Abstract
Background
A household approach to decolonization decreases skin and soft tissue infection (SSTI) incidence, though this is burdensome and costly. As prior SSTI increases risk for SSTI, we hypothesized that the effectiveness of decolonization measures to prevent SSTI when targeted to household members with prior year SSTI would be noninferior to decolonizing all household members.
Methods
Upon completion of our 12-month observational Household Observation of Methicillin-resistant Staphylococcus aureus in the Environment (HOME) study, 102 households were enrolled in HOME2, a 12-month, randomized noninferiority trial. Pediatric index patients with community-associated methicillin-resistant Staphylococcus aureus (MRSA) SSTI, their household contacts, and pets were enrolled. Households were randomized 1:1 to the personalized (decolonization performed only by household members who experienced SSTI during the HOME study) or household (decolonization performed by all household members) approaches. The 5-day regimen included hygiene education, twice-daily intranasal mupirocin, and daily bleach-water baths. At 5 follow-up visits in participants’ homes, swabs to detect S. aureus were collected from participants, environmental surfaces, and pets; incident SSTIs were ascertained.
Results
Noninferiority of the personalized approach was established for the primary outcome 3-month cumulative SSTI: 23 of 212 (10.8%) participants reported SSTI in household approach households, while 23 of 236 (9.7%) participants reported SSTI in personalized approach households (difference in proportions, −1.1% [95% confidence interval, −6.7% to 4.5%]). In multivariable analyses, prior year SSTI and baseline MRSA colonization were associated with cumulative SSTI.
Conclusions
The personalized approach was noninferior to the household approach in preventing SSTI. Future studies should interrogate longer durations of decolonization and/or decontamination of the household environment to reduce household MRSA burden.
Clinical Trials Registration
Keywords: methicillin-resistant Staphylococcus aureus, skin and soft tissue infection, decolonization, mupirocin, bleach
In households affected by methicillin-resistant Staphylococcus aureus, a decolonization regimen targeted to household members with a history of skin and soft tissue infection (SSTI) in the prior year was noninferior in preventing SSTI vs decolonization of all household members.
(See the Editorial Commentary by Knox et al on pages e4578–80.)
Community-associated (CA) methicillin-resistant Staphylococcus aureus (MRSA) has caused an epidemic of infections in immunocompetent hosts. Skin and soft tissue infection (SSTI) is the most frequent entity caused by CA-MRSA, and up to 70% of patients will experience recurrent SSTI, engendering burden and frustration for patients and clinicians [1–4]. Moreover, CA-MRSA colonization is a demonstrated risk factor for the development of SSTI. In studies conducted in community settings, 26%–38% of individuals with CA-MRSA colonization experienced subsequent SSTI [5, 6]. To ameliorate this risk, early in the SSTI epidemic, preventive measures traditionally employed in healthcare settings—including decolonization with topical antimicrobials (eg, mupirocin, chlorhexidine, and dilute bleach-water baths)—began to be used in ambulatory patients [7–9]. Initial studies in community settings all focused on decolonization of the index patient exclusively [10–12]. However, MRSA SSTIs cluster in households, particularly in households with children [13–15]. Recent studies demonstrate that in addition to person-to-person transmission, environmental surfaces also serve as reservoirs for household MRSA transmission [16–18]. These observations raised the following question: In households affected by MRSA, who should be prescribed decolonization? To address this question, we previously conducted a randomized clinical trial comparing the effectiveness of decolonization targeted at the index patient alone to decolonization performed by all household members. That trial demonstrated a significantly reduced incidence of SSTI in index patients and household contacts within households performing household decolonization compared to index patient–only decolonization [19].
Though successful in reducing SSTI incidence, decolonizing all household members may pose substantial time and financial burden on families. Furthermore, widespread use may lead to the development of antimicrobial resistance [20–25]. These untoward consequences may be mitigated through targeting select household members. In deciding whom to decolonize, there are several possible approaches. The first is to screen all household members to identify MRSA carriers; this approach, however, is impractical. Additionally, some individuals may be intermittently colonized, or colonized in sites not routinely sampled using typical surveillance approaches, and thus would be falsely identified as “noncarriers.” As a history of SSTI predicts subsequent SSTI [26], a second, perhaps more practical approach might target decolonization toward those household members with a history of SSTI. To this end, we conducted a randomized clinical trial to test the primary hypothesis that a 5-day decolonization protocol performed only by household members with a history of SSTI in the prior year would be noninferior to decolonizing all household members in preventing SSTI 3 months following the intervention.
METHODS
Participants
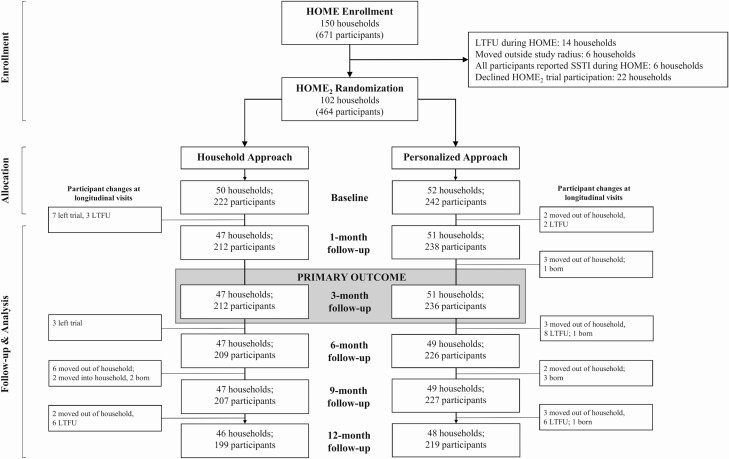
Index patients (N = 150) with CA-MRSA infection, their household contacts, and indoor dogs and cats were enrolled in the Household Observation of MRSA in the Environment (HOME) study and followed for 1 year [18, 27–30]; incidence of SSTI in household members was recorded. After this observational year, households were invited to enroll in the “HOME2 Decolonization Trial.” Eligibility required participation in HOME; index patients enrolled in HOME were ≤18 years old, residing ≤80 miles from St Louis Children’s Hospital, with a culture-confirmed CA-MRSA SSTI. Exclusion for HOME2 was incident SSTI in all household members. Of 150 HOME households, 130 completed 12-month follow-up (Figure 1). Six households were ineligible for HOME2 (all household members reported SSTI during HOME) and 22 declined participation. From April 2013 through November 2016, index patients, household contacts, and pets from 102 households were enrolled in HOME2 upon written, informed consent (and assent where appropriate) from each household member (and primary caretaker of pets). The Washington University Human Research Protection Office and Animal Studies Committee approved the study methods (ClinicalTrials.gov identifier NCT01814371).
Figure 1.
Consolidated Standards for Reporting Trials (CONSORT) flow diagram for the HOME2 decolonization trial, showing study participants with data available for analysis of cumulative skin and soft tissue infection at each time point. Abbreviations: HOME, Household Observation of Methicillin-Resistant Staphylococcus aureus in the Environment; LTFU, lost to follow-up; SSTI, skin and soft tissue infection.
Randomization and Decolonization Regimen
HOME2 was an open-label, randomized noninferiority trial. Households were randomized 1:1 to the “personalized approach,” in which decolonization was performed only by household members who experienced SSTI during the 12-month HOME study, or the “household approach,” in which decolonization was performed by all household members. All index patients (whose CA-MRSA SSTI prompted HOME enrollment) received decolonization. Block randomization was generated in the Research Electronic Data Capture (REDCap) web application [31] using a minimization algorithm [32] to ensure balanced arms; treatment approaches were balanced by number of household members with SSTI (1 vs >1) and household size (≤4 vs >4 household members). Distinct informed consent documents were used for each treatment approach to reduce risk of crossover (Supplementary Methods) [12].
The decolonization regimen consisted of twice-daily application of a pea-sized amount of 2% mupirocin ointment (Perrigo, Allegan, Michigan) to the anterior nares, plus daily 15-minute dilute bleach-water baths (one-quarter cup of bleach [Clorox, Clorox Company, Oakland, California] per one-quarter tub of water [33]) for 5 days. Participants <1 month of age were excluded from decolonization. All household members performed enhanced hygiene measures (Supplementary Methods). Participants recorded completion, ease or difficulty of the measures, and adverse reactions using a memory aid. Adherence was defined as completing 80% of the assigned regimen (≥4/5 bleach baths and ≥8/10 mupirocin applications).
Data Collection
After randomization, visits were conducted at 1, 3, 6, 9, and 12 months in participants’ homes. Trained study staff queried participants (or their guardians, when necessary) about incident SSTI, including date, body site, type of infection (abscess or cellulitis), medical care sought, drainage, and treatment. Surveys also included questions regarding healthcare exposure, antibiotic use, and additional decolonization measures.
To measure S. aureus colonization status at each visit, swabs (Eswab, Becton Dickinson, Franklin Lakes, New Jersey) were collected from the anterior nares, axillae, and inguinal folds of all household members and from the nares (minitip Eswab, Becton Dickinson) and dorsal fur (Eswab) of indoor dogs and cats [30]. Up to 21 environmental surfaces (Supplementary Methods) were sampled using Eswabs and contact plates (Baird Parker agar; Hardy Diagnostics, Santa Maria, California) [34].
Microbiological Methods
Broth-enrichment culture-based methods were used to detect S. aureus from Eswabs; colony morphology was used to select S. aureus from contact plates. Identification and antibiotic susceptibility testing of S. aureus isolates were conducted as previously described (Supplementary Methods) [35]. Strain typing was assessed through repetitive sequence-based polymerase chain reaction for isolates from baseline, 1-month, and 3-month samplings and assigned at the household level; strains with ≥95% similarity were considered identical [36, 37].
Sample Size Calculation for Primary Outcome
SSTI incidence 3 months postintervention was chosen as our primary outcome as this is a reasonable amount of time for individuals undergoing decolonization to reacquire the organism from other colonized household contacts and for a subsequent infection to develop. Based on a prior study [19], we anticipated a 9% 3-month SSTI incidence in household approach participants. An absolute difference of 10% is considered clinically equivalent and has been recommended for anti-infective trials [38]; thus, <19% SSTI incidence in personalized approach participants would be considered clinically equivalent. Noninferiority of the personalized approach was defined as the upper limit of the 2-sided 95% confidence interval (CI) for the difference in 3-month SSTI incidence between arms being <10% (Supplementary Methods) [38]. Considering the variance inflation factor due to clustering of participants within households (estimated to be 1.4 based on our prior studies [19]), a total sample of 344 participants (or 86 households) would provide 80% power with α = .05 to conclude noninferiority (generated using PASS software; NCSS, Kaysville, Utah). Anticipating 15% attrition between trial enrollment and 3-month follow-up, we enrolled 102 households.
Statistical Analyses of Secondary Outcomes
Analyses were conducted using SPSS version 25 for Windows (IBM SPSS, Chicago, Illinois). Baseline differences between arms were assessed with Fisher exact, Pearson χ 2, and Mann-Whitney U tests. Self-reported cumulative SSTI (ie, 1 or more SSTIs reported between initial sampling and each follow-up visit) were compared between arms using Fisher exact test. A Cox proportional hazards model was used, adjusting for MRSA colonization status at baseline sampling, to determine effect of regimen on cumulative SSTI. Staphylococcus aureus colonization of participants was compared between baseline and follow-up samplings within each arm with McNemar test and between arms using Fisher exact test. Mean differences in environmental contamination pressure (ie, the number of contaminated surfaces divided by the total number of surfaces sampled per household) were analyzed between baseline and follow-up samplings within each arm with paired t tests. Adherence was compared between arms using Fisher exact test. As exploratory analyses, S. aureus carriage in pets and prevalence of mupirocin-resistant S. aureus were compared between baseline and follow-up samplings within each arm with McNemar test and between arms using Fisher exact test.
Multivariable generalized mixed-effects logistic regression models were employed to estimate how treatment arm, along with individual and household attributes a priori posited to be associated with the outcomes, influenced cumulative SSTI and MRSA colonization in the year following decolonization assignment (Supplementary Methods). These individual-level models were fitted separately for each follow-up sampling using R library “MCMCglmm” [39, 40], with a random intercept for household included to control for clustering within households.
RESULTS
Study Population and Household Demographics
A cohort of 102 pediatric patients (median age, 3.9 years [range, 1.1–17.1 years]) with history of medically attended MRSA SSTI in the past year, household contacts (n = 372 [362 at baseline, 10 enrolled at longitudinal visits]; median age, 25.9 years [range, 0.02–79.0 years]), and pets (n = 95 [in 53 homes]; 79 dogs, 16 cats) were enrolled. Participants were white (331 [70%]), African American (126 [27%]), and multiracial (17 [4%]). Median household size was 4 (range, 2–13) (Table 1). Fifty-two households (n = 248 participants) were randomized to the personalized approach and 50 (n = 226 participants) to the household approach (Figure 1). Participants in the personalized approach arm were more frequently MRSA colonized (63 [26%]) at baseline sampling than participants in the household approach arm (40 [18%]) (P = .04).
Table 1.
Participant, Household, and Pet Characteristics by Treatment Approach
| Participant Characteristics | All Participants (N = 474) | Personalized Approach (n = 248) | Household Approach (n = 226) | P Value |
|---|---|---|---|---|
| Age, years, median (range) | 13.6 (0.02–79) | 13.4 (0.02–76) | 14.2 (0.03–79) | .95 |
| Male sex | 216 (46) | 114 (46) | 102 (45) | .93 |
| Race | ||||
| White | 331 (70) | 169 (68) | 162 (72) | .42 |
| African American or multiraciala | 143 (30) | 79 (32) | 64 (28) | |
| Insuranceb,c | ||||
| Private or military | 328 (71) | 166 (69) | 162 (73) | .42 |
| Medicaid or none | 135 (29) | 74 (31) | 61 (27) | |
| Chronic medical conditionc,d | 225 (50) | 107 (46) | 118 (54) | .11 |
| Eczemab,c | 86 (19) | 49 (21) | 37 (17) | .28 |
| Takes prescription medicationsb,c | 135 (30) | 68 (29) | 67 (31) | .76 |
| SSTI in past yearb,e | 124 (26) | 66 (27) | 58 (26) | .83 |
| Surgery (including I&D) in past yearb,e | 64 (14) | 32 (13) | 32 (14) | .79 |
| ED/urgent care visit in past yearb,e | 130 (28) | 70 (29) | 60 (27) | .61 |
| Hospitalization in past yearb,e | 34 (7) | 17 (7) | 17 (8) | .86 |
| Antibiotic use in past yeare | 202 (44) | 107 (45) | 95 (43) | .71 |
| Decolonization in past yearb,e | 210 (46) | 115 (48) | 95 (43) | .26 |
| Mupirocin in nares | 125 (27) | 72 (30) | 53 (24) | .14 |
| Chlorhexidine washes | 58 (13) | 30 (13) | 28 (13) | 1.00 |
| Bleach baths | 139 (30) | 78 (33) | 61 (28) | .26 |
| Colonized with Staphylococcus aureus at baselineb | 219 (47) | 105 (43) | 114 (51) | .09 |
| MRSA | 103 (22) | 63 (26) | 40 (18) | .04 |
| Axillae | 25 (5) | 11 (5) | 14 (6) | .42 |
| Nares | 61 (13) | 38 (16) | 23 (10) | .10 |
| Inguinal folds | 55 (12) | 37 (15) | 18 (8) | .02 |
| MSSA | 128 (28) | 49 (20) | 79 (36) | <.001 |
| Axillae | 38 (8) | 11 (5) | 27 (12) | .004 |
| Nares | 105 (23) | 42 (17) | 63 (28) | .005 |
| Inguinal folds | 50 (11) | 15 (6) | 35 (16) | .001 |
| Household characteristics | All Households (n = 102) | Personalized Approach (n = 52) | Household Approach (n = 50) | P Value |
| No. persons in household, median (range) | 4 (2–13) | 4 (2–13) | 4 (2–8) | .85 |
| People per 1000 sq ft, median (range) | 3.0 (0.4–8.7) | 2.9 (0.4–8.3) | 3.1 (1.1–8.7) | .22 |
| Owns homec | 69 (68) | 38 (73) | 31 (62) | .29 |
| Personal S. aureus colonization pressure at baselinef, mean ± SD | 0.23 ± 0.19 | 0.21 ± 0.16 | 0.26 ± 0.22 | .16 |
| MRSA | 0.10 ± 0.13 | 0.11 ± 0.13 | 0.08 ± 0.13 | .20 |
| MSSA | 0.14 ± 0.18 | 0.09 ± 0.12 | 0.18 ± 0.22 | .01 |
| Environmental S. aureus contamination pressure at baselineg, mean ± SD | 0.16 ± 0.18 | 0.14 ± 0.16 | 0.18 ± 0.21 | .24 |
| MRSA | 0.06 ± 0.12 | 0.06 ± 0.10 | 0.07 ± 0.14 | .84 |
| MSSA | 0.09 ± 0.16 | 0.08 ± 0.12 | 0.11 ± 0.19 | .22 |
| Pet characteristics | All Pets (n = 95) | Personalized Approach (n = 48) | Household Approach (n = 47) | P Value |
| Pet type | .79 | |||
| Dog | 79 (83) | 39 (81) | 40 (85) | |
| Cat | 16 (17) | 9 (19) | 7 (15) | |
| Carried S. aureus at baselineb | 21 (26) | 10 (24) | 11 (28) | .80 |
| MRSA | 14 (17) | 7 (17) | 7 (18) | 1.00 |
| Nares | 6 (7) | 4 (10) | 2 (5) | .68 |
| Dorsal fur | 10 (13) | 4 (10) | 6 (16) | .51 |
| MSSA | 8 (10) | 3 (7) | 5 (13) | .47 |
| Nares | 2 (2) | 0 (0) | 2 (5) | .23 |
| Dorsal fur | 6 (8) | 3 (8) | 3 (8) | 1.00 |
Data are presented as no. (%) unless otherwise indicated. Fisher exact test, Pearson χ 2 test, Mann-Whitney U test, and Student t test were used where appropriate. P values ≤.05 were considered significant and are highlighted with bold text.
Abbreviations: ED, emergency department; I&D, incision and drainage; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus; SD, standard deviation; SSTI, skin and soft tissue infection.
aMultiracial participants include African American/white (n = 14) and African American/white/American Indian (n = 3).
bVarious characteristics contain missing data: insurance, n = 463; SSTI in past year, surgery, ED visit, hospitalization, decolonization in past year, n = 459; chronic medical condition, takes prescription medications, eczema, n = 452; colonized with S. aureus at baseline, n = 464; carried S. aureus at baseline, n = 81.
cDenotes information retrieved at enrollment into the Household Observation of Methicillin-Resistant Staphylococcus aureus in the Environment (HOME) study.
dChronic medical conditions include asthma, seasonal allergies, seizures, heart disease, diabetes, cancer, kidney disease, liver disease, connective tissue disease, gastroesophageal reflux disease, inflammatory bowel disease, immune system problems, depression or bipolar, attention deficit disorder, sickle cell disease, cystic fibrosis, and emphysema.
eDenotes characteristics of participants during HOME study year; does not include their HOME enrollment SSTI or details of treatment for that SSTI.
fNumber of anatomic sites (3 per person) colonized with S. aureus, MRSA, or MSSA divided by the total number of anatomic sites sampled per household.
gNumber of environmental surfaces (up to 21 per house) contaminated with S. aureus, MRSA, or MSSA divided by the total number of environmental surfaces sampled per household.
SSTI
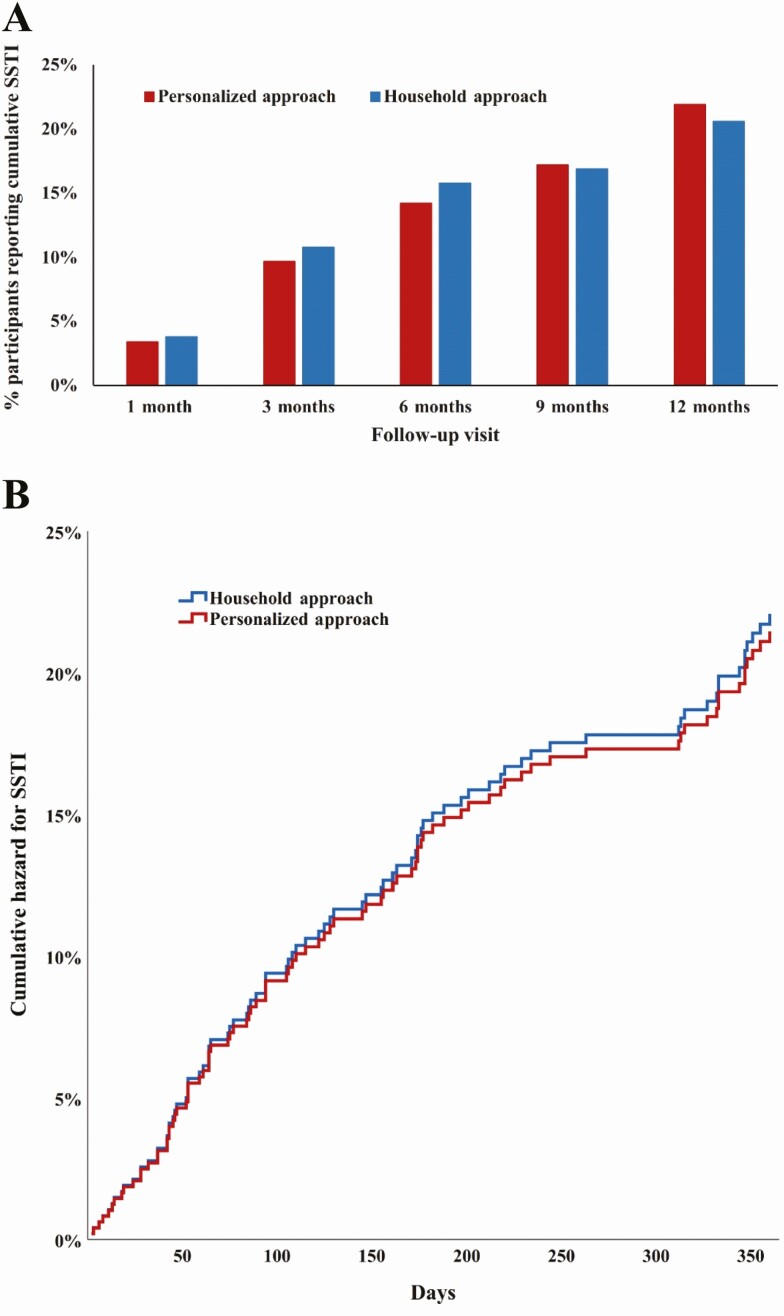
Cumulative SSTI incidence did not significantly differ between participants in the 2 arms (Figure 2A). Noninferiority of the personalized approach was established across all household members at the primary endpoint of 3-month cumulative SSTI: 23 of 212 (10.8%) participants reported SSTI in household approach households, while 23 of 236 (9.7%) participants reported SSTI in personalized approach households (difference in proportions, −1.1% [95% CI, −6.7% to 4.5%]). In total, 89 (19%) participants reported 175 SSTIs over 12 months of follow-up (details are shown in Supplementary Table 1).
Figure 2.
A, Cumulative skin and soft tissue infection (SSTI) self-reported by household members following decolonization intervention. Differences in cumulative SSTI over time between the personalized and household decolonization approaches were compared using Fisher exact test (P > .05 at each time point). Cumulative SSTI defined as 1 or more SSTIs reported between baseline sampling and each follow-up visit. B, Cox proportional-hazard regression analysis for SSTI for up to 1 year between household members assigned the household and personalized decolonization approaches, adjusting for baseline MRSA colonization status. Household members assigned the personalized approach were not more likely to report an SSTI than those assigned the household approach (adjusted hazard ratio [aHR], 1.0 [95% confidence interval {CI}, .6–1.5]). Household members who were MRSA-colonized at baseline sampling were more likely to report an SSTI than those not colonized with MRSA (aHR, 2.3 [95% CI, 1.5–3.5]).
In the Cox proportional hazards model, when adjusting for MRSA colonization status at baseline sampling, treatment arm had no effect on hazard of cumulative SSTI over time (adjusted hazard ratio [aHR], 1.0 [95% CI, .6–1.5]; Figure 2B). However, baseline MRSA colonization significantly increased hazard of SSTI (aHR, 2.3 [95% CI, 1.5–3.5]). Time to first SSTI did not differ between treatment arm (household approach: median, 89 days [interquartile range {IQR}, 44–179]; personalized approach: median, 110 days [IQR, 53–220]; P = .56).
In the multivariable generalized mixed-effects logistic regression model (Table 2), factors associated with cumulative SSTI included MRSA colonization at baseline sampling (at 6-, 9-, and 12-month follow-up), reporting an SSTI during the prior year (at 1-, 3-, 6-, 9-, and 12-month follow-up), being the index patient (vs a household contact; at 6-month follow-up), and African American race (vs white; at 6-month follow-up). Treatment arm was not significant in the cumulative SSTI model.
Table 2.
Factors Associated With Cumulative Skin and Soft Tissue Infection and Methicillin-Resistant Staphylococcus aureus Colonization at Longitudinal Study Visits, Multivariable Models
| Cumulative SSTIa, OR (95% CrI) | MRSA Colonization, OR (95% CrI) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Covariates | 1 mo | 3 mo | 6 mo | 9 mo | 12 mo | 1 mo | 3 mo | 6 mo | 9 mo | 12 mo |
| Personalized approach (vs household approach) | 0.89 (.26–2.73) | 0.87 (.51–1.55) | 0.93 (.63–1.36) | 1.05 (.74–1.52) | 1.06 (.74–1.52) | 1.75 (.81–3.64) | 1.69 (.96–3.04) | 1.49 (.70–3.52) | 2.49 (1.14–5.56) | 1.70 (.93–3.26) |
| MRSA colonized at baseline sampling | 1.35 (.59–3.27) | 1.67 (.96–2.81) | 2.16 (1.37–3.34) | 1.87 (1.24–2.83) | 2.05 (1.34–3.13) | 8.22 (4.60–14.93) | 4.29 (2.70–6.68) | 5.22 (3.02–9.11) | 3.79 (2.17–6.85) | 3.01 (1.86–4.92) |
| Age, y (increase of 1 SD, 16.2 y, from the mean 20.1 y) | 1.52 (.99–2.42) | 1.00 (.78–1.30) | 1.20 (.97–1.47) | 1.09 (.90–1.32) | 1.02 (.84–1.23) | 1.22 (.92–1.65) | 0.91 (.72–1.15) | 0.82 (.61–1.07) | 1.10 (.84–1.47) | 1.11 (.89–1.40) |
| Male sex | 0.88 (.43–1.75) | 0.84 (.55–1.28) | 1.02 (.73–1.46) | 1.03 (.74–1.41) | 1.10 (.79–1.49) | 1.42 (.89–2.23) | 1.31 (.87–1.92) | 1.54 (.96–2.49) | 1.22 (.75–1.96) | 1.14 (.77–1.68) |
| African American or multiracialb (vs white) | 1.20 (.22–5.37) | 1.46 (.70–2.94) | 1.84 (1.09–3.09) | 1.54 (.96–2.51) | 1.51 (.92–2.62) | 0.67 (.25–1.71) | 0.64 (.28–1.36) | 1.01 (.36–2.88) | 0.52 (.17–1.64) | 0.52 (.23–1.22) |
| Public or no health insurance (vs private or military) | 0.44 (.06–2.75) | 0.62 (.27–1.42) | 0.39 (.21–.72) | 0.63 (.36–1.05) | 0.75 (.44–1.31) | 0.88 (.32–2.51) | 1.06 (.48–2.43) | 0.61 (.19–1.95) | 0.92 (.29–2.89) | 0.81 (.32–1.97) |
| Rents home (vs owns) | 1.58 (.32–9.03) | 1.25 (.56–2.90) | 1.35 (.76–2.50) | 0.91 (.53–1.53) | 0.84 (.48–1.47) | 3.26 (1.07–10.00) | 2.42 (1.02–5.26) | 0.87 (.28–2.65) | 1.80 (.61–5.19) | 1.67 (.72–4.47) |
| People per 1000 sq ft (increase of 1 SD, 1.9 people, from the mean 3.6 people) | 1.32 (.65–2.78) | 1.12 (.80–1.59) | 1.11 (.87–1.42) | 1.15 (.91–1.43) | 1.11 (.89–1.41) | 0.91 (.57–1.46) | 0.95 (.66–1.36) | 0.86 (.52–1.48) | 0.96 (.60–1.53) | 1.25 (.88–1.83) |
| Environmental MRSA contamination pressurec at previous sampling (increase of 1 SD, 13.4%, from the mean 6.5%) | 0.99 (.54–1.80) | 0.93 (.68–1.27) | 0.93 (.75–1.12) | 0.97 (.81–1.18) | 0.93 (.77–1.13) | 1.18 (.78–1.79) | 1.37 (1.05–1.85) | 1.40 (1.03–1.97) | 1.78 (1.27–2.57) | 1.38 (1.06–1.85) |
| Index patient (vs household contact) | 2.18 (.86–5.96) | 1.62 (.93–2.74) | 1.71 (1.06–2.79) | 1.36 (.89–2.13) | 1.41 (.92–2.17) | 1.97 (.99–4.11) | 0.98 (.57–1.70) | 0.34 (.15–.74) | 0.71 (.34–1.41) | 1.15 (.66–2.00) |
| SSTI in past year (during HOME study) | 2.51 (1.13–5.84) | 2.65 (1.62–4.33) | 2.16 (1.47–3.16) | 1.95 (1.38–2.77) | 1.98 (1.38–2.87) | 0.79 (.45–1.41) | 1.17 (.72–1.85) | 1.17 (.68–2.06) | 1.55 (.92–2.78) | 1.14 (.71–1.82) |
| Systemic antibiotics since previous samplingd | … | … | … | … | … | 2.22 (.98–4.75) | 1.03 (.55–1.98) | 0.59 (.26–1.32) | 0.50 (.20–1.21) | 0.92 (.48–1.68) |
| Additional decolonization with mupirocin ointment to anterior nares since previous samplingd | … | … | … | … | … | 1.43 (.26–7.71) | 1.87 (.37–9.17) | 1.70 (.14–20.64) | 1.25 (.28–5.52) | 0.26 (.05–1.46) |
| Additional decolonization with chlorhexidine body wash or bleach baths since previous samplingd | … | … | … | … | … | 1.90 (.92–4.23) | 1.14 (.59–2.19) | 2.35 (.92–6.20) | 0.61 (.25–1.52) | 1.24 (.60–2.60) |
The cumulative SSTI and MRSA colonization models (Supplementary Methods) are individual-level, multivariable generalized mixed-effects logistic regression models fitted using R library “MCMCglmm”; eligible individuals for each model are those completing follow-up visit and with no missing data for any covariates: 1 month, n = 425; 3 months, n = 414; 6 months, n = 407; 9 months, n = 398; 12 months, n = 381. The odds ratio and 95% credible interval for covariates significantly associated with each outcome (credible interval does not cross 1) are highlighted with bold text.
Abbreviations: CrI, credible interval; HOME, Household Observation of Methicillin-Resistant Staphylococcus aureus in the Environment; MRSA, methicillin-resistant Staphylococcus aureus; OR, odds ratio; SD, standard deviation; SSTI, skin and soft tissue infection.
aDefined as 1 or more SSTIs reported between baseline sampling and each follow-up visit.
bMultiracial participants are African American/white and African American/white/American Indian.
cDefined as the number of environmental surfaces (up to 21 per house) contaminated with MRSA divided by the total number of environmental surfaces sampled per household; the cumulative SSTI model includes environmental MRSA contamination pressure at baseline, not previous, sampling (increase of 1 SD, 12.1%, from the mean 6.5%).
dSystemic antibiotics and additional decolonization since previous sampling are not included in cumulative SSTI model as temporality between SSTI and treatment for SSTI cannot be established.
MRSA Colonization
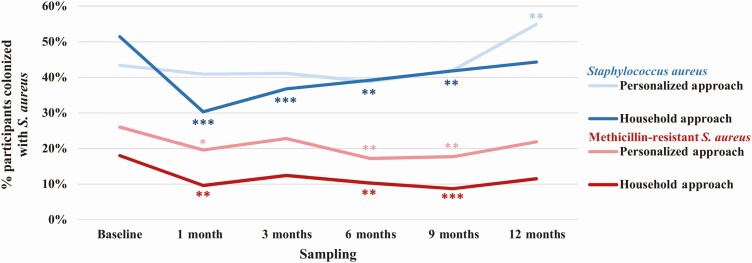
MRSA colonization significantly decreased at longitudinal samplings in households assigned the personalized approach and in households assigned the household approach (Figure 3). In the personalized approach arm, MRSA colonization decreased from 26.0% (63 of 242) of household members at baseline to 19.6% (45 of 230), 17.2% (39 of 227), and 17.7% (40 of 226) at 1-, 6-, and 9-month samplings (P = .03, P = .004, and P = .01, respectively). In the household approach arm, MRSA colonization decreased from 18.0% (40 of 222) of household members at baseline to 9.6% (20 of 208), 10.3% (21 of 204), and 8.7% (17 of 196) at 1-, 6-, and 9-month samplings (P = .01, P = .01, and P = .001, respectively). Comparing treatment approaches, the change in MRSA colonization from baseline did not differ significantly between arms at any sampling.
Figure 3.
Longitudinal household member Staphylococcus aureus colonization. Percentage of household members colonized with S. aureus (blue) and methicillin-resistant S. aureus (MRSA; red) at 6 sampling intervals over 12 months. 5-day decolonization intervention (personalized approach, light shade; household approach, dark shade) occurred immediately following baseline sampling. S. aureus includes MRSA and/or methicillin-susceptible S. aureus. Statistically significant changes in colonization at longitudinal samplings compared to baseline sampling within the decolonization approach represented by asterisks (using McNemar test: *P < .05, **P ≤ .01, ***P ≤ .001). Change in colonization between decolonization approaches was compared using Fisher exact test: for S. aureus, P = .01, P = .04, and P = .003 at 1-, 3-, and 12-month samplings, respectively; for MRSA: P > .05 at all samplings.
In the multivariable generalized mixed-effects logistic regression model (Table 2), factors associated with MRSA colonization at longitudinal samplings included MRSA colonization at baseline (at 1-, 3-, 6-, 9-, and 12-month follow-up), living in a rented home (at 1- and 3-month follow-up), and increasing environmental MRSA contamination pressure (at 3-, 6-, 9-, and 12-month follow-up). Longitudinal MRSA colonization did not differ between participants in households randomized to the personalized or household approaches (at 1-, 3-, 6-, and 12-month follow-up).
Molecular Epidemiology of Longitudinal Colonization
Of 124 S. aureus–colonized participants at baseline who remained colonized at the 1-month sampling despite the decolonization protocol, 82 remained colonized with the same strain, 39 were colonized with a distinct strain, and 3 were colonized with both. Of 31 participants S. aureus–colonized at baseline who were not colonized at 1-month sampling but became recolonized at 3-month sampling, 19 were recolonized with their original strain and 12 with a distinct strain. Treatment arm was neither associated with strain persistence nor acquisition of a distinct strain.
Adherence, Adverse Effects, and Mupirocin Resistance
Of 309 participants assigned decolonization measures, 191 of 297 (64%) with follow-up data were adherent with decolonization. Sixty-one of 86 (71%) participants in personalized approach households adhered to the decolonization intervention, compared with 130 of 211 (62%) participants in household approach households (P = .14). Minor adverse effects were reported by 143 (48%) participants overall, most commonly runny (41 [14%]) or itchy (25 [8%]) nose with mupirocin application and dry (81 [27%]) or itchy (42 [14%]) skin with bleach baths.
At baseline, 9 (1.9%) participants were colonized with mupirocin-resistant S. aureus (Supplementary Table 2); at 1 and 3 months following the intervention, 10 (2.3%) and 8 (1.9%) participants, respectively, were colonized with mupirocin-resistant S. aureus (P = 1.0 for each). There was no difference in longitudinal prevalence of mupirocin-resistant S. aureus colonization between treatment arms.
Effects of Personal Decolonization on Environmental Contamination and Pet Carriage
Within households assigned to either the personalized or household approaches, environmental MRSA contamination pressure did not significantly change after the decolonization intervention (Supplementary Figure 1). Across all households, environmental MRSA contamination pressure was 6.3% (±12.1%) at baseline and 6.3% (±12.3%) at 1-month sampling (P = .9). In household approach households, pet MRSA carriage declined postintervention; at baseline sampling, 7 of 39 (18%) pets carried MRSA, decreasing to 0 of 37 (0%) by the 9-month visit (P = .02; Supplementary Figure 2). Reductions in pet MRSA carriage in personalized approach households over the same time interval did not reach statistical significance (from 7 of 42 [17%] to 5 of 34 [15%]; P = 1.0).
DISCUSSION
Although decolonization with topical antimicrobials reduces the incidence of recurrent SSTIs, particularly when performed by all members of a household compared to the index patient alone, these measures can be cumbersome [19, 41]. Moreover, broad use of these agents may increase selective antimicrobial pressure and may disrupt the microbiota [42]. To decrease this burden, we aimed to compare the effectiveness of these measures, specifically a 5-day regimen of intranasal mupirocin application and dilute bleach-water baths, when performed by only those household members with history of SSTI in the past year (personalized approach) vs all household members (household approach). The personalized approach was noninferior to the household approach in preventing SSTI, while simultaneously reducing the burden for families. Last, environmental MRSA contamination pressure was unaffected by the 5-day decolonization protocol, though higher environmental MRSA contamination pressure was associated with longitudinal MRSA colonization of household members.
Prior studies have demonstrated that MRSA colonization is a predisposing factor for SSTI [5, 6]. Moreover, many individuals with SSTI will experience SSTI recurrences [19, 26, 43]. Indeed, in the present study, both baseline MRSA colonization status and history of SSTI were important predictors of SSTI. While some clinicians prescribe decolonization only for colonized household members [7, 9], routine culturing of all household members is infeasible in a community setting. Thus we posited that a more pragmatic approach would target decolonization measures solely to household members reporting prior SSTI. The success of this personalized approach is amenable to widespread implementation in clinical practice (ie, requiring only history taking) while reducing burden on households.
While both approaches reduced MRSA colonization in the months following the 1-time, 5-day intervention, this effect waned over time. Interestingly, among those from whom carriage was eradicated at the 1-month sampling, one-third who became recolonized at 3 months had acquired a new strain. Furthermore, despite the current intervention, 33% of index patients experienced recurrent SSTI during the 12 months of HOME2. While this SSTI incidence is lower than that experienced by the index patients in the year before HOME2 enrollment (50% [29]), the burden of recurrent SSTI was not entirely eliminated. Taken together, these findings reflect ongoing community exposure to MRSA reservoirs, both within and outside of the household, posing a risk for reacquisition and SSTI. This suggests that a 1-time decolonization regimen performed in homes affected by MRSA is inadequate to prevent SSTI, regardless of who is targeted [10, 11, 19]. One consideration is to prescribe more prolonged or periodic decolonization interventions; prior such trials have enrolled varying populations and examined different decolonization regimens, yielding disparate results [12, 41, 44]. Ultimately, further studies and approaches are needed to fully abate community transmission.
The most successful decolonization regimens have included intranasal mupirocin [10, 20, 23], underscoring the importance of persistent nasal colonization [29]. Increasing prevalence of resistance to topical antimicrobials has been associated with their widespread use for treating skin infections and for decolonization in community settings [22, 25, 45]. Additionally, high-level mupirocin resistance predicts failure of decolonization efforts with mupirocin, resulting in persistent staphylococcal carriage [20–24]. While the present trial resulted in no rise in the recovery of mupirocin-resistant strains, monitoring resistance to topical antimicrobials remains a priority.
This study has limitations. The prevalence of MRSA colonization at baseline was higher among participants randomized to the personalized (vs household) approach. To account for this, all analyses of longitudinal MRSA colonization and SSTI adjusted for baseline colonization. Incident SSTI as primary outcome was self-reported, which may have overestimated overall incidence. However, this practice of reporting is common in community-based studies [43, 46]; in addition, all enrolled households had a history of medically attended, culture-confirmed MRSA SSTI, increasing confidence in their ability to recognize and report interval SSTIs. Our study was designed to evaluate effectiveness; accordingly, in this real-world setting, adherence was not optimal, though it was statistically equivalent between study arms. Additionally, our findings may not be generalizable to households affected by methicillin-susceptible S. aureus SSTI.
In this pragmatic trial, we aimed to decrease the incidence of SSTI in households affected by MRSA while also decreasing the burden on families. A targeted approach of prescribing decolonization only for those household members with SSTI in the prior year was noninferior to decolonization of all household members. MRSA colonization and prior SSTI were strong predictors of incident SSTI, which may be taken into consideration when counseling families. Last, in the present study, the burden of household environmental MRSA surface contamination was associated with longitudinal MRSA colonization. Thus, future studies are needed to interrogate longer durations of decolonization, decontamination of the household environment, or the integration of both as novel approaches to reduce the burden of MRSA in households.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Meghan Wallace for assistance with molecular strain typing.
Disclaimer. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH) or the Agency for Healthcare Research and Quality (AHRQ).
Financial support. This work was supported by the Children’s Discovery Institute of Washington University and St Louis Children’s Hospital (to S. A. F.); NIH/National Institute of Allergy and Infectious Diseases (grant number K23-AI091690) to S. A. F.; the National Center for Advancing Translational Sciences at the NIH (grant number UL1-TR002345) to S. A. F.; the AHRQ (grant numbers R01-HS021736 and R01-HS024269) to S. A. F.; and the Burroughs Wellcome Foundation Investigators in the Pathogenesis of Infectious Disease Award (to J. B. W.). The computational analysis was partially funded by the Defense Advanced Research Projects Agency Big Mechanism program (Army Research Office contract W911NF1410333) to A. R.; by the NIH (grant numbers R01HL122712, 1P50MH094267, and U01HL108634) to A. R.; and by a gift from Liz and Kent Dauten) to A. R.
Potential conflicts of interest. D. A. H. reports personal fees from BioVersys AG, outside the submitted work. J. B. W. reports a financial agreement with Aridis Pharmaceutics related to patents owned by the University of Chicago. C. D. B. reports grants from Cepheid, bioMérieux, Luminex, and BioFire, and personal fees from Thermo Fisher Scientific, outside the submitted work. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Fritz SA, Garbutt J, Elward A, Shannon W, Storch GA. Prevalence of and risk factors for community-acquired methicillin-resistant and methicillin-sensitive Staphylococcus aureus colonization in children seen in a practice-based research network. Pediatrics 2008; 121:1090–8. [DOI] [PubMed] [Google Scholar]
- 2. Kaplan SL, Hulten KG, Gonzalez BE, et al. Three-year surveillance of community-acquired Staphylococcus aureus infections in children. Clin Infect Dis 2005; 40:1785–91. [DOI] [PubMed] [Google Scholar]
- 3. Lina G, Piémont Y, Godail-Gamot F, et al. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis 1999; 29:1128–32. [DOI] [PubMed] [Google Scholar]
- 4. Fritz SA, Shapiro DJ, Hersh AL. National trends in incidence of purulent skin and soft tissue infections in patients presenting to ambulatory and emergency department settings, 2000–2015. Clin Infect Dis 2020; 70:2715–8. [DOI] [PubMed] [Google Scholar]
- 5. Ellis MW, Hospenthal DR, Dooley DP, Gray PJ, Murray CK. Natural history of community-acquired methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clin Infect Dis 2004; 39:971–9. [DOI] [PubMed] [Google Scholar]
- 6. Fritz SA, Epplin EK, Garbutt J, Storch GA. Skin infection in children colonized with community-associated methicillin-resistant Staphylococcus aureus. J Infect 2009; 59:394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Creech CB, Beekmann SE, Chen Y, Polgreen PM. Variability among pediatric infectious diseases specialists in the treatment and prevention of methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Pediatr Infect Dis J 2008; 27:270–2. [DOI] [PubMed] [Google Scholar]
- 8. Liu C, Bayer A, Cosgrove SE, et al. Infectious Diseases Society of America . Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52:e18–55. [DOI] [PubMed] [Google Scholar]
- 9. Parrish KL, Salwan NK, Thompson RM, et al. Skin and soft tissue infection treatment and prevention practices by pediatric infectious diseases providers [manuscript published online ahead of print 26 November 2019]. J Pediatric Infect Dis Soc 2019. doi:10.1093/jpids/piz080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ellis MW, Griffith ME, Dooley DP, et al. Targeted intranasal mupirocin to prevent colonization and infection by community-associated methicillin-resistant Staphylococcus aureus strains in soldiers: a cluster randomized controlled trial. Antimicrob Agents Chemother 2007; 51:3591–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fritz SA, Camins BC, Eisenstein KA, et al. Effectiveness of measures to eradicate Staphylococcus aureus carriage in patients with community-associated skin and soft-tissue infections: a randomized trial. Infect Control Hosp Epidemiol 2011; 32:872–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaplan SL, Forbes A, Hammerman WA, et al. Randomized trial of “bleach baths” plus routine hygienic measures vs. routine hygienic measures alone for prevention of recurrent infections. Clin Infect Dis 2014; 58:679–82. [DOI] [PubMed] [Google Scholar]
- 13. Fritz SA, Hogan PG, Hayek G, et al. Staphylococcus aureus colonization in children with community-associated Staphylococcus aureus skin infections and their household contacts. Arch Pediatr Adolesc Med 2012; 166:551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miller LG, Eells SJ, Taylor AR, et al. Staphylococcus aureus colonization among household contacts of patients with skin infections: risk factors, strain discordance, and complex ecology. Clin Infect Dis 2012; 54:1523–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Knox J, Sullivan SB, Urena J, et al. Association of environmental contamination in the home with the risk for recurrent community-associated, methicillin-resistant Staphylococcus aureus infection. JAMA Intern Med 2016; 176:807–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mollema FP, Richardus JH, Behrendt M, et al. Transmission of methicillin-resistant Staphylococcus aureus to household contacts. J Clin Microbiol 2010; 48:202–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ng W, Faheem A, McGeer A, et al. Community- and healthcare-associated methicillin-resistant Staphylococcus aureus strains: an investigation into household transmission, risk factors, and environmental contamination. Infect Control Hosp Epidemiol 2017; 38:61–7. [DOI] [PubMed] [Google Scholar]
- 18. Mork RL, Hogan PG, Muenks CE, et al. Longitudinal, strain-specific Staphylococcus aureus introduction and transmission events in households of children with community-associated meticillin-resistant S aureus skin and soft tissue infection: a prospective cohort study. Lancet Infect Dis 2020; 20: 188–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fritz SA, Hogan PG, Hayek G, et al. Household versus individual approaches to eradication of community-associated Staphylococcus aureus in children: a randomized trial. Clin Infect Dis 2012; 54:743–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Robicsek A, Beaumont JL, Thomson RB Jr, Govindarajan G, Peterson LR. Topical therapy for methicillin-resistant Staphylococcus aureus colonization: impact on infection risk. Infect Control Hosp Epidemiol 2009; 30:623–32. [DOI] [PubMed] [Google Scholar]
- 21. Lee AS, Macedo-Vinas M, François P, et al. Impact of combined low-level mupirocin and genotypic chlorhexidine resistance on persistent methicillin-resistant Staphylococcus aureus carriage after decolonization therapy: a case-control study. Clin Infect Dis 2011; 52:1422–30. [DOI] [PubMed] [Google Scholar]
- 22. Patel JB, Gorwitz RJ, Jernigan JA. Mupirocin resistance. Clin Infect Dis 2009; 49:935–41. [DOI] [PubMed] [Google Scholar]
- 23. Simor AE, Phillips E, McGeer A, et al. Randomized controlled trial of chlorhexidine gluconate for washing, intranasal mupirocin, and rifampin and doxycycline versus no treatment for the eradication of methicillin-resistant Staphylococcus aureus colonization. Clin Infect Dis 2007; 44:178–85. [DOI] [PubMed] [Google Scholar]
- 24. Fritz SA, Hogan PG, Camins BC, et al. Mupirocin and chlorhexidine resistance in Staphylococcus aureus in patients with community-onset skin and soft tissue infections. Antimicrob Agents Chemother 2013; 57:559–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McNeil JC, Hulten KG, Kaplan SL, Mason EO. Decreased susceptibilities to retapamulin, mupirocin, and chlorhexidine among Staphylococcus aureus isolates causing skin and soft tissue infections in otherwise healthy children. Antimicrob Agents Chemother 2014; 58:2878–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen AE, Cantey JB, Carroll KC, Ross T, Speser S, Siberry GK. Discordance between Staphylococcus aureus nasal colonization and skin infections in children. Pediatr Infect Dis J 2009; 28:244–6. [DOI] [PubMed] [Google Scholar]
- 27. Hogan PG, Mork RL, Boyle MG, et al. Interplay of personal, pet, and environmental colonization in households affected by community-associated methicillin-resistant Staphylococcus aureus. J Infect 2019; 78:200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mork RL, Hogan PG, Muenks CE, et al. Comprehensive modeling reveals proximity, seasonality, and hygiene practices as key determinants of MRSA colonization in exposed households. Pediatr Res 2018; 84:668–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hogan PG, Mork RL, Thompson RM, et al. Environmental methicillin-resistant Staphylococcus aureus contamination, persistent colonization, and subsequent skin and soft tissue infection. JAMA Pediatr 2020; 174:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fritz SA, Hogan PG, Singh LN, et al. Contamination of environmental surfaces with Staphylococcus aureus in households with children infected with methicillin-resistant S aureus. JAMA Pediatr 2014; 168:1030–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Scott NW, McPherson GC, Ramsay CR, Campbell MK. The method of minimization for allocation to clinical trials: a review. Control Clin Trials 2002; 23: 662–74. [DOI] [PubMed] [Google Scholar]
- 33. Fisher RG, Chain RL, Hair PS, Cunnion KM. Hypochlorite killing of community-associated methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J 2008; 27:934–5. [DOI] [PubMed] [Google Scholar]
- 34. Hogan PG, Burnham C-AD, Singh LN, et al. Evaluation of environmental sampling methods for detection of Staphylococcus aureus on fomites. Ann Public Health Res 2015; 2:1013. [PMC free article] [PubMed] [Google Scholar]
- 35. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. 28th ed. CLSI supplement M100. Wayne, PA: CLSI, 2018. [Google Scholar]
- 36. Del Vecchio VG, Petroziello JM, Gress MJ, et al. Molecular genotyping of methicillin-resistant Staphylococcus aureus via fluorophore-enhanced repetitive-sequence PCR. J Clin Microbiol 1995; 33:2141–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rodriguez M, Hogan PG, Satola SW, et al. Discriminatory indices of typing methods for epidemiologic analysis of contemporary Staphylococcus aureus strains. Medicine 2015; 94:e1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. D’Agostino RB Sr, Massaro JM, Sullivan LM. Non-inferiority trials: design concepts and issues—the encounters of academic consultants in statistics. Stat Med 2003; 22:169–86. [DOI] [PubMed] [Google Scholar]
- 39. R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2017. Available at: https://www.R-project.org/. Accessed 20 September 2018. [Google Scholar]
- 40. Hadfield J. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J Stat Softw 2010; 33:1–22.20808728 [Google Scholar]
- 41. Ellis MW, Schlett CD, Millar EV, et al. Hygiene strategies to prevent methicillin-resistant Staphylococcus aureus skin and soft tissue infections: a cluster-randomized controlled trial among high-risk military trainees. Clin Infect Dis 2014; 58:1540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. SanMiguel AJ, Meisel JS, Horwinski J, Zheng Q, Bradley CW, Grice EA. Antiseptic agents elicit short-term, personalized, and body site-specific shifts in resident skin bacterial communities. J Invest Dermatol 2018; 138:2234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Miller LG, Eells SJ, David MZ, et al. Staphylococcus aureus skin infection recurrences among household members: an examination of host, behavioral, and pathogen-level predictors. Clin Infect Dis 2015; 60:753–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Raz R, Miron D, Colodner R, Staler Z, Samara Z, Keness Y. A 1-year trial of nasal mupirocin in the prevention of recurrent staphylococcal nasal colonization and skin infection. Arch Intern Med 1996; 156:1109–12. [PubMed] [Google Scholar]
- 45. Shahbazian JH, Hahn PD, Ludwig S, et al. Multidrug and mupirocin resistance in environmental methicillin-resistant Staphylococcus aureus (MRSA) collected from the homes of people diagnosed with a community-onset MRSA infection. Appl Environ Microbiol 2017; 83:e01369–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cluzet VC, Gerber JS, Metlay JP, et al. CDC Prevention Epicenters Program . The effect of total household decolonization on clearance of colonization with methicillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol 2016; 37:1226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.