacokinetics and biopharmaceutical studies are needed to verify whether rat is a good in vivo model to predict chrysin disposition in humans. Another finding is that oxidative metabolites have never been reported in in vivo PK studies although hydroxyl-chrysin was detected as the metabolites when chrysin was incubated with microsomes in in vitro studies (see metabolism section below).
3.2. Systemic and intestinal bioavailability
Many studies indicated that the oral bioavailability of chrysin is extremely poor [25,37]. Usually, only one digit of μM of chrysin can be detected in the plasma regardless the doses when chrysin was administered through oral route (Table 2). The absolute oral bioavailability (F%) is < 1%. For example, in a PK study in adult male SD rats, chrysin F% was 1% and the majority of the chrysin was detected in the plasma as the glucuronide form [23]. Multiple factors limited chrysin’s oral bioavailability including low aqueous solubility, moderate permeability, and rapid metabolism. Approaches intervening these factors can be developed in order to improve chrysin oral bioavailability. Increasing water solubility using formulations to enhance chrysin absorption have been attempted. Example approaches include chrysin-folate conjugated micelles, chrysin-loaded PLGA-PEG nanoparticles, polymeric chrysin nanocapsules, lipid-core nanocapsules [33,42–44]. The results from these studies were encouraging because chrysin’s absorption can be enhanced and in vivo exposure can be increased as showed in the PK studies listed in Table 2 [33]. Another approach is to inhibit metabolism for higher in vivo chrysin exposure. For example, it was reported that when chrysin was administered together with sodium oleate, chrysin metabolism was significantly inhibited and in vivo exposure (AUC) was significantly increased in a PK study in SD rats [37].
Although chrysin’s systemic bioavailability is poor, its local bioavailability in the intestine may not be necessarily poor. Due to low solubility and poor absorption, a large proportion of the chrysin dosed through the oral route may exceed its MAD. Although, this proportion of chrysin cannot be absorbed into the systemic circulation, it may exert certain pharmacological functions locally in the intestine, such as optimizing intestinal environment through interact with microbiome [45]. In addition, due to enterohepatic recycling, chrysin conjugates (i.e., chrysin-glucuronide, chrysin-sulfate) are secreted into the bile from the hepatocytes and enter intestine, where the conjugates can be rapidly hydrolyzed to release chrysin by intestinal microbial beta-glucuronidases [46]. Thus, chrysin can be “reused” in the intestine, resulting in enhanced its local bioavailability. It is not known how many times can chrysin be reused in the intestine due to recycling, which depends on the recycling efficient controlled by many factors including metabolic rate, efflux efficient, and hydrolysis rate. It was estimated that a bile acid molecule can be reused for about 20 times before being eliminated through the body due to enterohepatic recycling [47]. For chrysin, we expected that one molecule can be reused for “several” times in the intestine. This feature renders chrysin as an ideal compound to treat intestinal diseases with limited systemic impact.
4. Biopharmaceutical mechanisms
4.1. Physical and chemical property
Chrysin (5,7-dihydroxyflavone) is a heterocyclic diphenol with two hydroxyl group and its chemical name is 5,7-Dihydroxyflavone or 5,7-Dihydroxy-2-phenyl-4H-1-benzopyran-4-one. Chrysin is a lipophilic compound (Log P = 2.29) and has a low molecular weight (MW=254.24). Chrysin’s aqueous solubility is 0.06 ± 0.1 mg/mL at pH 6.5, and 0.058 ± 0.04 mg/mL at pH 7.4 [48]. It was estimated that the minimum aqueous solubility (MAS) of a medium permeable drug should be higher than 0.05 μg/mL to achieve a good absorption when given at 1 mg/kg dose [49]. Chrysin’s permeability is medium and its solubility is on this border if given at 1 mg/kg dose. However, in most of the animal and human studies, chrysin’s doses were higher than 1 mg/kg (Tables 1 and 2), therefore, a large proportion of the dose was not absorbable. This could partially explain why chrysin’s Tmax was longer when given at higher doses (e.g., 10 mg/kg) than those at lower doses (<1 mg/kg) in animal studies (Table 2). More studies are needed to determine the impact of dosing regimens on chrysin’s pharmacokinetics and pharmacodynamics.
4.2. Absorption
Chrysin’s molecular weight is 254 and it has two hydrogen bond donors (i.e., hydroxyl groups) and four hydrogen bond acceptors (i.e., 4 oxygens) in the structure. In addition, chrysin’s logP is 2.2. All these features fit the Lipinski’s rule of five [50,51]. Theoretically, chrysin should have a good absorption. In term of permeability, in the parallel artificial membrane permeability (PAMPA) model, chrysin’s apical to basolateral permeability is ~ 5 × 10−6 cm/sec, suggesting that the passive diffusion rate of chrysin is relatively fast. In the Caco-2 cell culture model, the apparent absorption permeability of chrysin was 5–7 × 10−6 cm/sec reported by different research groups [48,52]. These findings revealed that chrysin intestinal absorption was moderate because Caco-2 permeability at 10 × 10−6 cm/sec range usually predicts a > 75% of intestinal absorption in humans [53]. Bi-directional transport studies in the Caco-2 monolayer showed that the efflux ratio of chrysin is ~0.87–2.0 at a concentration range of 5–25 μM [48,52]. It seems like efflux transporter(s) is/are involved in chrysin’s absorption. Our study showed that in the presence of Ko-143, a specific inhibitor of BCRP, chrysin concentration in the basolateral side in a Caco-2 study was slightly increased [54], suggesting Bcrp could be the involved transporter. However, we further found that in Bcrp knockout (Bcrp (−/−)) mice, in vivo exposure of chrysin and its metabolites was not affected [54]. These findings revealed that chrysin mainly follows passive diffusion transport mechanism and Bcrp might also be involved in chrysin’s transport, but manipulation of BCRP does not significantly increase chrysin’s absorption and bioavailability.
Chrysin’s intestinal absorption in in vivo study is affected by its low aqueous solubility as mentioned above. Approaches focusing on enhancing chrysin’s solubility have been attempted to improve chrysin’s intestinal absorption. For example, it was reported that solubilizers, such as cyclodextrin, can enhance both chrysin’s solubility and permeability in the Caco-2 cell culture model [55]. Another report showed that chrysin in vivo absorption can be enhanced by certain formulations such as nanoemulsion [37].
4.3. Distribution
The tissue distribution of chrysin has not been well studied. Effect on gastric mucosa protection suggests that chrysin should have a sufficient distribution in the stomach [56]. In addition, chrysin was reported to be active in preventing cancers in different organs including mouth, colon, liver, breast, kidney, prostate, and skin, suggesting that chrysin is broadly distributed throughout these organs [57]. However, the proportion of chrysin distributed in these organs are unknown. It is expected that chrysin has a relatively higher tissue distribution in the liver and the GI tract due to efficient enterohepatic recycling. Clinical studies have been conducted using chrysin to modulate UGTs in the GI tract to prevent irinotecan-induced diarrhea. The results showed that systemic exposure of SN-38 was not altered significantly when chrysin was combined with irinotecan [21]. In addition, diarrhea severity seems to be reduced. These findings may reveal that UGT1A1, the enzyme catalyzing SN-38 metabolism, was only regulated in the intestine but not in the liver by chrysin. If this hypothesis is correct, chrysin could have a higher distribution in the GI tract to exert a better UGT1A1 regulation when compared to the liver and other organs. Tissue bio-distribution studies are highly expected to explain chrysin’s local efficacy in the GI tract. This feature render chrysin a good locally bioavailable drug for the treatment of local diseases without or with limited off-target side effects. On the other hand, target delivery of chrysin should be paid special attention to if the disease organ is not in the GI tract so that enough chrysin can be delivered to that organ to exert its pharmacological efficacy.
4.4. Metabolism
In vitro and in vivo studies showed that chrysin undergoes both phase I and II metabolism. The metabolism pathway is illustrated in Fig. 1. Chrysin-7-O-glucuronide and Chrysin-7-O-sulfate are the major phase II metabolites mediated by UGTs or SULTs and 5,6,7-trihydroxyl-flavone and 5,7,4′-trihydroxy-flavone are the major phase I metabolites mediated by CYPs. Usually, liver is believed to be the major metabolic organ due to high metabolic enzyme expression. For phase II metabolism, enzymes (e.g., UGTs and SULTs) are also highly expressed in the other organs such as the GI tract and the kidney [58]. Therefore, other than the liver, some other organs, especially the GI tract, can also metabolize chrysin. Extensive metabolism in the GI tract and the liver is one of the reasons to cause low oral bioavailability for chrysin.
4.4.1. Glucuronidation
Chrysin can be rapidly metabolized by UGTs to produce chrysin-7-O-glcuruonide as the major metabolites. Our studies demonstrated that when incubated with liver and intestinal microsome, chrysin was metabolized into chrysin-7-O-glucuronide at rates higher than those of genistein, which is a well-known rapid metabolized isoflavone [59], suggesting that chrysin glucuronidation is rapid. Other research groups have found that chrysin-7-O-glucuronide is the major glucuronide in in vitro and in vivo studies [23,60]. Multiple UGT isoforms are involved in chrysin’s glucuronidation reaction when chrysin was incubated with recombinant human UGT isoforms with the following order of potency: UGT1A3 > 1A9 > 1A6 > 1A1 > 1A8 > 2B7 > 1A10 [61].
4.4.2. Sulfonation
Sulfate is another major phase II metabolite. In vitro study showed that when chrysin was incubated with Caco-2 cells, both chrysin-7-O-glucuronide and chrysin-7-O-sulfate were detected as the major metabolites [62]. Further studies showed that chrysin is a good substrate of SULT1A3 and SULT1A1 and can be conjugated into chrysin-7-O-sulfate [63].
Few studies have been done comparing chrysin glucuronidation in different tissues such as in the intestine and the liver. Organ specific metabolism may be important when using chrysin to treat diseases in different organs. In additional to organ specificity, species difference of chrysin metabolism is also important. It was reported that both chrysin-7-O-glucuronide and chrysin-7-O-sulfate were detected in mice plasma when chrysin was administered through oral gavage in animal PK studies [54], while in rats, chrysin-7-O-glucuronide is the major metabolite detected in the plasma as shown in Table 2. Human PK studies results showed that chrysin-7-O-sulfate is the major metabolite detected in the plasma and chrysin-7-O-glucuronide is mainly found in the urine. These results revealed that chrysin disposition is specie-dependent, thus specie-dependent metabolism studies using both in vitro and in vivo models are suggested in future studies.
Some researchers think that sulfonation is the major metabolism pathway for chrysin due to high sulfate concentration in the plasma in humans [64]. We believe that more studies are needed to support such a conclusion. Although sulfate is the major metabolite in the plasma, high concentration of glucuronide was detected in the urine in humans [25]. In vivo exposure is a combination of both metabolism and elimination. It was reported that chrysin-7-O-glucuronide is a good substrate of Bcrp efflux transporter [60] and could be rapidly pumping out of the plasma, resulting in low exposure level in the plasma.
4.4.3. Oxidation
When chrysin is incubated with CYP enzymes, it can be oxidized. It was reported that chrysin was converted into tri-hydroxyl-flavone when incubated with human liver microsomes and recombinant CYP isoforms [65]. Additionally, chrysin was converted into 5,6,7-trihydroxyl-flavone by CYP1A1, 1A2, 1B1.1, and 1B1.3. Metabolism rates ranged from 1 to 3 nmol/min/nmol CYP protein, which is a relatively low metabolic rate. In addition, chrysin can also be converted into 5,7,4′-trihydroxy-flavone by CYP2A6 and CYP2A13 at an even lower rate [65].
Although in vitro study demonstrated that chrysin can be oxidized into hydroxyl-chrysin, these oxidized metabolites have never been reported in in vivo studies. One of the possible reasons is that the concentrations of these phase I metabolites are too low to be detected in vivo, the other possible reason is that these hydroxyl-flavones can be further conjugated into phase II metabolites. The other more probable reason is that these oxidized metabolites may not exist in vivo because chrysin can be rapidly conjugated into phase II metabolites and the chance for chrysin to interact with CYPs is very low.
4.4.4. Ring fission by microflora
In vitro and in vivo studies have demonstrated that ring fission mediated by intestinal microflora is a common metabolism pathway for many flavonoids [66]. Usually, the double bond between position 2 and 3 in a flavonoid structure is hydrogenated to form dihydroflavonoids, followed by the middle ring being broken. The final products of ring fission degradation are usually hydroxyphenylacetic/phenylpropionic acid and phloroglucinol [67]. However, chrysin appeared to be resistant to ring fission both in vivo and in vitro. In an early study, when chrysin was incubated with intestinal microflora from male Wistar rats, unlike other flavonoids, ring fission products were not detected [68]. Lack of hydroxy groups in the B-ring was proposed as the rationale for ring fission resistance. Human microflora metabolism study also suggested that ring fission products of chrysin were barely detected [69].
4.5. Elimination
Clinical studies showed that chrysin was eliminated as glucuronide through urine and chrysin appeared to be the major form eliminated through feces [25]. When chrysin was administered to healthy volunteers at 400 mg through the oral route, the total urine recoveries of chrysin and chrysin-7-O-glucurnide were 1.0 ± 0.4 mg and 11.3 ± 3.0 mg, respectively. The total recovery in the urine was less than 7%. Most of the dose appeared in feces and the total recovery in the feces was more than 90% as the aglycone form. High aglycone elimination could be because of enterohepatic recycling. In addition, since chrysin’s aqueous solubility is poor, the insoluble and unabsorbed portion of chrysin will be eliminated through feces directly. Rapid Tmax (Table 2) when given at lower doses suggests the existence of unabsorbed chrysin aglycone. Mechanism studies for chrysin elimination have not been reported in preclinical studies. Further studies are needed to reveal the mechanism of chrysin’s elimination.
4.6. Transporters facilitate disposition of chrysin
Caco-2 cell culture model, which is recognized by the FDA as a standard model to study drug absorption, is believed to be a good cell-based model to identify transporters. In a transport study using the Caco-2 model, the permeability of chrysin from basolateral to apical (Pba) and from apical to basolateral (Pab) was slightly different with efflux ratio (Pba/Pab) close to 2, suggesting that efflux transporters might be involved in chrysin’s transport [52]. However, this evidence is not enough to conclude that chrysin is a substrate of certain efflux transporter(s) because: (1) efflux ratio is not high enough; and (2) efflux could be because of metabolism-efflux interplay.
On the other hand, chrysin phase II conjugates are reported to be good substrates of efflux transporters. For example, in the Caco-2 transport study, it was found that as much as 90% of chrysin conjugates (i.e., chrysin-7-O-glucuronide, chrysin-7-O-sulfate) were detected in the apical side when chrysin was loaded in the apical side [52]. Intestinal perfusion studies also demonstrated that when chrysin was perfused through the rats intestine, high concentration of chrysin conjugates were detected in the perfusate, suggesting that chrysin conjugations were pumped back into the intestinal lumen [59]. Efficient efflux of chrysin conjugates is another reason for its low intestinal absorption and poor oral bioavailability. Efflux intervention could be a direction to improve chrysin’s systemic and local bioavailability. Since efflux transporters are expressed with different levels in different organs, the role of efflux transporters in the elimination of chrysin conjugates are different in these organs as illustrated in Fig. 2. So far, no evidence supports the involvement of any uptake transporters in chrysin or its conjugate transport.
Fig. 2.
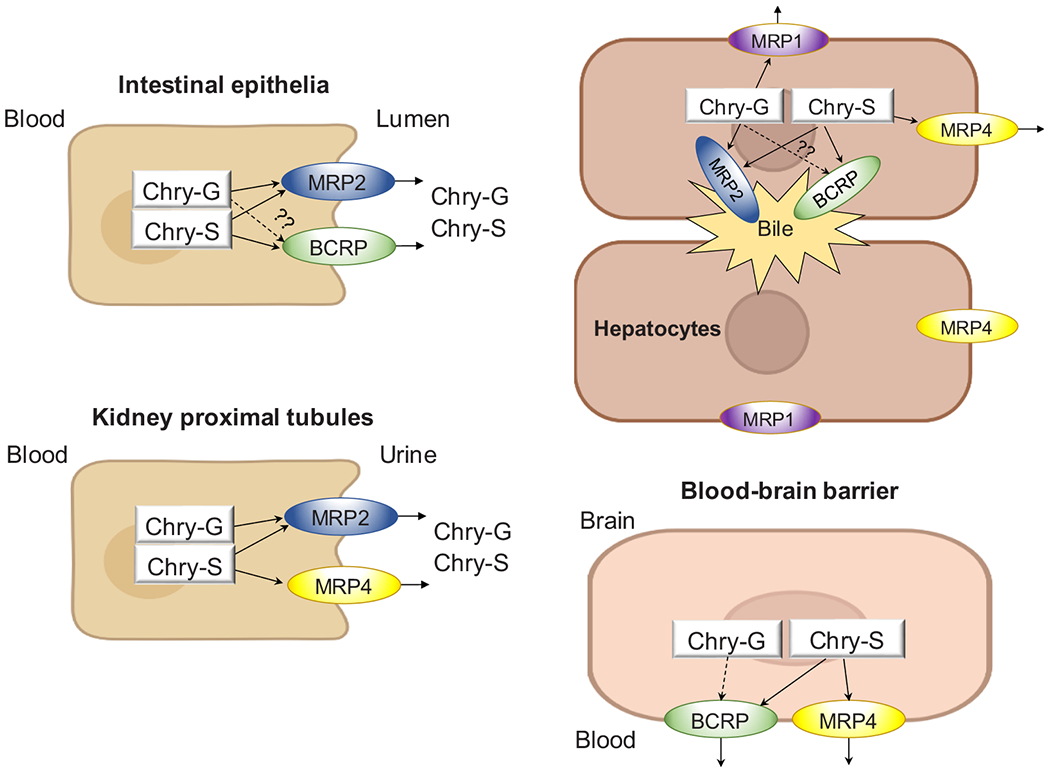
The role of efflux transporters in the disposition of chrysin conjugates.
4.7. Breast Cancer Resistance Protein (BCRP)
BCRP, which is highly expressed in many tissues including the intestine, liver, and blood-brain barrier, is an essential efflux transporter involved in many drug’s efflux. Other than normal tissues, BCRP is also expressed in certain types of cancer cells and overexpression of BCRP is proved to be an important reason for drug resistance in chemotherapy [70]. In vitro studies using Caco-2 cell culture model showed that in the presence of Ko-143, a specific chemical inhibitor for BCRP, chrysing-7-O-sulfate efflux was significantly inhibited, while efflux of chrysin-7-O-glucuronide was inhibited slightly, indicating that chrysin sulfate, but not glucuronide, is a good substrate of BCRP while glucuronide is not (Fig. 2) [54]. However, RNA-mediated silencing results in HeLa cells showed that when BCRP was silenced, chrysin-7-O-glucuronide secretion was significantly decreased, suggesting that glucuronide is a substrate of BCRP [60]. While in an animal study, we found that the impact of Bcrp on chrysin and its conjugates is limited as the PK parameters were not altered significantly in Bcrp knockout mice compared to those in wild-type mice [54]. Current findings suggested that chrysin conjugates are substrates of Bcrp, but the impact of BCRP on in vivo chrysin and chrysin conjugates might be minor. More PK studies are needed to evaluate the impact of BCRP on chrysin in vivo exposure.
4.7.1. Multidrug resistance-associated proteins (MRPs)
MRPs are very common efflux transporters participating in the efflux of many flavonoid conjugates, resulting in low systemic bioavailability of the flavonoids. In an in vitro study using the Caco-2 cell culture model, apical efflux of both chrysin glucuronide and sulfate were decreased in the presence of MK-571, which is a specific MRP2 inhibitor, suggesting that both glucuronide and sulfate are substrate of MPR2 [52]. Another in vitro study using HEK293 cell suggested that in the presence of MK-571, the efflux of chrysin-7-O-sulfate was inhibited and the intracellular level of sulfate was increased significantly [63]. These are probably the only two evidences to show that MPR2 is involved in chrysin conjugates efflux. Interestingly, it was reported that chrysin-7-O-sulfates is a good substrate of MRP4 transporter. Evidence to support this conclusion is that when MPR4 was knocked out in HEK293 cells, secretion of chrysin-7-O-sulfate was decreased significantly and intracellular accumulation was increased significantly (125–135%) [63]. MPR4 is located on the apical or basolateral side of different organs (Fig. 2). For example, in the kidney and blood brain barrier (BBB), MPR4 is expressed on the apical side, pumping chrysin-sulfate into the urine in the kidney or blood in the brain. While in the hepatocytes, MPR4 is located on the basolateral side transporting chrysin-sulfate back into the blood from the hepatocytes [71]. MPR1 was also reported to be a transporter facilitating chrysin-7-O-glucuronide efflux in Hela cells [60].
4.8. Enterohepatic recycling
Like many other flavonoids, chrysin undergoes efficient enterohepatic recycling after oral administration, where the aglycone is absorbed in the small intestine and reaches to the liver through the portal vein. Then, chrysin can be rapidly metabolized into glucuronide and sulfate by hepatic UGTs and SULTs, followed by biliary secretion facilitated by efflux transporters (e.g., BCRP, MPR2). The secreted conjugates (e.g., chrysin-7-O-glucuronide and chrysin-7-O-sulfate) will reach the colon through the small intestine to meet with intestinal microbiome and can be efficiently hydrolyzed back into chrysin by microbial beta-glucuronidases (GUS). Then, the newly released chrysin can be absorbed and reach the liver to form a recycle, resulting in multiple absorption peaks and long half-life in PK profile. Due to recycling, chrysin will be presented in the colon and terminal ileum multiple times like a shuttle before being directly eliminated through feces or urine via systemic circulation. In PK studies in rats, chrysin showed two absorption peaks at around 2 and 5–10 hrs with a half-life at 4–9 h reported by different research groups [35,38]. PK studies in mice also found double absorption peaks at a similar time [54]. In human, there are three absorption peaks at around 2, 6, and 24 h. All these PK studies in different species revealed that chrysin participates in recycling.
Although recycling was observed in chrysin’s PK studies, the recycling mechanism is not entirely understood. In an in situ study in rats, we have found that when chrysin was perfused through the intestine, about 1/3 of chrysin was detected in the portal vein as glucuronide form [59]. In addition, when chrysin-7-O-glucuronide was infused through the portal vein, the majority amount of chrysin-7-O-glucuronide was detected in the bile, indicating that hepatocytes can uptake chrysin-7-O-glucuronide, which is then secreted into the bile and then to the intestine. These findings suggest that chrysin can also be metabolized in the GI tract and enter the liver through portal vein as the glucuronide form, followed by secretion from the bile to participate in recycling. This is different from traditional enterohepatic recycling, in which aglycone is absorbed and metabolized in the liver. More studies are expected to explain and further investigate this observation.
4.9. Interaction with the host
4.9.1. UGT induction
Many studies have demonstrated that chrysin could induce UGT1A1 in different cell lines including Caco-2 and HepG2 cells with different sensitivity. For example, in an in vitro study, it was demonstrated that when Caco-2 cells were exposed to 50 μM of chrysin, glucuronidation function increased 3.8-fold in the intact cells or 14-fold in cell lysate [72]. Further studies showed that in Caco-2 cells, chrysin (25 μM) significantly induced UGT1A1 expression as shown by Northern blot analysis [73]. In addition, in vitro studies using HepG cells demonstrated that chrysin induced UGT1A1 at mRNA, protein, and functional levels [74]. Mechanism studies revealed that chrysin induces UGT1A1 in a MEK-1-dependent manner involving the Ah receptor [75].
As abovementioned, chrysin is a dietary component mainly in honey and is relatively safe. Therefore, it has been suggested to use chrysin as a UGT1A1 inducer to enhance detoxification via glucuronidation or to treat inheritance diseases such as Gilbert’s syndrome. Clinical studies have been conducted using chrysin to alleviate irinotecan-induced intestinal injury and diarrhea, where intestinal UGT1A1 is expected to be induced to enhance intestinal glucuronidation of SN-38, the toxic compound causing local damage. The results turned out to be promising [21]. However, chrysin is also a UGT1A1 substrate. It has been suggested that the metabolic stability of chrysin would limit its ability to induce UGT1A1 in vivo [76]. Future studies should focus on dynamic changes of chrysin concentration when using chrysin as a UGT1A1 inducer. Additionally, GI tract is theoretically the best organ for UGT induction using chrysin because its oral bioavailability is poor and in vivo exposure is usually low.
4.9.2. BCRP inhibition
Chrysin is a strong BCRP inhibitor as demonstrated in in vitro studies using cell lines [77]. A recent comprehensive study showed that chrysin BCRP inhibition IC50 was 0.4 μM [78]. Molecular docking analysis showed that chrysin binds with BCRP through Pi-Pi stacked interaction with Phe 439 of BCRP or Pi-Alkyl interactions with Val546 of BCRP, instead of conventional hydrogen bonds [79]. However, some in vivo studies showed that chrysin played a minor role in substrate efflux [80,81]. Solubility could be one of the primary reasons to causing inconsistency between in vitro and in vivo studies when evaluating chrysin’s BCRP inhibition. When chrysin was administered through a solid dispersion formulation, which significantly improves chrysin’s solubility, oral bioavailability of BCRP substrates (e.g., topotecan, doxorubicin, temozolomide, mitoxantrone) were significantly enhanced [79,82]. Since the water solubility of chrysin is low, in vivo achievable concentrations of chrysin should be taken into consideration in future preclinical and clinical studies.
4.9.3. Other interactions
In vitro studies demonstrated that chrysin and chrysin conjugates also inhibit CYPs [83,84] and transporters including OATPs, MRP2 [81,85] as shown in Table 3.
Table 3.
Transporters and metabolic enzymes inhibition (IC50, μM) by Chrysina.
| Transporters and enzymes | Chrysin | Chrysin-glucuronide | Chrysin-sulfate |
|---|---|---|---|
| OATP1A2 | >100 | 24.1 | 18.3 |
| OATP1B1 | >100 | 4.4 | 0.8 |
| OATP1B3 | >100 | 14.3 | 1.7 |
| OATP2B1 | 4.8 | 0.3 | 0.5 |
| BCRP | 0.4 | 19.8 | 0.6 |
| MPR2 | >100 | 11.2 | >100 |
| CYP2C9 | 3.2 | >100 | 2.7 |
| CYP2C19 | 4.6 | >100 | >100 |
Data from reference [78].
5. Remarks
Chrysin is a dietary component possessing multiple beneficial activities. Clinical trials using chrysin as an adjuvant agent to treat several diseases (e.g. hormone homeostasis, drug-induced GI toxicity) have been conducted and the results turned out to be very interesting. Biopharmaceutical studies showed that chrysin absorption is moderate due to its low solubility and moderate permeability. Low oral bioavailability is the challenge for developing chrysin as a therapeutic drug. Multiple metabolic enzymes (e.g., UGTs, SULTs, CYPs) and transporters (e.g., BCRP, MRP2/MRP4) are involved in chrysin’s disposition, which can be further studied as potential targets to enhance chrysin’s bioavailability. Further studies on tissue and species variation in ADME of chrysin would be helpful for developing chrysin as a therapeutic agent using animal models and in clinical studies.
Acknowledgments
This work was supported by a grant from the Cancer Prevention Research Institute of Texas, USA (CPRIT, RP190672) and National Institute of General Medical Sciences, NIH, USA (1R15GM126475–01A1) for Song Gao. This work was also made possible, in part, by the GCC Center for Comprehensive PK/PD and Formulation (CCPF) with CPRIT, USA grant number of RP180748 and RCMI grant with National Institute of Minority Health and Health Disparity, USA (U54MD007605).
Footnotes
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Perkin A, Berichte der dLuteolin, Part 2, Journal of the Chemical Society, Transactions, 1896. 〈https://pubs.rsc.org/en/content/articlepdf/1896/ct/ct8966900799?casa_token=yZhxRQrX-H0AAAAA:Wo3HIAy8yFYSzu7oumF4wFA8EZ1HnlgC0uSEE7lor849a_hhplndYkvTZ23JzDEUb008_caIC7O7〉. [Google Scholar]
- [2].Naz S, Imran M, Rauf A, Orhan IE, Shariati MA, Iahtisham Ul H, Iqra Y, Shahbaz M, Qaisrani TB, Shah ZA, Plygun S, Heydari M, Chrysin: pharmacological and therapeutic properties, Life Sci. 235 (2019), 116797. [DOI] [PubMed] [Google Scholar]
- [3].Kalogeropoulos N, Yanni AE, Koutrotsios G, Aloupi M, Bioactive microconstituents and antioxidant properties of wild edible mushrooms from the island of Lesvos, Greece, Food Chem. Toxicol 55 (2013) 378–385. [DOI] [PubMed] [Google Scholar]
- [4].Mani R, Natesan V, Chrysin: sources, beneficial pharmacological activities, and molecular mechanism of action, Phytochemistry 145 (2018) 187–196. [DOI] [PubMed] [Google Scholar]
- [5].Beszterda M, Franski R, Detection of flavone C-glycosides in the extracts from the bark of Prunus avium L. and Prunus cerasus L, Eur. J. Mass Spectrom. (Chichester) 26 (5) (2020) 369–375. [DOI] [PubMed] [Google Scholar]
- [6].Xie J, Wang Y, Jiang WW, Luo XF, Dai TY, Peng L, Song S, Li LF, Tao L, Shi CY, Hao RS, Xiao R, Tian Y, Sheng J, Moringa oleifera leaf petroleum ether extract inhibits lipogenesis by activating the AMPK signaling pathway, Front Pharm. 9 (2018) 1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Berthier A, Girard C, Grandvuillemin A, Muyard F, Skaltsounis AL, Jouvenot M, Delage-Mourroux R, Effect of 7-O-beta-D-glucopyranosylchrysin and its aglycone chrysin isolated from Podocytisus caramanicus on estrogen receptor alpha transcriptional activity, Planta Med. 73 (14) (2007) 1447–1451. [DOI] [PubMed] [Google Scholar]
- [8].El-Askary HI, Haggag MY, Abou-Hussein DR, Hussein SM, Sleem AA, Bioactivity-guided study of Passiflora caerulea L. leaf extracts, Iran. J. Pharm. Res 16 (Suppl) (2017) 46–57. [PMC free article] [PubMed] [Google Scholar]
- [9].Baell JB, Nissink JWM, Seven year itch: pan-assay interference compounds (PAINS) in 2017-utility and limitations, ACS Chem. Biol 13 (1) (2018) 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Samarghandian S, Farkhondeh T, Azimi-Nezhad M, Protective effects of chrysin against drugs and toxic agents, Dose Response 15 (2) (2017), 1559325817711782, 1559325817711782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Angelopoulou E, Pyrgelis ES, Piperi C, Neuroprotective potential of chrysin in Parkinson’s disease: molecular mechanisms and clinical implications, Neurochem. Int 132 (2020), 104612. [DOI] [PubMed] [Google Scholar]
- [12].Pingili RB, Pawar AK, Challa SR, Kodali T, Koppula S, Toleti V, A comprehensive review on hepatoprotective and nephroprotective activities of chrysin against various drugs and toxic agents, Chem. Biol. Inter 308 (2019) 51–60. [DOI] [PubMed] [Google Scholar]
- [13].Farkhondeh T, Samarghandian S, Bafandeh F, The cardiovascular protective effects of chrysin: a narrative review on experimental researches, Cardiovasc Hematol. Agents Med. Chem 17 (1) (2019) 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zeinali M, Rezaee SA, Hosseinzadeh H, An overview on immunoregulatory and anti-inflammatory properties of chrysin and flavonoids substances, Biomed. Pharm 92 (2017) 998–1009. [DOI] [PubMed] [Google Scholar]
- [15].Li Z, Chu S, He W, Zhang Z, Liu J, Cui L, Yan X, Li D, Chen N, A20 as a novel target for the anti-neuroinflammatory effect of chrysin via inhibition of NF-kappaB signaling pathway, Brain Behav. Immun 79 (2019) 228–235. [DOI] [PubMed] [Google Scholar]
- [16].Filho CB, Jesse CR, Donato F, Del Fabbro L, de Gomes MG, Goes ATR, Souza LC, Giacomeli R, Antunes M, Luchese C, Roman SS, Boeira SP, Neurochemical factors associated with the antidepressant-like effect of flavonoid chrysin in chronically stressed mice, Eur. J. Pharm 791 (2016) 284–296. [DOI] [PubMed] [Google Scholar]
- [17].Moghadam ER, Ang HL, Asnaf SE, Zabolian A, Saleki H, Yavari M, Esmaeili H, Zarrabi A, Ashrafizadeh M, Kumar AP, Broad-spectrum preclinical antitumor activity of chrysin: current trends and future perspectives, Biomolecules 10 (10) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lee S, Lee SK, Jung J, Potentiating activities of chrysin in the therapeutic efficacy of 5-fluorouracil in gastric cancer cells, Oncol. Lett 21 (1) (2021) 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lim HK, Kim KM, Jeong SY, Choi EK, Jung J, Chrysin increases the therapeutic efficacy of docetaxel and mitigates docetaxel-induced edema, Integr. Cancer Ther 16 (4) (2017) 496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sultana S, Verma K, Khan R, Nephroprotective efficacy of chrysin against cisplatin-induced toxicity via attenuation of oxidative stress, J. Pharm. Pharm 64 (6) (2012) 872–881. [DOI] [PubMed] [Google Scholar]
- [21].Tobin PJ, Beale P, Noney L, Liddell S, Rivory LP, Clarke S, A pilot study on the safety of combining chrysin, a non-absorbable inducer of UGT1A1, and irinotecan (CPT-11) to treat metastatic colorectal cancer, Cancer Chemother. Pharm 57 (3) (2006) 309–316. [DOI] [PubMed] [Google Scholar]
- [22].Ali N, Rashid S, Nafees S, Hasan SK, Sultana S, Beneficial effects of Chrysin against Methotrexate-induced hepatotoxicity via attenuation of oxidative stress and apoptosis, Mol. Cell Biochem 385 (1–2) (2014) 215–223. [DOI] [PubMed] [Google Scholar]
- [23].Noh K, Oh do G, Nepal MR, Jeong KS, Choi Y, Kang MJ, Kang W, Jeong HG, Jeong TC, Pharmacokinetic interaction of chrysin with caffeine in rats, Biomol. Ther. (Seoul.) 24 (4) (2016) 446–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Farkhondeh T, Samarghandian S, Roshanravan B, Impact of chrysin on the molecular mechanisms underlying diabetic complications, J. Cell Physiol 234 (10) (2019) 17144–17158. [DOI] [PubMed] [Google Scholar]
- [25].Walle T, Otake Y, Brubaker JA, Walle UK, Halushka PV, Disposition and metabolism of the flavonoid chrysin in normal volunteers, Br. J. Clin. Pharm 51 (2) (2001) 143–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Brown GA, Vukovich MD, Martini ER, Kohut ML, Franke WD, Jackson DA, King DS, Effects of androstenedione-herbal supplementation on serum sex hormone concentrations in 30- to 59-year-old men, Int J. Vitam. Nutr. Res 71 (5) (2001) 293–301. [DOI] [PubMed] [Google Scholar]
- [27].Kohut ML, Thompson JR, Campbell J, Brown GA, Vukovich MD, Jackson DA, King DS, Ingestion of a dietary supplement containing dehydroepiandrosterone (DHEA) and androstenedione has minimal effect on immune function in middle-aged men, J. Am. Coll. Nutr 22 (5) (2003) 363–371. [DOI] [PubMed] [Google Scholar]
- [28].Brown GA, Vukovich MD, Martini ER, Kohut ML, Franke WD, Jackson DA, King DS, Endocrine and lipid responses to chronic androstenediol-herbal supplementation in 30 to 58 year old men, J. Am. Coll. Nutr 20 (5) (2001) 520–528. [DOI] [PubMed] [Google Scholar]
- [29].Brown GA, Vukovich MD, Reifenrath TA, Uhl NL, Parsons KA, Sharp RL, King DS, Effects of anabolic precursors on serum testosterone concentrations and adaptations to resistance training in young men, Int J. Sport Nutr. Exerc Metab 10 (3) (2000) 340–359. [DOI] [PubMed] [Google Scholar]
- [30].Gambelunghe C, Rossi R, Sommavilla M, Ferranti C, Rossi R, Ciculi C, Gizzi S, Micheletti A, Rufini S, Effects of chrysin on urinary testosterone levels in human males, J. Med. Food 6 (4) (2003) 387–390. [DOI] [PubMed] [Google Scholar]
- [31].Arafa MG, Ghalwash D, El-Kersh DM, Elmazar MM, Propolis-based niosomes as oromuco-adhesive films: a randomized clinical trial of a therapeutic drug delivery platform for the treatment of oral recurrent aphthous ulcers, Sci. Rep 8 (1) (2018) 18056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ding X, Rose JP, Van Gelder J, Developability assessment of clinical drug products with maximum absorbable doses, Int. J. Pharm 427 (2) (2012) 260–269. [DOI] [PubMed] [Google Scholar]
- [33].Baidya D, Kushwaha J, Mahadik K, Patil S, Chrysin-loaded folate conjugated PF127-F68 mixed micelles with enhanced oral bioavailability and anticancer activity against human breast cancer cells, Drug Dev. Ind. Pharm 45 (5) (2019) 852–860. [DOI] [PubMed] [Google Scholar]
- [34].Roberts MS, Magnusson BM, Burczynski FJ, Weiss M, Enterohepatic circulation: physiological, pharmacokinetic and clinical implications, Clin. Pharm 41 (10) (2002) 751–790. [DOI] [PubMed] [Google Scholar]
- [35].Zhao X, Su X, Liu C, Jia Y, Simultaneous determination of chrysin and tectochrysin from Alpinia oxyphylla fruits by UPLC-MS/MS and its application to a comparative pharmacokinetic study in normal and dementia rats, Molecules 23 (7) (2018) 1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sanchez R, Villagran E, Concha M, Cornejo R, Ultrastructural analysis of the attachment sites of Escherichia coli to the human spermatozoon after in vitro migration through estrogenic cervical mucus, Int. J. Fertil 34 (5) (1989) 363–367. [PubMed] [Google Scholar]
- [37].Dong D, Quan E, Yuan X, Xie Q, Li Z, Wu B, Sodium oleate-based nanoemulsion enhances oral absorption of chrysin through inhibition of UGT-mediated metabolism, Mol. Pharm 14 (9) (2017) 2864–2874. [DOI] [PubMed] [Google Scholar]
- [38].Tong L, Wan M, Zhang L, Zhu Y, Sun H, Bi K, Simultaneous determination of baicalin, wogonoside, baicalein, wogonin, oroxylin A and chrysin of Radix Scutellariae extract in rat plasma by liquid chromatography tandem mass spectrometry, J. Pharm. Biomed. Anal 70 (2012) 6–12. [DOI] [PubMed] [Google Scholar]
- [39].Zhang J, Zhang S, Teng S, Zhai L, An LC-MS/MS method for simultaneous determination of four flavonoids from Semen Oroxyli in rat plasma and its application to a pharmacokinetic study, J. Chromatogr. B Anal. Technol. Biomed. Life Sci 1020 (2016) 96–102. [DOI] [PubMed] [Google Scholar]
- [40].Zhu Y, Tong L, Zhou S, Sun H, Bi K, Zhang B, Simultaneous determination of active flavonoids and alkaloids of Tang-Min-Ling-Pill in rat plasma by liquid chromatography tandem mass spectrometry, J. Chromatogr. B Anal. Technol. Biomed. Life Sci 904 (2012) 51–58. [DOI] [PubMed] [Google Scholar]
- [41].Qi Y, Cheng X, Jing H, Yan T, Xiao F, Wu B, Bi K, Jia Y, Comparative pharmacokinetic study of the components in Alpinia oxyphylla Miq.-Schisandra chinensis (Turcz.) Baill. herb pair and its single herb between normal and Alzheimer’s disease rats by UPLC-MS/MS, J. Pharm. Biomed. Anal 177 (2020), 112874. [DOI] [PubMed] [Google Scholar]
- [42].Anari E, Akbarzadeh A, Zarghami N, Chrysin-loaded PLGA-PEG nanoparticles designed for enhanced effect on the breast cancer cell line, Artif. Cells Nanomed. Biotechnol 44 (6) (2016) 1410–1416. [DOI] [PubMed] [Google Scholar]
- [43].El-Hussien D, El-Zaafarany GM, Nasr M, Sammour O, Chrysin nanocapsules with dual anti-glycemic and anti-hyperlipidemic effects: chemometric optimization, physicochemical characterization and pharmacodynamic assessment, Int. J. Pharm 592 (2021), 120044. [DOI] [PubMed] [Google Scholar]
- [44].Giacomeli R, de Gomes MG, Reolon JB, Haas SE, Colome LM, Jesse CR, Chrysin loaded lipid-core nanocapsules ameliorates neurobehavioral alterations induced by (β-amyloid1-42 in aged female mice, Behav. Brain Res 390 (2020), 112696. [DOI] [PubMed] [Google Scholar]
- [45].Andrade N, Marques C, Andrade S, Silva C, Rodrigues I, Guardao L, Guimaraes JT, Keating E, Calhau C, Martel F, Effect of chrysin on changes in intestinal environment and microbiome induced by fructose-feeding in rats, Food Fund. 10 (8) (2019) 4566–4576. [DOI] [PubMed] [Google Scholar]
- [46].Yang G, Ge S, Singh R, Basu S, Shatzer K, Zen M, Liu J, Tu Y, Zhang C, Wei J, Shi J, Zhu L, Liu Z, Wang Y, Gao S, Hu M, Glucuronidation: driving factors and their impact on glucuronide disposition, Drug Metab. Rev 49 (2) (2017) 105–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hofmann AF, The enterohepatic circulation of bile acids in man, Adv. Intern Med 21 (1976) 501–534. [PubMed] [Google Scholar]
- [48].Rastogi H, Jana S, Evaluation of physicochemical properties and intestinal permeability of six dietary polyphenols in human intestinal colon adenocarcinoma Caco-2 cells, Eur. J. Drug Metab. Pharm 41 (1) (2016) 33–43. [DOI] [PubMed] [Google Scholar]
- [49].Lipinski CA, Drug-like properties and the causes of poor solubility and poor permeability, J. Pharm. Toxicol. Methods 44 (1) (2000) 235–249. [DOI] [PubMed] [Google Scholar]
- [50].Lipinski CA, Lombardo F, Dominy BW, Feeney PJ, Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings, Adv. Drug Deliv. Rev 46 (1–3) (2001) 3–26. [DOI] [PubMed] [Google Scholar]
- [51].Lipinski CA, Lead- and drug-like compounds: the rule-of-five revolution, Drug Disco Today Technol. 1 (4) (2004) 337–341. [DOI] [PubMed] [Google Scholar]
- [52].Walle UK, Galijatovic A, Walle T, Transport of the flavonoid chrysin and its conjugated metabolites by the human intestinal cell line Caco-2, Biochem. Pharm 58 (3) (1999) 431–438. [DOI] [PubMed] [Google Scholar]
- [53].Artursson P, Karlsson J, Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells, Biochem. Biophys. Res. Commun 175 (3) (1991) 880–885. [DOI] [PubMed] [Google Scholar]
- [54].Ge S, Gao S, Yin T, Hu M, Determination of pharmacokinetics of chrysin and its conjugates in wild-type FVB and Bcrp1 knockout mice using a validated LC-MS/MS method, J. Agric. Food Chem 63 (11) (2015) 2902–2910. [DOI] [PubMed] [Google Scholar]
- [55].Fenyvesi F, Nguyen TLP, Haimhoffer A, Rusznyak A, Vasvari G, Bacskay I, Vecsernyes M, Ignat SR, Dinescu S, Costache M, Ciceu A, Hermenean A, Varadi J, Cyclodextrin complexation improves the solubility and Caco-2 permeability of chrysin, Materials (Basel) 13 (16) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Fagundes FL, de Morais Piffer G, Perico LL, Rodrigues VP, Hiruma-Lima CA, Dos Santos RC, Chrysin modulates genes related to inflammation, tissue remodeling, and cell proliferation in the gastric ulcer healing, Int. J. Mol. Sci 21 (3) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kasala ER, Bodduluru LN, Madana RM, V AK, Gogoi R, Barua CC, Chemopreventive and therapeutic potential of chrysin in cancer: mechanistic perspectives, Toxicol. Lett 233 (2) (2015) 214–225. [DOI] [PubMed] [Google Scholar]
- [58].Yang N, Sun R, Liao X, Aa J, Wang G, UDP-glucuronosyltransferases (UGTs) and their related metabolic cross-talk with internal homeostasis: a systematic review of UGT isoforms for precision medicine, Pharm. Res 121 (2017) 169–183. [DOI] [PubMed] [Google Scholar]
- [59].Zeng M, Sun R, Basu S, Ma Y, Ge S, Yin T, Gao S, Zhang J, Hu M, Disposition of flavonoids via recycling: direct biliary excretion of enterically or extrahepatically derived flavonoid glucuronides, Mol. Nutr. Food Res 60 (5) (2016) 1006–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Quan E, Wang H, Dong D, Zhang X, Wu B, Characterization of chrysin glucuronidation in UGT1A1-overexpressing HeLa cells: elucidating the transporters responsible for efflux of glucuronide, Drug Metab. Dispos 43 (4) (2015) 433–443. [DOI] [PubMed] [Google Scholar]
- [61].Robotham SA, Brodbelt JS, Identification of flavone glucuronide isomers by metal complexation and tandem mass spectrometry: regioselectivity of uridine 5′-diphosphate-glucuronosyltransferase isozymes in the biotransformation of flavones, J. Agric. Food Chem 61 (7) (2013) 1457–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Galijatovic A, Otake Y, Walle UK, Walle T, Extensive metabolism of the flavonoid chrysin by human Caco-2 and Hep G2 cells, Xenobiotica 29 (12) (1999) 1241–1256. [DOI] [PubMed] [Google Scholar]
- [63].Li W, Sun H, Zhang X, Wang H, Wu B, Efflux transport of chrysin and apigenin sulfates in HEK293 cells overexpressing SULT1A3: the role of multidrug resistance-associated protein 4 (MRP4/ABCC4), Biochem. Pharm 98 (1) (2015) 203–214. [DOI] [PubMed] [Google Scholar]
- [64].Ancuceanu R, Dinu M, Dinu-Pirvu C, Anuta V, Negulescu V, Pharmacokinetics of B-ring unsubstituted flavones, Pharmaceutics 11 (8) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Nagayoshi H, Murayama N, Kakimoto K, Tsujino M, Takenaka S, Katahira J, Lim YR, Kim D, Yamazaki H, Komori M, Guengerich FP, Shimada T, Oxidation of flavone, 5-hydroxyflavone, and 5,7-dihydroxyflavone to mono-, di-, and tri-hydroxyflavones by human cytochrome p450 enzymes, Chem. Res. Toxicol 32 (6) (2019) 1268–1280. [DOI] [PubMed] [Google Scholar]
- [66].Murota K, Nakamura Y, Uehara M, Flavonoid metabolism: the interaction of metabolites and gut microbiota, Biosci. Biotechnol. Biochem 82 (4) (2018) 600–610. [DOI] [PubMed] [Google Scholar]
- [67].Gao S, Hu M, Bioavailability challenges associated with development of anticancer phenolics, Mini Rev. Med. Chem 10 (6) (2010) 550–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Griffiths LA, Smith GE, Metabolism of apigenin and related compounds in the rat. Metabolite formation in vivo and by the intestinal microflora in vitro, Biochem. J 128 (4) (1972) 901–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Simons AL, Renouf M, Hendrich S, Murphy PA, Human gut microbial degradation of flavonoids: structure-function relationships, J. Agric. Food Chem 53 (10) (2005) 4258–4263. [DOI] [PubMed] [Google Scholar]
- [70].Mao Q, Unadkat JD, Role of the breast cancer resistance protein (BCRP/ABCG2) in drug transport–an update, Aaps J. 17 (1) (2015) 65–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].International Transporter C, Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, Hofffmaster KA, Ishikawa T, Keppler D, Kim RB, Lee CA, Niemi M, Polli JW, Sugiyama Y, Swaan PW, Ware JA, Wright SH, Yee SW, Zamek-Gliszczynski MJ, Zhang L, Membrane transporters in drug development, Nat. Rev. Drug Disco 9 (3) (2010) 215–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Galijatovic A, Walle UK, Walle T, Induction of UDP-glucuronosyltransferase by the flavonoids chrysin and quercetin in Caco-2 cells, Pharm. Res 17 (1) (2000) 21–26. [DOI] [PubMed] [Google Scholar]
- [73].Galijatovic A, Otake Y, Walle UK, Walle T, Induction of UDP-glucuronosyltransferase UGT1A1 by the flavonoid chrysin in Caco-2 cells–potential role in carcinogen bioinactivation, Pharm. Res 18 (3) (2001) 374–379. [DOI] [PubMed] [Google Scholar]
- [74].Walle T, Otake Y, Galijatovic A, Ritter JK, Walle UK, Induction of UDP-glucuronosyltransferase UGT1A1 by the flavonoid chrysin in the human hepatoma cell line hep G2, Drug Metab. Dispos 28 (9) (2000) 1077–1082. [PubMed] [Google Scholar]
- [75].Bonzo JA, Belanger A, Tukey RH, The role of chrysin and the ah receptor in induction of the human UGT1A1 gene in vitro and in transgenic UGT1 mice, Hepatology 45 (2) (2007) 349–360. [DOI] [PubMed] [Google Scholar]
- [76].Smith CM, Graham RA, Krol WL, Silver IS, Negishi M, Wang H, Lecluyse EL, Differential UGT1A1 induction by chrysin in primary human hepatocytes and HepG2 Cells, J. Pharm. Exp. Ther 315 (3) (2005) 1256–1264. [DOI] [PubMed] [Google Scholar]
- [77].Zhang S, Yang X, Morris ME, Flavonoids are inhibitors of breast cancer resistance protein (ABCG2)-mediated transport, Mol. Pharm 65 (5) (2004) 1208–1216. [DOI] [PubMed] [Google Scholar]
- [78].Mohos V, Fliszar-Nyul E, Ungvari O, Bakos E, Kuffa K, Bencsik T, Zsido BZ, Hetenyi C, Telbisz A, Ozvegy-Laczka C, Poor M, Effects of chrysin and its major conjugated metabolites chrysin-7-sulfate and chrysin-7-glucuronide on cytochrome p450 enzymes and on OATP, P-gp, BCRP, and MRP2 transporters, Drug Metab. Dispos 48 (10) (2020) 1064–1073. [DOI] [PubMed] [Google Scholar]
- [79].Fan X, Bai J, Zhao S, Hu M, Sun Y, Wang B, Ji M, Jin J, Wang X, Hu J, Li Y, Evaluation of inhibitory effects of flavonoids on breast cancer resistance protein (BCRP): From library screening to biological evaluation to structure-activity relationship, Toxicol. Vitr 61 (2019), 104642. [DOI] [PubMed] [Google Scholar]
- [80].Zhao R, Raub TJ, Sawada GA, Kasper SC, Bacon JA, Bridges AS, Pollack GM, Breast cancer resistance protein interacts with various compounds in vitro, but plays a minor role in substrate efflux at the blood-brain barrier, Drug Metab. Dispos 37 (6) (2009) 1251–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Zhang S, Wang X, Sagawa K, Morris ME, Flavonoids chrysin and benzoflavone, potent breast cancer resistance protein inhibitors, have no significant effect on topotecan pharmacokinetics in rats or mdr1a/1b (−/−) mice, Drug Metab. Dispos 33 (3) (2005) 341–348. [DOI] [PubMed] [Google Scholar]
- [82].Lee SH, Lee YS, Song JG, Han HK, Improved in vivo effect of chrysin as an absorption enhancer via the preparation of ternary solid dispersion with Brij(R)L4 and aminoclay, Curr. Drug Deliv 16 (1) (2019) 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Quintieri L, Palatini P, Nassi A, Ruzza P, Floreani M, Flavonoids diosmetin and luteolin inhibit midazolam metabolism by human liver microsomes and recombinant CYP 3A4 and CYP3A5 enzymes, Biochem Pharm. 75 (6) (2008) 1426–1437. [DOI] [PubMed] [Google Scholar]
- [84].Kim HJ, Lee SB, Park SK, Kim HM, Park YI, Dong MS, Effects of hydroxyl group numbers on the B-ring of 5,7-dihydroxyflavones on the differential inhibition of human CYP 1A and CYP1B1 enzymes, Arch. Pharm. Res 28 (10) (2005)1114–1121. [DOI] [PubMed] [Google Scholar]
- [85].Navratilova L, Ramos Mandikova J, Pavek P, Mladenka P, Trejtnar F, Honey flavonoids inhibit hOATP2B1 and hOATP1A2 transporters and hOATP-mediated rosuvastatin cell uptake in vitro, Xenobiotica 48 (7) (2018) 745–755. [DOI] [PubMed] [Google Scholar]


