Abstract
Endocervical mucus changes play a key role in regulating fertility throughout the menstrual cycle and in response to hormonal contraceptives. Non-human primates (NHP) provide the most translational animal model for reproductive tract studies, as they have hormonally-regulated menstrual cycles and mucus changes, similar to women. We used TMT labelling and LC-LC/MS to compare the proteins found in the mucus of the rhesus macaque to the mucus of the human endocervix. Data are available via ProteomeXchange with identifier PXD021710. We found 3,048 total proteins present in both rhesus mucus and human mucus, and of these, 57% showed a similar expression pattern. An even higher similarity occurred in the top 500 most prevalent proteins, with overlap in 341 (68%) proteins. Mucin MUC5B was the most highly expressed mucin protein (top 10 expressed proteins in both) but other key proteins related to mucus structure were present in both samples. We find that the mucus proteome of the endocervical mucus is highly conserved in NHP and women. This supports use of the NHP model system for studies of the endocervix and trials of novel fertility treatments targeting the cervix.
Endocervical mucus changes play a key role in regulating fertility throughout the menstrual cycle and in response to hormonal contraceptives [1]. Under the influence of estrogens, mucus becomes abundant, fluid, and watery, and facilitates sperm entry through the cervix into the upper female reproductive tract. Under the influence of progestogens, mucus becomes thick and viscous, and acts as a natural barrier for movement of sperm or pathogens to the upper tract. While the fluctuations due to hormonal changes are well recognized, we have a limited understanding of the compositional changes to mucus that drive fertile and non-fertile conditions.
Cervical mucus is a highly complex mixture of water, lipids, cholesterols, carbohydrates, organic and inorganic ions, and proteins. The proteins have a variety of purpose such as the structure and hydration of mucus (i.e. mucins), immune function (i.e. immunoglobulins), and enzymatic reactions (i.e. elastases). While many mucus proteins have direct roles in fertility such as sperm capacitation[2], the vast majority do not have a defined function [3].
The practical difficulties of obtaining carefully timed cervical tissue biopsies and mucus samples in women greatly limit human studies examining the regulation of mucus. Proof-of-concept studies of novel mucus altering agents as candidate drugs also require an appropriate animal model [4]. Non-human primates (NHP) provide an ideal model for studying the reproductive tract as they have comparable anatomy and hormonally-mediated responses that include menstrual and cervical mucus cycles, similar to those of women [5]. While we hypothesize that NHPs provide an ideal pre-clinical model for novel agents that target the cervix, we do not know how closely mucus secreted by the NHP endocervix resembles mucus from women. Experiments in NHPs examining sperm movement through the reproductive tract have not verified that NHP secreted cervical mucus has similar proteins and properties to human mucus. Here, we used quantitative proteomic methods to show that the proteins found in the mucus of the rhesus macaque are similar to women.
Figure 1 describes the overall study design. We collected human samples (n=3) from a donors undergoing a clinical trial at Oregon Health and Science University examining the effects of the progestin-only mini-pill in women who had undergone ovarian suppression with add-back hormonal therapy [6]. We used a vaginal speculum to expose the cervix and a scopette to clear the external os of any fluid or debris. We then inserted a mucus aspirette (Unimar Aspirette device, Cooper Surgical, Trumbull, CT, USA) into the external os, approximately one centimeter to obtain a sample. The Oregon Health and Science Institutional Review Board approved this study and we registered this trial on clincaltrials.gov (NCT02969590).
Figure 1:
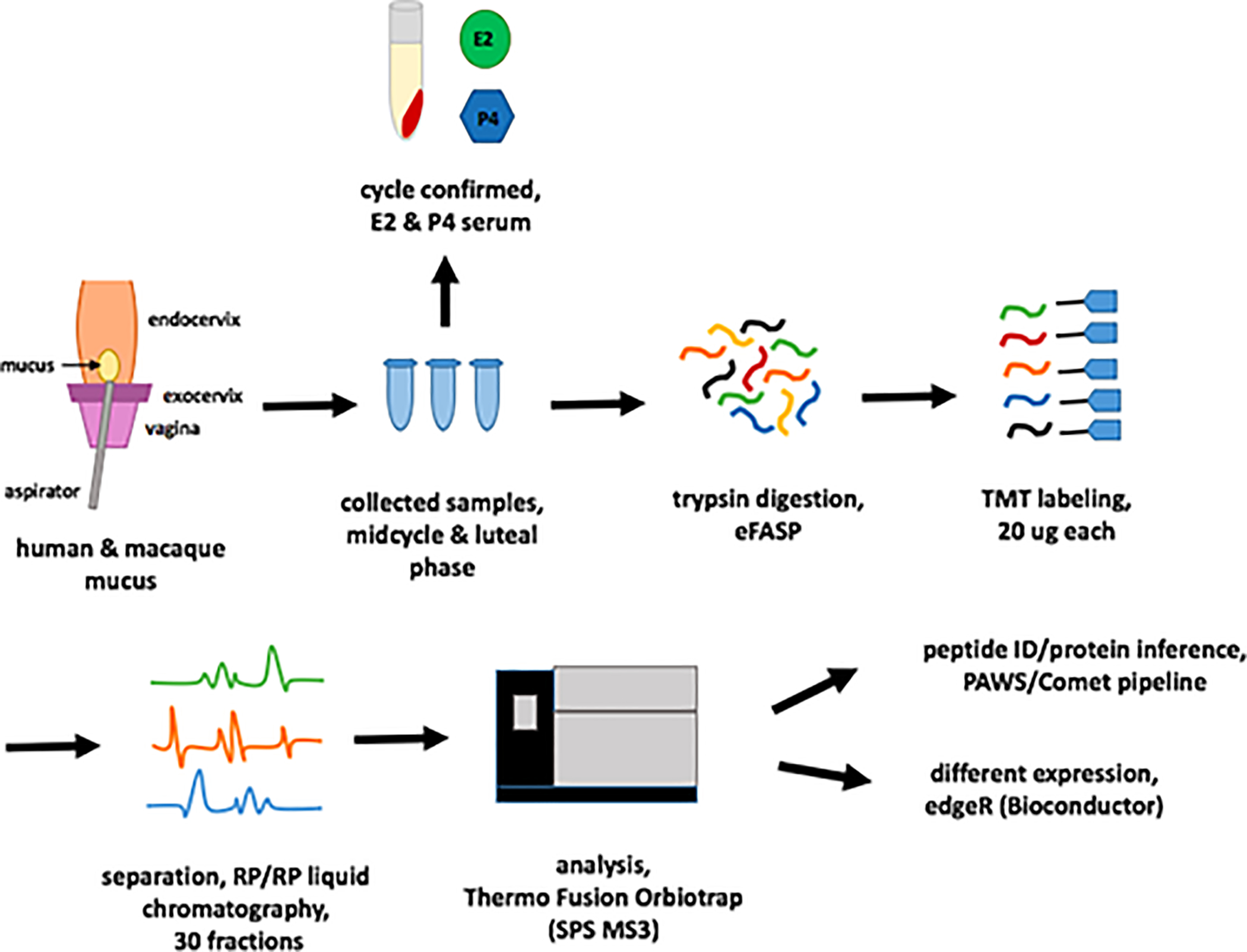
Study design. Mucus was collected from the endocervix of participants in a clinical trial or from fresh necropsy specimens of rhesus macaques. Collected samples were trypsin digested, 20ug each labeled with tandem mass tags (TMT), separated with RP/RP LC (30 fractions), and analyzed on a Thermo Fusion Orbitrap (SPS MS3). Peptide ID and protein inference used the PAW/Comet pipeline.
We collected rhesus mucus samples from reproductive-aged female rhesus macaques (Macaca mulatta) (n=2) already undergoing necropsy at the Oregon National Primate Research Center (ONPRC) for unrelated reasons. We bi-valved the endocervix specimens and washed and aspirated the luminal surface with 200 μl of phosphate buffered saline (PBS) using a 1 ml slip-tip insulin syringe. We also collected serum samples from both the woman and macaques to measure estradiol (E2) and progesterone (P4) levels to verify their hormonal status at the time of the collection. Based on E2 and P4, macaque samples corresponded to early follicular (E2=16 pg/ml, P4 = .13ng/ml) and luteal phase (E2=21, P4= 1.64 ng/ml). We collected human samples under hormonal suppression with leuprolide with add-back hormonal therapy using estradiol patches and oral progestogens. [E2=undetectable pg/ml, P4=.13 ng/ml, mucus score=1 (out of 15)], high estradiol conditions (E2=356 pg/ml, P4= .11 ng/ml, mucus score=13) and conditions where we co-administered high estradiol and oral norethindrone (NET) (E2=303.8pg/ml, P4=.08 ng/ml, NET=.52 ng/ml, mucus score 6).
We probe sonicated approximately 60–200ul of each sample using 4% SDS, 0.2% Deoxycholic acid, and 100mM TEAB. We then quantified each sample using a BCA protein assay, and used 55μg of digested sample [7]. We labeled 20μg of peptide digest from each sample with tags (TMT10plex™ Isobaric Label Reagent Set and TMT11–131C Label Reagent, Thermo Scientific) from an 11-plex TMT kit (see Supplementary Methods for full descriptions of sample preparation, peptide detection and analysis).
We ran a pre-analysis normalization run to determine final mixing volumes, then fractionated the multiplexed sample with high pH reverse phase (30-fractions), followed by conventional low pH reverse phase, ionized with nano-electrospray, and analyzed on a Thermo Fusion Tribrid mass spectrometer. We collected MS2 spectra with CID using the linear ion trap. The reporter ions were generated using HCD after SPS MS3 enrichment [8].
We used the Comet [9] and the PAW pipeline [10] to identify proteins and peptides. We used canonical UniProt reference human (20,960 sequences, UP000005640, release 2019.06) or rhesus monkey (21,211 sequences, UP000006718, release 2019.07) protein databases. We obtained confident peptide identifications using accurate mass conditional score histograms and the target/decoy method. We used the PAW pipeline to infer proteins, perform homologous protein grouping, establish peptide uniqueness to the inferred proteins, and sum unique PSM (peptide spectrum matches) reporter ions into protein intensity totals. We conducted differential expression testing using edgeR[11] from Bioconductor. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD021710 [12].
Protein recovery from the samples (human, n=3; macaque, n=2) analyzed ranged from 290ug to 5.7mg with an average recovery of 2.3mg. Average peptide recovery was 53μg after eFASP digestion of 55μg of protein. Searching the data against the human protein database resulted in 93K accepted PSMs (1% FDR) and 3103 protein identifications (0.2% FDR). Using the monkey database resulted in 97K PSM identifications (1% FDR) and also 3137 proteins. Use of the human database for analyses of the monkey channels decreased intensity by only 7% compared to use of the monkey database. In contrast, use of the monkey database on the human channel decreased totals by 55% compared to the human database. Therefore, we used the search results from the human database when comparing human and monkey samples.
In total, we found 3048 proteins present in both rhesus mucus and human mucus. Table 1 shows the most abundant proteins for each species. Macaque and human shared 341 (68%) of the top 500 proteins, and 10 (40%) of the top 25 proteins. The top two proteins on each list, serum albumin [UniProt: P02768] and protein S100-A9 [UniProt: P06702], were identical. MUC5B [UniProt: Q9HC84] was the most prevalent mucin protein in both samples and also one of the most prevalent proteins overall. We observed 26.8% sequence coverage of Mucin 5B including high coverage of both the N-terminal and C-terminal regions (full sequence coverage presented in supplemental methods). Other endocervical mucins, in particular MUC5AC [UniProt: P98088] and MUC16 [UniProt: Q8WXI7], were highly present. Using a mucin western blot, we confirmed the presence of MUC5B in both rhesus and human mucus samples (see supplementary figure 1, supplementary methods). Other key proteins related to mucus structure present in both samples included leukocyte elastase [UniProt: P30740] and secretory leukocyte proteinase inhibitor (SLPI) [UniProt: P03973]. We used a proteomics result annotation tool (https://github.com/pwilmart/annotations) to provide the Gene Ontology (GO) biological process terms associated for each protein. The most prevalent GO processes were immunity, metabolism, cell-cell signaling, transport, and hemostasis.
Table 1:
Top 25 most abundant proteins identified in macaque and human mucus samples. Samples in red are present in both lists.
| Macaque | UniProt Accession | Avg. reporter ion intensity (in millions) | GO: Biological Process | Human | UniProt Accession | Avg. reporter ion intensity (in millions) | GO: Biological Process | |
|---|---|---|---|---|---|---|---|---|
| 1 | Serum albumin | P02768 | 54.7 | metabolism, post translational protein modification | Serum albumin | P02768 | 819 | metabolism, post translational protein modification |
| 2 | Protein S100-A9 | P06702 | 16.2 | immune response, cell death, cell-cell signaling | Protein S100-A9 | P06702 | 121 | immune response, cell-cell signaling |
| 3 | Hemoglobin subunit alpha | P69905 | 9.59 | transport, stress response | Protein S100-A8 | P05109 | 104 | immune response |
| 4 | Pyruvate kinase PKM | P14618 | 9.24 | metabolism | Serotransferrin (Transferrin) | P02787 | 89.4 | transport, iron ion homeostasis, protein regulation |
| 5 | Complement C3c alpha' chain fragment 2 | P01024 | 9.09 | immune response, cell-cell signaling | Complement C3c alpha' chain fragment 2 | P01024 | 53.8 | immune response, cell-cell signaling |
| 6 | Alpha-enolase | P06733 | 8.85 | metabolism, immune response | Immunoglobulin kappa constant | P01834 | 52.0 | immune response |
| 7 | Annexin A2 | P07355 | 8.53 | cell growth, collagen fibril organization | Truncated apolipoprotein A-I | P02647 | 46.6 | metabolism, transport, hormone regulation |
| 8 | Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | P04406 | 8.31 | immune response | Short peptide from AAT | P01009 | 37.4 | acute phase response, hemostasis |
| 9 | Mucin-5B (MUC-5B) | Q9HC84 | 8.21 | Immune response | Mucin-5B (MUC-5B) | Q9HC84 | 30.8 | Immune response |
| 10 | Protein S100-A8 | P05109 | 7.66 | immune response | Lactoferroxin-C | P02788 | 23.4 | immune response, transport |
| 11 | Heat shock cognate 71 kDa protein | P11142 | 7.09 | transport, immune response, stress response, mRNA regulation | Immunoglobulin heavy constant gamma 1 | P01857 | 23.3 | immune response |
| 12 | Serotransferrin (Transferrin) | P02787 | 6.98 | transport, iron ion homeostasis, protein regulation | Immunoglobulin heavy constant alpha 1 | P01876 | 18.5 | immune response |
| 13 | Gelsolin | P06396 | 6.84 | metabolism, cilia biogenesis/degradation | Small proline-rich protein 3 | Q9UBC9 | 17.7 | stress response, keratinization |
| 14 | Elongation factor 1-alpha 1 (EF-1-alpha-1) | P68104 | 6.68 | protein regulation | Secretory component | P01833 | 17.4 | cell-cell signaling |
| 15 | Actin, cytoplasmic 1, N-terminally processed | P60709 | 6.36 | motility | Neutrophil defensin 2 | P59665 | 16.8 | immune response |
| 16 | Myosin-9 | P35579 | 6.33 | intracellular organization | Fibrinogen gamma chain | P02679 | 16.1 | cell-cell signaling, hemostasis |
| 17 | Protein S100-A6 | P06703 | 6.18 | cell growth, cell-cell signaling | Fibrinogen beta chain | P02675 | 12.6 | immune response, hemostasis |
| 18 | Anterior gradient protein 2 homolog (AG-2; hAG-2) | O95994 | 5.77 | cell growth | Actin, cytoplasmic 1, N-terminally processed | P60709 | 12.3 | motility |
| 19 | Short peptide from AAT | P01009 | 5.42 | acute phase response, hemostasis | Desmoplakin (DP) | P15924 | 11.3 | cell-cell signaling, keratinization |
| 20 | Profilin-1 | P07737 | 5.24 | motility | Histone H4 | P62805 | 11.0 | metabolism, gene regulation |
| 21 | Annexin A1 | P04083 | 4.7 | immune response, cell growth, hormone regulation | Vitamin D-binding protein (DBP; VDB) | P02774 | 10.9 | transport |
| 22 | Triosephosphate isomerase (TIM) | P60174 | 4.63 | metabolism | Myeloperoxidase heavy chain (MPO) | P05164 | 10.9 | immune response |
| 23 | Histone H4 | P62805 | 4.44 | metabolism, gene regulation | BPI fold-containing family B member 1 | Q8TDL5 | 10.6 | immune response |
| 24 | Protein disulfide-isomerase A3 | P30101 | 4.35 | immune response | Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | P04406 | 10.3 | immune response |
| 25 | Protein-glutamine gamma-glutamyltransferase 2 | P21980 | 4.29 | cell growth | Neutrophil gelatinase-associated lipocalin (NGAL) | P80188 | 10.2 | immune response, transport |
Overall, we found 1349 proteins to be differentially expressed (FDR <0.05); 635 proteins with increased relative abundance in the macaque and 713 proteins with increased relative abundance in the human (Figure 2). We performed a functional annotation analysis using the Database for Annotation, Visualization, and Integrated Discovery (DAVID, https://david.ncifcrf.gov/) on the differently regulated proteins. DAVID functional annotation analysis top keywords for proteins with increased abundance in macaque mucus included phosphoprotein (59.2%), alternative splicing (57.2%), acetylation (52.3%), cytoplasm (44.3%), disease mutation (19.1%), and transport (17.5%). The top keywords for proteins with increased abundance in human mucus were polymorphism (65.4%), phosphoprotein (49.9%), membrane (40.7%), signal (38.6%), glycoprotein (35.3%), and disulfide bonds (33.3%). Additionally, we found immunoglobulins chains to be more abundant in human samples.
Figure 2:
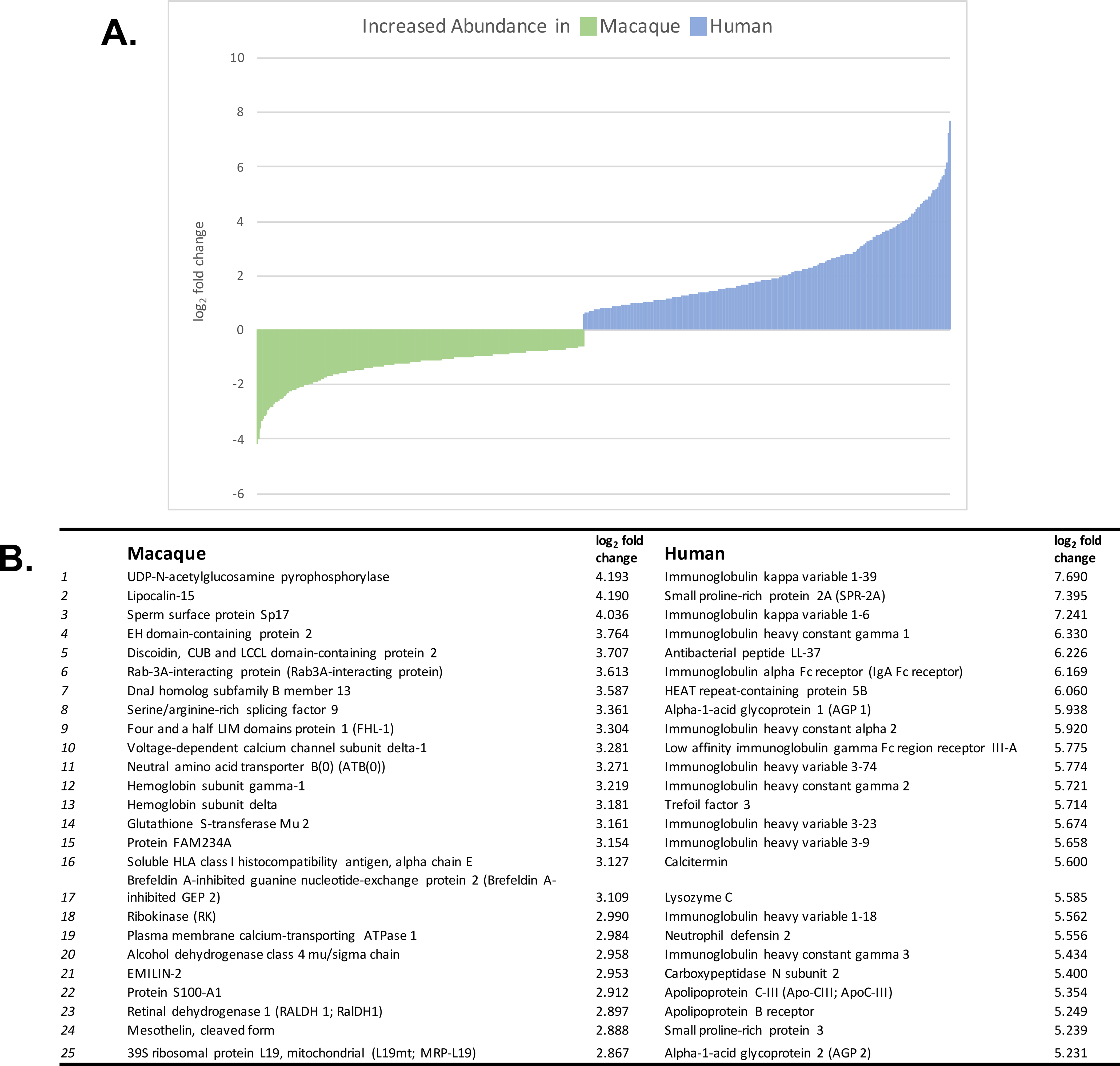
A. All differently expressed proteins identified in macaque and human mucus samples graphed by their log2 fold change. B. Top 25 differentially expressed proteins identified in macaque and human mucus samples.
The highly conserved mucus proteome between human and macaque mucus is consistent with the close genetic, anatomic, and physiologic similarities between women and female NHPs. Of note, human and macaque samples contained similar relative quantities of mucins proteins (MUC5B, MUC5AC, MUC 16, MUC4, MUC1) which serve as the backbone of the mucus gel network [13]. Of these, MUC5B is the most important structural element as it is the most prevalent mucus in human endocervical mucus [14]. Along with other secreted proteins found in both human and macaque samples, these proteins play critical roles in the rheological characteristics of mucus involved in fertility and immunity.
The major biological process based on proteins we found in both human and macaque mucus relates to immunity. In our panel, complement proteins, immunoglobulins, and defensins were all highly secreted in both human and macaque sample. These findings support the existing use of NHPs to study lower reproductive tract infections. Infectious pathogens such as HIV or Chlamydia have relied on NHP studies to find biological mechanisms of disease entry and transmission. Secreted immune elements are thought to fluctuate significantly through the menstrual cycle and under hormonal contraception in order to keep out bacterial infection, and also possibly sperm itself through an immune mechanism [15].
The proteomic similarities have importance particularly for contraceptive discovery and pre-clinical contraceptive studies. Current clinical trials of mucus-altering drugs use Insler or Mucus Scores for appraising the physical characteristics of human cervical mucus in order to determine its fertility potential. These elements include rheological characteristics of the gel itself (spinnbarkeit, viscosity), evaluation of ion content (ferning), cellular content (cellularity) and overall volume. While our proteomics study includes both secreted and intracellular protein, based on the high similarity in found proteins, we would presume these to have similar qualities in NHP studies. As clinical trials in women are limited to approved investigational drugs, translating clinical measures to NHPs would be useful for proof-of-concept studies. Results from our proteomic study support use of NHPs to perform mechanistic assessments of mucus protein changes under experimental conditions.
Performing a mixed species TMT experiment is complicated. The common strategy of only using unique peptides for quantification fails when two similar species FASTA databases are concatenated. Many peptides would be identical between species and not usable for quantification. This would reduce quantification sensitivity but could also bias the data. Performing species specific-database searches yields the best total protein intensities for each species. However, comparisons of channels between species is complicated because the data come from two different searches of different FASTA files. In order to compare the intensities of the same proteins between monkey and human, we compared the protein identifications from each search and used the database with the least amount of change in the cross-species peptide intensity.
Since we did not design this pilot to compare mucus under different conditions, we cannot determine whether proteomic changes seen in NHPs during the menstrual cycle are similar to those in human. Insight into the differential regulation between species is limited by our small sample size. Both biological variability and hormonal changes could have masked or amplified differences we observed. In this study, the more notable finding was the large overlap in secreted proteins found in human and NHP mucus. Future studies could consider more closely examining the cyclic changes of macaque mucus and compare them to existing human studies [16].
In summary, this study found major compositional similarities between human and monkey cervical mucus and suggests that NHP systems could be excellent experimental models for studies of the endocervix and trials of novel fertility treatments targeting the cervix.
Supplementary Material
Acknowledgements
This research received support from the grant K12 HD000849, awarded to the Reproductive Scientist Development Program by the NICHD. In addition, this work received funding from The March of Dimes Foundation, American Society for Reproductive Medicine and American Board of Obstetrics and Gynecology as part of the RSDP, as well as the OHSU-School of Medicine, Medical Foundation of Oregon and ONPRC core grant number P51 OD011092
Mass spectrometry was done at the OHSU Proteomics Shared Resource with partial support from NIH grants P30EY010572, P30CA069533, and S10OD012246.
Footnotes
Conflict of Interest Statement
The authors have declared no conflict of interests
References
- [1].Han L, Taub R, Jensen JT, Cervical mucus and contraception: what we know and what we don’t. Contraception 2017, 96, 310–321. [DOI] [PubMed] [Google Scholar]
- [2].De Jonge C, Biological basis for human capacitation—revisited. Hum Reprod Update 2017, 23, 289–299. [DOI] [PubMed] [Google Scholar]
- [3].Andersch-Björkman Y, Thomsson KA, Larsson JMH, Ekerhovd E, Hansson GC, Large Scale Identification of Proteins, Mucins, and Their O-Glycosylation in the Endocervical Mucus during the Menstrual Cycle. Mol Cell Proteomics 2007, 6, 708–716. [DOI] [PubMed] [Google Scholar]
- [4].Han L, Slayden O, Edelman A, Jensen J, Appraising Cervical Mucus: A New Approach to Evaluating Contraceptives” 2016. [DOI] [PubMed]
- [5].Brenner RM, Rudolph L, Matrisian L, Slayden OD, Non-human primate models: artificial menstrual cycles, endometrial matrix metalloproteinases and s.c. endometrial grafts. Hum Reprod 1996, 11, 150–164. [DOI] [PubMed] [Google Scholar]
- [6].Han L, Padua E, Hart KD, Edelman A, Jensen JT, Comparing cervical mucus changes in response to an oral progestin or oestrogen withdrawal in ovarian-suppressed women: a clinical pilot. The European Journal of Contraception & Reproductive Health Care 2019, 24, 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Erde J, Loo RRO, Loo JA, Enhanced FASP (eFASP) to Increase Proteome Coverage and Sample Recovery for Quantitative Proteomic Experiments. J. Proteome Res. 2014, 13, 1885–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].McAlister GC, Nusinow DP, Jedrychowski MP, Wühr M, et al. , MultiNotch MS3 enables accurate, sensitive, and multiplexed detection of differential expression across cancer cell line proteomes. Anal. Chem. 2014, 86, 7150–7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Eng JK, Jahan TA, Hoopmann MR, Comet: An open-source MS/MS sequence database search tool. PROTEOMICS 2013, 13, 22–24. [DOI] [PubMed] [Google Scholar]
- [10].Wilmarth PA, Riviere MA, David LL, Techniques for accurate protein identification in shotgun proteomic studies of human, mouse, bovine, and chicken lenses. J Ocul Biol Dis Infor 2009, 2, 223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Robinson MD, McCarthy DJ, Smyth GK, edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Perez-Riverol Y, Csordas A, Bai J, Bernal-Llinares M, et al. , The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Res. 2019, 47, D442–D450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gipson IK, Mucins of the human endocervix. Front. Biosci. 2001, 6, D1245–1255. [DOI] [PubMed] [Google Scholar]
- [14].The Amount of MUC5B Mucin in Cervical Mucus Peaks at Midcycle. The Journal of Clinical Endocrinology & Metabolism 2001, 86, 594–600. [DOI] [PubMed] [Google Scholar]
- [15].Ambrosini G, Andrisani A, Fiore C, Faggian D, et al. , Anti-Helicobacter pylori antibodies in cervical mucus: a new cause of infertility. European Journal of Obstetrics & Gynecology and Reproductive Biology 2011, 155, 157–160. [DOI] [PubMed] [Google Scholar]
- [16].Grande G, Milardi D, Vincenzoni F, Pompa G, et al. , Proteomic characterization of the qualitative and quantitative differences in cervical mucus composition during the menstrual cycle. Mol. BioSyst. 2015, 11, 1717–1725. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


