Abstract
Importance:
Evidence on the relative benefits and harms of primary high-risk HPV testing is needed to inform guidelines.
Objective:
To inform the US Preventive Services Task Force by modeling the benefits and harms of various cervical cancer screening strategies.
Design, Setting, and Participants:
Microsimulation model of a hypothetical cohort of women initiating screening at age 21 years.
Exposures:
Screening with cytology, high-risk HPV testing, and cytology and HPV cotesting, varying age to switch from cytology to HPV testing or cotesting (25, 27, 30 years), rescreening interval (3, 5 years), and triage options for HPV-positive results (16/18 genotype, cytology testing). Complete adherence for all 19 strategies was assumed.
Main Outcomes and Measures:
Lifetime number of tests, colposcopies, disease detection, false positives, cancer cases and deaths, life-years, and efficiency ratios expressing the tradeoff of harms (i.e., colposcopies, tests) versus benefits (life-years gained, cancer cases averted). Efficient strategies were those that yielded more benefit and less harm than another strategy or a lower harm-to-benefit ratio than a strategy with less harms.
Results:
Compared to no screening, all modeled cervical cancer screening strategies were estimated to result in substantial reductions in cancer cases and deaths, and gains in life-years. The effectiveness of screening across the different strategies was estimated to be similar, with primary HPV-based and alternative cotesting strategies having slightly higher effectiveness and greater harms than current guidelines-based cytology testing. For example, cervical cancer deaths associated with the guidelines-based strategies ranged from 0.30 to 0.76 deaths per 1000 women, whereas new strategies involving primary HPV testing or cotesting had fewer cervical cancer deaths, ranging from 0.23 to 0.29 deaths per 1000 women. In all analyses, primary HPV testing strategies occurring at 5-year intervals were efficient. For example, 5-year primary HPV testing (cytology triage) based on switching from cytology to HPV screening at ages 30 years, 27 years, and 25 years had ratios per life-year gained (LYG) of 73, 143 and 195 colposcopies, respectively. In contrast, strategies involving 3-year HPV testing had much worse ratios, ranging from 2188 to 3822 colposcopies per LYG. In most analyses, strategies involving cotesting were not efficient.
Conclusions and Relevance:
In this microsimulation modeling study, it was estimated that primary HPV screening may represent a reasonable balance of harms and benefits when done every 5 years. Switching from cytology to HPV testing at age 30 yielded the most efficient harm-to-benefit ratio when using colposcopy as a proxy for harms.
In 2012, cervical cancer screening guidelines were harmonized across several major guidelines-making organizations, including the US Preventive Services Task Force (USPSTF),1–3 recommending routine cytology screening every 3 years starting at age 21, with an option to switch to cytology and HPV “cotesting” every 5 years starting at age 30 (A recommendation). Screening is recommended to end at age 65, provided a history of regular screening without abnormalities in the past 10–20 years.1–3 Since 2012, new evidence on primary human papillomavirus (HPV) testing has emerged, contributing to the FDA-approval of the first stand-alone high-risk HPV test for primary screening in women ages 25 years and older. Interim clinical guidance on the use of primary HPV testing has been issued from several professional organizations.4
While empirical studies such as randomized clinical trials provide high-quality evidence on the effectiveness of screening, outcomes are usually based on intermediate endpoints (e.g., precancer detection or colposcopy rates) after limited rounds of screening. Mathematical disease simulation models can complement such evidence by extrapolating data beyond the trial period to project long-term outcomes of screening (e.g., life expectancy), over multiple rounds of screening. Models can also explore alternative scenarios that have not been examined in empirical studies. This decision analysis using a cervical cancer disease simulation model accompanied an evidence report for the USPSTF to update the evidence and address gaps in the expected benefits and harms of cervical cancer screening strategies in primary care.5
METHODS
The full decision analysis technical report is available at https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cervical-cancer-screening2. The full report contains additional model calibration and validation results, as well as results from additional scenario and sensitivity analyses.
Model Description
The disease microsimulation model has a natural history component and a screening component that are used to project the life histories of simulated women under different screening strategies.6,7 In the natural history model, each simulated woman faces monthly transitions between health states that describe underlying disease status, including HPV infection, precancer (i.e., cervical intraepithelial neoplasia, CIN grades 2 or 3), and invasive cancer (i.e., local, regional, distant stages) (Figure 1). States are further stratified by oncogenic HPV types 16, 18, 31, 33, 45, 52, and 58, each considered separately; pooled other high-risk types; and pooled low-risk types. Each month, women have risks of hysterectomy and death from all causes8,9 as well as death from cervical cancer based on survival data from the Surveillance, Epidemiology, and End Results (SEER) Program.10 Transition probabilities can vary by age, HPV type, duration of infection or lesion status, and a woman’s history of prior HPV infection and CIN treatment. Uncertain parameters, such as HPV incidence, CIN progression and regression, and HPV natural immunity were calibrated to data on HPV prevalence and type distribution among women with and without cervical disease.11–13 The model focuses on squamous cell carcinoma, the most common histologic subtype of cervical cancer.
Figure 1.
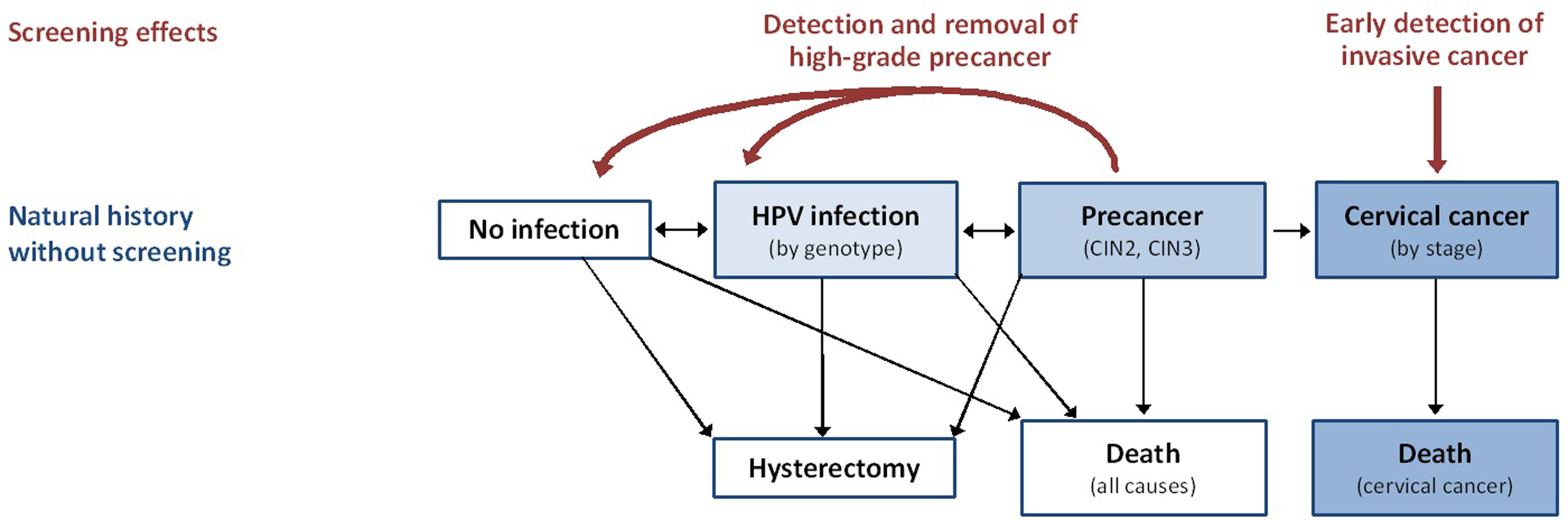
Model Schematic. The main health states of the natural history model comprise no infection, HPV infection (by genotype), precancer (cervical intraepithelial neoplasia or CIN, grades 2 and 3), invasive cancer (by stage), hysterectomy, and death (from all-causes or from cervical cancer). Movement between these health states occur as monthly transitions. The model focuses on squamous cell carcinoma, the most common histologic subtype of cervical cancer. Screening is used to detect the presence of CIN 2 or 3, which can be treated and removed before progressing to cancer, as well as for early detection of invasive cancer.
Screening is used to detect the presence of high-grade precancers, which can be treated before progressing to cancer, or for the earlier detection of invasive cancer. Screening assumptions in the model can vary by screening start and stop ages, frequency, coverage, follow-up (i.e., triage) testing, and adherence to recommended follow-up. Tests for primary screening and triage include cytology, high-risk HPV DNA testing, and cytology and HPV cotesting, with varying test characteristics (Table 1).14–18 Diagnostic colposcopy and biopsy were assumed to be 100% accurate in confirmation of histologic status, and the effectiveness of precancer treatment (i.e., loop electrosurgical excisional procedure (LEEP)) was assumed to be 100%, but both assumptions were varied in sensitivity analysis. Details on the selection of input data and assumptions are available in the full report.
Table 1.
Screening Test Characteristics
| Test characteristica | Base-Case Value | Source | Worst-Case Value | Best-Case Value | Source |
|---|---|---|---|---|---|
| Cytologyb | 14,15 | ||||
| HPVc | 15–18 | ||||
| Cotestc | 15–18 |
Sensitivity (specificity) for all tests defined as probability to detect presence (absence) of cervical intraepithelial neoplasia, grade 2 (CIN 2) or worse.
For cytology testing, positivity threshold is atypical squamous cells of undetermined significance (ASC-US).
For HPV testing and cotesting, given the wide variation in absolute test characteristics across studies due to differences in protocols and populations, we elected to utilize relative sensitivity and specificity values, compared with cytology testing (positivity threshold of ASC-US). For example, the base-case test sensitivity values for primary HPV testing was 0.901 (0.727 × 1.24) and for cotesting was 0.952 (0.727 × 1.31).
The model was validated against data from SEER cancer registries (years 2000–2013), under assumptions of current screening practice patterns (eFigure 1 in the Supplement).19–21 Additional model validation exercises included comparing model projections against reported outcomes from the HPV for cervical cancer screening (HPV FOCAL) trial, a randomized trial evaluating stand-alone HPV testing for primary screening.22
Screening Strategies
The analysis focused on the comparative effectiveness and harms of primary HPV testing, compared to currently recommended screening strategies. Table 2 summarizes the 19 main strategies evaluated. Guideline-based screening strategies comprised cytology alone every 3 years from ages 21–65 years (strategy 1), and cytology alone every 3 years from ages 21–29 years, with a switch to cytology and HPV cotesting every 5 years from ages 30–65 years (strategy 2).1–3 Management of women with equivocal or abnormal tests were assumed to follow established guidelines.2,23 For cotesting, HPV-positive/cytology-negative women were managed by repeat cotesting at 12 months, with referral to colposcopy for any positive result.
Table 2.
Cervical Cancer Screening Strategiesa
| # | Strategy Name | Screen (1) test, interval | Screen (1) start age | Screen (2) test, interval | Screen (2) start age | Triage strategies for HPV-pos results |
|---|---|---|---|---|---|---|
| 1 | CYTO-3Y, 21 b | Cytology, 3y | 21 | -- | -- | HPV for ASC-US |
| 2 | CYTO-3Y, 21/COTEST-5Y, 30 b | Cytology, 3y | 21 | Cotest, 5y | 30 | Repeat cotest, 12 mos |
| 3 | CYTO-4Y, 21/HPV-3Y (16/18), 25 | Cytology, 4y | 21 | HPV, 3y | 25 | HPV-16/18 genotype |
| 4 | CYTO-3Y, 21/HPV-3Y (16/18), 27 | Cytology, 3y | 21 | HPV, 3y | 27 | HPV-16/18 genotype |
| 5 | CYTO-3Y, 21/HPV-3Y (16/18), 30 | Cytology, 3y | 21 | HPV, 3y | 30 | HPV-16/18 genotype |
| 6 | CYTO-4Y, 21/HPV-5Y (16/18), 25 | Cytology, 4y | 21 | HPV, 5y | 25 | HPV-16/18 genotype |
| 7 | CYTO-3Y, 21/HPV-5Y (16/18), 27 | Cytology, 3y | 21 | HPV, 5y | 27 | HPV-16/18 genotype |
| 8 | CYTO-3Y, 21/HPV-5Y (16/18), 30 | Cytology, 3y | 21 | HPV, 5y | 30 | HPV-16/18 genotype |
| 9 | CYTO-4Y, 21/HPV-3Y (cyto), 25 | Cytology, 4y | 21 | HPV, 3y | 25 | Cytology triage |
| 10 | CYTO-3Y, 21/HPV-3Y (cyto), 27 | Cytology, 3y | 21 | HPV, 3y | 27 | Cytology triage |
| 11 | CYTO-3Y, 21/HPV-3Y (cyto), 30 | Cytology, 3y | 21 | HPV, 3y | 30 | Cytology triage |
| 12 | CYTO-4Y, 21/HPV-5Y (cyto), 25 | Cytology, 4y | 21 | HPV, 5y | 25 | Cytology triage |
| 13 | CYTO-3Y, 21/HPV-5Y (cyto), 27 | Cytology, 3y | 21 | HPV, 5y | 27 | Cytology triage |
| 14 | CYTO-3Y, 21/HPV-5Y (cyto), 30 | Cytology, 3y | 21 | HPV, 5y | 30 | Cytology triage |
| 15 | CYTO-4Y, 21/COTEST-3Y, 25 | Cytology, 4y | 21 | Cotest, 3y | 25 | Repeat cotest, 12 mos |
| 16 | CYTO-3Y, 21/COTEST-3Y, 27 | Cytology, 3y | 21 | Cotest, 3y | 27 | Repeat cotest, 12 mos |
| 17 | CYTO-3Y, 21/COTEST-3Y, 30 | Cytology, 3y | 21 | Cotest, 3y | 30 | Repeat cotest, 12 mos |
| 18 | CYTO-4Y, 21/COTEST-5Y, 25 | Cytology, 4y | 21 | Cotest, 5y | 25 | Repeat cotest, 12 mos |
| 19 | CYTO-3Y, 21/COTEST-5Y, 27 | Cytology, 3y | 21 | Cotest, 5y | 27 | Repeat cotest, 12 mos |
Abbreviations: Cyto, cytology; HPV, human papillomavirus.
Cotest strategies involve cytology and high-risk HPV testing; management of women with abnormal screening results was assumed to follow clinical guidelines2,23 and includes: for cytology testing, reflex HPV testing for women with atypical squamous cells of undetermined significance (ASC-US) and referral to colposcopy for women with more severe abnormal results; for cotesting, repeat cotesting in 12 months for women with cytology-negative, HPV-positive results; for HPV testing, two triage options were evaluated: “HPV (16/18)” strategies involved referral to colposcopy for women positive on HPV-16/18 genotype testing and cytology triage for women positive for other (non-16/18) high-risk HPV, and “HPV (cyto)” strategies involved cytology triage for all high-risk HPV-positive women. Analysis assumes screening end age of 65 years.
The primary HPV testing strategies (strategies 3–14) were varied by: (1) age to switch from cytology to HPV screening, (2) rescreening interval following an HPV-negative result, and (3) triage options for HPV-positive results. Age to switch to HPV screening was evaluated at ages 25, 27, and 30 years, following cytology-only screening starting at age 21. The rescreening interval for primary HPV testing was evaluated at every 3 years and every 5 years, consistent with current guidelines for cytology-only and cotesting. Two triage strategies for HPV-positive screening results were examined (eFigure 2 in the Supplement): (a) assuming HPV-16/18 genotype information is available, 16/18-positive women are referred to colposcopy, whereas women positive for other high-risk HPV types receive cytology triage (those with a cytology result of ASCUS or worse are referred to colposcopy; those with a cytology-negative result receive a follow-up test in 12 months); (b) all women with high-risk HPV receive cytology triage. Additional cotesting strategies (strategies 15–19) were also included varying the age to switch and rescreening interval.
In the base-case analysis, the age to stop screening was 65 years, assuming no recent history of abnormal results, consistent with current guidelines; sensitivity analysis was used to evaluate the effect of extending the age threshold at which to terminate screening to 70 and 75 years. The analysis assumed full adherence to screening initiation, rescreening interval, and follow-up for both diagnostic and pre-cancer treatment referrals. Furthermore, the base-case analysis focused on women who did not receive HPV vaccination.
Screening Outcomes
The model was used to generate a number of outcomes associated with each screening strategy, reflecting both health benefits and harms over the lifetime of screening starting at age 21 years. Harms included total number of cytology and HPV tests (including screening, triage, and surveillance), colposcopies, and false positive screening results (defined as total number of colposcopies without underlying CIN2, CIN3 or cancer); benefits included CIN2 and CIN3 detected, CIN3+ detected (including CIN3 and cervical cancers detected through screening), cervical cancer cases and deaths averted, and life-years gained. These measures were calculated as the cumulative number of events or time spent in the different health states, which were then modified by the interventions, over the selected time horizon (i.e., lifetime).
Analysis
The relative efficiency of each screening strategy was evaluated to examine the tradeoff of harms versus benefits for the general population of women being screened and was expressed as the incremental number of colposcopies per year of life gained (life-year gained, LYG). This efficiency ratio was defined as the additional number of colposcopies divided by the additional life-years of a specific strategy compared to the strategy with the next fewer colposcopies. Strategies with more harms (colposcopies) and less benefits (life-years) than an alternative strategy, or with a higher harm-to-benefit ratio than a strategy with more harms, were considered “inefficient” and eliminated from the calculation; all other strategies were considered “efficient.” Because there is no consensus on the appropriate metric to assess efficiency, results are also presented in terms of (1) the incremental number of total screening tests per life-year gained, and (2) the incremental number of colposcopies per cervical cancer case averted.
Sensitivity Analyses
The effects of uncertainty and alternative assumptions on the results were also assessed. Data uncertainty included screening test characteristics, colposcopy/biopsy performance, and precancer treatment effectiveness. Alternative screening scenarios included variations in management of HPV-positive women, including cytology triage with a colposcopy referral threshold of LSIL (base-case assumed ASC-US), varying intervals for follow-up testing from 6 months to 24 months (base-case assumed 12 months), and immediate colposcopy for all HPV-positive women. To reflect a low-risk population, screening in HPV-vaccinated women was evaluated, assuming that 100% of women were vaccinated with the three-dose HPV-16/18 vaccine in pre-adolescence and that vaccination conferred 100% protection against HPV-16 and −18 infections over the lifetime. The model was programmed in C++, and model output were analyzed in R version 1.0.136.
RESULTS
In the absence of screening, the lifetime risk of cervical cancer incidence was 1.9% and lifetime risk of cervical cancer mortality was 0.83%, resulting in a life expectancy of 63.9 years (Table 3) for 20-year-old women. Compared to no screening, all modeled cervical cancer screening strategies were estimated to result in substantial reductions in cancer cases and deaths, and gains in life-years. However, the effectiveness of screening across the different strategies was estimated to be similar, with primary HPV-based and alternative cotesting strategies having slightly higher effectiveness in terms of life-years gained and cancer cases and deaths averted than current guidelines-based cytology testing. For example, cervical cancer deaths associated with the guidelines-based strategies (strategies 1 and 2) ranged from 0.30 to 0.76 deaths per 1,000 women, whereas the new strategies involving primary HPV testing or cotesting varying switch age, interval and triage option (strategies 3–19) had fewer cervical cancer deaths, ranging from 0.23 to 0.29 deaths per 1,000 women (an improvement of 0.01 to 0.53 lives saved per 1,000 women screened).
Table 3.
Outcomes for Cervical Cancer Screening Strategies Over the Lifetime of Screeninga
| Per 1,000 women | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| # | Strategy | Cyto tests | HPV tests | Total testsb | Colpos | CIN2,3 detected | CIN3+ detectedc | False positivesd | CC cases | CC deaths | Life-years |
| 0 | No screening | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 18.86 | 8.34 | 63921.34 |
| 1 | CYTO-3Y, 21–65 | 13877 | 786 | 14662 | 645 | 160 | 46 | 484 | 2.34 | 0.76 | 64181.89 |
| 2 | CYTO-3Y, 21 / COTEST-5Y, 30–65 | 11425 | 8380 | 19806 | 1630 | 201 | 54 | 1429 | 1.08 | 0.30 | 64192.97 |
| 3 | CYTO-4Y, 21 / HPV-3Y (16/18), 25–65 | 1905 | 14807 | 16712 | 2530 | 218 | 57 | 2312 | 0.74 | 0.23 | 64195.61 |
| 4 | CYTO-3Y, 21 / HPV-3Y (16/18), 27–65 | 2876 | 13772 | 16648 | 2278 | 214 | 56 | 2063 | 0.83 | 0.25 | 64195.08 |
| 5 | CYTO-3Y, 21 / HPV-3Y (16/18), 30–65 | 3824 | 12428 | 16252 | 1978 | 205 | 54 | 1773 | 1.01 | 0.27 | 64193.51 |
| 6 | CYTO-4Y, 21 / HPV-5Y (16/18), 25–65 | 1706 | 10065 | 11771 | 2068 | 211 | 55 | 1857 | 0.79 | 0.25 | 64195.39 |
| 7 | CYTO-3Y, 21 / HPV-5Y (16/18), 27–65 | 2697 | 9290 | 11987 | 1861 | 207 | 55 | 1655 | 0.89 | 0.28 | 64194.69 |
| 8 | CYTO-3Y, 21 / HPV-5Y (16/18), 30–65 | 3675 | 8476 | 12151 | 1635 | 199 | 53 | 1435 | 1.05 | 0.29 | 64193.38 |
| 9 | CYTO-4Y, 21 / HPV-3Y (cyto), 25–65 | 2277 | 14790 | 17067 | 2209 | 217 | 56 | 1992 | 0.75 | 0.23 | 64195.53 |
| 10 | CYTO-3Y, 21 / HPV-3Y (cyto), 27–65 | 3205 | 13738 | 16943 | 1992 | 213 | 56 | 1779 | 0.85 | 0.25 | 64194.82 |
| 11 | CYTO-3Y, 21 / HPV-3Y (cyto), 30–65 | 4102 | 12397 | 16499 | 1734 | 203 | 54 | 1530 | 1.04 | 0.28 | 64193.19 |
| 12 | CYTO-4Y, 21 / HPV-5Y (cyto), 25–65 | 1993 | 10049 | 12042 | 1826 | 209 | 55 | 1617 | 0.81 | 0.25 | 64195.35 |
| 13 | CYTO-3Y, 21 / HPV-5Y (cyto), 27–65 | 2950 | 9273 | 12223 | 1648 | 205 | 54 | 1443 | 0.91 | 0.28 | 64194.44 |
| 14 | CYTO-3Y, 21 / HPV-5Y (cyto), 30–65 | 3888 | 8459 | 12348 | 1452 | 198 | 53 | 1254 | 1.08 | 0.29 | 64193.07 |
| 15 | CYTO-4Y, 21 / COTEST-3Y, 25–65 | 15723 | 14693 | 30416 | 2535 | 223 | 57 | 2312 | 0.76 | 0.23 | 64195.50 |
| 16 | CYTO-3Y, 21 / COTEST-3Y, 27–65 | 15765 | 13723 | 29488 | 2303 | 218 | 57 | 2084 | 0.83 | 0.25 | 64194.75 |
| 17 | CYTO-3Y, 21 / COTEST-3Y, 30–65 | 15456 | 12411 | 27867 | 2021 | 209 | 55 | 1812 | 1.03 | 0.27 | 64193.17 |
| 18 | CYTO-4Y, 21 / COTEST-5Y, 25–65 | 10944 | 9914 | 20859 | 2029 | 213 | 55 | 1816 | 0.82 | 0.26 | 64195.26 |
| 19 | CYTO-3Y, 21 / COTEST-5Y, 27–65 | 11275 | 9233 | 20508 | 1846 | 209 | 55 | 1637 | 0.89 | 0.27 | 64194.40 |
Abbreviations: CC, cervical cancer; CIN, cervical intraepithelial neoplasia; Colpos, colposcopies; Cyto, cytology; HPV, Human papillomavirus.
Outcomes calculated from age 20 to 100 years and expressed per 1,000 women; analysis assumes screening end age of 65 years.
Total number of tests, irrespective of primary, triage or surveillance context.
CIN3+ includes cases of CIN3 and cervical cancers detected through screening (excludes clinically detected cancers).
Total number of colposcopies that did not result in CIN2, CIN3 or cancer detection.
In terms of harms, more frequent interval (i.e., 3-year versus 5-year) and cotesting strategies were generally associated with a greater number of lifetime total tests, whereas the age to switch from cytology to HPV testing or cotesting did not have much effect on the estimates. Using cytology triage for HPV-positive women was associated with increased lifetime total tests slightly (1–2%) compared to 16/18 genotype triage. With cotesting, the number of lifetime total tests were 60% to 82% greater than that with the analogous strategy involving HPV testing alone.
Cytology testing alone every 3 years from age 21 to 65 years was associated with the lowest number of lifetime colposcopies (i.e., 645 per 1,000 women) but also the lowest number of CIN2,3 and CIN3+ detected. All other strategies were associated with higher colposcopies, especially for cotesting (strategies 2, 15–19), followed by primary HPV testing with 16/18 genotype triage (strategies 3–8). HPV testing with 16/18 genotype triage had 12–14% greater colposcopies than HPV testing with cytology triage. Consistent with the trend of colposcopies, the number of false positives increased from cytology testing every 3 years to HPV testing or cotesting.
Relative Efficiency Analysis
Three different metrics were calculated to reflect different ways of capturing the harm-to-benefit tradeoff (Figure 2).
Figure 2.
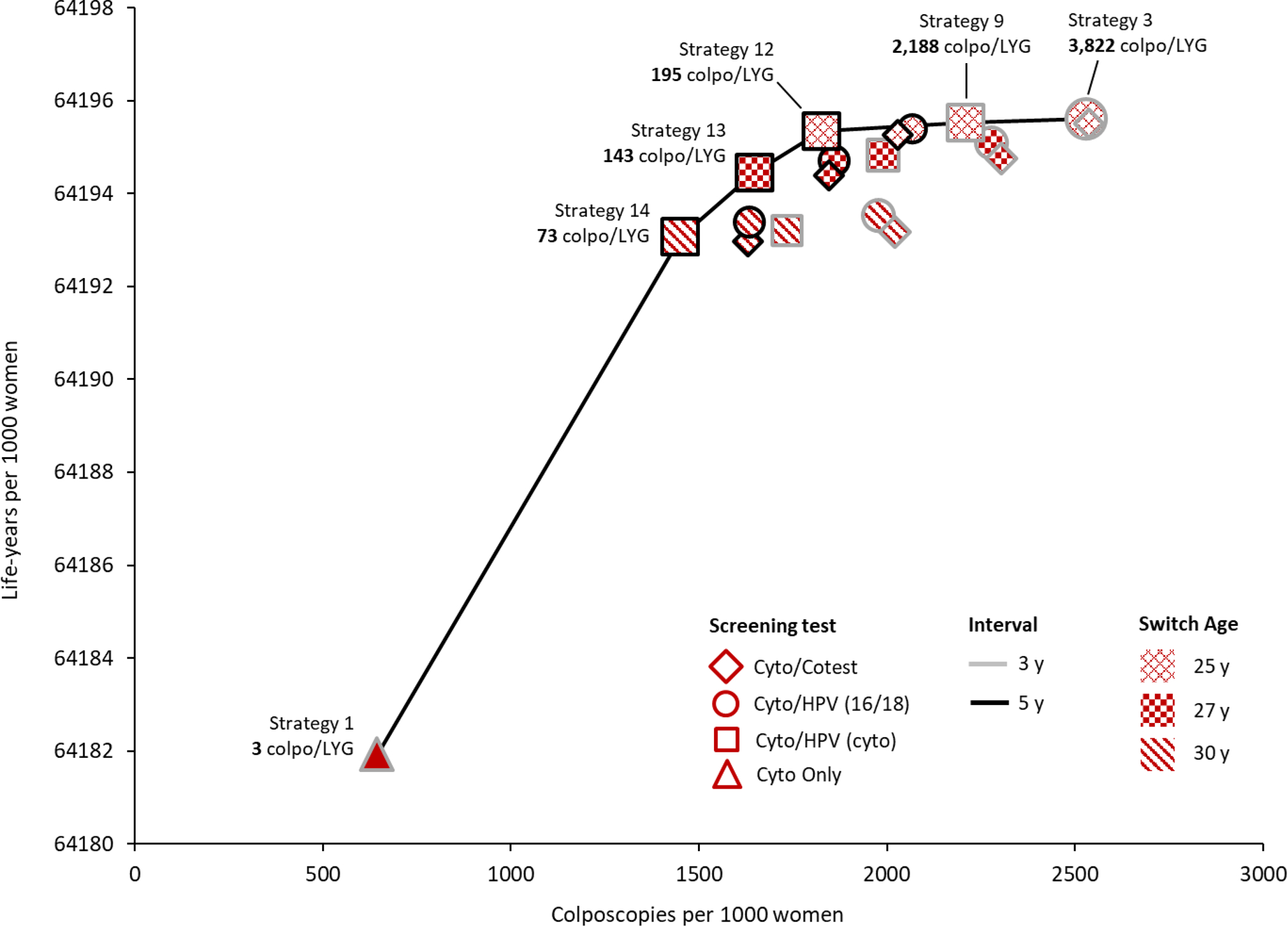
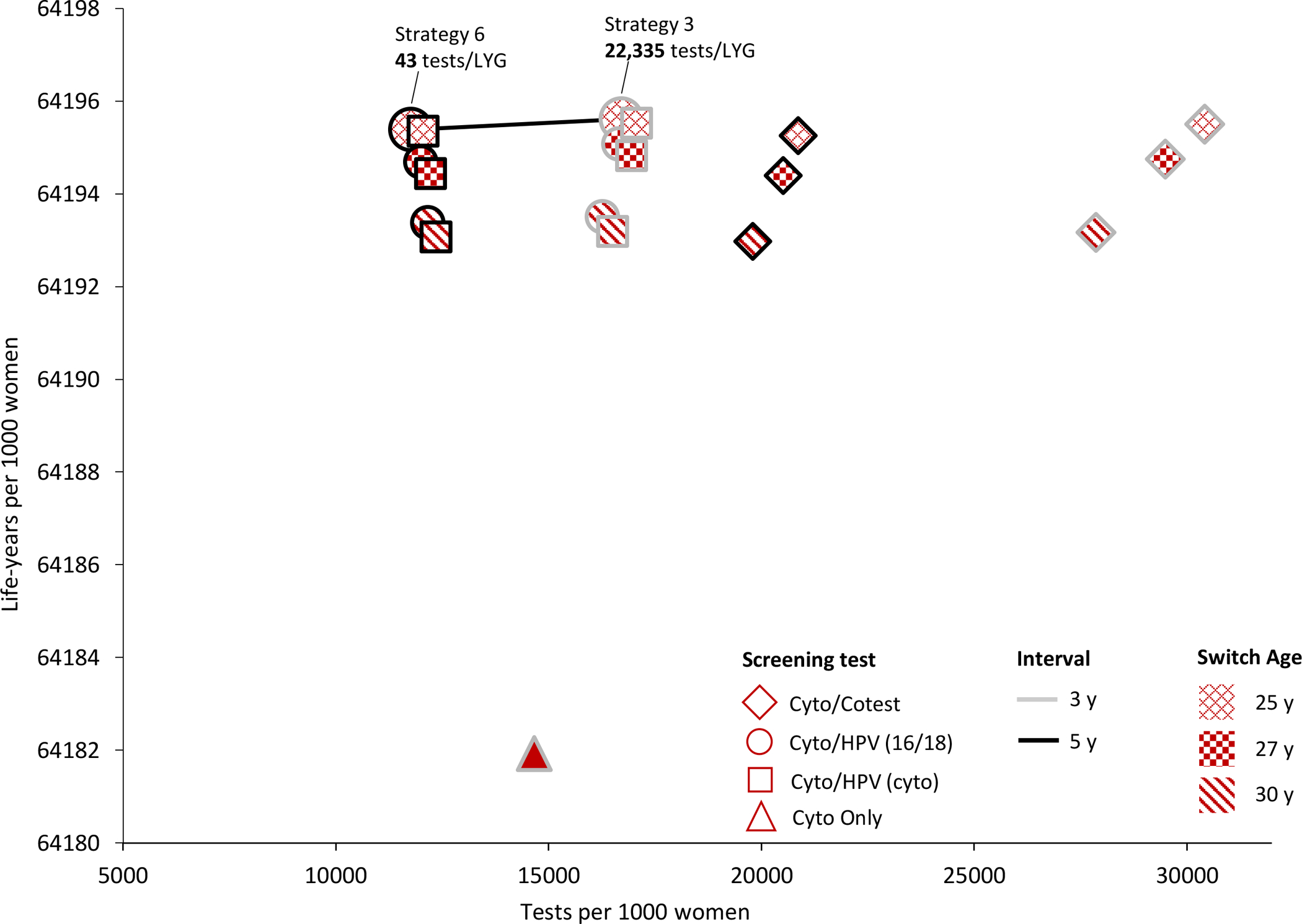
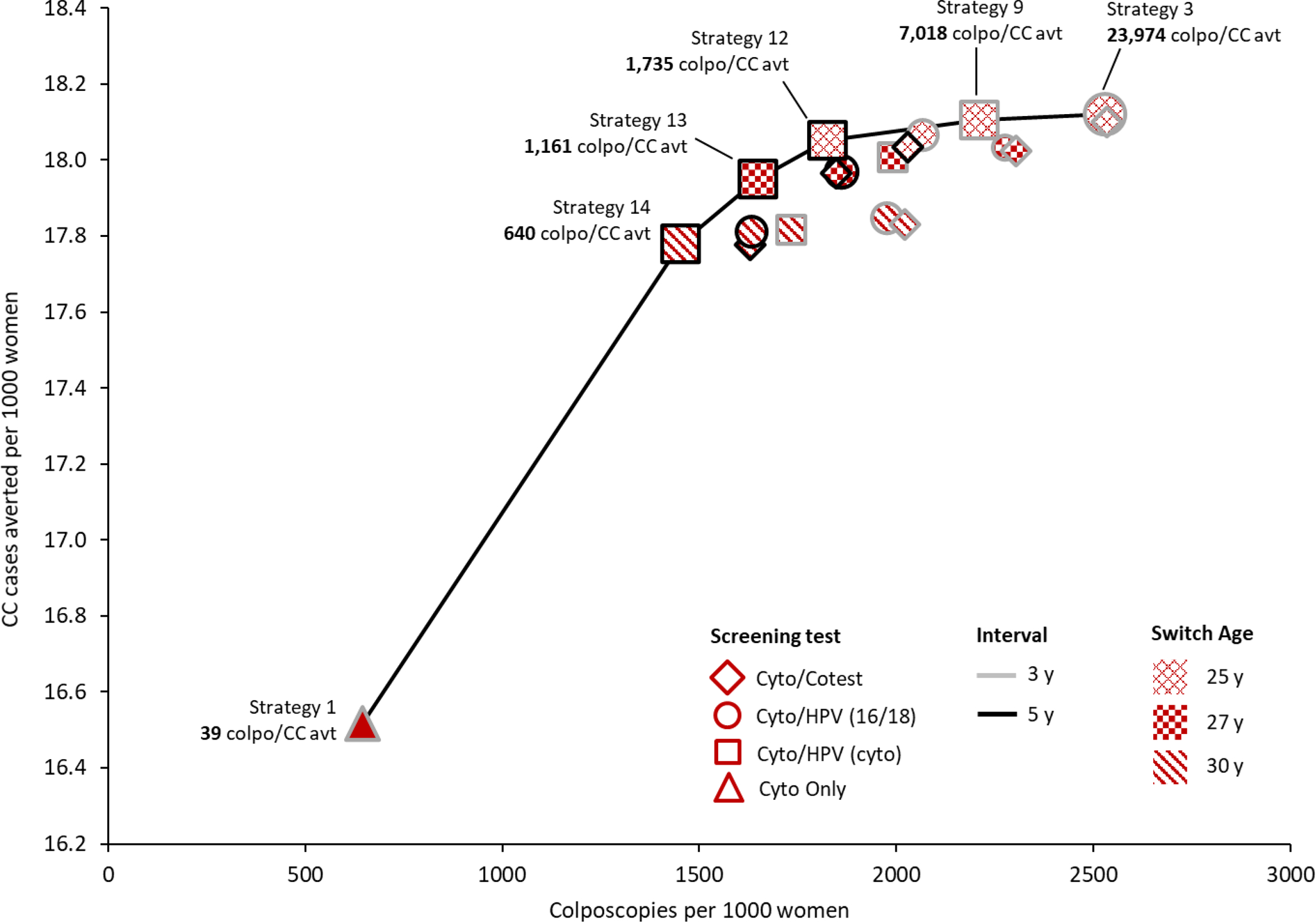
Efficiency Frontiers. These graphs show results from the efficiency analysis in terms of colposcopies per life-year gained (colpo/LYG) (panel A), tests per life-year gained (tests/LYG) (panel B), and colposcopies per cervical cancer case averted (colpos/(CC avt) (panel C) for all 19 cervical cancer screening strategies. Strategies varied in terms of primary screening test (shape: diamond=cytology with a switch to cotesting; circle=cytology with a switch to primary HPV testing with HPV-16/18 genotype testing for triage of HPV-positive women; square=cytology with a switch to primary HPV testing with cytology triage of HPV-positive women; triangle=cytology only); screening interval (shape border: grey=3-year; black=5-year); switch age from cytology to primary HPV testing or cotesting (pattern: x=25 years; checker=27 years; diagonal=30 years). The efficiency ratios were calculated as the additional number of harms (i.e., colposcopies or tests) divided by the additional benefits (i.e., LYG or CC averted) of a specific strategy compared to the strategy with the next fewer harms. Efficient strategies (i.e., that lie on the efficiency frontier) were those that yielded more benefit and less harm than another strategy, or a lower harm-to-benefit ratio than a strategy with less harms; all other strategies (i.e., that do not lie on the efficiency frontier) were considered “inefficient.” All strategies assume cytology alone starting at age 21 years and screening end age of 65 years. Tests include the total number of screening tests over the lifetime of screening, including both routine screening and any surveillance testing but not diagnostic (i.e., colposcopy/biopsy) testing.
Colposcopies per life-year gained
The strategy with the lowest number of colposcopies per LYG was the current guidelines-based strategy of cytology testing alone every 3 years from ages 21 to 65 (strategy 1), with 3 colposcopies per LYG compared to no screening (Figure 2A). By comparison, primary HPV and cotesting strategies were associated with both increased life-years and number of colposcopies. Primary HPV testing with cytology triage every 5 years at a switch age of 30, 27 and 25 years (strategies 14, 13, 12) were associated with 73, 143 and 195 colposcopies per LYG, respectively. Increasing the frequency of screening to 3-year primary HPV testing and switching at age 25 required a much greater number of colposcopies per LYG, ranging from 2,188 (cytology triage, strategy 9) to 3,822 (16/18 genotype triage, strategy 3). All other strategies, including cotesting (strategies 2, 15–19) were not efficient, given the similar gains in life-years but much higher number of colposcopies.
Tests per life-year gained
When the analysis was expressed in terms of tests (i.e., cytology and HPV tests) per LYG, the only efficient strategies were primary HPV testing with 16/18 genotype triage at a switch age of 25 (Figure 2B); the efficiency ratio was 43 tests per LYG for 5-year screening (strategy 6) and increased substantially to 22,335 tests per LYG for 3-year screening (strategy 3). Both cytology only and cotesting strategies (strategies 1–2, 15–19) were either equally or less effective but had higher numbers of tests than primary HPV testing strategies and were therefore not efficient.
Colposcopies per cervical cancer case averted
Efficient strategies were consistent with those identified in the analysis of colposcopies per LYG. Cytology-only screening every 3 years (strategy 1) had the lowest ratio of 39 colposcopies per CC averted (Figure 2C). Switching from cytology to 5-year primary HPV testing at age 30 (strategy 14) was associated with a ratio of 640 colposcopies per CC averted; earlier switch ages required a greater number of colposcopies per CC averted, ranging from 1,161 for switch age 27 (strategy 13) and 1,735 for switch age 25 (strategy 12). HPV testing every 3 years at a switch age of 25, increased the ratio to 7,018 colposcopies per CC averted (cytology triage, strategy 9) and 23,974 colposcopies per CC averted (16/18 genotype triage, strategy 3). As with colposcopies per LYG, cotesting strategies (strategies 2, 15–19) were not efficient given the much higher rate of colposcopies.
Sensitivity Analysis
When the age to end screening was extended to 70 or 75 years, the efficiency results were similar to the base-case analyses (i.e., age to end screening at 65 years) for all three efficiency outcomes (eFigure 3 and eTables 2–4 in the Supplement). The corresponding ratios increased as the end age increased, indicating that when screening is continued to later ages, it becomes less efficient.
Sensitivity analyses assessed the effect of test performance characteristics on the main results (eTables 2–4 in the Supplement). When test sensitivity for cytology was increased to the upper-bound (with a corresponding decrease in specificity), the efficient strategies remained the same for all three efficiency metrics, but the ratios generally increased (i.e., became less efficient) given the increase in downstream colposcopies and tests. In contrast, when specificity for cytology was increased (with a decrease in sensitivity), the effectiveness of all strategies decreased – especially for screening with cytology alone – but given the corresponding decrease in colposcopies, the ratios using this measure decreased (became more efficient) for all strategies.
The lower-bound (worst-case) relative sensitivity of HPV testing was varied, affecting both HPV testing alone and cotesting. Despite a decrease in the effectiveness of the primary HPV testing strategies, these strategies were still associated with greater benefits than the current guidelines-based strategies. Since the decrease in effectiveness was also accompanied by a decrease in colposcopies, the ratios among efficient strategies improved and more strategies involving 3-year screening with HPV testing alone (strategies 3, 9, 10) became efficient, likely due to an offset from the lower sensitivity value.
In analyses that introduced error in the performance of colposcopy/biopsy in classifying a woman’s true histologic status, or assumed the effectiveness of precancer treatment (i.e., LEEP) was decreased to 82%, the base-case results of the efficiency analyses remained stable under both sensitivity analyses, with slight decreases in the ratios due to the relatively greater reductions in harms (i.e., colposcopies and tests) than benefits (i.e., life-years and cases averted).
Alternative follow-up algorithms based on protocols from empirical studies were examined, including, for women who receive cytology triage, a more stringent cutoff of LSIL as the threshold for colposcopy referral (i.e., ASC-US in the base case), as well as varying the time to repeat testing after a normal cytology triage result to 6 months or 24 months (i.e., 12 months in the base case). Each of these sensitivity analyses resulted in similar efficient strategies as in the base-case analysis, and the ratios for the strategies across the different efficiency outcomes changed only marginally.
A third alternative triage option was evaluated in which all HPV-positive women are referred directly to colposcopy. The number of colposcopies and false positives was much greater with only a small increase in effectiveness. For ratios that used colposcopies as a measure of harm, all strategies that referred HPV-positive women directly to colposcopy without further testing were not efficient.
For women assumed to be completely protected from HPV-16/18 infections over the lifetime due to vaccination, the same strategies were identified as efficient as in the base case; however, the harm-to-benefit ratios for these strategies (assumed for unvaccinated women) became less favorable given the considerably lower cervical cancer risk in HPV-vaccinated women.
DISCUSSION
In this analysis, consistent with short-term evidence from clinical studies, the model projected that strategies involving primary HPV testing or cotesting were associated with greater health benefits compared to current guidelines-based cytology testing alone but come at a harm of greater testing, colposcopies, and false positives. In all analyses, across three different efficiency measures, primary HPV testing strategies occurring at 5-year intervals were efficient, with the harm-to-benefit ratios decreasing (i.e., becoming more efficient) as the switch age extended from 25 to 30 years. By comparison, strategies involving 3-year HPV testing generally had much higher ratios. The efficiency of triage options for HPV-positive women depended on which outcome was used as a proxy for harm: cytology triage was more efficient than 16/18 genotype triage for the two efficiency metrics that used colposcopy as the proxy for harm (per LYG and per CC averted); however, 16/18 genotype testing was the preferred triage option when using screening tests as the proxy for harm (per LYG).
Cytology alone every 3 years from ages 21 to 65, had the lowest benefit in terms of life-years gained and cancer cases averted, as well as the lowest number of colposcopies. When colposcopies was used as the measure of harm, cytology testing alone every 3 years was associated with very low (i.e., efficient) ratios; however, when using total tests as the measure of harm, cytology testing was inefficient. Cotesting strategies, including one that is currently recommended in the United States (consisting of cytology testing every 3 years starting at age 21, switching to cotesting every 5 years at age 30; strategy 2), were predominantly inefficient compared to strategies involving HPV testing alone across all analyses.
This analysis, used to inform the USPSTF recommendations for cervical cancer screening, extends the 2012 decision analysis, which primarily evaluated cytology-based strategies.24 The current analysis focused specifically on HPV testing for primary screening and included variations in age to switch from cytology-only screening to HPV testing, the rescreening interval, and triage options for HPV-positive women. For strategies that overlapped in both reports, the results from the current analysis were similar to the findings from the previous analysis.
When extending age to end screening from 65 to 70 or 75 years (assuming no recent abnormal results), the model projected slight increases in each of the ratios due to decreased efficiency of screening in older ages. However, given the uncertainties regarding the natural history of HPV infection and screening effectiveness in older women – which were not extensively explored in the current analysis – the findings of screening end age should be viewed as exploratory and interpreted with caution.
When multiple strategies are identified as efficient, selecting the “optimal” strategy depends on a threshold ratio that would be considered a reasonable balance of harms and benefits. The desired thresholds for each of the three efficiency measures is not clear when using intermediate metrics such as colposcopies or tests as a proxy for harm as it is difficult to directly compare against other (non-cervical cancer) health interventions.
Limitations
This study has several limitations. First, the analysis was based on assumptions of perfect adherence to screening intervals and management of screen-positive women; however, it is well-documented that screening practice is not perfect and quite variable across the United States. How loss-to-follow up might differ across testing modalities, age, and interval is uncertain but could affect the overall effectiveness and relative efficiency of the screening strategies. Second, although a number of unique strategies were analyzed, there may be other strategies that could lead to a more attractive balance of harms and benefits. For example, the rescreening interval was restricted to not extend beyond every 5 years, but extending intervals longer (e.g., 7 or 10 years) may be more efficient without compromising on effectiveness. Third, the analyses did not explore different assumptions regarding the natural history of HPV infection in older women, nor did they examine other strategies or criteria to determine when to stop screening. There is much uncertainty regarding the prevalence and clinical importance of a newly-acquired HPV infection versus re-activation of a previously-acquired infection in older ages, which may affect the optimal age at which to stop screening. Two studies have indicated that the incidence and mortality rates from cervical cancer are underestimated by Surveillance, Epidemiology, and End Results Program (SEER) given high rates of hysterectomies in US women, and suggest that the current recommendation for terminating screening may not be optimal.25,26 The findings from the microsimulation model, which do correct for hysterectomy rates by age in the population, indicate efficiency and greater effectiveness by extending the screening end age to 70 or 75; however, other screening exit criteria and strategies should be further explored in analyses under various assumptions of disease risk and screening effect at older ages.
Fourth, issues regarding HPV-negative cancers and the implications for the relative effectiveness of HPV testing alone versus cytology alone or cotesting were not fully addressed.27 The sensitivity analysis in which HPV relative test sensitivity (compared to cytology) was decreased to a lower bound estimate mimics a scenario of greater missed disease due to HPV negativity; this scenario was the only one in which strategies involving 3-yearly HPV screening became more efficient with ratios comparable to 5-yearly HPV screening in the base-case analysis. In assessing screening in a low-risk population, only one very specific subset of low-risk women was represented, those who receive protection against HPV-16/18 infection and disease from vaccination. While there are other low-risk segments of the population, this question will become increasingly more pertinent as vaccinated women enter screening age. Fifth, the results from the model represent average outcomes across the whole population and are intended to inform guidelines at the population level, not at an individual level.
CONCLUSIONS
In this microsimulation modeling study, it was estimated that primary HPV screening may represent a reasonable balance of harms and benefits when done every 5 years. Switching from cytology to HPV testing at age 30 yielded the most efficient harm-to-benefit ratio when using colposcopy as a proxy for harms.
Supplementary Material
ACKNOWLEDGEMENTS
Funding/Support:
This research was funded under contract number HHSA-290-2012-00015-I Task Order 6 from the Agency for Healthcare Research and Quality (AHRQ), U.S. Department of Health and Human Services. JJK, EAB, CR, and SS was also supported in part by a National Cancer Institute grant (U01CA199334).
Role of Sponsor:
Investigators worked with USPSTF members and AHRQ staff to develop the scope and key questions for this analysis. AHRQ staff provided project oversight; reviewed the report to ensure that the analysis met methodological standards, and distributed the draft for peer review. Otherwise, AHRQ had no role in the conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript findings. The opinions expressed in this document are those of the authors and do not reflect the official position of AHRQ or the U.S. Department of Health and Human Services.
Additional Contributions:
The authors gratefully acknowledge the following individuals for their contributions to this project: the AHRQ staff, the EPC team, and the U.S. Preventive Services Task Force members for comments on earlier versions of this research; Shalini Kulasingam, PhD, MPH, Emily A. Groene, MA, and Aanjaneya Shukla, MS (from the University of Minnesota), and the National Cancer Institute-funded Cancer Intervention and Surveillance Modeling Network (CISNET) cervical cancer working group for intellectual support and feedback throughout the project. Dr Kulasingam, Ms Groene, and Mr Shukla received salary support for their contributions.
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Additional Information: A draft version of this evidence report underwent external peer review from 3 content experts (Diana Petitti, MD, MPH, University of Arizona College of Medicine; Gillian Sanders Schmidler, PhD, Duke University; Alan Waxman, MD, MPH, University of New Mexico). Comments were presented to the USPSTF during its deliberation of the evidence and were considered in preparing the final report.
Editorial Disclaimer: This modeling study is presented as a document in support of the accompanying USPSTF Recommendation Statement. It did not undergo additional peer review after submission to JAMA.
REFERENCES
- 1.Moyer VA. Screening for Cervical Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2012;156:880–891. [DOI] [PubMed] [Google Scholar]
- 2.Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ACOG Practice Bulletin Number 131: Screening for cervical cancer. Obstet Gynecol. 2012;120(5):1222–1238. [DOI] [PubMed] [Google Scholar]
- 4.Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: Interim clinical guidance. Gynecol Oncol. 2015; 136(2):178–82. [DOI] [PubMed] [Google Scholar]
- 5.Melnikow J, Henderson JT, Burda BU, et al. Screening for Cervical Cancer With High-Risk Human Papillomavirus Testing: A Systematic Evidence Review for the U.S. Preventive Services Task Force. Evidence Synthesis No. 158. AHRQ Publication No. 17–05231-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; 2017 [PubMed] [Google Scholar]
- 6.Campos NG, Burger EA, Sy S, et al. An updated natural history model of cervical cancer: derivation of model parameters. Am J Epidemiol. 2014;180(5):545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim JJ, Campos NG, Sy S, et al. Inefficiencies and High-Value Improvements in U.S. Cervical Cancer Screening Practice: A Cost-Effectiveness Analysis. Ann Intern Med. 2015;163(8):589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Center for Health Statistics. 2010 National Hospital Discharge Survey (NHDS) public use micro-data file and documentation. Hyattsville, MD. 2012. Available at https://www.cdc.gov/nchs/nhds/nhds_questionnaires.htm. (Last accessed January 31, 2017). [Google Scholar]
- 9.University of California Berkeley. Berkeley Mortality Database. Available at http://demog.berkeley.edu/~bmd. (Data downloaded on August 2, 2016).
- 10.National Cancer Institute. Surveillance, Epidemiology, End Results (SEER) Cancer Statistics Review, 1975–2013. https://seer.cancer.gov/csr/1975_2013/. Accessed January 31, 2017. http://seer.cancer.gov/csr/1975_2011/.
- 11.Wheeler CM, Hunt WC, Cuzick J, et al. A population-based study of human papillomavirus genotype prevalence in the United States: baseline measures prior to mass human papillomavirus vaccination. Int J Cancer. 2013;132(1):198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joste NE, Ronnett BM, Hunt WC, et al. Human papillomavirus genotype-specific prevalence across the continuum of cervical neoplasia and cancer. Cancer Epidemiol Biomarkers Prev. 2015;24(1):230–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saraiya M, Unger ER, Thompson TD, et al. US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines. J Natl Cancer Inst. 2015;107(6):djv086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koliopoulos G, Arbyn M, Martin-Hirsch P, Kyrgiou M, Prendiville W, Paraskevaidis E. Diagnostic accuracy of human papillomavirus testing in primary cervical screening: a systematic review and meta-analysis of non-randomized studies. Gynecol Oncol. 2007;104(1):232–246. [DOI] [PubMed] [Google Scholar]
- 15.Cox JT, Castle PE, Behrens CM, Sharma A, Wright TC Jr., Cuzick J. Comparison of cervical cancer screening strategies incorporating different combinations of cytology, HPV testing, and genotyping for HPV 16/18: results from the ATHENA HPV study. Am J Obstet Gynecol. 2013;208(3):184 e181–184 e111. [DOI] [PubMed] [Google Scholar]
- 16.Arbyn M, Ronco G, Anttila A, et al. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine. 2012;30 Suppl 5:F88–99. [DOI] [PubMed] [Google Scholar]
- 17.Ronco G, Giorgi-Rossi P, Carozzi F, et al. Human papillomavirus testing and liquid-based cytology in primary screening of women younger than 35 years: results at recruitment for a randomised controlled trial. Lancet Oncol. 2006;7(7):547–555. [DOI] [PubMed] [Google Scholar]
- 18.Ronco G, Segnan N, Giorgi-Rossi P, et al. Human papillomavirus testing and liquid-based cytology: results at recruitment from the new technologies for cervical cancer randomized controlled trial. J Natl Cancer Inst. 2006;98(11):765–774. [DOI] [PubMed] [Google Scholar]
- 19.Cuzick J, Myers O, Hunt WC, et al. A population-based evaluation of cervical screening in the United States: 2008–2011. Cancer Epidemiol Biomarkers Prev. 2014;23(5):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cuzick J, Myers O, Hunt WC, et al. Human papillomavirus testing 2007–2012: Co-testing and triage utilization and impact on subsequent clinical management. Int J Cancer. 2015;136(12):2854–2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinney W, Hunt WC, Dinkelspiel H, et al. Cervical excisional treatment of young women: a population-based study. Gynecol Oncol. 2014;132(3):628–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogilvie GS, Krajden M, van Niekerk D, et al. HPV for cervical cancer screening (HPV FOCAL): Complete Round 1 results of a randomized trial comparing HPV-based primary screening to liquid-based cytology for cervical cancer. Int J Cancer. 2017;140(2):440–448. [DOI] [PubMed] [Google Scholar]
- 23.Massad LS, Einstein MH, Huh WK, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17(5 Suppl 1):S1–S27. [DOI] [PubMed] [Google Scholar]
- 24.Kulasingam SL, Havrilesky LJ, Ghebre R, Myers ER. Screening for cervical cancer: a modeling study for the US Preventive Services Task Force. J Low Genit Tract Dis. 2013;17(2):193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer. 2017;123(6):1044–1050. [DOI] [PubMed] [Google Scholar]
- 26.Rositch AF, Nowak RG, Gravitt PE. Increased age and race-specific incidence of cervical cancer after correction for hysterectomy prevalence in the United States from 2000 to 2009. Cancer. 2014;120(13):2032–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blatt AJ, Kennedy R, Luff RD, Austin RM, Rabin DS. Comparison of cervical cancer screening results among 256,648 women in multiple clinical practices. Cancer Cytopathol. 2015;123(5):282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


