Abstract
The in vitro and in vivo efficacy of caspofungin was determined in combination with isavuconazole against Candida auris. Drug–drug interactions were assessed utilizing the fractional inhibitory concentration indices (FICIs), the Bliss independence model and an immunocompromised mouse model. Median planktonic minimum inhibitory concentrations (pMICs) of 23 C. auris isolates were between 0.5 and 2 mg/l and between 0.015 and 4 mg/l for caspofungin and isavuconazole, respectively. Median pMICs for caspofungin and isavuconazole in combination showed 2–128-fold and 2–256-fold decreases, respectively. Caspofungin and isavuconazole showed synergism in 14 out of 23 planktonic isolates (FICI range 0.03–0.5; Bliss cumulative synergy volume range 0–4.83). Median sessile MICs (sMIC) of 14 biofilm-forming isolates were between 32 and >32 mg/l and between 0.5 and >2 mg/l for caspofungin and isavuconazole, respectively. Median sMICs for caspofungin and isavuconazole in combination showed 0–128-fold and 0-512-fold decreases, respectively. Caspofungin and isavuconazole showed synergistic interaction in 12 out of 14 sessile isolates (FICI range 0.023–0.5; Bliss cumulative synergy volume range 0.13–234.32). In line with the in vitro findings, synergistic interactions were confirmed by in vivo experiments. The fungal kidney burden decreases were more than three log volumes in mice treated with combination of 1 mg/kg caspofungin and 20 mg/kg isavuconazole daily; this difference was statistically significant compared with control mice (P < 0.001). Despite the favorable effect of isavuconazole in combination with caspofungin, further studies are needed to confirm the therapeutic advantage of this combination when treating an infection caused by C. auris.
Keywords: Candida auris, biofilms, synergy, isavuconazole, mouse, echinocandin
Lay Summary
Candida auris poses a continuous therapeutic challenge. We demonstrate an approach where the combination of caspofungin and isavuconazole showed a potent activity against planktonic cells and biofilms. This synergism helps to expand the therapeutic options against C. auris.
Introduction
Since its first identification more than 10 years ago, Candida auris has emerged as a global public health threat due to its ability to cause nosocomial outbreaks of invasive infections in health care facilities worldwide.1 Previously, four major phylogenetically distinct lineages (South Asian, East Asian, South African and South American) emerged simultaneously, a phenomenon that highlights the global dissemination of this pathogen. In addition, a potential fifth clade (Iranian origin) has also been described in the recent past.2,3
C. auris can colonize a variety of body sites and medical implants such as central venous catheters, where biofilm development is one of the most important complications.4 An interesting, understudied trait showed by some isolates of C. auris is their ability to form aggregates, which exhibits significantly lower pathogenicity compared to non-aggregating counterparts.4 Previous studies have reported higher biofilm-forming ability in case of non-aggregative strains, however, other results did not confirm this association.5,6 Clinical studies have shown that indwelling devices were the source in 89% of C. auris bloodstream infections; these data emphasize the clinical importance of these sessile communities.7–9 Nevertheless, it is clear that C. auris has exceptionally high minimum inhibitory concentrations (MICs) against the three main classes of antifungals;8,10,11 therefore, the potential biofilm-forming ability further complicates treatment.12 For example, echinocandins – including caspofungin – are frequently administered for the treatment of invasive C. auris infections.13,14 However, these drugs are not expected to be effective in biofilm-related C. auris diseases due to the 2–512-fold higher sessile MIC values to echinocandins.12 The need for novel therapeutic approaches against C. auris is increasing, but the development of new antifungal drugs has decelerated. Therefore, a promising treatment strategy would be to administer antifungals in combination, an approach that can reduce the toxicity and improve the pharmacokinetics and the antifungal effect of drugs used, ultimately improving the prognosis of patients.15,16
In 2016, a new broad-spectrum antifungal drug, isavuconazole, was introduced in clinical practice; it has a favorable safety profile with high activity against a wide variety of fungal pathogens, but the activity of isavuconazole against C. auris is variable.17 Nevertheless, a multicenter study revealed that isavuconazole was not inferior relative to caspofungin for the primary treatment of candidemia and invasive candidiasis.18 Whether combinations of isavuconazole with echinocandins possess synergistic interactions against C. auris, especially against biofilms, has been poorly studied. Hence, we examined in vitro and in vivo combinations of isavuconazole and caspofungin against C. auris isolates derived from the four main clades.
Material
Isolates
Isolates of four different C. auris clades (South Asian, n = 9; East Asian, n = 4; South African, n = 5; South American, n = 5) were tested; their origin is listed in Supplementary Table 1. The isolates showing aggregation are presented in Table 1. Biofilm-forming ability was evaluated with the crystal violet assay as previously described by O'Toole (2011).19 Biofilm development were considered if the O.D. value at 540 nm is higher than 0.15. All isolates were identified to the species level by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Clade delineation was conducted by polymerase chain reaction (PCR) amplification and sequencing of the 28S ribosomal DNA (rDNA) gene and the internal transcribed spacer region 1, as described previously.20,21
Table 1.
Minimum inhibitory concentrations (MICs) of caspofungin alone and in combination with isavuconazole against Candida auris planktonic cells and one-day-old biofilms
| Planktonic cells | Biofilms | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median MIC (range) of drug used (50% O.D. reduction in turbidity) | Median MIC (range) of drug used (50% O.D. reduction in metabolic activity) | |||||||||
| Alone | In combination | Alone | In combination | |||||||
| Clades | Isolates | Phenotype | Caspofungin (mg/l) | Isavuconazole (mg/l) | Caspofungin (mg/l) | Isavuconazole (mg/l) | Caspofungin (mg/l) | Isavuconazole (mg/l) | Caspofungin (mg/l) | Isavuconazole (mg/l) |
| South Asian | 10 | Non-aggregative | >1a | >2b (2– >2) | 0.25 (0.25–0.5) | 0.015 (0.008–0.015) | >32c | >2b | 0.5 | 0.015 (0.015–0.06) |
| 12 | Non-aggregative | 1 | 1 (0.5–1) | 0.5 (0.015–0.5) | 0.015 (0.015–0.25) | >32c | >2b | 0.5 | 0.015 (0.008–0.015) | |
| 20 | Non-aggregative | >1a | 2 (2- >2) | 1 | 0.015 (0.015–1) | >32c | >2b | 0.5 | 0.03 | |
| 27 | Non-aggregative | >1a (1- >1) | 1 (0.5– >2) | 0.25 (0.25–0.5) | 0.015 (0.015–0.06) | >32c | >2b | 0.5 | 0.015 (0.008–0.015) | |
| 33 | Non-aggregative | >1a | 0.06 | 0.015 (0.015–0.03) | 0.008 | NA | NA | NA | NA | |
| 82 | Non-aggregative | >1a | 2 | 0.25 (0.25–1) | 0.25 (0.03–0.25) | >32c | >2b | 4 (4–8) | 0.06 (0.008–0.06) | |
| 164 | Non-aggregative | >1a | 0.5 | 0.5 (0.5–1) | 0.06 (0.03–0.06) | NA | NA | NA | NA | |
| 174 | Non-aggregative | >1a | 0.06 | 0.015 (0.015–0.25) | 0.008 | NA | NA | NA | NA | |
| 196 | Non-aggregative | >1a | 0.06 | 0.06 (0.06–0.125) | 0.008 (0.008–0.015) | NA | NA | NA | NA | |
| East Asian | 15 | Aggregative | 0.5 | 0.5 (0.25–0.5) | 0.25 | 0.25 | >32c | >2b | 0.5 | 0.125 |
| 12 372 | Aggregative | 1 | 0.5 | 0.125 | 0.008 (0.008–0.015) | >32c | >2b | 4 | 1 | |
| 12 373 | Aggregative | 0.5 | 0.5 | 0.25 | 0.25 (0.25–0.125) | NA | NA | NA | NA | |
| Type strain (NCPF 13 029) | Non-aggregative | 0.5 | 0.03 | 0.015 | 0.008 | NA | NA | NA | NA | |
| South African | 2 | Aggregative | 1 | 0.125 (0.06–0.125) | 0.015 | 0.008 (0.008–0.015) | NA | NA | NA | NA |
| 185 | Aggregative | 1 | 0.25 (0.125–0.25) | 0.015 | 0.03 (0.03–0.015) | NA | NA | NA | NA | |
| 204 | Aggregative | 0.5 | 0.125 (0.06–0.125) | 0.015 | 0.03 | 32 (16–32) | >2b | 0.5 | 0.008 | |
| 206 | Aggregative | 1 | 0.25 (0.125–0.25) | 0.015 | 0.008 | NA | NA | NA | NA | |
| 228 | Aggregative | 1 | 0.06 | 0.03 (0.015–0.06) | 0.008 (0.008–0.015) | 32 | >2b | 0.5 | 0.008 (0.008–0.015) | |
| South American | I-24 | Non-aggregative | >1a | 1 (0.5–1) | 0.015 (0.015–1) | 0.008 (0.008–0.03) | >32c | 0.5 | 4 | 0.125 |
| I-172 | Non-aggregative | 1 | 0.5 | 0.5 | 0.25 | >32c | 0.5 (0.5–2) | 16 | 0.06 | |
| 13 108 | Non-aggregative | >1a | 2 | 1 | 0.008 | >32c | >2b | >32c | >2b | |
| 13 112 | Non-aggregative | 0.5 | 2 | 0.015 (0.015–0.03) | 0.5 | >32c | >2b | >32c | >2b | |
| 16 565 | Non-aggregative | 0.5 | 0.015 | 0.015 | 0.008 | 2 | 1 (0.5–1) | 0.5 | 0.06 | |
MIC is off-scale at >1 mg/l, 2 mg/l (one dilution higher than the highest tested concentration) was used for FICI analysis.
MIC is off-scale at >2 mg/l, 4 mg/l (one dilution higher than the highest tested concentration) was used for FICI analysis.
MIC is off-scale at >32 mg/l, 64 mg/l (one dilution higher than the highest tested concentration) was used for FICI analysis.
Determination of the planktonic minimal inhibitory concentration
The planktonic MIC (pMIC) was determined according to the recommendations proposed by the Clinical Laboratory Standards Institute M27-A3 protocol.22 Susceptibility to caspofungin pure powder (Molcan, Toronto, Canada) and isavuconazole pure powder (Merck, Budapest, Hungary) was determined in RPMI-1640 (with l-glutamine and without bicarbonate, pH 7.0, and with MOPS; Merck, Budapest, Hungary). The drug concentrations tested ranged from 0.008 to 4 mg/l for isavuconazole and from 0.03 to 2 mg/l for caspofungin. pMICs were determined as the lowest drug concentration that produces at least 50% growth reduction compared with the growth control. pMICs represent three independent experiments per isolate and are expressed as the median. Candida parapsilosis ATCC 22 019 and Candida krusei ATCC 6258 were used as quality control strains.
Biofilm development
C. auris isolates were subcultured on Sabouraud dextrose agar (Lab M Ltd., Bury, United Kingdom). After 48 h, fungal cells were harvested by centrifugation (3000 g for 5 min) and were washed three times in sterile physiological saline. After the final washing step, pellets were resuspended in physiological saline (ca. 5–6 ml) and were counted using a Bürker chamber (Hirschmann Laborgeräte GmbH & Co. KG, Eberstadt, Germany). The final density of inoculums was adjusted in RPMI-1640 broth to 1 × 106 cells/ml and 100 μl aliquots were inoculated onto flat-bottom 96-well sterile microtitre plates (TPP, Trasadingen, Switzerland) and then incubated statically at 37°C for 24 h to produce one-day-old biofilms.23,25
Determination of the minimal inhibitory concentration of one-day-old biofilms
The examined caspofungin concentrations for sessile MIC (sMIC) determination ranged from 1 to 32 mg/l, while the examined isavuconazole concentrations ranged from 0.008 to 2 mg/l. One-day-old biofilms were washed three times with sterile physiological saline. Subsequently, sMICs were determined in RPMI-1640 using a metabolic activity change–based XTT assay. The percentage change in metabolic activity was calculated based on absorbance (A) at 492 nm as 100% × (Awell – Abackground)/(Adrug-free well – Abackground). sMICs were defined as the lowest drug concentration resulting in at least a 50% metabolic activity decrease compared with untreated control cells.23–25 sMICs represent three independent experiments per isolate and are expressed as the median.
Evaluation of interactions by fractional inhibitory concentration index and the Bliss independence model
Interactions between caspofungin and isavuconazole were assessed by a two-dimensional broth microdilution chequerboard assay.23–25 Interactions were then analyzed by determining the fractional inhibitory concentration index (FICI) and using the Bliss independence model.16,23–26 In the case of planktonic cells, the tested concentration ranged from 0.008 to 2 mg/l for isavuconazole and from 0.015 to 1 mg/l for caspofungin. For biofilms, the examined caspofungin concentrations ranged from 1 to 32 mg/l, while the tested isavuconazole concentrations ranged from 0.008 to 2 mg/l. FICIs were calculated with the widely used following formula: ΣFIC = FICA + FICB = [(MICAcomb/MICAalone)] + [(MICBcomb/MICBalone)], where MICAalone and MICBalone stand for MICs of drugs A and B when used alone, and MICAcomb and MICBcomb represent the MICs of drugs A and B in combination at isoeffective combinations, respectively.16,23–26 FICIs were determined as the lowest ΣFIC. MICs of the drugs alone and of all isoeffective combinations were determined as the lowest concentration resulting in at least 50% metabolic activity reduction compared with the untreated control biofilms. If the obtained MIC was higher than the highest tested drug concentration, the next highest twofold concentration was considered the MIC. FICIs ≤ 0.5 were defined as synergistic, between >0.5 and 4 as indifferent, and >4 as antagonistic.16,23–26 FICIs were determined in three independent experiments and are presented as the median.
To further evaluate caspofungin–isavuconazole interactions, MacSynergy II analysis was applied; this approach employs the Bliss independence algorithm in a Microsoft Excel–based interface to determine synergy.23–27 The Bliss independence algorithm is a well-described method for the examination of the nature of drug–drug interactions. Briefly, the Bliss independence algorithm calculates the difference (ΔE) in the predicted percentage of growth (Eind) and the experimentally observed percentage of growth (Eexp) to define the interaction of the drugs used in combination. Eind is calculated with the equation Eind = EA × EB, where Eind is the predicted percentage of growth that defines the effect of combination when the drugs are acting alone. EA and EB are the experimental percentages of growth with each drug acting alone. The MacSynergy II model uses interaction volumes and defines positive volumes as synergistic and negative volumes as antagonistic. The obtained E values of each combination are presented on the z-axis in the three-dimensional plot. Synergy or antagonism is significant if the interaction log volumes are higher than 2 or lower than 2, respectively.16,27
Log volume values between >2 and 5, between >5 and 9, and >9 should be considered as minor synergy, moderate synergy and strong synergy, respectively. The corresponding negative values define minor, moderate and strong antagonism, respectively. The synergy volumes were calculated at the 95% confidence level.27
Infection model
Pathogen-free female BALB/c mice weighing 22 to 24 g were used for the in vivo experiments. The Guidelines for the Care and Use of Laboratory Animals were strictly followed during the maintenance of mice. Animals were allowed access to food and water ad libitum. In vivo experiments were approved by the Animal Care Committee of the University of Debrecen (permission number is 12/2014). An immunocompromised mouse disseminated model was used for the studies. The animals were rendered neutropenic by intraperitoneal injection of cyclophosphamide (Endoxan, Baxter, Deerfield, IL, USA) 4 days (150 mg/kg body weight) and 1 day (100 mg/kg body weight) before infection and then 2 and 4 days postinfection (100 mg/kg body weight).28 Mice were infected intravenously through the lateral tail vein with 1–1.3 × 107 colony-forming units (CFU) in 200 μl physiological saline.28 The inoculum density was confirmed by plating serial dilutions on Sabouraud dextrose agar. Mice were divided into four groups (8 mice per group): (i) untreated control; (ii) 1 mg/kg/day caspofungin; (iii) 20 mg/kg/day isavuconazole; and (iv) 1 mg/kg/day caspofungin + 20 mg/kg/day isavuconazole. Cresemba© intravenous formulation (Basilea Pharmaceutica Ltd., Basel, Switzerland) was used for isavuconazole treatment. All treatment arms were given intraperitoneally and started 24 h postinfection. In the case of caspofungin–isavuconazole combination, isavuconazole doses were administered 1 h after the caspofungin treatments. Control mice were given 0.5 ml sterile physiological saline intraperitoneally. At 6 days postinfection, animals were euthanized; subsequently, the kidneys of each mouse were removed, weighed and homogenized aseptically. Homogenates were serially diluted tenfold and 100 μl aliquots were plated onto Sabouraud dextrose agar for viable fungal colony counts after incubation for 48 h at 37°C.28 The lower limit of detection was 500 CFU/kidney. The kidney burden was analyzed using the Kruskal–Wallis test with Dunn's post-test (GraphPad Prism 6.05.). Significance was defined as P < 0.05.
Results
The median and the range of MICs for planktonic and sessile C. auris isolates are shown in Table 1. The planktonic form of the tested isolates was considered to be susceptible to caspofungin based on the tentative MIC breakpoint recommended by the Centers for Disease Control and Prevention (≥2 mg/l).29 By the microdilution method, the 23 isolates exhibited pMICs for caspofungin alone from 0.5 to 2 mg/l, with a pMIC50, pMIC90 and geometric mean pMIC of 1, 2 and 1.13 mg/l, respectively. In the case of isavuconazole, pMICs were from 0.015 to 4 mg/l, with a pMIC50, pMIC90 and geometric mean pMIC of 0.5, 2 and 0.33 mg/l, respectively. Fourteen out of 23 isolates formed biofilms, which showed significantly higher resistance to caspofungin and isavuconazole compared with planktonic cells (Table 1). sMICs for caspofungin alone were from 2 to >32 mg/l, with sMIC50, sMIC90 and geometric mean sMIC of >32, > 32 and 45.25 mg/l, respectively (64 mg/l was used for geometric mean sMIC analysis in the case of sMIC >32 mg/l). The biofilm-forming isolates exhibited sMICs for isavuconazole alone from 0.5 to >2 mg/l, with sMIC50, sMIC90 and geometric mean sMIC of >2, > 2 and 3.12 mg/l, respectively (4 mg/l was used for geometric mean sMIC analysis in the case of sMIC >2 mg/l) (Table 1).
The median pMICs observed in combination showed a 2–128-fold and a 2–256-fold reduction for caspofungin and isavuconazole, respectively. A similarly marked reduction in median sMICs was observed for biofilms (a 0–128-fold and a 0–512-fold decrease for caspofungin and isavuconazole, respectively) (Table 1).
Table 2 summarizes the in vitro interactions between caspofungin and isavuconazole based on the median FICIs. An antagonistic interaction was never observed (all FICIs ≤ 4). Using a two-dimensional broth microdilution chequerboard assay and FICI calculation, the nature of the caspofungin–isavuconazole interaction was synergistic for 61% of the planktonic isolates, with median FICIs from 0.03 to 0.5 and a mean of the median FICI of 0.34. In the case of sessile cells, synergism was observed for 86% of the 14 biofilm-forming isolates, with median FICIs from 0.029 to 0.5 and a mean of the median FICI of 0.14 (Table 2).
Table 2.
In vitro interactions by fractional inhibitory concentration indices (FICIs) of caspofungin in combination with isavuconazole against Candida auris planktonic cells and one-day-old biofilms
| Planktonic cells | Biofilms | ||||
|---|---|---|---|---|---|
| FICI | FICI | ||||
| Clades | Isolate | Median (range) of FICI | Interaction | Median (range) of FICI | Interaction |
| South Asian | 10 | 0.28 (0.28–0.312) | Synergy | 0.037 (0.037–0.068) | Synergy |
| 12 | 0.03 (0.03–1) | Synergy | 0.076 (0.023–0.076 | Synergy | |
| 20 | 0.51 (0.51–0.75) | Indifferent | 0.023 | Synergy | |
| 27 | 0.5 (0.25–0.5) | Synergy | 0.029 (0.029–0.038) | Synergy | |
| 33 | 0.313 (0.258–0.375) | Synergy | NA | NA | |
| 82 | 0.375 (0.25–0.51) | Synergy | 0.155 (0.133–0.155) | Synergy | |
| 164 | 0.62 (0.56–0.75) | Indifferent | NA | NA | |
| 174 | 0.383 (0.375–0.5) | Synergy | NA | NA | |
| 196 | 0.53 (0.5–0.625) | Indifferent | NA | NA | |
| East Asian | 15 | 1 (0.75–1) | Indifferent | 0.5 | Synergy |
| 12 372 | 0.37 (0.31–0.5) | Synergy | 0.375 (0.187–0.375) | Synergy | |
| 12 373 | 0.5 (0.5–1) | Synergy | NA | NA | |
| Type strain (NCPF 13 029) | 0.296 | Synergy | NA | NA | |
| South African | 2 | 0.37 (0.255–0.49) | Synergy | NA | NA |
| 185 | 0.245 (0.245–0.255) | Synergy | NA | NA | |
| 204 | 0.51 (0.49–0.54) | Indifferent | 0.019 | Synergy | |
| 206 | 0.255 (0.255–0.3) | Synergy | NA | NA | |
| 228 | 0.53 (0.5–0.625) | Indifferent | 0.03 (0.03–0.06) | Synergy | |
| South American | I-24 | 0.51 | Indifferent | 0.5 (0.5–0.56) | Synergy |
| I-172 | 1 | Indifferent | 0.31 (0.28–0.37) | Synergy | |
| 13 108 | 0.5 | Synergy | 2 | Indifferent | |
| 13 112 | 0.37 (0.37–0.5) | Synergy | 2 | Indifferent | |
| 16 565 | 0.563 (0.563–0.593) | Indifferent | 0.31 | Synergy | |
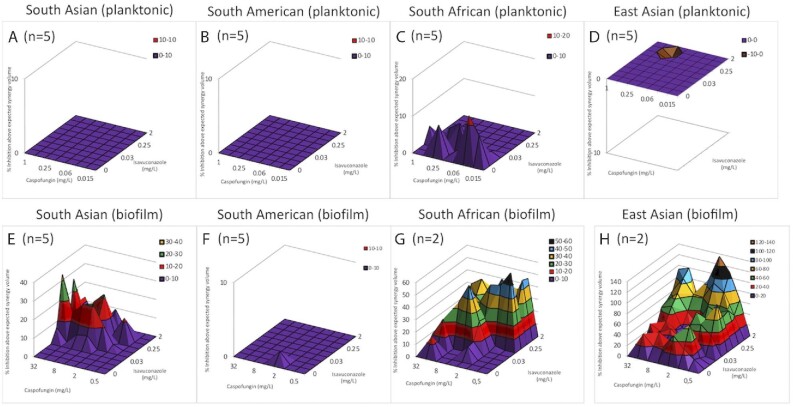
FICI calculation involves Loewe additivity-based analysis assuming that both drugs have the same mechanism of action, while the Bliss independence–based MacSynergy II program does not have this assumption. Figure 1 shows the dose-response surfaces for caspofungin–isavuconazole generated with MacSynergy II. Based on clade-specific cumulative log volumes, the combination of caspofungin and isavuconazole exerted minor synergy (the synergy log volume was 4.83) against planktonic isolates derived from the South African clade (Fig. 1C). For the South Asian, South American and East Asian clades, the synergy log volumes were zero, indicating indifferent interactions (Fig. 1A, B and D). In the case of biofilms, 77.2, 23.21 and 234.32 cumulative synergy log volumes were observed for South African, South Asian and East Asian clades, respectively, indicating strong synergistic interactions (Fig. 1E, G and H). By contrast, the South American clade exhibited an indifferent interaction, with a cumulative synergy log volume of 0.13 (Fig. 1F). Based on the evaluation of in vitro combinations, the data derived from the FICI calculation correlate with the MacSynergy analysis primarily in the case of biofilms. Although the combination of caspofungin and isavuconazole was synergistic or considerably reduced the amount of drug needed in some instances, the observed results may show strain specificity within clades, especially in the case of planktonic cells.
Figure 1.
Effect of caspofungin in combination with isavuconazole against planktonic (A-D) and sessile (E-H) Candida auris isolates using MacSynergy II analysis. Positive values show synergy, while negative values indicate antagonism at given concentrations. The volumes are calculated at the 95% confidence interval.
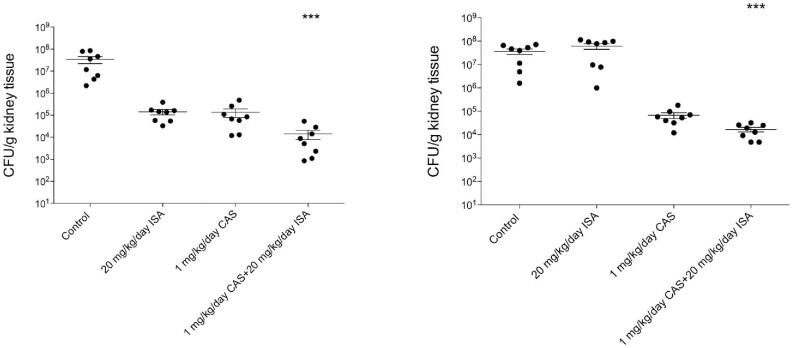
To further evaluate the in vivo applicability of the caspofungin and isavuconazole combination, representative isolates were chosen where synergistic and indifferent interactions were observed in vitro, respectively. The results of the in vivo experiments are shown in Fig. 2. A total of 1 mg/kg daily caspofungin treatment decreased the fungal kidney burden in the case of the tested isolates; however, this therapeutic strategy was not statistically different compared with untreated control mice (P > 0.05). The 20 mg/kg/day isavuconazole treatment proved to be statistically ineffective against the tested C. auris isolates, especially in the case of isolate 13 112 (P > 0.05). It is noteworthy that the fungal tissue burden decreases were higher than the three log decreases in mice treated with a daily combination of 1 mg/kg caspofungin and 20 mg/kg isavuconazole, which was statistically significant compare with control mice (P < 0.001) (Fig. 2).
Figure 2.
Kidney tissue burden of deeply neutropenic BALB/c mice infected intravenously with Candida auris 12 (A) and 13 112 (B) isolates. Daily intraperitoneal caspofungin (CAS) (1 mg/kg/day) and isavuconazole (ISA) (20 mg/kg/day) treatment was started 24 h after the infection. Tissue burden experiments were performed on day 6 post-infection. Bars represent means ± standard error of mean. *** corresponds to P < 0.001 compared with the control population.
Discussion
The impending challenge of antifungal resistance and newly emerged fungal pathogens necessitates bold and innovative therapeutic solutions.30 In recent years, combination-based antifungal treatments have become a promising therapeutic approach, especially against multidrug-resistant fungal species such as C. auris. Based on previous susceptibility studies against C. auris, the efficacy of in vitro combinations has shown high variability; in addition, the degree of activity is highly strain – or rather clade – specific.31–33
Isavuconazole is recommended primarily for the treatment of invasive aspergillosis and mucormycosis; however, it has also exerted variable in vitro activity against several Candida species.17 Sanglard and Coste (2016) reported that the activity range of isavuconazole is similar to that of voriconazole against the Candida strains they tested, findings that were confirmed by Marcos-Zambrano et al. (2018), who showed high in vitro activity of isavuconazole against clinically relevant Candida species, particularly against C. albicans.34–35 Desnos-Ollivier et al. (2019) reported an isavuconazole MIC of 0.015 mg/l against planktonic C. auris; however, they tested only two strains.36 Regarding clinical findings, the ACTIVE trial compared intravenous isavuconazole to intravenous caspofungin followed by oral isavuconazole in a phase 3 randomized, double-blind clinical trial for patients with Candida bloodstream infection. These results support the use of isavuconazole as a potential therapy for candidiasis.18
To the best of our knowledge, this is the first study to examine the in vitro and in vivo combined effect of isavuconazole and caspofungin against C. auris strains derived from four different lineages focusing on both planktonic and sessile susceptibility. In the case of Aspergillus spp., this combination showed synergistic interaction in 13% of tested strains.37 In our study, we found in vitro synergy for the caspofungin–isavuconazole combination using chequerboard microdilution, especially based on FICI determination, which was definitely pronounced in the case of one-day-old biofilms. It is noteworthy, that synergistic interaction was observed in 100 and 80% of the biofilms formed by aggregative and non-aggregative strains, respectively, while this ratio was comparable in case of planktonic forms. Interestingly, the highest synergy volumes obtained by Bliss algorithm were observed in case of biofilms formed by aggregative strains. Brown et al. (2020) described that several key cell membrane and cell wall components were upregulated in aggregative biofilms.38 We hypothesize that the isavuconazole-caspofungin combination may interfere with these enhanced cell wall and cell membrane related pathways, which are essential for aggregative biofilms explaining the higher synergy volumes observed.38
Katragkou et al. (2017) showed synergistic interactions between isavuconazole and micafungin against C. albicans, Candida parapsilosis and Candida krusei using the Bliss independence model (the degree of synergy ranged from 1.8 to 16.7%), which was confirmed by time-kill curves, especially against C. albicans and C. parapsilosis.39 Voriconazole exerted synergistic interaction with caspofungin or other echinocandins against C. auris isolates using the FICI.40 In a recent study, Pfaller et al. (2021) examined the in vitro activity of voriconazole or isavuconazole in combination with anidulafungin; synergy or partial synergy was observed in 14 and 61% of the isolates with the combination of anidulafungin plus voriconazole and in 19 and 53% of isolates for the combination of anidulafungin plus isavuconazole.41 It is noteworthy that O'Brien et al. (2020) examined four pan-resistant C. auris strains derived from a New York outbreak to evaluate whether they are susceptible to combinations of antifungals. Based on their results, flucytosine combinations with either amphotericin B, azoles or echinocandins exhibited the highest efficacy.32 However, the combination of azoles with echinocandins had no superior effect compared with monotherapy.32
The number of in vivo experiments focusing on combination-based therapy against C. auris is strongly limited. In the only published in vivo combination-based experiments, Eldesouky et al. (2018) observed that the examined sulfamethoxazole–voriconazole combination enhanced the survival of Caenorhabditis elegans nematodes infected with C. auris by nearly 70%.42 Our study is the first that has examined the effect of caspofungin in combination with isavuconazole in vivo at clinically relevant concentrations using an immunocompromised mouse model. Although caspofungin alone produced a remarkable reduction in the kidney fungal burden, only its combination with isavuconazole was statistically superior compared with the untreated control (P < 0.001).
The multidrug resistance phenotype is a well-known characteristic for C. auris; it may be more pronounced in biofilms and further complicate treatment.9,10 Based on previous susceptibility testing, amphotericin B, fluconazole, voriconazole, anidulafungin, micafungin and caspofungin could not completely eradicate C. auris biofilms in vitro, increasing the need for effective combination therapies.43 Certain non-antifungal agents in combination with traditional antifungal drugs have been tested to eradicate C. auris biofilms with variable efficacy.24,25,44 However, to date there is no experimental evidence about the efficacy of antifungal drug–drug combinations against C. auris biofilms. In our study, we found a prominent synergistic interaction between caspofungin and isavuconazole against biofilms for three out of the four clades examined. We observed indifferent interaction only in the case of two hospital-derived isolates from the South American clade (13 112, 13 108). The origin of these strains may explain the significantly higher resistance against drugs tested and the observed indifferent interaction compared to other isolates.
It should be pointed out that our study had a limitation, namely the choice of the endpoint for FICI-based assessment of antifungal combinations. To date, there is no solid consensus about which endpoint should be used.26,27,33 In addition, for MacSynergy-based evaluation, there is no endpoint at all, and the nature of the interaction is calculated only based on the percentage of growth at given concentrations.27,33 Despite this limitation, the therapeutic potential of the caspofungin and isavuconazole in combinations is unquestionable, which was definitely confirmed against C. auris biofilms and our in vivo experiments.
In summary, the presented synergistic combinations correspond to clinically achievable and safe drug concentrations. Our findings suggest that administration of the caspofungin–isavuconazole combination may help to expand the therapeutic options against C. auris. Nevertheless, the more extensive in vivo correlation and significant clinical relevance of these in vitro and in vivo results warrants further studies, especially in the case of biofilms.
Supplementary Material
Acknowledgments
The authors thank Dr. Shawn R. Lockhart and Dr. Ronen Ben-Ami for their generous gifts of Candida auris strains from South America and Israel, respectively.
Contributor Information
Fruzsina Nagy, Department of Medical Microbiology, Faculty of Medicine, University of Debrecen, Nagyerdei krt. 98., 4032 Debrecen, Hungary; Doctoral School of Pharmaceutical Sciences, University of Debrecen, Nagyerdei krt. 98, 4032 Debrecen, Hungary.
Zoltán Tóth, Department of Medical Microbiology, Faculty of Medicine, University of Debrecen, Nagyerdei krt. 98., 4032 Debrecen, Hungary; Doctoral School of Pharmaceutical Sciences, University of Debrecen, Nagyerdei krt. 98, 4032 Debrecen, Hungary; Department of Pharmacology and Pharmacotherapy, Faculty of Medicine, University of Debrecen, Nagyerdei krt. 98, 4032 Debrecen, Hungary.
Fanni Nyikos, Department of Medical Microbiology, Faculty of Medicine, University of Debrecen, Nagyerdei krt. 98., 4032 Debrecen, Hungary.
Lajos Forgács, Department of Medical Microbiology, Faculty of Medicine, University of Debrecen, Nagyerdei krt. 98., 4032 Debrecen, Hungary; Doctoral School of Pharmaceutical Sciences, University of Debrecen, Nagyerdei krt. 98, 4032 Debrecen, Hungary.
Ágnes Jakab, Department of Molecular Biotechnology and Microbiology, Faculty of Science and Technology, Institute of Biotechnology, University of Debrecen, Nagyerdei krt. 98, 4032 Debrecen, Hungary.
Andrew M Borman, UK National Mycology Reference Laboratory, Public Health England, Science Quarter, Southmead Hospital, Bristol BS10 5NB, UK; Medical Research Council Centre for Medical Mycology, University of Exeter, Exeter EX4 4QD, UK.
László Majoros, Department of Medical Microbiology, Faculty of Medicine, University of Debrecen, Nagyerdei krt. 98., 4032 Debrecen, Hungary.
Renátó Kovács, Department of Medical Microbiology, Faculty of Medicine, University of Debrecen, Nagyerdei krt. 98., 4032 Debrecen, Hungary; Department of Metagenomics, University of Debrecen, Nagyerdei krt. 98, 4032 Debrecen, Hungary.
Funding
Renátó Kovács was supported by the EFOP-3.6.3-VEKOP-16-2017-00009 program, OTKA Bridging Fund and FEMS Research and Training Grant (FEMS-GO-2019-502). Zoltán Tóth and Fruzsina Nagy were supported by the ÚNKP-19-3 and ÚNKP-20-3 New National Excellence Program of the Ministry for Innovation and Technology. The publication is supported by the GINOP-2.3.4-15-2020-00008 project.
Declaration of interest
László Majoros has received conference travel grants from MSD, Astellas, Pfizer and Cidara. All other authors declare no competing interests.
Ethical approval
Not required.
References
- 1.Meis JF, Chowdhary A. Candida auris: a global fungal public health threat. Lancet Infect Dis. 2018; 18: 1298–1299. [DOI] [PubMed] [Google Scholar]
- 2.Lockhart SR, Etienne KA, Vallabhaneni Set al. . Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis. 2017; 64: 134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chow NA, de Groot T, Badali H, Abastabar M, Chiller TM, Meis JF. Potential fifth clade of Candida auris, Iran, 2018. Emerg Infect Dis. 2019; 25: 1780–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kean R, Ramage G. Combined antifungal resistance and biofilm tolerance: the global threat of Candida auris. mSphere. 2019; 4: e00458–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sherry L, Ramage G, Kean Ret al. . Biofilm-forming capability of highly virulent, multidrug-resistant Candida auris. Emerg Infect Dis. 2017; 23: 328–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh R, Kaur M, Chakrabarti A, Shankarnarayan SA, Rudramurthy SM. Biofilm formation by Candida auris isolated from colonising sites and candidemia cases. Mycoses. 2019; 62: 706–709. [DOI] [PubMed] [Google Scholar]
- 7.Sayeed MA, Farooqi J, Jabeen K, Mahmood SF. Comparison of risk factors and outcomes of Candida auris candidemia with non-Candida auris candidemia: a retrospective study from Pakistan. Med Mycol. 2020; 58: 721–729. [DOI] [PubMed] [Google Scholar]
- 8.Horton MV, Nett JE. Candida auris infection and biofilm formation: going beyond the surface. Curr Clin Microbiol Rep. 2020; 7: 51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arensman K, Miller JL, Chiang Aet al. . Clinical outcomes of patients treated for Candida auris Infections in a Multisite Health System, Illinois, USA. Emerg Infect Dis. 2020; 26: 876–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lockhart SR. Candida auris and multidrug resistance: defining the new normal. Fungal Genet Biol. 2019; 131: 103243. [DOI] [PubMed] [Google Scholar]
- 11.Chowdhary A, Prakash A, Sharma Cet al. . A multicentre study of antifungal susceptibility patterns among 350 Candida auris isolates (2009-17) in India: role of the ERG11 and FKS1 genes in azole and echinocandin resistance. J Antimicrob Chemother. 2018; 73: 891–899. [DOI] [PubMed] [Google Scholar]
- 12.Romera D, Aguilera-Correa JJ, Gadea I, Viñuela-Sandoval L, García-Rodríguez J, Esteban J. Candida auris: a comparison between planktonic and biofilm susceptibility to antifungal drugs. J Med Microbiol. 2019; 68: 1353–1358. [DOI] [PubMed] [Google Scholar]
- 13.Singhal T, Kumar A, Borade P, Shah S, Soman R. Successful treatment of C. auris shunt infection with intraventricular caspofungin. Med Mycol Case Rep. 2018; 22: 35–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cortegiani A, Misseri G, Fasciana T, Giammanco A, Giarratano A, Chowdhary A. Epidemiology, clinical characteristics, resistance, and treatment of infections by Candida auris. J Intensive Care. 2018; 6: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kontoyiannis DP, Lewis RE. Toward more effective antifungal therapy: the prospects of combination therapy. Br J Haematol. 2004; 126: 165–175. [DOI] [PubMed] [Google Scholar]
- 16.Bidaud AL, Schwarz P, Herbreteau G, Dannaoui E. Techniques for the assessment of in vitro and in vivo antifungal combinations. J Fungi (Basel). 2021; 7: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellsworth M, Ostrosky-Zeichner L. Isavuconazole: mechanism of action, clinical efficacy, and resistance. J Fungi (Basel). 2020; 6: 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kullberg BJ, Viscoli C, Pappas PGet al. . Isavuconazole versus caspofungin in the treatment of Candidemia and other invasive Candida infections: the ACTIVE Trial. Clin Infect Dis. 2019; 68: 1981–1989. [DOI] [PubMed] [Google Scholar]
- 19.O'Toole GA. Microtiter dish biofilm formation assay. J Vis Exp. 2011;2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borman AM, Szekely A, Johnson EM. Comparative pathogenicity of United Kingdom isolates of the emerging pathogen Candida auris and other key pathogenic Candida species. mSphere. 2016; 1: e00189–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borman AM, Szekely A, Johnson EM. Isolates of the emerging pathogen Candida auris present in the UK have several geographic origins. Med Mycol. 2017; 55: 563–567. [DOI] [PubMed] [Google Scholar]
- 22.Clinical and Laboratory Standards Institute . Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. Approved Standard, 3rd ed.; M27-A3; CLSI: Wayne, PA, USA, 2008. [Google Scholar]
- 23.Nagy F, Tóth Z, Daróczi Let al. . Farnesol increases the activity of echinocandins against Candida auris biofilms. Med Mycol. 2020; 58: 404–407. [DOI] [PubMed] [Google Scholar]
- 24.Nagy F, Vitális E, Jakab Áet al. . In vitro and in vivo effect of exogenous farnesol exposure against Candida auris. Front Microbiol. 2020; 11: 957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kovács R, Nagy F, Tóth Zet al. . The Neosartorya fischeri antifungal protein 2 (NFAP2): a new potential weapon against multidrug-resistant Candida auris biofilms. Int J Mol Sci. 2021; 22: 771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meletiadis J, Verweij PE, TeDorsthorst DT, Meis JF, Mouton JW. Assessing in vitro combinations of antifungal drugs against yeasts and filamentous fungi: comparison of different drug interaction models. Med Mycol. 2005; 43: 133–152. [DOI] [PubMed] [Google Scholar]
- 27.Prichard MN, Shipman C Jr. A three-dimensional model to analyze drug-drug interactions. Antiviral Res. 1990; 14: 181–205. [DOI] [PubMed] [Google Scholar]
- 28.Forgács L, Borman AM, Prépost Eet al. . Comparison of in vivo pathogenicity of four Candida auris clades in a neutropenic bloodstream infection murine model. Emerg Microbes Infect. 2020; 9: 1160–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention . Antifungal Susceptibility Testing and Interpretation. 2020. Available online: https://www.cdc.gov/fungal/candida-auris/c-auris-antifungal.html (accessed on 29 May 2020). [Google Scholar]
- 30.Roemer T, Krysan DJ. Antifungal drug development: challenges, unmet clinical needs, and new approaches. Cold Spring Harb Perspect Med. 2014; 4: a019703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Y, Totten M, Memon W, Ying C, Zhang SX. In vitro antifungal susceptibility of the emerging multidrug-resistant pathogen Candida auris to miltefosine alone and in combination with amphotericin B. Antimicrob Agents Chemother. 2020; 64: e02063–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Brien B, Chaturvedi S, Chaturvedi V. In vitro evaluation of antifungal drug combinations against multidrug-resistant Candida auris isolates from New York outbreak. Antimicrob Agents Chemother. 2020; 64: e02195–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwarz P, Bidaud AL, Dannaoui E. In vitro synergy of isavuconazole in combination with colistin against Candida auris. Sci Rep. 2020; 10: 21448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanglard D, Coste AT. Activity of isavuconazole and other Azoles against Candida clinical isolates and yeast model systems with known azole resistance mechanisms. Antimicrob Agents Chemother. 2016; 60: 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marcos-Zambrano LJ, Gómez A, Sánchez-Carrillo Cet al. . Isavuconazole is highly active in vitro against Candida species isolates but shows trailing effect. Clin Microbiol Infect. 2018; 24: 1343.e1–1343.e4. [DOI] [PubMed] [Google Scholar]
- 36.Desnos-Ollivier M, Bretagne S, Boullié A, Gautier C, Dromer F, Lortholary O; French Mycoses Study Group. Isavuconazole MIC distribution of 29 yeast species responsible for invasive infections (2015-2017). Clin Microbiol Infect. 2019; 25: 634.e1–634.e4. [DOI] [PubMed] [Google Scholar]
- 37.Raffetin A, Courbin V, Jullien V, Dannaoui E. In vitro combination of isavuconazole with echinocandins against azole-susceptible and -resistant Aspergillus spp. Antimicrob Agents Chemother. 2017; 62: e01382–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown JL, Delaney C, Short Bet al. . Candida auris phenotypic heterogeneity determines pathogenicity in vitro. mSphere. 2020; 5: e00371–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katragkou A, McCarthy M, Meletiadis Jet al. . In vitro combination therapy with isavuconazole against Candida spp. Med Mycol. 2017; 55: 859–868. [DOI] [PubMed] [Google Scholar]
- 40.Fakhim H, Chowdhary A, Prakash Aet al. . In vitro interactions of echinocandins with triazoles against multidrug-resistant Candida auris. Antimicrob Agents Chemother. 2017; 61: e01056–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pfaller MA, Messer SA, Deshpande LM, Rhomberg PR, Utt EA, Castanheira M. Evaluation of synergistic activity of isavuconazole or voriconazole plus anidulafungin and the occurrence and genetic characterisation of Candida auris detected in a surveillance program. Antimicrob Agents Chemother. 2021; 65: AAC.02031-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eldesouky HE, Li X, Abutaleb NS, Mohammad H, Seleem MN. Synergistic interactions of sulfamethoxazole and azole antifungal drugs against emerging multidrug-resistant Candida auris. Int J Antimicrob Agents. 2018; 52: 754–761. [DOI] [PubMed] [Google Scholar]
- 43.Vargas-Cruz N, Reitzel RA, Rosenblatt Jet al. . Nitroglycerin-citrate-ethanol catheter lock solution is highly effective for in vitro eradication of Candida auris biofilm. Antimicrob Agents Chemother. 2019; 63: e00299–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wall G, Chaturvedi AK, Wormley FL Jret al. . Screening a repurposing library for inhibitors of multidrug-resistant Candida auris identifies ebselen as a repositionable candidate for antifungal drug development. Antimicrob Agents Chemother. 2018; 62: e01084–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.