Abstract
Introduction:
Currently, various destructive and ablative treatment options are conventionally used for warts, but all of them are limited in some form by their adverse effects, high recurrences, suboptimal effectiveness, and the need to treat every wart. Lately, immunotherapy has emerged as a safe treatment relying on biological substances that modulate the immune system to achieve disease control.
Aims and Objectives:
We aimed at conducting a placebo-controlled study to compare the rate of efficacy of intralesional MMR vaccine with vitamin D3 in the management of recalcitrant extragenital warts in immune-competent adults. Follow-up was done at third and sixth month.
Materials and Methods:
Patients were divided into three groups, namely, group A, B, and C. Groups A, B, and C received intralesional MMR vaccine, vitamin D3 and normal saline, respectively, in the largest wart. The injections were repeated every 2 weeks, for a maximum of four injections.
Results:
Among injected warts, in group A, complete clearance was seen in 29 (87.8%) patients, partial clearance in two (6.1%) and no response in two (6.1%) patients. In group B, 24 (77.4%) patients, five (16.1%) patients, and two (6.5%) patients showed signs of complete, partial, and no clearance, respectively, in injected warts. Complete response in distant warts was seen in 25 (75.7%) patients in group A and 20 (64.5%) patients in group B. There was no statistically significant difference between responses of the two groups. In group C, only three (12.5%) patients had complete clearance in injected warts, and none in distant warts. Recurrence was seen in two (6.4%) patients, each in group B and C. However, for management of verruca plana MMR was found to be superior to vitamin D3.
Limitations:
Our study was limited by a small sample size, absence of immunological analysis, and limited follow-up period.
Conclusion:
MMR vaccine and vitamin D3 are equally effective and safe treatment option for multiple, recalcitrant warts, as well as warts on difficult to treat sites with minimal recurrence.
Keywords: Cutaneous warts, immunotherapy, MMR vaccine, placebo controlled, vitamin D3
Introduction
Warts are caused by infection of keratinized and non-keratinized epithelia with Human papilloma viruses (HPV).[1] Benign cutaneous warts are most common in childhood and into the 20s.[2,3] Depending upon their site they can be painful, like palmoplantar warts, or an emabrassing entity if they are present on face. Currently, various destructive and ablative treatment options that are conventionally used for warts (cauterization, excision, cryosurgery, salicylic acid, trichloroacetic acid, etc.) are limited in some form by their adverse effects, high recurrences, suboptimal effectiveness, and the need to treat every wart. Therefore, there exists a need to develop an agent, which specifically targets the HPV.
Keeping the lacunae of conventional therapies in use, immunotherapy has emerged as a novel treatment using biological substances that modulate the immune system to achieve disease control. It utilizes various intralesional antigens like measles, mumps, and rubella (MMR) vaccine, tuberculin purified protein derivative (PPD), Bacillus of Clamette and Guerin (BCG) vaccine, Mycobacterium w (Mw) vaccine, Candida albicans antigen, and so on.[3,4,5,6,7,8] Other antigens include cytokines and biologically important compounds like vitamin D3. The principle of immunotherapy is activation of delayed hypersensitivity and humoral immunity response against intralesional antigens as well as the viruses causing warts, which destroy both locally treated and distant warts while also preventing recurrences.[9]
The mechanism of action of MMR vaccine as an immunotherapy is by mounting Th1 immune response, increase in TNF α, IL-2, IL-4, IL-5, and IFN-γ as well as propogation of delayed hypersensitivity reaction against both MMR viral antigens and HPV.[6]
However, Vitamin D3 has multiple mechanism-like regulation of epidermal proliferation and cytokine production. This dual action gives it a leverage over other immunotherapies. It downregulates IL-1a, IL-6, and activates the toll-like receptor of human macrophages, which induce antimicrobial peptide formation in injected and distant warts.[10,11]
So far, only two studies have compared vitamin D3 with MMR vaccine as immunogens, in which one study was conducted by our group in pediatric patients.[12,13] However, both studies were limited by the lack of a placebo group. Herein, we are expanding our previously published work in the pediatric patients and present our observation in treating recalcitrant warts in adults. We aimed at comparing the efficacy of these two intralesional immunogens against a placebo in the management of recalcitrant verruca plana, verruca vulgaris, priungual, and palmoplantar warts in immune-competent adults.
Materials and Methods
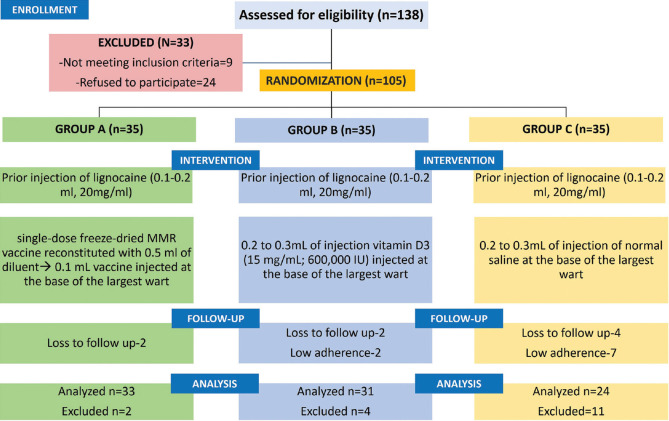
This prospective randomized comparative and placebo-controlled study was initiated after taking due approval from institutional ethical committee and conducted between October 2017 and April 2019. Immunocompetent adults attending our out-patients' department (OPD) of Dermatology, Venereology, and Leprosy were recruited for the study from January 2018 to July 2018 [Figure 1]. Subjects aged between 18 and 65 years with clinical diagnosis of two or more recalcitrant extragenital warts of any duration at various sites of the body and not taking any concurrent systemic or topical treatment for warts over the period of the past 1 month were enrolled. Only those patients who had warts not responding to at least two other forms of treatment in the past were included.
Figure 1.
Gantt chart depiction of the timeline of study
Patients aged less than 18 years or more than 65 years were excluded from the study. Additionally, patients presenting with genital warts or both genital and extragenital warts, pregnant and lactating women, patients with any systemic illness, secondary infections, acute febrile illness, those with prior allergic response to any component of MMR vaccine or injection vitamin D3 or any past history of asthma or allergic skin disorders such as generalized eczema or urticaria, past history of meningitis or convulsions, iatrogenic or primary immunosuppression were excluded from the study, irrespective of their age. Additionally, immunocompromised patients (e.g., HIV positive patients) or those with keloidal tendencies and patients with unpredictable behavior, who cannot be followed up, were excluded.
A record of previous treatments received for the warts and presence of warts on distant sites (defined as those present on anatomic sites, different from the wart receiving intralesional therapy) were also noted.
After explaining about the procedure and taking a formal written consent from all participants, the subjects were randomly allocated into three groups, namely, group A (MMR group), group B (vitamin D3), and group C (normal saline) with 35 patients each.
Sample size for each group was calculated using the probability of 90% with a significant result considered at 5%, giving the sample size to be 25 per group. However, we kept the sample size to be 35 per group in order to avoid any further reduction in size due to possible drop outs.
Randomization
The study was participant blinded and unstratified randomization was done using an open list of computer-generated random number chosen by the participant. The random number was mentioned on the patient's prescription for further visits.
In Group A, Measles, Mumps, and Rubella (MMR) vaccine (live) I. P. (freeze-dried) TRESIVAC® (Manufacturer: Serum Institute of India Ltd., Pune, Maharashtra, India) was used without any pre-sensitization testing. The MMR vaccine was reconstituted with 0.5 mL distilled water, and 0.2-0.3 mL of this solution was injected intralesionally into the largest wart.
In Group B, the patients received 0.2-0.3 mL (600,000 IU; 15 mg/mL) of intralesional Vitamin D3 or Cholecalciferol (ARACHITOL-6L® (Manufacturer-Akums drugs and pharmaceuticals Ltd.) in the largest wart.
In Group C, 0.2-0.3 mL of normal saline was injected at the base of the largest wart. In all the groups injections were given at 2 weeks intervals with maximum of four injections.
In all groups, an injection of 0.1-0.2 mL Lignocaine (20 mg/mL) was given beforehand around the lesion.
Injections were repeated every 2 weeks, in the same wart, till a maximum of four injections or till the complete clearance of warts, whatever was achieved first. In case, only the injected wart cleared after first/second visit itself, but distant warts didn't resolve completely, the next largest wart was chosen for subsequent injections. Results were assessed after each injection and at follow-up period of third and sixth month. The primary outcome measure was complete disappearance of all the lesions, which is said to occur when the thickening and hyperkeratosis is no more evident and the normal skin markings return.
Assessment of response rates
The responses of injected warts to the treatment were graded as: 100% resolution-complete response (CR), 50-99% reduction in size-partial response (PR), and <50% reduction in size-inadequate or no response (NR).
Similarly, the resolution of distant warts was graded as: CR (resolution of all distant warts), PR (≤99% reduction in size and number of distant warts with presence of a few residual warts), and NR (<50% resolution in size or number of distant warts was categorized as inadequate or no resolution). Persistence of warts beyond 12 weeks of completion of therapy was considered to be treatment failure.
Statistical analysis
Descriptive statistics was performed by calculating the mean and standard deviation for the continuous variables. Categorical variables were presented as absolute numbers and percentage. Nominal categorical data between the groups were compared using Chi-square goodness-to-fit test.
The sample size was calculated using 90% power (probability of achieving significant result at 5% level), 80% preference rate for vitamin D3 (number of samples in each group 25), and a significance level of 5%. The preference rate for vitamin D3, which is a new immunogen, was taken at 80% and a significant level of 5% was kept.
Chi-square test was used to investigate whether distributions of categorical variables differ from one another, and P value was taken significant when less than 0.05 (P < 0.05) and a confidence interval of 95% was taken. The χ2-static follows a χ2 distribution with degrees of freedom as (r-1) x (C- 1), where r is the number of rows and c is the number of columns in the tabulated date. The calculated value was then compared with the theoretical value of χ2 distribution for the given degree of freedom to obtain the level of significance.
Z-score was used to compare the sample mean and standard deviations of group A, B, and C to estimate the population values. The P value was calculated using the Z score for comparison of te baseline characters of the three groups.
Data were entered, checked, and analyzed through Statistical package for social science for personal computers v 22.0. Chi-square test and Z scores were calculated wherever necessary. P value <0.05 was considered statistically significant.
Results
Out of a total 105 patients, only 88 completed the study (33 patients in group A, 31 in group B, and 24 in group C). However, the remaining two patients in group A, four patients in group B, and 11 patients in group C discontinued at different times due to different reasons, such as failure to follow-up and low adherence [Figure 2]. Demographic data of the patients has been tabulated in Table 1.
Figure 2.
Consort flow diagram
Table 1.
Baseline and demographic characteristics of the subjects among the three groups
| Group A (MMR vaccine) n=33 | Group B (Vitamin D3) n=31 | Group C (Normal saline) n=24 | P | |
|---|---|---|---|---|
| Age distribution in years | ||||
| Range | 18-55 yrs | 18-56 yrs | 18-52 yrs | |
| Mean | 31.60±6.94 yrs | 33.16±7.81 yrs | 33.02±6.28 yrs | Group A vs B=0.4 Group A vs C=0.43 Group B vs C=0.94 |
| Gender distribution | ||||
| Male | 19 | 18 | 15 | |
| Female | 14 | 13 | 9 | |
| Male:female | 1.35:1 | 1.38:1 | 1.66:1 | |
| Duration of warts (in months) | ||||
| Mean | 9.07±6.03 | 8.37±5.87 | 9.13±5.91 | Group A vs B=0.85 Group A vs C=0.98 Group B vs C=0.83 |
| Mean number of warts | ||||
| 6.23±3.80 | 6.02±3.16 | 5.73±3.24 | Group A vs B=0.91 Group A vs C=0.88 Group B vs C=0.96 |
|
| Conclusion | The difference between the three groups is statistically non-significant | |||
In group A, there were 12, seven, 11, and three patients with verruca vulgaris (VV), verruca plana (VP), palmoplantar warts (PP), and periungual warts (PU) respectively. Similarly, in group B, 13, nine, eight, and one patient had VV, VP, PP, and PU. Also, there were six, eight, and 10 patients with VV, VP, and PP, in group C.
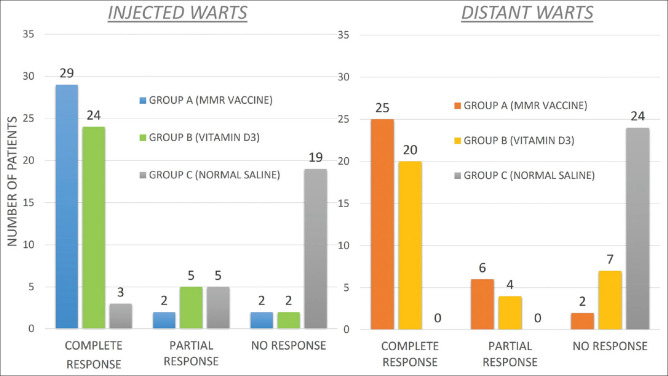
Patients started showing significant improvement in total number and size of warts after first injection itself in Group A and B in both injected [Figures 3-5] as well as distant warts. However, only marginal response was seen in group C with no response in any of the distant warts. Among the three groups in injected warts, there was a higher rate of complete response in Group A where complete clearance was seen in 29 (87.8%) patients, partial clearance was seen in two (6.1%) patients, and there was no response in the remaining two (6.1%) patients [Figures 6 and 7]. Similarly, in group B, 24 (77.4%) patients, five (16.1%) patients and two (6.5%) patients showed signs of complete, partial, and no clearance, respectively [Figures 8 and 9]. Though the results among the two groups were comparable, the difference in response was statistically non-significant (P value = 0.42). Interestingly, in group C as well three (12.5%) patients showed complete clearance in injected warts. But, after statistical analysis, its response was found to be extremely lower compared to group A and B (P value = 0.0001) [Table 2 and Figure 4].
Figure 3.
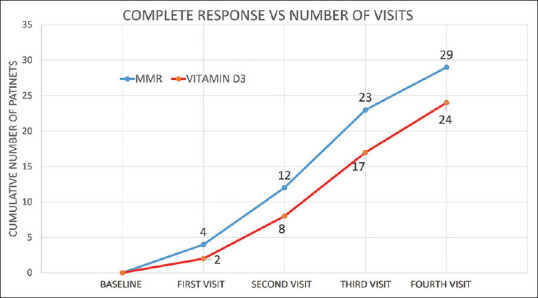
A cumulative rate of complete clearance in injected warts versus number of visits
Figure 5.
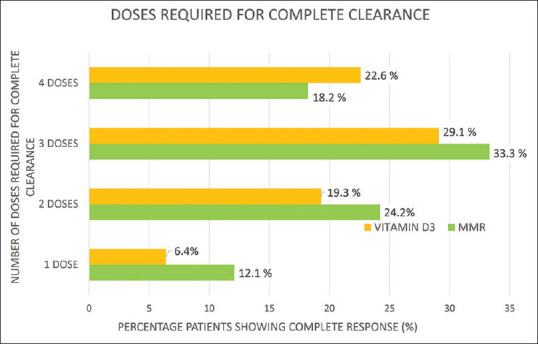
Number of patients with complete clearance in all warts versus number of injections required
Figure 6.
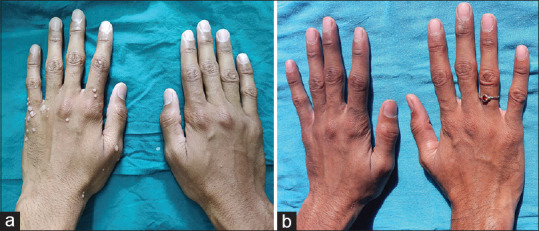
(a) Multiple verruca vulgaris on bilateral dorsum of hands at baseline; (b) complete response after second dose of MMR vaccine
Figure 7.
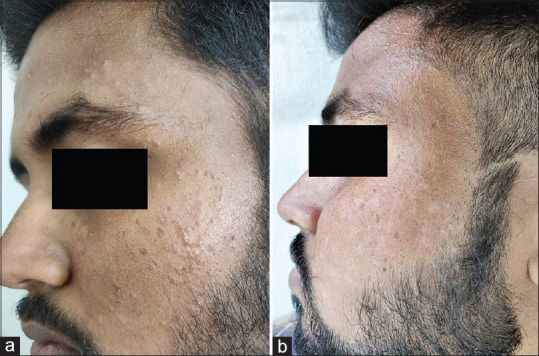
(a) multiple verruca plana on face; (b) complete response after one dose of MMR vaccine
Figure 8.
(a) Periungual warts; (b) mild response after first dose; (c) complete clearance after four doses of vitamin D3
Figure 9.
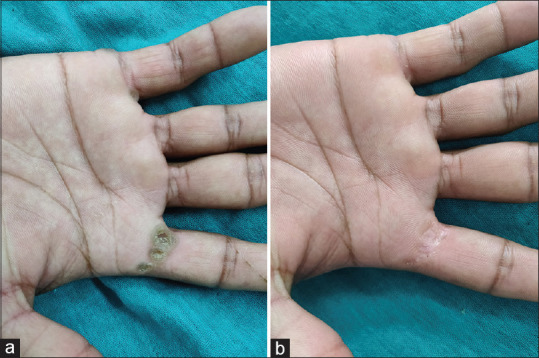
(a) Multiple verruca on right palm at baseline; (b) complete response after third dose of vitamin D3
Table 2.
Comparison of response in injected warts in three groups after four applications
| Injected warts | Complete reponse | Partial response | No response | Total |
|---|---|---|---|---|
| Group A (MMR vaccine; n=33) | 29 (87.8%) | 2 (6.1%) | 2 (6.1%) | 33 (100%) |
| Group B (Vitamin D3; n=31) | 24 (77.4%) | 5 (16.1%) | 2 (6.5%) | 31 (100%) |
| Group C (Normal saline; n=24) | 3 (12.5%) | 5 (20.8%) | 16 (66.7%) | 24 (100%) |
In group A vs B- χ2=1.696, P=0.42. Inference-the response between the two active groups is statistically non-significant. Group A vs C- χ2=35.93, P<0.0001, Group B vs C- χ2=29.96, P<0.0001. Inference-the rate of response of both MMR as well as Vitamin D3 against normal saline is extremely significant
Figure 4.
Comparison of clearance rate of injected and distant warts in patients in all three groups
In distant warts, there was a higher rate of complete response in Group A where complete clearance was seen in 25 (75.7%) patients, partial clearance in six (18.2%) patients, and no clearance in two (6.1%). In distant warts in group B, 20 (64.5%) patients, four (12.9%) patients and seven (22.6%) patients displayed signs of complete, partial, and no clearance, respectively. None of the patients showed any response in distant warts in group C [Table 3 and Figure 4].
Table 3.
Comparison of response in distant warts in three groups after four applications
| Distant warts | Complete response | Partial response | No response | Total |
|---|---|---|---|---|
| Group A (MMR vaccine) | 25 (75.7%) | 6 (18.2%) | 2 (6.1%) | 33 (100%) |
| Group B (Vitamin D3) | 20 (64.5%) | 4 (12.9%) | 7 (22.6%) | 31 (100%) |
| Group C (Normal saline) | 0 (0.00%) | 0 (0.00%) | 24 (100%) | 24 (100%) |
In group A vs B- χ2=3.674, P=0.15. Inference-the response between the two active groups is statistically non-significant
As far as types of warts were concerned, there was a statistically significant difference between the complete clearance rate of verruca plana between groups A and B (P value = 0.049). MMR vaccine was found to be superior to vitamin D in management of VP [Table 4]. All other types of warts were equally susceptible to both immunogens.
Table 4.
Complete clearance rate in various type of warts
| Type of wart | MMR vaccine | Vitamin D3 | P | ||
|---|---|---|---|---|---|
|
|
|
||||
| Total number of patients | Complete responders (%) | Total number of patients | Complete responders (%) | ||
| Verruca vulgaris | 12 | 9 (75%) | 13 | 11 (83.3%) | 0.548 |
| Verruca plana | 7 | 5 (71.4%) | 9 | 2 (22.2%) | 0.049 |
| Palmoplantar warts | 11 | 9 (81.8%) | 8 | 6 (75%) | 0.718 |
| Periungual | 3 | 3 (100%) | 1 | 1 (100%) | |
The average number of injections required for clearance of injected warts were 2.6 and 2.8 in group A and B, respectively. Similarly, 3.2 and 3.6 injections were required for clearance of all (injected plus distant) warts in group A and B, respectively [Figure 5].
In our study, the complete response was significantly more amongsubjects with pseudokoebner phenomenon in Group A and B. Pseudokoebner phenomenon was present in 14 patients in Group A. Out of which, 13 (92.9%) patients had complete response and one (7.1%) had partial response. Similarly, in group B, 13 patients had pseudokoebner phenomenon. Out of these, 10 (76.9%) had complete response and three (23.1%) had partial response.
The side-effect profile of our patients was minimal with only tolerable pain in nine (27.3%) patients in group A and 10 (32.3%) patients in group B. Other side-effects included injection site erythema and swelling in five (15.2%) patients each in group A, and seven (22.6%) and eight (25.8%) patients in group B, respectively. One unique side-effect observed only in group B was injection site pruritus in four (12.9%), which lasted for around 3–4 days before resolving on its own. No significant correlation between disease duration and treatment response was seen.
In the ensuing 6 months' follow-up period, none of the warts in patients from Group A showed any recurrence whereas in Group B, there were signs of recurrence among distant warts in two (6.5%) patients, who had previously been reported to have partial clearance. In group C, out of the three patients who had complete clearance in injected warts, two (8.3%) displayed recurrence in their lesions. The treatment failure patients in group C (n = 21) after 12 weeks of follow-up were later treated with appropriate immunotherapy as part of routine out-patient department procedures.
Discussion
Due to the suboptimal response of available therapies used in treating extragenital warts, their treatment is a frustrating battle for clinicians. As none of them is 100% effective, the search for novel and efficient therapeutic options is still an elusive battle.[4] One of the most frustrating domain in management of warts is their notorious recurrence due to the recrudescence of virus from the surrounding tissue reservoir.
Immunotherapy overcomes the limitations of traditional ablative therapy by enhancing the body's own immune system to clear the virus-infected tissue. In this sense, they can target lesions situated remotely from the site of immunotherapy, making it a preferred option in multiple warts, warts on inaccessible or difficult-to-treat sites (like sub- or periungual region), or in cosmetically sensitive areas (facial warts).[3,14,15,16]
Both MMR vaccine and vitamin D3 being easily and widely available, appear to be promising options. In our study, in group A, among injected warts complete response was seen in 29 (87.8%) patients. These results were fairly similar to the pioneer study conducted by Nofal et al.[7] who had reported complete cure in 57 (81.4%) patients as compared with 11 (27.5%) patients in placebo group with intralesional MMR vaccine and antigens. However, our results were slightly better than Agrawal et al.[17] who reported complete response in 18 of 30 (60%) patients in injected warts. However, results similar to ours were reported by Mohamad et al.[18] and Zamanian et al.[19] separately observing complete clearance in 41 (82%) patients and 18 (75%) patients, respectively.
In group A, as far as distant warts were concerned, complete response was seen in 23 (76.7%) patients. Our results were slightly better than those observed by Mahajan et al.[20] who had evaluated the effect of MMR vaccine in pediatric age group and reported 58.7% complete clearance. Recently, Chauhan et al.'s[21] study has reported an impressive complete resolution rate of 82.4%. Also, Agrawal et al.[17] has reported complete clearance in 16 (69.5%) patients with distant warts. As all three of the above-mentioned studies, similar to ours, have been conducted in India where MMR vaccination is part of the vaccine program, the confounding factors could be reduced.
In our study four (12.1%) patients showed complete response after first dose itself. This observation was similar to Chauhan et al.'s[21] study who had also reported complete response in 7.8% (n = 4) patients after first dose.
With consideration to group B, among injected warts complete response was seen in 24 (77.42%) patients and in distant warts 20 (64.5%)patients showed complete response. Unlike MMR, only a handful of studies have evaluated the efficacy of vitamin D3 for warts with complete response rates ranging from 40% to 90%.[12,13,22,23,24,25,26,27] Our results are fairly similar with the first published study of intralesional vitamin D3 conducted by Aktaş et al.[22] who had also reported complete clearance in 80% patients. However, unlike us, they had used a higher dose and injected upto five warts.
The forerunner study was followed by Kavya et al.[23] and Raghukumar et al.[24] who reported 78.57% and 90% complete clearance. They had also injected vitamin D3 in more than one wart. Several studies in the past have compared the response of intralesional vitamin D3 against other immunotherapies like PPD, candida antigen and MMR vaccine.[11,12,13,14,25,26] In their study Singh et al.[25] had compared Vitamin D3 with PPD tuberculin and revealed that 72.5% in the former group showed complete response. Our response rate was found to be higher than theirs. In another comparative saline controlled-study, similar to ours, Kareem et al.[27] compared vitamin D3 with candida antigen where 70% patients showed excellent response.
The importance of placebo in any randomized trial like this cannot be overstated. While our previously published study was limited by the absence of same (owing to the ethical considerations in pediatric group),[12] we made our best efforts to include a control group in this one. Concerning the control group (group C) using saline, complete response was seen only in three (12.5%) patients, partial response in five (20.8%) patients and no response in 19 (66.7%) patients. The results clearly demonstrate a highly significant difference between the therapeutic response of extra genital warts to MMR vaccine and vitamin D3 compared with normal saline (P < 0.001). Similar findings have also been reported by other studies that compared intralesional antigen therapy with normal saline as the control group.[7,14,17,18,19,26,27,28]
Although, there is no unanimity on the indication of immunotherapy, a review by Thappa et al.[16] has laid down the following indications: recalcitrant warts, recurrent warts, extensive warts, and warts on difficult-to-treat area. We found both immunogens to be excellent and equally efficaceous for treatment of recalcitrant warts and those on difficult-to-treat sites, that is. periungual and palmoplantar [Table 4]. However, interestingly we found MMR vaccine to be superior to vitamin D3 in management of verruca plana.
In the present study, the complete response was significantly more among subjects with pseudokoebner phenomenon. To the best of our knowledge there is no study published showing clinical response to MMR vaccine or vitamin D3 in cases with presence of pseudokoebner phenomenon. This could possibly be explained by the fact that in patients with localized recalcitrant warts only the local immune response is subdued, whereas the levels of circulating antibodies against viral coat proteins are already high.[29] Over the course of time, such warts become resistant to these immune mechanisms. Because immunotherapy also works by eliciting similar immune response, it might not hold any added advantage for such patients. On the contrary, in individuals with active disseminated warts (pseduokoebnerization) the level of such circulating antibodies are low, and employment of immunotherapy in them could provide just the right amount of boost in immune response required for clearance. Additionally, as pseudukoebnerization is seen most commonly in verruca plana, such patients in group A responded better to MMR.[30] However, these findings might just be incidental and require further exploration.
The side-effect profile of our patients was minimal with only slight pain, erythema, and swelling, all self-resolving in nature. One unique side-effect observed only in group B was injection site pruritus in four (12.9%) patients which lasted for around 3-4 days before resolving on its own. This finding was similar to the study conducted by Abou-Taleb et al.[26] The possible culprit, for such pruritic reactions in case of injection vitamin D3, could be a local inflammatory reaction against the arachis oil present in the oil base of injection.[31] No flu-like symptoms, or post-inflammatory pigmentation, or vasovagal attack, the commonly reported adverse effects of MMR vaccine in other studies, were observed.[18,19,32,33,34] Another interesting observation made by use was that a majority of patients who had developed persistent erythema, swelling, or itching after the first injection in both the groups A and B showed a quicker resolution in lesions after first or second dose itself.
Because of the prior use of local anesthetic, most of the patients felt no discomfort while administration of intralesional therapies in either group. However, lesions on finger/toe tips and face were prone to be slightly uncomfortable while injecting intralesional vitamin D3 owing to its oily base.
Our study had some limitations including a small sample size, no immunological measurement, and a limited follow-up period. Additionally, the effect of these immunogens in anogenital warts was not assessed.
Conclusion
To the best of our knowledge, no placebo-controlled study has been conducted to compare these two immunogens together against normal saline. Our findings reinforce the fact that both MMR and vitamin D3 are equally effective, safe, and tolerable modalities for treatment of recalcitrant cutaneous warts with extremely low rates of recurrence. However, MMR was found to be superior to vitamin D3 in the management of verruca plana. We suggest both of them to be employed as a first line treatment for multiple, recurrent, recalcitrant warts, and warts located on difficult-to-treat sites.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Braaten KP, Laufer MR. Human Papillomavirus (HPV), HPV-Related Disease, and the HPV Vaccine. Rev Obstet Gynecol. 2008;1:2–10. [PMC free article] [PubMed] [Google Scholar]
- 2.Bruggink SC, Eekhof JAH, Egberts PF, van Blijswijk SC, Assendelft WJ, Gussekloo J. Natural course of cutaneous warts among primary schoolchildren: A prospective cohort study. Ann Fam Med. 2013;11:437–41. doi: 10.1370/afm.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clifton MM, Johnson SM, Roberson PK, Kincannon J, Horn TD. Immunotherapy for recalcitrant warts in children using intralesional mumps or Candida antigens. Pediatr Dermatol. 2003;20:268–71. doi: 10.1046/j.1525-1470.2003.20318.x. [DOI] [PubMed] [Google Scholar]
- 4.Lipke MM. An armamentarium of wart treatments. Clin Med Res. 2006;4:273–93. doi: 10.3121/cmr.4.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta S, Malhotra AK, Verma KK, Sharma VK. Intralesional immunotherapy with killed Mycobacterium w vaccine for the treatment of ano-genital warts: An open label pilot study. J Eur Acad Dermatol Venereol. 2008;22:1089–93. doi: 10.1111/j.1468-3083.2008.02719.x. [DOI] [PubMed] [Google Scholar]
- 6.Scott M, Nakagawa M, Moscicki AB. Cell-mediated immune response to human papillomavirus infection. Clin Diagn Lab Immunol. 2001;8:209–20. doi: 10.1128/CDLI.8.2.209-220.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nofal A, Nofal E. Intralesional immunotherapy of common warts: Successful treatment with mumps, measles and rubella vaccine. J Eur Acad Dermatol Venereol. 2010;24:1166–70. doi: 10.1111/j.1468-3083.2010.03611.x. [DOI] [PubMed] [Google Scholar]
- 8.Maronn M, Salm C, Lyon V, Galbraith S. One-year experience with candida antigen immunotherapy for warts and molluscum. Pediatr Dermatol. 2008;25:189–92. doi: 10.1111/j.1525-1470.2008.00630.x. [DOI] [PubMed] [Google Scholar]
- 9.Chandrashekar L. Intralesional immunotherapy for the management of warts. Indian J Dermatol Venereol Leprol. 2011;77:261–3. doi: 10.4103/0378-6323.79694. [DOI] [PubMed] [Google Scholar]
- 10.Moscarelli L, Annunziata F, Mjeshtri A, Paudice N, Tsalouchos A, Zanazzi M, et al. Successful treatment of refractory wart with a topical activated vitamin D in a renal transplant recipient. Case Rep Transplant. 2011;2011:368623. doi: 10.1155/2011/368623. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 12.Mohta A, Kushwaha RK, Gautam U, Sharma P, Nyati A, Jain SK. A comparative study of the efficacy and safety of intralesional measles, mumps, and rubella vaccine versus intralesional vitamin D3 for the treatment of warts in children. Pediatr Dermatol. 2020;37:853–9. doi: 10.1111/pde.14280. [DOI] [PubMed] [Google Scholar]
- 13.Shaldoum DR, Hassan GFR, El-Maadawy EH, El-Maghraby GM. Comparative clinical study of the efficacy of intralesional MMR vaccine vs intralesional vitamin D injection in treatment of warts. J Cosmet Dermatol. 2020;19:2033–40. doi: 10.1111/jocd.13272. [DOI] [PubMed] [Google Scholar]
- 14.Fathy G, Sharara MA, Khafagy AH. Intralesional vitamin D3 versus Candida antigen immunotherapy in the treatment of multiple recalcitrant plantar warts: A comparative case–control study? Dermatol Ther. 2019;32:e12997. doi: 10.1111/dth.12997. doi: 10.1111/dth.12997. [DOI] [PubMed] [Google Scholar]
- 15.Garg S, Baveja S. Intralesional immunotherapy for difficult to treat warts with Mycobacterium w vaccine. J Cutan Aesthet Surg. 2014;7:203–8. doi: 10.4103/0974-2077.150740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thappa DM, Chiramel MJ. Evolving role of immunotherapy in the treatment of refractory warts. Indian Dermatol Online J. 2016;7:364–70. doi: 10.4103/2229-5178.190487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agrawal C, Vyas K, Mittal A, Khare AK, Gupta LK. A randomized double blind controlled study comparing the efficacy of intralesional MMR vaccine with normal saline in the treatment of cutaneous warts. Indian Dermatol Online J. 2018;9:389–93. doi: 10.4103/idoj.IDOJ_111_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohamad NS, Badran F, Yakout E. Evaluation of the efficacy of a combination-measles, mumps and rubella vaccine in the treatment of plantar warts. Our Dermatol Online. 2013;4:463–7. [Google Scholar]
- 19.Zamanian A, Mobasher P, Jazi GA. Efficacy of intralesional injection of mumps-measles-rubella vaccine in patients with wart. Adv Biomed Res. 2014;3:107. doi: 10.4103/2277-9175.129701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahajan VK, Chauhan PS, Sharma A, Mehta KS, Rawat R, Sharma V. Evaluation of efficacy and safety of intralesional Measles-Mumps-Rubella virus vaccine for the treatment of common warts in children and adolescents. Indian J Paediatr Dermatol. 2019;20:231–5. doi: 10.4103/idoj.IDOJ_142_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chauhan PS, Mahajan VK, Mehta KS, Rawat R, Sharma V. The efficacy and safety of intralesional immunotherapy with measles, mumps, rubella virus vaccine for the treatment of common warts in adults. Indian Dermatol Online J. 2019;10:19–26. doi: 10.4103/idoj.IDOJ_142_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aktaş H, Ergin C, Demir B, Ekiz Ö. Intralesional Vitamin D injection may be effective treatment option for warts. J Cutan Med Surg. 2016;20:118–22. doi: 10.1177/1203475415602841. [DOI] [PubMed] [Google Scholar]
- 23.Kavya M, Shashikumar BM, Harish MR, Shweta BP. Safety and efficacy of intralesional vitamin D3 in cutaneous warts: An open uncontrolled trial. J Cutan Aesthet Surg. 2017;10:90–4. doi: 10.4103/JCAS.JCAS_82_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raghukumar S, Ravikumar BC, Vinay KN, Suresh MR, Aggarwal A, Yashovardhan DP. Intralesional Vitamin D3 injection in treatment of recalcitrant warts: A novel proposition. J Cutan Med Surg. 2017;21:320–4. doi: 10.1177/1203475417704180. [DOI] [PubMed] [Google Scholar]
- 25.Singh SK, Mohan A, Gupta AK, Pandey AK. A comparative study between intralesional PPD and vitamin D3 in treatment of viral warts. Int J Res Dermatol. 2018;4:197–201. [Google Scholar]
- 26.Abou-Taleb DAE, Abou-Taleb HA, El-Badawy O, Ahmed AO, Hassan AET, Awad SM. Intralesional vitamin D3 versus intralesional purified protein derivative (PPD) in treatment of multiple warts: A comparative clinical and immunological study. Dermatol Ther. 2019;29:e13034. doi: 10.1111/dth.13034. [DOI] [PubMed] [Google Scholar]
- 27.Kareem IMA, Ibrahim IM, Mohammed SFF, Ahmed AA. Effectiveness of intralesional vitamin D3 injection in the treatment of common warts: Single-blinded placebo-controlled study. Dermatol Ther. 2019;32:e12882. doi: 10.1111/dth.12882. [DOI] [PubMed] [Google Scholar]
- 28.Shaheen MA, Salem SA, Fouad DA, Abd El-Fatah AA. Intralesional tuberculin (PPD) versus measles, mumps, rubella (MMR) vaccine in treatment of multiple warts: A comparative clinical and immunological study. Dermatol Ther. 2015;28:194–200. doi: 10.1111/dth.12230. [DOI] [PubMed] [Google Scholar]
- 29.Villa LL, Costa RL, Petta CA, Andrade RP, Ault KA, Giuliano AR, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: A randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6:271–8. doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- 30.Masatkar V, Gupta LK, Khare AK, Mehta S, Mittal A. Clinicoepidemiologic study of verruca plana at a tertiary care center. Indian J Dermatol Venereol Leprol. 2018;84:247. doi: 10.4103/ijdvl.IJDVL_1028_15. [DOI] [PubMed] [Google Scholar]
- 31.Al-Ahmed N, Alsowaidi S, Vadas P. Peanut allergy: An overview. Allergy Asthma Clin Immunol. 2008;4:139–43. doi: 10.1186/1710-1492-4-4-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gamil H, Elgharib I, Nofal A, Abd-Elaziz T. Intralesional immunotherapy of plantar warts: Report of a new antigen combination. J Am Acad Dermatol. 2010;63:40–3. doi: 10.1016/j.jaad.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 33.Nofal A, Nofal E, Yosef A, Nofal H. Treatment of recalcitrant warts with intralesional measles, mumps, and rubella vaccine: A promising approach. Int J Dermatol. 2015;54:667–71. doi: 10.1111/ijd.12480. [DOI] [PubMed] [Google Scholar]
- 34.Saini S, Dogra N, Dogra D. A prospective randomized open label comparative study of efficacy and safety of intralesional measles, mumps, rubella vaccine versus 100% trichloroacetic acid application in the treatment of common warts. Int J Res Med Sci. 2016;4:1529–33. [Google Scholar]