Abstract
Background:
There has been an upsurge in the cases of cutaneous leishmaniasis over the past few years in the pediatric population of Jammu and Kashmir, hitherto a nonendemic area for the disease., The aim of this study was to describe the clinico-epidemiological profile and therapeutic outcome of pediatric cutaneous leishmaniasis (PCL) over a 10-year period in J and K.
Materials and Methods:
An observational study was conducted at two tertiary care hospitals of Jammu and Kashmir over a period of 10 years (July 2010–June 20). Children presenting to the outpatient department with lesions suggestive of CL were enrolled. Patients suspected of having CL based on clinical criteria were subjected to slit skin smears (SSS) and histopathological examination (HPE) for validation of the diagnosis. Intralesional or systemic sodium stibogluconate (SSG) was the treatment modality used for the management of patients. Clinical follow-up was done at intervals of 2 weeks for the first 2 months and monthly thereafter.
Results:
A total of 376 cases of CL in children aged 1.5–15 years (mean age 8.4 ± 1.4 years) were included in the study. The duration of the disease ranged from 8 to 52 weeks (mean 22.52 ± 1.5 weeks). Lesions were noted mainly on exposed parts of the body, with face being the most commonly affected site (89.0%). Nodulo-ulcerative plaques were the predominant clinical presentation (62.76%). The diagnosis was confirmed by the demonstration of Leishman Donovan (LD) bodies in 54.25% on SSS- and 25.79% on hematoxylin and eosin -stained tissue sections. In cases where diagnosis could not be confirmed by demonstration of LD body, a histological pattern conforming to CL and response to a therapeutic trial of SSG provided evidence of leishmanial infection. Complete healing was achieved in 95.02% of the cases at the end of treatment.
Conclusion:
CL is an emerging health problem in the pediatric population of Jammu and Kashmir. Awareness among pediatric health workers regarding this disease and recognition among the differential diagnosis of ulcerated papules or plaques in the pediatric population is imperative.
Keywords: Cutaneous leishmaniasis, intralesional, Jammu and Kashmir, LD bodies, non-endemic, pediatric, sodium stibogluconate
Introduction
Leishmaniasis is a group of tropical neglected diseases caused by various species of protozoa of genus Leishmania transmitted by the bite of female sandflies. Being viscerotropic as well as dermotropic, an infection can lead to a wide disease spectrum ranging from cutaneous and mucocutaneous variants which are cosmetically disfiguring to the devastating and fatal visceral form.[1] Cutaneous leishmaniasis (CL) is the most common variant with a worldwide annual incidence of 2–2.5 million cases and an estimated disease burden of 12 million.[2]
In the old world, Leishmania tropica (urban/dry type) and Leishmania major (rural/wet type) predominantly cause CL, and infected female sandfly of the Phlebotomus genus acts as the vector.[3,4]
Rajasthan, Bikaner, and Gujarat are the known endemic areas in India owing to their dry and hot climate. Cases from other parts of the country including Himachal Pradesh, Kerala, Assam, and Haryana have also been reported recently.[5,6,7] L. tropica has been identified as the main causative species along with L.Donovani in the Indian scenario, and Phlebotomus papatasii remains the most common vector recognized.[7,8]
Rising incidence in recent years has been attributed to activities which damage the natural habitat of the vectors and events which result in large-scale displacement of populations.[8,9,10]
In recent years, Jammu and Kashmir (J and K), the northern-most union territory of the Indian subcontinent, has emerged as a new focus of CL with a predilection for the pediatric age group.
Most of the cases are reported from the Chenab Valley and Poonch and Rajouri districts in the Jammu division; and Kupwara and Baramulla districts in the Kashmir division. Severe harsh winters with heavy snow and rainfall in the Kashmir division; and extremely hot summers of the Jammu division might provide a favorable climate for the sustenance of Leishmania species and an optimal breeding ground.
In areas endemic to CL, children account for 60%–70% of the total disease burden. The higher incidence could be attributed to the fact that children are exposed to the parasite at an early age when there is a lack of CL-specific immunity.
The increasing number of cases among the pediatric population in J and K points towards the possibility of a previously unrecognized focus of endemicity. Thus, a comprehensive study evaluating the clinico-epidemiological profile and therapeutic outcome of CL in the pediatric group in our region becomes imperative.
Materials and Methods
This was a bi-centric, hospital-based, cohort study conducted at two main tertiary care hospitals of Jammu and Kashmir (Government Medical College Jammu and Government Medical College Srinagar) over a period of 10 years (July 2010– June 2020).
Children (15 years and younger) presenting with lesions suggestive of CL (one or more discrete nodules, plaques, or nonhealing ulcers on exposed sites or any atypical lesion anywhere on the body not responding to repeated courses of antibiotics and antitubercular drugs) were enrolled. Children who had been treated for CL in the past and those with cough, fever, or other systemic illnesses were excluded from the study. After taking informed consent from the parent/guardian, the clinico-demographic data was recorded on a preformed questionnaire. The patients were diagnosed as having CL on the basis of clinical criteria proposed by Kubba et al.[11]
Routine investigations, erythrocyte sedimentation rate, Mantoux test, X-ray chest, polymerase chain reaction (PCR) for tuberculosis, acid-fast bacillus (AFB) staining and culture for fungus and AFB, and ELISA for HIV were carried out in all. Demonstration of Leishman Donovan (LD) bodies on Giemsa-stained slit skin smear or elliptical biopsy sections stained with hematoxylin and eosin (H and E) was taken as confirmatory for a diagnosis of CL. In cases where demonstration of LD bodies was not possible by either method, a histological pattern conforming to CL and response to a therapeutic trial of SSG provided evidence of leishmanial infection. The patients were followed up fortnightly for the first 2 months, and monthly thereafter.
Ethical Clearance: The study was approved by the local Clinical Research Ethics Committee of Govt. Medical Colleges, Srinagar and Jammu.
Administration of treatment: Intralesional sodium stibogluconate (SSG) at a dose of 0.5 mL/cm2 (100 mg/mL solution) was administered thrice a week to all the patients until a cure was achieved or for a maximum duration of 10 weeks. For larger lesions (>5 cm in largest diameter or cumulative area >10 cm2), the drug was administered, intravenously or intramuscular, as a once-daily dose of 20 mg/kg/day for 3 weeks.
Cure criteria: Complete resolution of induration and erythema along with complete re-epithelialization was taken as the point of cure.
Statistical analysis: The data were tabulated in the form of a master chart and analyzed using the statistical software package SPSS 22. Discrete variables were assessed using the Chi-square test, and a P value <0.05 was taken as significant.
Results
A total of 376 cases of pediatric CL were presented during the study period from July 2010 to June 2020. The age of the children ranged from 1.5 to 15 years with a mean age of 8.4 ± 3.4 years. The majority of the children belonged to the 7 to 9 year age group. The demographic and clinical profile of the patients has been shown in Table 1. The incidence of the disease increased during summer (59.53%) as compared to winter season (40.47%).
Table 1.
Demographic and clinical profile in patients of Pediatric Cutaneous leishmaniasis (n=376)
| Characteristics | Number (n) | Percentage |
|---|---|---|
| Age (Years) | ||
| 0-3 | 12 | 3.19% |
| 4-6 | 23 | 6.11% |
| 7-9 | 68 | 32.97% |
| 10-12 | 149 | 39.62% |
| 13-15 | 124 | 18.08% |
| Gender | ||
| Males | 178 | 47.34% |
| Females | 198 | 52.65% |
| Residence | ||
| Rural | 369 | 98.13% |
| Urban | 7 | 1.86% |
| Family history of similar lesions | ||
| Yes | 38 | 10.11% |
| No | 338 | 89.89% |
| History of sleeping outdoors | ||
| Yes | 76 | 20.21% |
| No | 300 | 79.79% |
| Number of lesions | ||
| Single | 289 | 76.86% |
| Multiple | 87 | 23.13% |
| Duration of lesions (months) | ||
| 0-3 | 4 | 1.06% |
| 4-6 | 99 | 26.30% |
| 7-9 | 174 | 46.27% |
| 10-12 | 58 | 15.43% |
| >12 | 41 | 10.90% |
| Site of lesions* | ||
| Face | 349 | 92.82% |
| Nose | 286 | 81.94% |
| Cheeks | 229 | 65.62% |
| Eyelids involvement | 56 | 16.05% |
| Forehead | 42 | 12.03% |
| Chin | 33 | 9.45% |
| Mucocutaneous (lips/nose) | 1 | 0.002% |
| Arms | 9 | 2.39% |
| Dorsum of hands | 14 | 3.72% |
| Trunk and lower limbs | 4 | 1.06% |
| Total | 376 | 100% |
*Many patients had large-sized and multiple lesions involving more than one site on the face
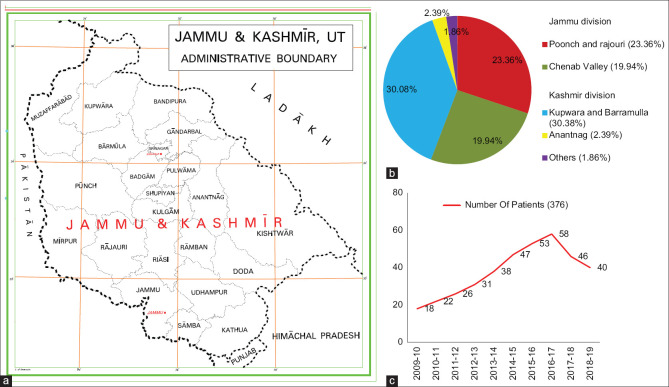
Figure 1a, b and c shows the geographic and year-wise distribution of our study population. A total of 56.65% of the patients belonged to the Kashmir division, whereas 43.35% were from the Jammu division. In the Kashmir division, Kupwara and Baramulla districts contributed the maximum number of cases (52.39%), followed by Anantnag district (2.39%), and others (1.86%). In the Jammu division, most of the cases hailed from Poonch and Rajouri districts (23.4%), followed by the Chenab Valley (19.96%) (comprising three districts; Doda, Kishtwar, and Ramban situated along the banks of the River Chena b). None of our patients had a history of travel to any endemic areas in the last 5 years preceding the disease onset.
Figure 1.
(a) Geographic map of Jammu and Kashmir and its boundaries. (b) Area-wise distribution of pediatric cutaneous leishmaniasis cases in Jammu and Kashmir. (c) Year wise distribution of pediatric cutaneous leishmaniasis cases in Jammu and Kashmir
Family history of similar lesions was elicited in 10.10%, and 20.21% of the patients had a habit of sleeping outdoors. The duration of disease ranged from 8 to 52 weeks (mean 22.52 ± 11.5 weeks). The time lag between symptom onset and diagnosis varied greatly among patients. Majority of the patients (46.27%) presented between 7 to 9 months of disease onset.
Lesions were noted mainly on exposed parts of the body, with the face being the most commonly affected site (92.82%), followed by the dorsa of hands (3.72%), and arms (2.39%). Mucocutaneous lesions involving the lower lip were seen in one child only. Relevant clinical characteristics are tabulated in Table 1. The total number of lesions ranged from 1 to 6. A solitary lesion was seen in the majority of cases (76.86%), whereasmultiple lesions were seen in 23.13%.
Nodulo-ulcerative plaques were the predominant lesion type observed in 62.76% of the cases, followed by impetigo-like lesions (13.82%), ecthymatous (6.91%), and furuncle-like (5.85%). Less common types seen were vegetating lesions (2.13%), rhinopymatous (2.13%), chancriform (1.59%), verrucous (1.59%), DLE-like (1.33%), eczematous (1.06%), zosteriform (0.26%), and sporotrichoid (0.26%), and mucocutaneous form (0.26%). [Figure 2a-l] and [Figure 3a-l]. In patients presenting with DLE-like lesions, tin tack sign could be elicited in 2 out of 5 patients (40%). Lymph node enlargement was present in 10.37%, and satellite lesions were found in 22.34% of the patients.
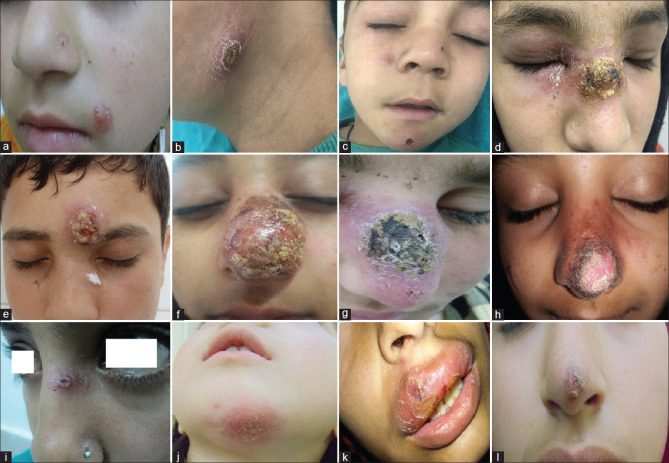
Figure 2.
Clinical spectrum of localized cutaneous leishmaniasis in study population. The usual course of disease involved (a) erythematous indurated nodule on the left cheek, (b) crusted nodule on the chin, (c) multiple papules and nodules showing crusting, (d) Plaque with crusting and edema of eyelids, (e) Impetiginized lesions on the forehead, (f) Vegetating lesion over the nose, (g) Ecthymatous plaque on the nose. Less common presentations seen: (h) Verrucous plaque on the nose, (i) Chancriform lesion, (j) Eczema-like, (k) Lip leishmaniasis, (l) DLE-like lesion
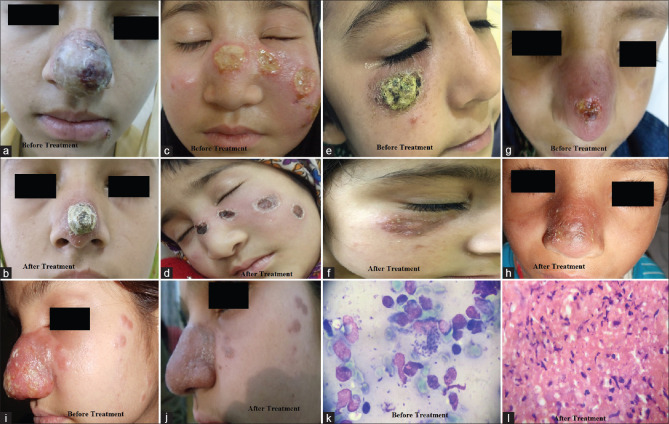
Figure 3.
(a) Rhinophyma-like cutaneous leishmaniasis (b) post-treatment at 4 weeks, (c) zoosteriform cutaneous leishmaniasis, (d) post systemic treatment at 18 days of IV SSG, (e) Crusted plaque on the right cheek, near the lower eyelid, (f) post-treatment scar and milia can be seen, (g) Ulcerated plaque on tip of the nose, (h) post-treatment scarring and hyperplgmentation (i) Indurated plaque on the nose and multiple papules on the cheek, (j) Post-treatment hyperpigmentation and mild scarring, (k) Amastigote form of the parasite in Giemsa-stained slit skin smears (×1000), (l) Chronic lymphomononuclear, plasmacytic infiltrate, and occasional LD bodies. (H and E, ×400)
Slit Skin Smear: Out of 376 clinically diagnosed cases, LD bodies were demonstrated in 54.25% on SSS.
Histopathological examination: Well-defined epithelioid cell granulomas interspersed with Langhans giant cells were the most common finding observed in 53.99% of cases. Non-specific lympho-histiocytic infiltrate in the dermis was seen in the rest (46.01%). LD bodies were demonstrated in tissue sections in 25.79%.
Clinical diagnosis was confirmed by demonstration of the parasite in 80.04% of the cases (LD body demonstration on SSS or HPE). In the remaining 19.96% cases, the presence of a recognizable histological pattern conforming to CL and response to a therapeutic trial of SSG was considered to be confirmatory of CL. Positivity for LD bodies was significantly more likely if the disease duration was less than 6 months as compared to older lesions for both SSS (P value <0.001) and HPE (P value = 0.001) [Table 2].
Table 2.
Laboratory and therapeutic characteristics with statistical relevance (n=376)
| Characteristics | Sub-groups | % age | Number | P |
|---|---|---|---|---|
| LD body positivity | Slit skin smear | 54.25% | n=204/376 | |
| Histopathology | 25.79% | n=97/376 | ||
| Both | 19.42% | n=73/376 | ||
| Only on HPE | 6.38% | n=24/376 | ||
| LD body demonstration | Duration of disease | |||
| Slit skin smear | <6 months | 77.67% | n=80/103 | P<0.001 |
| HPE | >6 months | 45.42% | n=124/273 | |
| Both | <6 months | 31.07% | n=32/103 | P=0.001 |
| >6 months | 16.48% | n=45/273 | ||
| <6 months | 32.04% | n=33/103 | P<0.001 | |
| >6 months | 14.65% | n=40/273 | ||
| Mode of administration of SSG | Intralesional | 91.22% | n=343/376 | |
| Intravenous/Intramuscular | 8.78% | n=33/376 | ||
| Response to treatment | Intralesional SSG | 91.84% | n=315/343 | P=0.44 |
| Intravenous/Intramuscular SSG | 87.88% | n=29/33 | ||
| Complete resolution in relation to the number of lesions and treatment modality | ||||
| Intralesional SSG | Single lesion | 93.01% | n=253/272 | P=0.06 |
| Multiple lesions | 86.11% | n=62/72 | ||
| IM/IV SSG | Single lesion | 88.89% | n=16/18 | P=0.84 |
| Multiple lesions | 86.67% | n=13/15 |
LD: Leishman-Donovan; HPE: histopathological examination; SSG: sodium stibogluconate
Intralesional SSG was the most commonly employed modality of treatment used in 343 patients (91.22%) and was well tolerated in all patients. A total of 33 (8.78%) patients received the drug intravenously or intramuscularly. Complete resolution was obtained in 91.49% of all patients with the average time till clearance being 6.6 ± 1.8 weeks (range 4 to 10 weeks). No significant difference was observed in response to intralesional or systemic mode of administration (P value = 0.44) except for a shorter duration of treatment in the latter. The time taken for the lesions to resolve with systemic administration was shorter (2.4 ± 0.6 weeks) compared to local administration (6.4 ± 2.6 weeks) (P <0.001).
A complete response to intralesional SSG was comparable in patients with a single lesion (93.01%) and those with multiple lesions (86.11%) (P value = 0.06). The same applied to systemic administration of SSG for single versus multiple lesions (88.89% vs 86.67%, P value = 0.84). All the patients tolerated the treatment well with negligible side effects, and no recurrences were observed during the follow-up period. During the first few days following the administration of SSG, almost all patients developed increased erythema, edema, and induration in the lesions which subsided on their own and did not warrant withdrawal of treatment. Pain related to injection was a commonly reported side effect with both modes of administration. Most patients demonstrated hypo- or hyperpigmented scars at the end of the treatment [Table 3].
Table 3.
Complications related to treatment****
| IL SSG | Systemic SSG | |
|---|---|---|
| Occurrence of side effects | 77.26% (n=265/343) | 72.72% (n=24/33) |
| Pain +++ | Pain++Raised liver enzymes +++ | |
| Hyperpigmentation+++ | Headache & Giddiness+++ | |
| Hypertrophic & atrophic scars+++ | Fainting+Myalgias +++ | |
| Milia++ | Hyperpigmentation +++ | |
| Raised liver enzymes - | Hypertrophic & atrophic scars +++ | |
| Postural hypotension+ | ||
| Headache & Giddiness - | ||
| Myalgias - | ||
| Relapse of disease | None at 6 months | None at 6 months |
****Many patients experienced more than one side effect of therapy. +++ Very common; ++ Common; + Less common; Nil. SSG: Sodium stibogluconate
Discussion
Although few studies have highlighted Jammu and Kashmir as a new focus of cutaneous leishmaniasis, there is no comprehensive study on the affliction of the pediatric age group by CL in our region.[12,13] Most of our patients belonged to northwest districts of Kashmir province that shares climatic similarities with neighboring areas where CL is highly endemic.[14,15]
The incidence of CL is higher among the pediatric population in endemic areas, accounting for 60%–70% of the cases.[16,17] Similar to earlier studies, the 7 to 12 year age group was most commonly affected in our study. This could be accounted for by the greater risk of sandfly bites consequent to the increased involvement in outdoor playing activities as well as lack of immunity to CL in this age group.
The mean disease duration in our study was 22.52 weeks. This is in contrast to studies from Tunisia and Turkey where the mean duration of the disease was reported as 12.71 weeks and 8.58 weeks, respectively[17,18] This difference could be attributed to the difference in the prevalent Leishmania strains in our geographic belt. This is supported by the finding of similar results (4.6 months) in a study on CL in adults by Wani et al.[12] from the Kashmir Valley. There was a female preponderance in our study. This is in contrast to the studies by Kumar,[16] Agrawal[19], and Bari et al.[20] where a male preponderance was reported. Multiple lesions were encountered in 23.13% of our patients. Bari et al.[20] observed multiple lesions in 25% of cases. Lesser and greater numbers of cases with multiple lesions have been described by Zaraa et al.[17] (8.4%) and Talaei et al.[21] (48.7%). Positive family history in 10.10% of patients in our study was in line with the findings of Kharfi et al.,[22] and Qasmi et al.[23] Average time lag between the appearance of CL lesion and the first diagnosis have been reported as 12.71 months and 8 months by Zaraa and Kharfi, respectively.[17,22] We found a shorter time interval of approximately 5 months. This could be due to increased concern regarding the health issues of children and early consultation by the parents. The face was the most common site of involvement affected in 92.82% of the patients, compatible with the established clinical picture of the disease.[18,19,20]
In the current study, the most common lesions were nodulo-ulcerative. An impetigo-like or impetiginized lesion with purulence was another common observation in our pediatric population. Bacterial contamination of CL lesions has been reported previously,[24] and such cases are likely to get misdiagnosed as bacterial infections of the skin. This underlines the importance of a high index of suspicion among clinicians who may be confronted with a myriad of clinical presentations of CL. Uncommon variants such as verrucous, rhinophymatous, and zosteriform lesions were seen as well.[15,25,26] The role of both parasite-related factors and the host's immune responses is responsible for this varied clinical picture.[15,25,26,27] Mucosal lesions were a trifling occurrence seen in only one patient.[6,15] The disease was acquired locally as there was no history of travel to any endemic areas. This points towards the possibility of a local vector as well as a reservoir in our region.
In the present study, SSS and histopathology were confirmatory in 54.25% and 25.79%, respectively. This incongruity between SSS and histopathology could be because Leishmania organisms appear smaller and flimsier on histopathology owing to repeated processing of specimens. Agrawal et al.[19] and Kumar et al.[16] in their respective studies on PCL reported a tissue smear positivity in 70% and 62.5% cases, respectively, whereas the positivity rates on histopathology were found to be 50% in both studies. Similar findings were reported by Gupta M in his study on 10 children with CL.[28] The lower positivity rates on histopathology in our study could be attributed to the increased duration of the lesions, which are less likely to yield the parasite. Moreover, a lesser proficiency with the technique owing to the nonendemic status of our region could account for the lower positivity rates. The advanced diagnostic modalities for leishmaniasis including immunofluorescence, use of monoclonal antibodies, DNA probes, and polymerase chain reaction were not available at our center.[29,30]
The pentavalent compounds have yielded the best results in all age groups among the different treatment modalities utilized for the treatment of CL. In our study, a complete cure was achieved in 91.49% of the patients over an average period of 6.6 weeks following the WHO protocol. This is similar to the findings of Zaraa et al.[17] and Agrawal et al.[19] who found 86·25% and 84.4% efficacy of intralesional antimonial treatment in PCL patients, respectively. Sharma et al.[6] and Tallab et al. administered three intralesional injections of SSG (1–5 mL) given on an alternate day schedule till 5–7 doses.[29]
The treatment efficacy with the intralesional or systemic mode of administration was found to be comparable. Both modalities of treatment gave better results in patients with a single lesion although the difference was not found to be statistically significant. Overall, intralesional treatment is the preferred treatment for PCL, so as to avoid the toxicity associated with systemic administration of antimonials.[31]
Limitations
Species determination was not done in this study due to the nonavailability of PCR in our hospital setup.
Conclusion
This study provides an epidemiological background of cutaneous leishmaniasis in thepediatric population of Jammu and Kashmir over a period of 10 years. This data can serve as a pivot to guide future studies related to this topic and fill the void that exists in the literature with regards to the characteristics of pediatric cutaneous leishmaniasis in this part of the world. This will further help us to direct the most appropriate laboratory evaluation and careful management in an effort to minimize the morbidity due to disease in this vulnerable age group.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Akhoundi M, Kuhls K, Cannet A, Votýpka J, Marty P, Delaunay P, et al. A historical overview of the classification, evolution, and dispersion of leishmania parasites and sandflies. PLoS Negl Trop Dis. 2016;10:e0004349. doi: 10.1371/journal.pntd.0004349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burza S, Croft SL, Boelaert M. Leishmaniasis. Lancet. 2018;392:951–70. doi: 10.1016/S0140-6736(18)31204-2. [DOI] [PubMed] [Google Scholar]
- 3.Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis world-wide and global estimates of its incidence. PLoS One. 2012;7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. Leishmaniasis. Geneva: World Health Organization; [Last accessed on 2017 Jun 09]. Available from: http://www.who.int/mediacentre/factsheets/fs375/en/ [Google Scholar]
- 5.Kumar R, Bumb RA, Ansari NA, Mehta RD, Salotra P. Cutaneous leishmaniasis caused by L.tropica in Bikaner, India: Parasite identification and characterization using molecular and immunologic tools. Am J Trop Med Hyg. 2007;76:896–901. [PubMed] [Google Scholar]
- 6.Sharma RC, Mahajan VK, Sharma NL, Sharma A. A new focus of cutaneous leishmaniasis in Himachal Pradesh (India) Indian J Dermatol Venereol Leprol. 2003;69:170–2. [PubMed] [Google Scholar]
- 7.Simi SM, Anish TS, Jyothi R, Vijaykumar K, Philip RR, Paul N. Searching for cutaneous leishmaniasis in tribals from Kerala, India. J Glob Infect Dis. 2010;2:95–100. doi: 10.4103/0974-777X.62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma NL, Mahajan VK, Kanga A, Sood A, Katoch VM, Mauricio I, et al. Localized cutaneous leishmaniasis due to Leishmania donovani and Leishmania tropica: Preliminary findings of the study of 161 new cases from a new endemic focus in Himachal Pradesh, India. Am J Trop Med Hyg. 2005;72:819–24. [PubMed] [Google Scholar]
- 9.Alawieh A, Musharrafieh U, Jaber A, Berry A, Ghosn N, Bizri AR. Revisiting leishmaniasis in the time of war: The Syrian conflict and the Lebanese outbreak. Int J Infect Dis. 2014;29:115–9. doi: 10.1016/j.ijid.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 10.Alvar J, Aparicio P, Aseffa A, Den Boer M, Canavate C, Dedet JP. The relationship between leishmaniasis and AIDS: The second 10 years. Clin Microbiol Rev. 2008;21:334–59. doi: 10.1128/CMR.00061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kubba R, Al-Gindan Y, el-Hassan AM, Omer AH. Clinical diagnosis of cutaneous leishmaniasis (oriental sore) J Am Acad Dermatol. 1987;16:1183–9. doi: 10.1016/s0190-9622(87)70155-8. [DOI] [PubMed] [Google Scholar]
- 12.Wani GM, Ahmad SM, Khursheed B. Clinical study of cutaneous leishmaniasis in the Kashmir Valley. Indian Dermatol Online J. 2015;6:387–92. doi: 10.4103/2229-5178.169732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaul N, Gupta V, Bhardwaj S, Dogra D, Dogra N. A new focus of cutaneous leishmaniasis in Jammu division of Jammu and Kashmir State, India. Indian J Dermatol Venereol Leprol. 2016;82:145–50. doi: 10.4103/0378-6323.175930. [DOI] [PubMed] [Google Scholar]
- 14.Ul Bari A, Rahman S. Correlation of clinical, histopathological, and microbiological findings in 60 cases of cutaneous leishmaniasis. Indian J Dermatol Venereol Leprol. 2006;72:28–32. doi: 10.4103/0378-6323.19714. [DOI] [PubMed] [Google Scholar]
- 15.Bari A, Rahman S. Many faces of cutaneous leishmaniasis. Indian J Dermatol Venereol Leprol. 2008;74:23–7. doi: 10.4103/0378-6323.38402. [DOI] [PubMed] [Google Scholar]
- 16.Kumar P, Garg A, Kumari S, Negi A, Tegta GR. One-year prospective study of pediatric cutaneous leishmaniasis: A neglected tropical disease in sub Himalayan region, India. Int J Contemp Pediatr. 2018;5:1546–50. [Google Scholar]
- 17.Zaraa I, Ishak F, Kort R, El Euch D, Mokni M, Chaker E, et al. Childhood and adult cutaneous leishmaniasis in Tunisia. Int J Dermatol. 2010;49:790–3. doi: 10.1111/j.1365-4632.2010.04467.x. [DOI] [PubMed] [Google Scholar]
- 18.Aksoy M, Doni N, Ozkul HU, Yesilova Y, Ardic N, Yesilova A, et al. Pediatric cutaneous leishmaniasis in an endemic region in Turkey: A retrospective analysis of 8786 cases during 1998-2014. PLoS Negl Trop Dis. 2016;10:e004835. doi: 10.1371/journal.pntd.0004835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agrawal S, Khandelwal K, Bumb RA, Oghumu S, Salotra P, Satoskar AR. Short report: Pediatric cutaneous leishmaniasis in an endemic region in India. Am J Trop Med Hyg. 2014;91:901–4. doi: 10.4269/ajtmh.13-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bari A U. Childhood cutaneous leishmaniasis. J ClinDiag Res. 2008;2:973–8. [Google Scholar]
- 21.Talaei R, Shajari G, Vakili Z, Taghaviardakani A. Childhood cutaneous leishmaniasis: Report of 117 cases from Iran. Korean J Parasitol. 2006;44:355–60. doi: 10.3347/kjp.2006.44.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kharfi M, Benmously R, El Fekih N, Daoud M, Fitouri Z, Mokhtar I, et al. Childhood leishmaniasis: Report of 106 cases. Dermatol Online J. 2004;10:6. [PubMed] [Google Scholar]
- 23.Qasmi S, Elguelbazouri N, Belgnaoui FZ, Marcil T, Bouhllab J, Senouci K, et al. Childhood cutaneous leishmaniasis: Experience of a Moroccan unit of dermatology. Dermatol Online J. 2008;14:18. [PubMed] [Google Scholar]
- 24.Layegh P, Ghazvini K, Moghiman T, Hadian F, Zabolinejad N, Pezeshkpour F. Bacterial contamination in cutaneous leishmaniasis: Its effect on the lesions healing course. Indian J Dermatol. 2015;60:211. doi: 10.4103/0019-5154.152560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iftikhar N, Bari I, Ejaz A. Rare variants of Cutaneous Leishmaniasis: Whitlow, paronychia, and sporotrichoid. Int J Dermatol. 2003;42:807–9. doi: 10.1046/j.1365-4362.2003.02015.x. [DOI] [PubMed] [Google Scholar]
- 26.Meireles CB, Maia LC, Soares GC, Teodoro IPP, Gadelha MDSV, da Silva CGL, et al. Atypical presentations of cutaneous leishmaniasis: A systematic review. Acta Trop. 2017;172:240–54. doi: 10.1016/j.actatropica.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 27.Kafetzis DA. An overview of paediatric leishmaniasis. J Postgrad Med. 2003;49:31–8. doi: 10.4103/0022-3859.930. [DOI] [PubMed] [Google Scholar]
- 28.Gupta M. Cutaneous leishmaniasis in children: A case series. Indian J Paediatr Dermatol. 2018;19:37–9. [Google Scholar]
- 29.Tallab TM, Bahamdam KA, Mirdad S, Johargi H, Mourad MM, Ibrahim K, et al. Cutaneous leishmaniasis: Schedules for intralesional treatment with sodium stibogluconate. Int J Dermatol. 1996;35:594–7. doi: 10.1111/j.1365-4362.1996.tb03669.x. [DOI] [PubMed] [Google Scholar]
- 30.Jara M, Adaui V, Valencia BM, Martinez D, Alba M, Castrillon C, et al. Real-time PCR assay for detection and quantification of Leishmania (Viannia) organisms in skin and mucosal lesions: Exploratory study of parasite load and clinical parameters. J Clin Microbiol. 2013;51:1826–33. doi: 10.1128/JCM.00208-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aronson N, Herwaldt BL, Libman M, Pearson R, Lopez-Velez R, Weina P, et al. Diagnosis and treatment of leishmaniasis: Clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH) Clin Infect Dis. 2016;63:1539–57. doi: 10.1093/cid/ciw742. [DOI] [PubMed] [Google Scholar]