Abstract
Background
Respiratory syncytial virus (RSV) is a major cause of childhood medically attended respiratory infection (MARI).
Methods
We conducted a randomized, double-blind, placebo-controlled phase 3 trial in 1154 preterm infants of 1 or 2 doses of suptavumab, a human monoclonal antibody that can bind and block a conserved epitope on RSV A and B subtypes, for the prevention of RSV MARI. The primary endpoint was proportion of subjects with RSV-confirmed hospitalizations or outpatient lower respiratory tract infection (LRTI).
Results
There were no significant differences between primary endpoint rates (8.1%, placebo; 7.7%, 1-dose; 9.3%, 2-dose). Suptavumab prevented RSV A infections (relative risks, .38; 95% confidence interval [CI], .14–1.05 in the 1-dose group and .39 [95% CI, .14–1.07] in the 2-dose group; nominal significance of combined suptavumab group vs placebo; P = .0499), while increasing the rate of RSV B infections (relative risk 1.36 [95% CI, .73–2.56] in the 1-dose group and 1.69 [95% CI, .92–3.08] in the 2-dose group; nominal significance of combined suptavumab group vs placebo; P = .12). Sequenced RSV isolates demonstrated no suptavumab epitope changes in RSV A isolates, while all RSV B isolates had 2–amino acid substitution in the suptavumab epitope that led to loss of neutralization activity. Treatment emergent adverse events were balanced across treatment groups.
Conclusions
Suptavumab did not reduce overall RSV hospitalizations or outpatient LRTI because of a newly circulating mutant strain of RSV B. Genetic variation in circulating RSV strains will continue to challenge prevention efforts.
Clinical Trials Registration
Keywords: respiratory syncytial virus, infants, safety, efficacy
In a randomized, double-blind, placebo-controlled phase 3 trial in 1154 preterm infants, 1 or 2 doses of suptavumab did not reduce overall respiratory syncytial virus (RSV) hospitalizations or outpatient LRTI because of a newly circulating mutant strain of RSV-B.
(See the Editorial Commentary by Langedijk and Bont on pages e4409–10.)
Respiratory syncytial virus (RSV) is the most common viral cause of acute lower respiratory tract infection (LRTI) and LRTI-related deaths in children younger than 5 years of age [1], but no preventative RSV vaccine is licensed [2]. Passive immunization with monthly administered anti-RSV monoclonal antibody, palivizumab, has been used since 1998 [3, 4], but position statements such as that from the American Academy of Pediatrics recommend its use only in the highest-risk category [4].
Suptavumab is a fully human monoclonal antibody that targets the prefusion F-protein–binding epitope [5]. Compared with synthesized palivizumab (manufactured at Regeneron from published sequences), suptavumab was 10- and 5-fold more potent in RSV-A and RSV-B neutralization in microneutralization assays, respectively. Serum levels associated with a 2-log or higher (99%) reduction (EC99) in RSV-A and RSV-B pulmonary viral titer in cotton rats were 4.9 mg/L and 27.8 mg/L, respectively [6]. The suptavumab epitope was highly conserved on interrogation of published data [7], neutralizing approximately 90 clinical RSV isolates from 1995 to 2015. Suptavumab was well tolerated in healthy adults [8]. Here we report the results of a global (18 countries), randomized, placebo-controlled phase 3 study of suptavumab in preterm infants to prevent medically attended RSV infection.
METHODS
Study Design
This phase 3 trial assessed the efficacy of suptavumab 30 mg/kg intramuscular as 1 dose or 2 doses administered 8 weeks apart in preventing medically attended RSV infections in preterm infants who were ineligible or without access to palivizumab. The study was conducted between November 2015 through September 2017 over 3 RSV seasons in both hemispheres. All participants’ parents or legal guardians provided written informed consent before participating in the trial.
Participants
The study participants consisted of healthy infants with a chronological age of younger than 6 months at the time of first dose and a gestational age of less than 36 weeks, and who were not eligible, recommended for, nor had access to palivizumab by standard practice, local guidelines, or their healthcare provider. Inclusion and exclusion criteria are available in Supplementary Appendix 1.
Interventions and Procedures
Eligible infants were randomly assigned in a 1:1:1 ratio into 3 groups: 1 dose of suptavumab 30 mg/kg and 1 dose of placebo, 2 doses of suptavumab 30 mg/kg, or 2 doses of placebo. Randomization was stratified by region (North America vs rest of world) and gestational age (≤31 weeks, 6 days; or 32 weeks, 0 days, to <36 weeks).
The efficacy follow-up period was 150 days after the first dose of study drug, with data collected on any acute medically attended respiratory illness (MARI). Following enrollment, parents/guardians were asked to contact study staff when attending a medical provider for MARI. Study personnel evaluated the subject during an unscheduled visit less than 72 hours following the onset of signs and symptoms of the respiratory illness, including medical history and nasal swab collections for testing using reverse transcriptase–polymerase chain reaction (RT-PCR) and genotyping assays for RSV identification and subtyping.
Endpoints
The primary endpoint was the proportion of individuals with a medically attended RSV infection (hospitalization for RSV infection or outpatient visit with RSV LRTI) during the 150-day efficacy follow-up period. Subjects who did not complete the 150-day period and had no observed primary endpoint event were imputed to the rate of the primary endpoint estimated using the placebo group. An RSV LRTI in an infant was defined as an RSV-confirmed infection with parent/guardian report of cough or difficulty breathing and with one of the following signs of LRTI, as assessed by a healthcare provider: lower chest wall indrawing, hypoxemia (peripheral capillary oxygen saturation <95% breathing room air) or wheezing, or crackles on auscultation of the chest. A key secondary endpoint was the proportion of subjects who had RSV-confirmed hospitalization or outpatient visit (for upper or lower tract infections), by RSV subgroup. Additional secondary endpoints are listed in Supplementary Appendix 1.
Safety was evaluated for those receiving 1 or more doses of an investigational product or placebo. Infants were followed by study investigators for 237 days. Additional details can be found in Supplementary Appendix 1.
Laboratory Assessments
Serum samples were analyzed for total suptavumab concentration using a validated enzyme-linked immunosorbent assay. The lower limit of quantification was 0.078 mg/L. Half-life was determined using population-based pharmacokinetic modeling [9].
Sparsely sampled suptavumab concentrations in serum were analyzed with a population pharmacokinetic model to predict drug concentration at time of first infection in those infants with a primary endpoint event during follow-up. Anti-suptavumab antibody status was assessed in serum samples using a validated electrochemiluminescence bridging immunoassay.
Methods for sequencing of RSV-F from clinical RSV isolates recovered from study participants and in vitro binding and neutralization assays conducted on these isolates are presented in Supplementary Appendix 4.
Statistical Analysis
The full statistical analysis plan can be found in Supplementary Appendix 2. Sample size was calculated assuming a primary endpoint event rate of 10% for the placebo group and 4% for either treatment arm of suptavumab (60% reduction), a 2-sided significance level of α = .025, and a 5% early dropout rate. We estimated that 1515 randomized subjects (505 subjects in each arm) were required to provide 90% power, based on a chi-square test with continuity correction and performed using nQuery version 7.0 (Statistical Solutions Ltd, Cork, Ireland). During the 2016–2017 Northern Hemisphere RSV season, the target sample size was reduced to approximately 1200 subjects due to the sponsor’s administrative decision. The Data and Safety Monitoring Board was informed of this decision and all other administrative changes to the protocol. To accommodate reduced sample size, overall type I error for the pairwise comparisons per suptavumab dose regimen to placebo was controlled using a prespecified hierarchical inferential approach. The comparison between the suptavumab 2-dose arm and placebo was the first test, and the comparison between the suptavumab 1-dose arm and placebo was the second. Statistical significance of the first test was required before drawing inferential conclusions about the second test at the 2-sided .05 α level (see also Supplementary Appendix 2). Assuming approximately 800 subjects (400 per arm) would be administered the study drug, the comparison between the suptavumab 2-dose regimen and placebo would have approximately 87% power to detect the previously planned 60% reduction in medically attended RSV infections with a 2-sided significance level of .05.
Efficacy analyses were performed in the full analysis set (FAS), which included all infants randomized and dosed with study drug. Any subject with a positive RT-PCR RSV result should not have received their second study drug dose; however, suptavumab 2-dose subject data were analyzed in the suptavumab 2-dose group (as randomized) for efficacy evaluation, whereas safety data were analyzed in the suptavumab 1-dose group (as treated) if the subject received only 1 dose. The absolute difference in the proportion of subjects with primary endpoint events was tested for the comparison between each suptavumab dose group and placebo, with the randomization stratum adjusted by the Mantel–Haenszel method. Exploratory analyses of primary endpoint events were performed for RSV-A and RSV-B. The study was not powered to show efficacy for each of the subtypes. Only observed primary endpoint events with available RSV subtype information were included in this analysis. For safety variables, subjects were analyzed as treated.
RESULTS
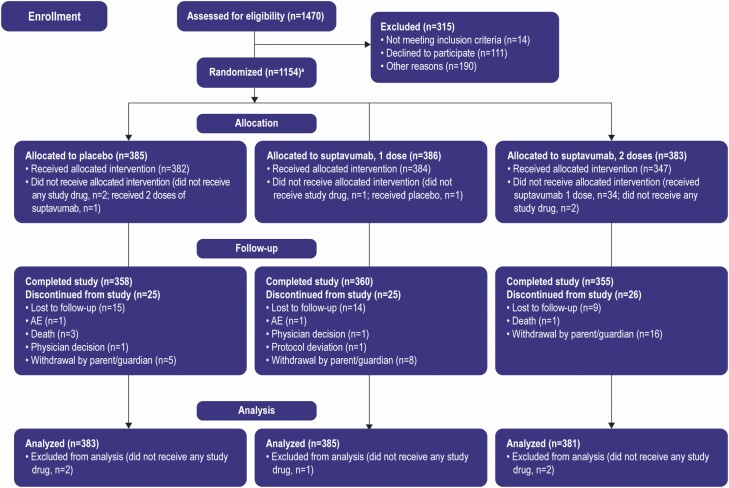
From November 2015 through September 2017, 1470 infants underwent screening for eligibility, 1154 were randomized, and 1149 of them received 1 or more study drug dose (FAS and safety population) (Figure 1). Twenty-five (6.5%) subjects in the suptavumab 1-dose group, 26 (6.8%) in the suptavumab 2-dose group, and 25 (6.5%) in the placebo group did not complete the study. Baseline demographic characteristics were similar between groups (Supplementary Table 1).
Figure 1.
Randomization, trial assignment, and follow-up. aOne subject eligible for the trial was not randomized but was dosed. Note: The full analysis set (all infants randomized and dosed with study drug) is used for analysis. Abbreviation: AE, adverse events.
Primary and Secondary Outcomes
The suptavumab 2-dose group did not show clinical benefit regarding RSV-related hospitalization or outpatient LRTI compared with placebo (9.3% vs 8.1%, respectively) (P = .58) (Table 1). Formal statistical testing was stopped after comparing the suptavumab 2-dose group with placebo according to the hierarchical testing procedure. Similar results were demonstrated in the suptavumab 1-dose group compared with placebo (7.7% vs 8.1%, respectively). Similar RSV hospitalization incidence (3.5% placebo vs 3.0% suptavumab 1-dose group and 4.4% suptavumab 2-dose group) and similar RSV outpatient LRTI incidence (4.6% vs 4.7% and 5.0%, respectively) were observed in all 3 treatment groups.
Table 1.
Incidence of Medically Attended Respiratory Syncytial Virus Infection in Preterm Infants, by Treatment Group
| Placebo (n = 383) | Suptavumab Single Dose (n = 385) | Relative Risk Reduction,% | P | Suptavumab 2 Doses (N = 381) | Relative Risk Reduction | P | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | % | ||||
| Primary endpoint (composite) | ||||||||||
| Subjects with an RSV hospitalization or outpatient LRTI | 31.1 | 8.1 | 29.7 | 7.7 | 1.00 | .8239a | 35.5 | 9.3 | −16.68 | .5773 |
| Secondary endpoint (composite) | ||||||||||
| RSV hospitalization or outpatient URTI or LRTI | 47.7 | 12.5 | 45.7 | 11.9 | 7.26 | .7778a | 55.3 | 14.5 | −14.81 | .4154a |
| Components of the primary and secondary endpoints | ||||||||||
| RSV hospitalization | 13.5 | 3.5 | 11.7 | 3.0 | 16.12 | NA | 16.7 | 4.4 | −23.78 | NA |
| RSV outpatient LRTI | 17.7 | 4.6 | 18.0 | 4.7 | 1.14 | NA | 19.0 | 5.0 | −11.27 | NA |
| RSV outpatient LRTI or URTI | 34.3 | 9.0 | 33.9 | 8.8 | 1.17 | NA | 38.9 | 10.2 | −11.78 | NA |
Patient numbers include both subjects with observed event or RSV-related death and imputed events for early terminated subjects with missing outcomes at the end of the 150-day period.
Abbreviations: LRTI, lower respiratory tract infection; NA, not applicable; RSV, respiratory syncytial virus; URTI, upper respiratory tract infection.
aNominal P value.
No reduction in the secondary efficacy endpoint (RSV hospitalization or RSV outpatient visits) for upper respiratory tract infection (URTI) or LRTI was seen (12.5% in placebo vs 11.9% suptavumab 1-dose group and 14.5% 2-dose group) (Table 1). Subgroup analyses of outcomes by geographic region or gestational age group revealed no notable differences between strata (Supplementary Figure 1).
Exploratory Outcomes
Overall, 142 participants had medically attended RSV infections (hospitalization or outpatient URTI or LRTI); 137 were subtyped (RSV-A: 41; RSV-B: 96). In the placebo group, 28 of 45 (62%) participants with subtyped RSV had RSV-B infection (Supplementary Table 2).
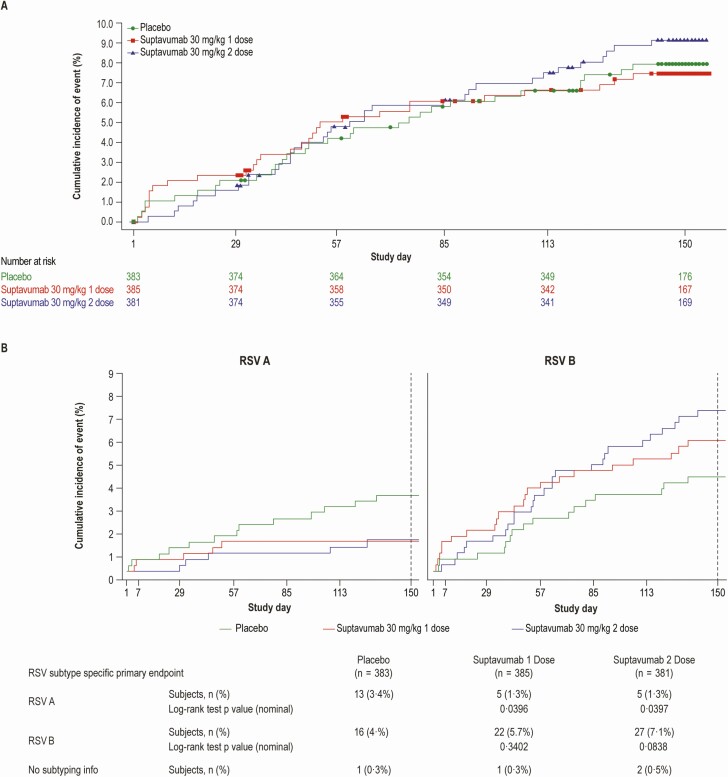
Primary endpoint analyses by RSV subtype demonstrated different treatment effects with RSV-A compared with RSV-B. Compared with placebo, relative risk for RSV-A hospitalization or outpatient LRTI was .38 (95% confidence interval [CI], .14–1.05) for the 1-dose cohort and .39 (95% CI, .14–1.07) for the 2-dose cohort. In contrast, the relative risk for RSV-B hospitalization or outpatient LRTI was 1.36 (95% CI, .73–2.56) and 1.69 (95% CI, .92–3.08) for the 1- and 2-dose cohorts, respectively (Supplementary Tables 3 and 4A and 4B). In a post-hoc analysis, compared with placebo, relative risk for RSV-A hospitalization or outpatient LRTI for the combined suptavumab 1- or 2-dose group versus placebo was .38 (95% CI, .17–.86) and the nominal P value for absolute risk difference was .0499. In contrast, the relative risk for RSV-B hospitalization or outpatient LRTI for the combined suptavumab 1- or 2-dose group versus placebo was 1.52 (95% CI, .88–2.64), and the nominal P value for absolute risk difference was .12.
In time-to-event analyses, suptavumab failed to demonstrate efficacy in reducing RSV hospitalization or outpatient LRTI overall (Figure 2A), but demonstrated efficacy related to RSV-A for the suptavumab 1-dose and 2-dose groups. By comparison, suptavumab led to increased RSV-B hospitalization and outpatient LRTI compared with placebo (Figure 2B). Similar efficacy results were seen for the secondary endpoint of RSV hospitalization or outpatient URTI or LRTI when analyzed by subtype (Supplementary Figure 2).
Figure 2.
Cumulative incidence of (A) overall RSV primary endpoint (RSV hospitalization or outpatient LRTI) over time, by treatment group, and (B) by RSV-A and RSV-B subtype primary endpoint, by treatment group. Subjects who had no event during the 150-day efficacy assessment period were censored at the last time point when their primary endpoint was assessed, ie, day 150 visit (day 150 ± 5 days) for completers of that visit, or the last visit (scheduled or unscheduled) completed by a subject up to day 150 for noncompleters of the day 150 visit. Abbreviations: LRTI, lower respiratory tract infection; RSV, respiratory syncytial virus.
Safety
Treatment-emergent adverse events (TEAEs) were similar across treatment groups. Treatment-emergent serious adverse events (SAEs) were reported in 11.5% of the placebo group, 12.9% of the suptavumab 1-dose group, and 8.3% of the suptavumab 2-dose group. Twenty-three subjects in the suptavumab 2-dose group contracted RSV before receiving the second dose (thus only received 1 dose of suptavumab), which is the main reason for the larger incidence of SAEs in the suptavumab 1-dose group (Table 2). Seventy-four treated subjects experienced TEAEs leading to discontinuation from the study drug—23 in the placebo group and 51 in the suptavumab 1-dose group—due to either RSV-related adverse events prior to the second dose (49 [11.7%]) or other adverse events (2 [0.5%]). None discontinued in the suptavumab 2-dose group as 34 subjects randomized to that group were analyzed as suptavumab 1-dose per protocol (Supplementary Table 5). Rates of hypersensitivity adverse events were similar among treatment groups (Supplementary Table 6).
Table 2.
Treatment-emergent Adverse Events in the Safety Analysis Set
| Suptavumab 30 mg/kg | |||
|---|---|---|---|
| Placebo (n = 384) | 1 dose (n = 418) | 2 doses (n = 348) | |
| Subjects with any TEAE, n (%) | 285 (74.2) | 296 (70.8) | 255 (73.3) |
| Subjects with any serious TEAE | 44 (11.5) | 54 (12.9) | 29 (8.3) |
| Subjects with any TEAE leading to death | 3 (.8) | 1 (.2) | 0 (.0) |
| Subjects with any TEAE leading to withdrawal of study drug | 23 (6.0) | 51 (12.2) | 0 (.0) |
| Subjects with TEAEs with severity of grade 3 or higher | 22 (5.7) | 22 (5.3) | 12 (3.4) |
| Subjects with TEAEs that occurred within 2 days after either the first dose or the second dose of study drug administrationa | 52 (13.5) | 56 (13.4) | 59 (17.0) |
| TEAEs ≥5% by actual treatment group, n (%) | |||
| Upper respiratory tract infection | 74 (19.3) | 92 (22.0) | 75 (21.6) |
| Otitis media | 29 (7.6) | 49 (11.7) | 26 (7.5) |
| Nasopharyngitis | 53 (13.8) | 30 (7.2) | 35 (10.1) |
| Bronchiolitis | 24 (6.3) | 29 (6.9) | 33 (9.5) |
| Pyrexia | 31 (8.1) | 35 (8.4) | 22 (6.3) |
| Gastroesophageal reflux disease | 31 (8.1) | 26 (6.2) | 25 (7.2) |
| Nasal congestion | 25 (6.5) | 24 (5.7) | 27 (7.8) |
| Cough | 14 (3.6) | 24 (5.7) | 23 (6.6) |
| Conjunctivitis | 18 (4.7) | 25 (6.0) | 18 (5.2) |
| Viral upper respiratory tract infection | 20 (5.2) | 19 (4.5) | 21 (6.0) |
| Bronchitis | 27 (7.0) | 24 (5.7) | 13 (3.7) |
| Diarrhea | 17 (4.4) | 24 (5.7) | 12 (3.4) |
| Rhinitis | 15 (3.9) | 17 (4.1) | 18 (5.2) |
| Constipation | 20 (5.2) | 11 (2.6) | 14 (4.0) |
| Summary of severity of TEAEs, n (%) | |||
| Grade 1—mild | 144 (37.5) | 136 (32.5) | 127 (36.5) |
| Grade 2—moderate | 119 (31.0) | 138 (33.0) | 116 (33.3) |
| Grade 3—severe | 19 (4.9) | 18 (4.3) | 10 (2.9) |
| Grade 4/5—potentially life-threatening/fatal | 3 (.8) | 4 (1.0) | 2 (.6) |
A total of 1150 subjects were included in the safety analysis. One subject was not randomized but received 2 doses of placebo. Therefore, this subject was added to the safety analysis set (n = 1150) but not the efficacy analysis set (n = 1149). Adverse events are coded according to the Medical Dictionary for Regulatory Activities (MedDRA), version 18.0, dictionary applied. The severity of adverse events was graded using the Common Terminology Criteria for Adverse Events (CTCAE), version 4.0, for allergic and anaphylactic reactions and the Modified Toxicity Grading Scale from Division of AIDS for Grading the Severity of Adult and Pediatric Adverse Events, versions 1.0 and 2.0, for other events. The table is sorted by descending order of frequency of preferred term. A subject with multiple TEAEs is counted once for the same preferred term. A subject who reported 2 or more TEAEs with different preferred terms within the same system organ class is counted only once in that system organ class. If a subject had more than 1 occurrence in the same event category, only the most severe occurrence was counted.
Abbreviation: TEAE, treatment-emergent adverse event.
aTEAEs with incomplete start date resulting in undetermined interval (≤ or >2 days) since the most recent dose are not included.
Immunogenicity
In the 1- and 2-dose groups, 3% and less than 1% of infants, respectively, produced a low-titered, anti-suptavumab antibody response compared with 4% in the placebo group (Supplementary Table 7).
Suptavumab Serum Concentrations
Maximal concentration (Cmax) of suptavumab was observed at day 29 for the 1-dose group (142 mg/L) and day 85 for the 2-dose group (238 mg/L). At the end of follow-up, mean total concentrations were 18.7 mg/L and 70.6 mg/L, respectively. The mean half-life of suptavumab was approximately 36 days (Supplementary Table 8).
Predicted total suptavumab concentrations at the time of first infection for all infected infants were above EC99 thresholds for RSV-A (4.9 mg/L) and RSV-B (27.8 mg/L) in cotton rats, with the majority of infections occurring when the drug concentration predicted by the population pharmacokinetic model were significantly above (>100 mg/L) the EC99 thresholds (Supplementary Figure 3).
RSV Isolate Sequencing of F-Protein, In Vitro Binding, Neutralization
In total, 137 nasal swabs from 137 infants in the 3 treatment arms were processed for RNA extraction and sequencing; 47 produced high-quality sequences (13 RSV-A, 34 RSV-B). The sequence of the suptavumab epitope in all RSV-A isolates perfectly matched the previously mapped sequence, whereas the suptavumab epitope in all RSV-B isolates contained substitutions in 2 amino acid positions in the F-protein (L172Q and S173L).
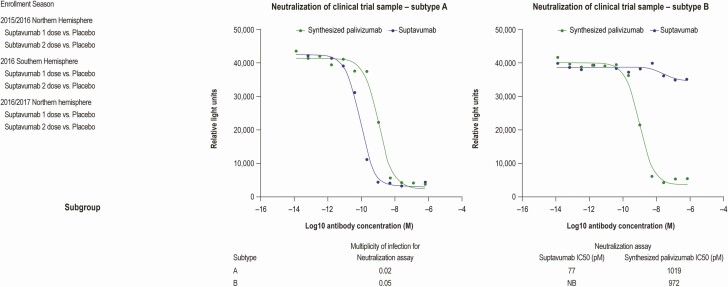
To assess whether suptavumab was able to bind to the sequenced RSV-B protein, surface plasmon resonance was used. Suptavumab-derived Fab had an affinity (KD) of approximately 2 nM for prefusion-stabilized RSV-F ectodomains derived from the RSV-A A2 strain. Its affinity for prefusion-stabilized RSV-F derived from subtype RSV-B B9320 was significantly weaker, with a KD of approximately 700 nM. This large difference in affinity is primarily due to the difference in dissociation rates, with suptavumab Fab having a much faster dissociation rate for the B9320 protein. In vitro binding experiments were performed using B9320 prefusion-stabilized F-protein with and without the L172Q/S173L substitutions. The 2–amino acid change resulted in loss of detectable binding using Biacore at the concentrations and capture densities tested (Supplementary Figure 4). In contrast, using an MSD-based assay, binding to both RSV-F variants was detected (Supplementary Figure 5), suggesting some residual binding of suptavumab to the L172Q/S173L variant that can only be detected in certain assay formats. In vitro neutralization assays were performed to test the ability of suptavumab to neutralize the strains isolated from the nasal swabs. Suptavumab potently neutralized all RSV-A isolates tested, but not RSV-B isolates (Figure 3).
Figure 3.
Neutralization assay indicating that suptavumab is able to neutralize RSV-A clinical isolates from trial participants but not RSV-B isolates. A neutralization assay using the indicated RSV strain was performed using suptavumab or palivizumab. To determine neutralization ability, each antibody was incubated with clinical trial sample subtype A (MOI: .02) or subtype B (MOI: .05) for 2 hours (37°C, 5% CO2). Virus-free and antibody-free controls were included. Postincubation, the antibody–virus mixture was added to the HEp-2 cells and the infection was maintained for 3 days. The degree of infection was determined by enzyme-linked immunosorbent assay. Luminescence values were analyzed by a 3-parameter logistic equation over an 11-point response curve (GraphPad Prism). Abbreviations: MOI, multiplicity of infection; RSV, respiratory syncytial virus.
DISCUSSION
Suptavumab was unable to provide protection against RSV hospitalization or outpatient medically attended LRTI in preterm infants, despite preclinical studies demonstrating high potency in vitro and in vivo in cotton rat models. We investigated several possible causes for this clinical failure.
Investigating serum concentrations of suptavumab dosed at 30 mg/kg intramuscularly, our data demonstrated that predicted serum concentrations of suptavumab in infants at the time of breakthrough infection were approximately 20- and 3-fold greater than the EC99 observed for RSV-A and RSV-B infections in cotton rats, respectively. A total of 10 breakthrough medically attended RSV infections occurred in both suptavumab groups. A review of the pharmacokinetic data (presented in Supplementary Figure 3) reveals that the majority of breakthrough RSV-A infections actually occurred within the first 60 days of the dose administration, suggesting that breakthrough infection is not due to subtherapeutic serum levels of suptavumab. While it is unclear what the mechanism of breakthrough infection is of RSV-A only early after dosing, it is interesting that, while there was ongoing infection in the placebo group, there were no breakthrough infections later after dosing. For RSV-B, as there was a mutation that abrogated binding of suptavumab to RSV-B F-protein, breakthrough infection was therefore seen throughout the at-risk period.
Lack of efficacy was not due to anti-suptavumab antibody responses, as the overall incidence rate and titers of immunogenicity were low across treatment groups. When investigating the RSV-F sequences, we found no changes in sequenced RSV-A isolates. In contrast, 100% of RSV-B isolates had identical 2–amino acid substitutions in the binding epitope, leading to subtype-specific loss of suptavumab binding and neutralization activity. Although amino acid mutations at positions 172 and 173 have been reported previously at frequencies of less than 1% (https://www.uniprot.org), it is unclear how frequently both mutations are found simultaneously in historical isolates. The recovery of these mutated strains of RSV-B occurred globally over 3 RSV seasons, and they were the majority subtype isolated during the study period. Indeed, RSV-B strains with L172Q and S173L changes were first reported to emerge in 2015 in China and comprised the majority of RSV-B strains in the United States by 2017 compared with 2013 reference strains [10, 11]. Palivizumab has been in use for the past 20 years [3, 12], and while resistance mutations in the RSV-F gene can be generated in vitro relatively easily producing attenuated viruses [13], circulating RSV viruses with these mutations are relatively uncommon [14–16]. This is in contrast to the RSV resistance mutations to suptavumab in current RSV-B strains.
Suptavumab had potential low-affinity residual binding to the mutant RSV-B, which may have provided the basis for the modest increase in primary endpoints among patients infected with RSV-B.
There are several strengths of this study, including the placebo-controlled design, use of a novel endpoint of medically attended RSV infection defined as hospitalization or outpatient LRTI rather than RSV hospitalization alone (which may not reflect the total burden of severe RSV infections in preterm infants), and conduct across multiple countries and over multiple RSV seasons. These aspects allow for generalization of our findings and suggest that circulation of the mutated RSV-B strain was a global phenomenon.
Findings from this study may inform development of other RSV investigational products. The F-protein on RSV has been the primary target for new monoclonal antibodies and RSV vaccine development, as it is thought to be well conserved across both RSV-A and RSV-B [17]. Suptavumab binds to the prefusion F conformation, where sequence variability has recently been described in clinical isolates of RSV [7]. Among prefusion antigenic sites, the suptavumab epitope is highly conserved compared with the more variable prefusion site 0, the target for next-generation RSV monoclonal antibodies and vaccines [18]. Although the 2 amino acid mutations found in clinical isolates of RSV-B subtype from this study have been described historically, they were only found in less than 1% of isolates for the L172Q mutation and the S173L mutation. However, strains with both mutations emerged in 100% of the RSV-B isolates collected over 3 RSV seasons. Therefore, targeting a single epitope on RSV-F, especially on the more variable prefusion conformation, carries risk, requiring ongoing molecular surveillance to ensure continued neutralization activity against contemporary strains. A potential approach to reducing this risk may be to combine monoclonal antibodies against nonoverlapping epitopes, a strategy that has been used for the treatment of Ebola virus disease [19, 20].
Another important finding is the occurrence of some breakthrough infections with RSV-A strains, whereby suptavumab still retained neutralization activity when drug levels were predicted to be greater than 100 mg/L, 20-fold above the EC99 in cotton rats. This suggests that cotton rat neutralizing antibody levels against RSV may be a poor “absolute” correlate of protection from severe disease in humans [21, 22].
In summary, this phase 3 study demonstrated that suptavumab did not prevent RSV hospitalization or outpatient medically attended LRTI. The main reason for failure was 2 amino acid mutations in the suptavumab epitope found on all circulating RSV-B strains, rendering suptavumab unable to bind and neutralize. This study highlights the need for a cocktail of nonoverlapping monoclonal antibodies to reduce the risk of treatment failure due to either escape viral variants during treatment or if a newly emergent variant starts circulating in future RSV seasons.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions . M. K., F. Y., F. M. M., L. L., N. S., G. D. Y., D. M. W., C. A. K., S. S., and S. L. B. were responsible for the study design. E. F.-N., H. C., R. R., E. D. F., E. W., J. M. N. P., F. M. M., C. A. K., and S. S. were responsible for the study conduct, implementation, enrollment of participants, and data collection. M. K., F. Y., Q. Z., and S. A. R. were responsible for immunogenicity and pharmacokinetic assays or analysis of the data. M. K. was responsible for preclinical data conducted at Regeneron and S. A. R. was responsible for binding studies of suptavumab against clinical strains conducted at his institution. E. F.-N., H. C., A. H., E. W., F. M. M., S. A. R., J. S. M., C. A. K., S. S., N. S., G. D. Y., and D. M. W. analyzed or interpreted the data. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. All authors contributed to data interpretation, as well as critical review, revision, and approval of the report.
Acknowledgments. The authors are indebted to the following contributors to this study: all study participants and their guardians; the NURSERY investigators (Supplementary Appendix 3); Yasmin Khan, Pamela Snodgrass, Ian Minns, and Karina Lippert of Regeneron Pharmaceuticals, Inc; and Kerren Davenport of Prime, Knutsford, United Kingdom, for editorial assistance on the manuscript. Qualified researchers may request access to study documents (including the clinical study report, study protocol with any amendments, blank case report form, and statistical analysis plan) that support the methods and findings reported in this manuscript. Individual anonymized participant data will be considered for sharing once the indication has been approved by a regulatory body, if there is legal authority to share the data and there is not a reasonable likelihood of participant reidentification. Submit requests to https://errs.regeneron.com/external.
Financial support. Employees of the study sponsor contributed to the study design, data collection, data analysis, data interpretation, and writing of the report.
Potential conflicts of interest. S. L. B., H. C., E. F.-N., G. P. G., M. R. H., M. K., C. A. K., L. L., R. R., S. S., N. S., D. W., E. W., G. D. Y., F. Y., and Q. Z. are employees and shareholders of Regeneron Pharmaceuticals, Inc. E. D. F. and A. H. are employees of Regeneron Genetics Center. F. M. M., J. S. M., J. M. N. P., S. A. R., and E. A. F. S. received nonfinancial support from Regeneron Pharmaceutical, Inc, during the conduct of the study. J. S. M. reports grants from Janssen, ReViral, Calder Biosciences, Mapp Biopharmaceutical, and MedImmune, outside the submitted work; has patents PCT/US2014/48086, 62/105667, PCT/EP2016/064218, 15/633,578, and 16/025,858 pending; and patents 9,738,689 and 10,017,543 issued. E. A. F. S. reports grants and nonfinancial support to the institution from AstraZeneca, Novavax, Pfizer, and Merck, and personal fees from Merck, Pfizer, Abbvie, Alere, Inc, and Roche, Inc, outside the submitted work. G. D. Y. reports that medical writing/editorial assistance was provided by Kerrin Davenport of Prime Medica funded by Regeneron Pharmaceuticals, Inc, during the conduct of the study. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Shi T, McAllister DA, O’Brien KL, et al. . Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 2017; 390:946–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mazur NI, Higgins D, Nunes MC, et al. ; Respiratory Syncytial Virus Network (ReSViNET) Foundation . The respiratory syncytial virus vaccine landscape: lessons from the graveyard and promising candidates. Lancet Infect Dis 2018; 18:e295–311. [DOI] [PubMed] [Google Scholar]
- 3. IMpact-RSV. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 1998; 102:531–7. [PubMed] [Google Scholar]
- 4. American Academy of Pediatrics Committee on Infectious Diseases; American Academy of Pediatrics Bronchiolitis Guidelines Committee. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics 2014; 134:e620–38. [DOI] [PubMed] [Google Scholar]
- 5. Adis Insight. Suptavumab. Available at: https://adisinsight.springer.com/drugs/800040470. Accessed 5 March 2019.
- 6. Chintha P, Wloga E, Kamat V, Duan X, Perez-Caballero D. REGN222, a potent neutralizing antibody. In: 9th International Respiratory Syncytial Virus Symposium 2014. 9–13 November, 2014. Capetown, South Africa. [Google Scholar]
- 7. Hause AM, Henke DM, Avadhanula V, Shaw CA, Tapia LI, Piedra PA. Sequence variability of the respiratory syncytial virus (RSV) fusion gene among contemporary and historical genotypes of RSV/A and RSV/B. PLoS One 2017; 12:e0175792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sivapalasingam S, Caballero-Perez D, Houghton M, et al. . Phase 1 study evaluating safety, tolerability, pharmacokinetics and immunogenicity of REGN2222 in healthy adults: a new human monoclonal RSV-F antibody for RSV prevention [poster]. In: ID Week 2015. 7–11 October, 2015. San Diego, CA. [Google Scholar]
- 9. Robbie GJ, Zhao L, Mondick J, Losonsky G, Roskos LK. Population pharmacokinetics of palivizumab, a humanized anti-respiratory syncytial virus monoclonal antibody, in adults and children. Antimicrob Agents Chemother 2012; 56:4927–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen X, Xu B, Guo J, et al. . Genetic variations in the fusion protein of respiratory syncytial virus isolated from children hospitalized with community-acquired pneumonia in China. Sci Rep 2018; 8:4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lu B, Liu H, Tabor DE, et al. . Emergence of new antigenic epitopes in the glycoproteins of human respiratory syncytial virus collected from a US surveillance study, 2015-17. Sci Rep 2019; 9:3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Simões EAF, Bont L, Manzoni P, et al. . Past, present and future approaches to the prevention and treatment of respiratory syncytial virus infection in children. Infect Dis Ther 2018; 7:87–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao X, Sullender WM. In vivo selection of respiratory syncytial viruses resistant to palivizumab. J Virol 2005; 79:3962–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhu Q, McAuliffe JM, Patel NK, et al. . Analysis of respiratory syncytial virus preclinical and clinical variants resistant to neutralization by monoclonal antibodies palivizumab and/or motavizumab. J Infect Dis 2011; 203:674–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oliveira DB, Iwane MK, Prill MM, et al. . Molecular characterization of respiratory syncytial viruses infecting children reported to have received palivizumab immunoprophylaxis. J Clin Virol 2015; 65:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Choi SH, Park KS, Kim YJ. Analysis of respiratory syncytial virus fusion protein from clinical isolates of Korean children in palivizumab era, 2009–2015. J Infect Chemother 2019; 25:514–9. [DOI] [PubMed] [Google Scholar]
- 17. Tapia LI, Shaw CA, Aideyan LO, et al. . Gene sequence variability of the three surface proteins of human respiratory syncytial virus (HRSV) in Texas. PLoS One 2014; 9:e90786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhu Q, McLellan JS, Kallewaard NL, et al. . A highly potent extended half-life antibody as a potential RSV vaccine surrogate for all infants. Sci Transl Med 2017; 9:eaaj1928. [DOI] [PubMed] [Google Scholar]
- 19. Sivapalasingam S, Kamal M, Slim R, et al. . Safety, pharmacokinetics, and immunogenicity of a co-formulated cocktail of three human monoclonal antibodies targeting Ebola virus glycoprotein in healthy adults: a randomised, first-in-human phase 1 study. Lancet Infect Dis 2018; 18:884–93. [DOI] [PubMed] [Google Scholar]
- 20. Pascal KE, Dudgeon D, Trefry JC, et al. . Development of clinical-stage human monoclonal antibodies that treat advanced Ebola virus disease in nonhuman primates. J Infect Dis 2018; 218:612–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Piedra PA, Jewell AM, Cron SG, Atmar RL, Glezen WP. Correlates of immunity to respiratory syncytial virus (RSV) associated hospitalization: establishment of minimum protective threshold levels of serum neutralizing antibodies. Vaccine 2003; 21:3479–82. [DOI] [PubMed] [Google Scholar]
- 22. Kulkarni PS, Hurwitz JL, Simões EAF, Piedra PA. Establishing correlates of protection for vaccine development: considerations for the respiratory syncytial virus vaccine field. Viral Immunol 2018; 31:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.