Abstract
Homo sapiens, as well as other primates, developed the evolutionary advantage of storing excess energy as body fat, primarily in the readily accessible visceral fat compartment when food is plentiful for use during scarcity. However, uniquely to female humans, a second transient dimorphic phenotypic change begins at menarche and is reversed by menopause. It is the diversion of visceral fat stores from the abdominal cavity to the gluteofemoral region. The evolutionary purpose for this remains unclear. The author proposes the gestational potential space hypothesis: that such fat diversion is for the reproductive purpose of increasing the potential abdominal space available for gestation and reducing the intra-abdominal pressure. This hypothesis is supported by the basic laws of physics and increased rates of maternal and fetal complications experienced by those with visceral adiposity.
Keywords: reproduction and hormones, obesity, nutrition, and metabolism, developmental origins of health and disease
Lay Summary
The author proposes that fat shifting from the abdominal cavity to the hips and thighs in women, during the childbearing period, is for the evolutionary benefit of reducing the intra-abdominal contents consequently increasing pregnancy potential space. Secondarily, it prevents intra-abdominal pressure elevation and reduces maternal and fetal complications associated with visceral fat in pregnancy.
INTRODUCTION
For the vast majority of their existence, Homo sapiens have been in the constant pursuit of scarce energy through food. Accordingly, our physiology and metabolism slowly adapted to favor energy conservation [1]. At some point during our evolution, humans and other primates developed the ability to store excess, ingested energy inside the body. The ingested energy surplus is stored as body fat and serves an evolutionary survival benefit, when food is plentiful it can be saved for future retrieval during times of food scarcity. The result has been labeled ‘energy capital’ [2]. As in all primates, human males preferentially store most of their excess energy in the central or visceral fat compartment primarily inside the abdominal cavity. However, unlike other primates, female humans exhibit a transient phase of dimorphism which occurs during the childbearing period. Beginning at menarche, the pelvis widens, and fat stores are mobilized from the abdominal cavity and central visceral compartment to the gluteofemoral region of the buttocks, hips, and thighs. This results in the characteristic gynecoid phenotype with a lower waist/hip ratio when compared to males [3].
Before puberty and after menopause, male and female pelvises demonstrate moderate sexual dimorphism with similar growth trajectories. However, by the time of puberty, the female pelvis exhibits rapid and significant widening [4]. This process has been plausibly hypothesized as an evolutionary reproductive strategy for the facilitation of the delivery of the fetus [5]. Mobilizing body fat from the visceral compartment to the gluteofemoral compartment is the second morphological change that takes place, in the body of women, during the childbearing period. The evolutionary purpose behind such reproductive strategy remains unknown.
Body fat stores can be divided into two main compartments. Peripheral or subcutaneous fat (located under the skin) and central or visceral fat (located inside and between the internal organs) [6]. Visceral fat occurs in the abdomen and also extends into the thoracic cavity [6]. Intra-abdominal fat is composed of retroperitoneal, intraperitoneal (mesocolon, lesser omentum, greater omentum) and mesenteric fat. Fat is also deposited inside and around the internal organs; termed ectopic fat, it can further occupy the valuable potential space inside the intra-abdominal cavity available for pregnancy. Additionally, intrathoracic visceral fat includes epicardial and intramyocardial fat and can impede the mechanical effect of cranial displacement of abdominal contents by the growing gravid uterus [6]. Furthermore, visceral fat is metabolically distinct from subcutaneous fat in being readily mobilizable and can act as a quickly accessible energy supply [7]. Accordingly, visceral fat became the primary energy depot for humans, and other primates, except in the transient interval during the childbearing years of women. During this time, fat is diverted from central fat depots to the gluteofemoral region. Such fat diversion appears at first glance to be an evolutionary energy disadvantage because the rate of lipolysis is slower in the gluteofemoral region, rendering it a markedly inefficient energy storage location [7]. Such a phenomenon could be described as an energy tradeoff to obtain a larger potential space for gestation.
Although the visceral fat compartment provides easy access to energy, it occupies prime real estate inside the gestational potential space (GPS) of the abdominal cavity of women during their childbearing years of life. As pregnancy progresses, the gravid uterus expands from the pelvis into the abdomen specifically inside the peritoneal cavity changing the geometry of the abdominal cavity which in turn pushes the diaphragm cranially pressuring the thoracic cavity [8]. The peritoneal cavity is a true potential space that is defined as one that is created without disrupting the normal structural or functional integrity of the tissues involved in its creation [9]. The potential space could be repeatedly created and obliterated without resulting in tissue damage or requiring tissue repair [9]. Moreover, the abdomen must expand in all directions to accommodate the growing gravid uterus, in addition to the anatomical reorganization of intra-abdominal organs. The expansion of the back is limited by the spine, while the diaphragm is pushed upwards, the abdominal wall stretches anteriorly and at the flanks. Therefore, higher subcutaneous fat of the abdomen could also impede abdominal wall compliance.
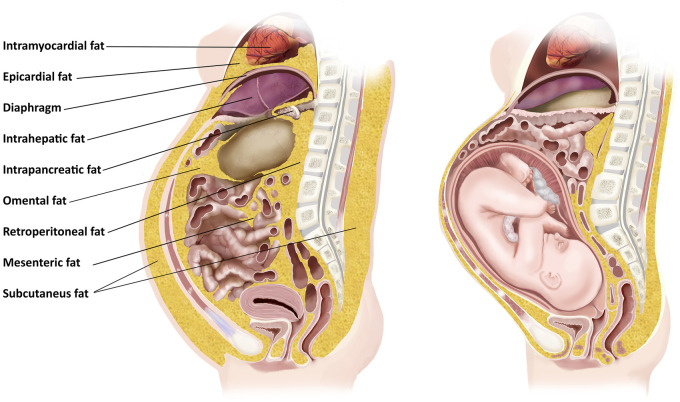
Accordingly, fat inside and outside the abdominal cavity has a significant potential for increasing the resistance against the growing and expanding gravid uterus. The location of fat that could impede the expansion of the gravid uterus includes three main categories: (i) subcutaneous fat of the abdomen increasing the resistance against the abdominal wall expansion (reduced abdominal wall compliance); (ii) intrathoracic fat, such as epicardial and intramyocardial, which could increase the resistance against the diaphragm especially during the last trimester; and (iii) intra-abdominal fat, which is further subdivided into (a) retroperitoneal; (b) intra and peri-organ fat (liver, spleen, pancreas and kidneys); and (c) intraperitoneal (lesser omental, greater omental and mesocolonic fat) (Fig. 1).
Figure 1.
Illustration of a sagittal section of the human female trunk depicting the GPS filled with a gravid uterus on the right and centrally deposited fat on the left.
Abdominal, pelvic and thoracic cavities are considered one cavity that is termed the ventral cavity. The diaphragm separates the thoracic cavity from the abdomino-pelvic cavity. The diaphragm is mobile and transmits pressure elevations. Therefore, the intrathoracic and intra-abdominal pressures (IAPs) are complementary [10]. Consequently the diversion of fat from the ventral cavity to the lower body reduces the intracavity area in both cavities. Given that pressure is defined as the amount of force exerted per area, P = F/A (pressure = unit of force/unit of area), the larger the area available (in this instance during pregnancy) the lower the pressure along with abdominal subcutaneous fat diversion leading to increased abdominal wall expandability and further reducing the IAP.
Intra-abdominal pressure during pregnancy has been an area of research interest, especially due to the increased prevalence of obesity, particularly visceral obesity [10]. IAP is analyzed by measuring the intravesical pressure of the urinary bladder through a Foley’s catheter [11]. IAP measurement through the rectal approach is also reported as an alternative method yet its reliability has not been determined [11]. There is a need to develop a direct and accurate method of measuring IAP since both methods used are performed in the pelvis.
The average normal IAP is measured at 5–7 mmHg. Intra-abdominal hypertension is defined as a sustained or repeated IAP ≥ 12 mmHg. However, in a state of morbid obesity with a large volume of intra-abdominal fat, IAP increases to 9–14 mmHg [10]. In a normal pregnancy, there is a physiologic increase to the average IAP measured at 22 mmHg in the third trimester, followed by a significant drop postpartum [11]. Interestingly, quadrupedal animals appear to use gravity to reduce IAP by pushing the gravid uterus down toward the abdominal wall. However, in bipedals, achieving proper erect posture imposes pressure anteriorly by the abdominal muscles and posteriorly by the lordotic spine [8]. Additionally, H. sapiens have a smaller abdominal cavity compared to non-human primates. For example, in gorillas, the pelvis represents about 3% of the whole abdominal cavity, while in humans it is 30% [8]. Therefore, the diversion of fat from the visceral compartment to the gluteofemoral region has potentially enabled the uterine distensibility, unrestricted growth of a fetus, and prevented maternal and fetal complications of intra-abdominal hypertension. To better understand the potential evolutionary advantage of this fat diversion, the author developed the GPS hypothesis.
ALTERNATIVE HYPOTHESES
Before presenting our hypothesis, it is prudent to explore some previously presented and common hypotheses forwarded in this field of inquiry. Gluteofemoral fat during pregnancy was hypothesized to evolve serving mechanical functions in several ways: (i) maintain bipedal balance and center of gravity and (ii) act as a counterbalance between the anterior gravid uterus and the posterior weight of the buttocks [12, 13]. A second hypothesis proposed that fat storage within the buttocks evolved as a signal to males that the female had a high amount of energy stored and that this made them competitive choice for a mate, as well as being able to invest fully into the raising of their offspring [14]. Furthermore, the gluteofemoral fat has been hypothesized to have a positive effect on the neurodevelopmental growth of the fetus and the delivery of healthy offspring [15]. The etiology of this advantage lies in its availability of long-chain polyunsaturated fatty acids, particularly omega-3 docosahexaenoic acid, that are critical for fetal neurodevelopment [15]. Another hypothesis has renamed lower body fat as reproductive fat based on the timing of the diversion of fat to the lower part of the body (initiated by menarche and reversed by menopause). This suggests a functional link to pregnancy and reproduction [16].
THE GPS HYPOTHESIS
I hypothesize that, in order to increase the potential space available for gestation and consequently reducing the IAP, evolution opted to divert fat mass deposition out of the abdominal cavity and shift abdominal subcutaneous adipose tissue to the gluteofemoral region. Furthermore, following menopause there is no potential need for the gestational space, thus evolution reinstates the preferential intra-abdominal and central fat storage. Simultaneously, the rate of lipolysis in gluteofemoral fat is significantly lower than that of visceral fat, rendering it an inefficient energy storage location. However, this energy disadvantage could serve as a reproductive tradeoff. Finally, women with less visceral fat during the childbearing period have decreased risk of cardiometabolic diseases. Further research is urgently needed to adequately measure and standardize normal visceral fat and IAP measurements among pregnant women with diverse body fat distributions in relation to the associated maternal and fetal health outcomes.
SUPPORTING EVIDENCE
The GPS hypothesis is strongly supported by two foundational pillars: (i) the laws of physics of the abdominal cavity and (ii) maternal fetal adverse outcomes associated with visceral adiposity in pregnancy. The advantage of the GPS hypothesis is that gluteofemoral fat has consistently been shown to be protective against a host of diverse conditions including cardiovascular disease, type-2 diabetes, as well as other diseases associated with visceral adiposity [17]. Furthermore, the proportion of abdominal to gluteofemoral fat, and the amount of abdominal fat present, also appear to be associated with obesity-related morbidity and mortality [18]. During the menopausal transition, women lose their reproductive ability that is accompanied by a narrowing of the pelvis. Fat diversion is also stopped and returns to the default state of intra-abdominal preference [19]. At this point, readily utilizable abdominal fat gains utility; however, in our culture of plenty, this becomes a disadvantage leading to a sharp rise in cardiometabolic diseases following menopause. [20].
Further evidence of the GPS hypothesis is obtained from the established consequences of the failure of such described evolutionary fat diversion out of the abdomen and its impact on pregnancy outcome and reproduction. It is crucial to emphasize the term “non-diseased” human state; in some diseases, such fat diversion is disabled or reversed, such as polycystic ovarian syndrome (PCOS), congenital lipodystrophy and Cushing syndrome, which are characterized by fat distribution to the visceral compartment instead of the gluteofemoral region resulting in a high risk of infertility [21]. The presence and amount of maternal visceral adipose tissue during the first half of pregnancy has been found to be predictive of gestational [22, 23] and neonatal complications [24]. It is important to point out that infertile women of normal weight, with PCOS and primary amenorrhea, exhibited a high amount of fat tissue, as well as a tendency toward visceral fat distribution [25, 26]. Furthermore, weight loss before pregnancy results in a better chance of conception and increases the percentage of live births for obese women with or without PCOS [21].
Visceral adiposity during pregnancy is an established risk factor for pre-eclampsia (pregnancy-induced hypertension) which has also been hypothesized as a state of pregnancy-induced intra-abdominal hypertension since the only definitive treatment of pre-eclampsia is decompression of the cavity through giving birth [27, 28]. The bigger the size of visceral fat, the shorter the duration and the higher the risk of preterm pregnancy. Higher amounts of visceral fat in pregnancy is a well-established risk factor for pre-eclampsia, including early-onset pre-eclampsia, which requires preterm delivery [29].
Intra-abdominal visceral fat increases the IAP which may be responsible, fully or in part, for the high risk of complications in pregnancy with visceral adiposity [30]. Conditions with increased intra-abdominal mass, such as visceral obesity and twin pregnancy, increase the risk of pregnancy complications [23, 24, 31]. Multiple gestation also imposes higher IAP, shorter pregnancy duration, higher maternal and fetal morbidity and mortality [31]. Moreover, the pregnancy complications timeline is parallel to the size of the gravid uterus, with the most severe at the end of the third trimester. It was also hypothesized that the rigid abdominal wall in muscular and primigravida women were prone to elevated IAPs and compromised abdominal perfusion pressure to the abdominopelvic viscera [32].
TESTING THE HYPOTHESIS
To verify the GPS hypothesis, the author suggests the future undertaking of a series of quantitative studies with a particular focus on the following four parameters. First, quantitative analysis of IAP in women during prepubertal, postmenopausal and childbearing periods to enable the precise definition of IAP. Second, consideration of the volumetric assessment of visceral and gluteofemoral fat via safe and accurate modalities such as MRI or ultrasonography is another important variable that requires adequate standardization during the various hormonal stages across the lifecourse. Third, both fat volume and IAP values could be tested for correlations and associations with android and/or gynecoid phenotypes. Finally, the aforementioned variables require further investigations to explore any potential associations with maternal and fetal health outcomes. Randomized controlled trials, comparing the same outcomes in similar populations, following certain interventions that are known to alter the body fat distribution, such as hormonal therapy and weight gain/loss before and during pregnancy. Determining a causal association will require further clinical investigation and research.
To date, normal values of IAP among pregnant women with visceral adiposity have not been adequately defined [33]. Furthermore, the medical implications of elevated IAP within this population are somewhat poorly understood. Further research on the health impact of visceral adiposity, and its association with IAP in pregnancy, is urgently needed [33].
If evidence was found to support the GPS hypothesis, it could not only reshape our understanding of the evolutionary purpose of body fat redistribution during the different hormonal stages that occur within the body and body shapes but could potentially predict gestational outcomes and enable the development of preventive and therapeutic interventions.
CONCLUDING REMARKS
In the non-diseased state, and during the years of reproduction, fat storage in the body of women transiently diverts from the abdominal cavity to the gluteofemoral region. This event reverts after menopause. It is proposed here that this function is to maximize the abdominal space available for gestation and consequently reduce the IAP. Further research, to better understand the role of the GPS in pregnancy and health outcomes, is much needed.
ACKNOWLEDGEMENTS
I thank Vanessa Gordon-Dseagu and Janna AbdelAziz for their excellent technical assistance and critical reading of the manuscript. I extend my gratitude to Nicolas Fernandez for creating such a detailed illustration.
FUNDING
None declared.
Conflict of interest: None declared.
REFERENCES
- 1.Neel JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am J Hum Genet 1962; 14:353–62. [PMC free article] [PubMed] [Google Scholar]
- 2.Wells JCK. The capital economy in hominin evolution: how adipose tissue and social relationships confer phenotypic flexibility and resilience in stochastic environments. Current Anthropology 2012; 53:S466–78. [Google Scholar]
- 3.Lassek WD, Gaulin SJC.. Menarche is related to fat distribution. Am J Phys Anthropol 2007; 133:1147–51. [DOI] [PubMed] [Google Scholar]
- 4.Huseynov A, Zollikofer CPE, Coudyzer W. et al. Developmental evidence for obstetric adaptation of the human female pelvis. Proc Natl Acad Sci USA 2016; 113:5227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pavličev M, Romero R, Mitteroecker P.. Evolution of the human pelvis and obstructed labor: new explanations of an old obstetrical dilemma. Am J Obstet Gynecol 2020; 222:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nauli AM, Matin S.. Why do men accumulate abdominal visceral fat? Front Physiol 2019; 10:1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arner P. Differences in lipolysis between human subcutaneous and omental adipose tissues. Ann Med 1995; 27:435–8. [DOI] [PubMed] [Google Scholar]
- 8.Abitbol MM. Growth of the fetus in the abdominal cavity. Am J Phys Anthropol 1993; 91:367–78. [DOI] [PubMed] [Google Scholar]
- 9.Haines DE. On the question of a subdural space. Anat Rec 1991; 230:3–21. [DOI] [PubMed] [Google Scholar]
- 10.De Keulenaer BL, De Waele JJ, Powell B. et al. What is normal intra-abdominal pressure and how is it affected by positioning, body mass and positive end-expiratory pressure? Intensive Care Med 2009; 35:969–76. [DOI] [PubMed] [Google Scholar]
- 11.Malbrain MLNG, De Laet IE, De Waele JJ. et al. Intra-abdominal hypertension: definitions, monitoring, interpretation and management. Best Pract Res Clin Anaesthesiol 2013; 27:249–70. [DOI] [PubMed] [Google Scholar]
- 12.Pawłowski B. The evolution of gluteal/femoral fat deposits and balance during pregnancy in bipedal Homo. Curr Anthropol 2001; 42:572–4. [Google Scholar]
- 13.Pawłowski B, Grabarczyk M.. Center of body mass and the evolution of female body shape. Am J Hum Biol 2003; 15:144–50. [DOI] [PubMed] [Google Scholar]
- 14.Cant JG. Hypothesis for the evolution of human breasts and buttocks. Am Nat 1981; 117:199–204. [Google Scholar]
- 15.Lassek W, Gaulin S.. Waist-hip ratio and cognitive ability: is gluteofemoral fat a privileged store of neurodevelopmental resources? Evol Hum Behav 2008; 29:26–34. [Google Scholar]
- 16.Lassek WD, Gaulin SJC.. Reproductive fat. In: Bolin A, Whelehan P (eds.). The International Encyclopedia of Human Sexuality. Oxford, UK: Wiley, 2015, 1059–114. [Google Scholar]
- 17.Karpe F, Pinnick KE.. Biology of upper-body and lower-body adipose tissue—link to whole-body phenotypes. Nat Rev Endocrinol 2015; 11:90–100. [DOI] [PubMed] [Google Scholar]
- 18.Manolopoulos KN, Karpe F, Frayn KN.. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes 2010; 34:949–59. [DOI] [PubMed] [Google Scholar]
- 19.Kirschner MA, Samojlik E, Drejka M. et al. Androgen-estrogen metabolism in women with upper body versus lower body obesity. J Clin Endocrinol Metab 1990; 70:473–9. [DOI] [PubMed] [Google Scholar]
- 20.Pu D, Tan R, Yu Q. et al. Metabolic syndrome in menopause and associated factors: a meta-analysis. Climacteric 2017; 20:583–91. [DOI] [PubMed] [Google Scholar]
- 21.Birdsall KM, vyas S, Khazaezadeh N. et al. Maternal obesity: a review of interventions. Int J Clin Prac 2009; 63:494–507. [DOI] [PubMed] [Google Scholar]
- 22.Rocha A da S, Bernardi JR, Matos S. et al. Maternal visceral adipose tissue during the first half of pregnancy predicts gestational diabetes at the time of delivery – a cohort study. PLoS One 2020; 15:e0232155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balani J, Hyer S, Shehata H. et al. Visceral fat mass as a novel risk factor for predicting gestational diabetes in obese pregnant women. Obstet Med 2018; 11:121–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindberger E, Sundström Poromaa I, Ahlsson F.. Impact of maternal central adiposity on infant anthropometry and perinatal morbidity: a systematic review. Eur J Obstet Gynecol Reprod Biol X 2020; 8:100117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirchengast S, Huber J.. Body composition characteristics and fat distribution patterns in young infertile women. Fertil Steril 2004; 81:539–44. [DOI] [PubMed] [Google Scholar]
- 26.Palomba S, de Wilde MA, Falbo A. et al. Pregnancy complications in women with polycystic ovary syndrome. Hum Reprod Update 2015; 21:575–92. [DOI] [PubMed] [Google Scholar]
- 27.Sugerman HJ. Hypothesis: preeclampsia is a venous disease secondary to an increased intra-abdominal pressure. Med Hypotheses 2011; 77:841–9. [DOI] [PubMed] [Google Scholar]
- 28.Sawchuck DJ, Wittmann BK.. Pre-eclampsia renamed and reframed: intra-abdominal hypertension in pregnancy. Med Hypotheses 2014; 83:619–32. [DOI] [PubMed] [Google Scholar]
- 29.Ray JG, De Souza LR, Park AL. et al. Preeclampsia and preterm birth associated with visceral adiposity in early pregnancy. J Obstet Gynaecol Can 2017; 39:78–81. [DOI] [PubMed] [Google Scholar]
- 30.Sugerman HJ. 2 Pathophysiology of obesity comorbidity: the effects of chronically increased intra-abdominal pressure. In: Brethauer SA, Schauer PR, Schirmer BD (eds.). Minimally Invasive Bariatric Surgery. New York: Springer New York, 2015, 7–13. [Google Scholar]
- 31.Conde-Agudelo A, Belizán JM, Lindmark G.. Maternal morbidity and mortality associated with multiple gestations. Obstet Gynecol 2000; 95:899–904. [PubMed] [Google Scholar]
- 32.Paramore RH. The intra-abdominal pressure in pregnancy. Proc R Soc Med 1913; 6:291–334. [PMC free article] [PubMed] [Google Scholar]
- 33.Kirkpatrick AW, Roberts DJ, De Waele J. et al. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med 2013; 39:1190–206. [DOI] [PMC free article] [PubMed] [Google Scholar]