Abstract
Chronic wound infections caused by biofilm-forming microorganisms represent a major burden to healthcare systems. Treatment of chronic wound infections using conventional antibiotics is often ineffective due to the presence of bacteria with acquired antibiotic resistance and biofilm-associated antibiotic tolerance. We previously developed an electrochemical scaffold that generates hydrogen peroxide (H2O2) at low concentrations in the vicinity of biofilms. The goal of this study was to transition our electrochemical scaffold into an H2O2-generating electrochemical bandage (e-bandage) that can be used in vivo. The developed e-bandage uses a xanthan gum-based hydrogel to maintain electrolytic conductivity between e-bandage electrodes and biofilms. The e-bandage is controlled using a lightweight, battery-powered wearable potentiostat suitable for use in animal experiments. We show that e-bandage treatment reduced colony-forming units of Acinetobacter buamannii biofilms (treatment vs. control) in 12 h (7.32 ± 1.70 vs. 9.73 ± 0.09 log10[CFU/cm 2]) and 24 h (4.10 ± 12.64 vs. 9.78 ± 0.08 log10[CFU/cm2]) treatments, with 48 h treatment reducing viable cells below the limit of detection of quantitative and broth cultures. The developed H2O2-generating e-bandage was effective against in vitro A. baumannii biofilms and should be further evaluated and developed as a potential alternative to topical antibiotic treatment of wound infections.
Keywords: biofilm, electrochemical bandage, hydrogen peroxide, wearable potentiostat, wound healing
1 |. INTRODUCTION
Chronic wounds can be recalcitrant as they may fail to progress through normal wound healing stages (Han & Ceilley, 2017). Chronic wounds are a significant burden to healthcare, affecting over 6.5 million people and costing $25 billion annually in the United States (Sen et al., 2009). Chronic wounds are often associated with the presence of biofilms on wound surfaces, with structural and chemical microenvironments that aid in pathogen survival and delay wound healing (Dowd et al., 2011; Metcalf & Bowler, 2013). Alleviating biofilm burden is challenging but necessary for chronic wounds to progress through normal wound healing stages.
Wound biofilm infections can be challenging to treat with topical antibiotics due to their structure and complex physicochemical microenvironments, low metabolic activity, and slow growth rate (Mah & O’Toole, 2001; Nguyen et al., 2011). The use of biocides as an alternative to antibiotics gained interest to reduce the selection of antibiotic-resistant microorganisms and due to their activity against metabolically active and inactive cells and the dearth of known resistance mechanisms. Hydrogen peroxide (H2O2) and hypochlorous acid (HOCl) are of particular interest, as they are natively released as a part of the native inflammatory wound response (Hampton et al., 1998; Wang et al., 2007; Zhu et al., 2017). H2O2 also plays a regulatory role in tissue proliferation and remodeling stages and promotes differentiation of keratinocytes and myofibroblasts (Zhao et al., 2013; Zhu et al., 2017). Only low concentrations of H2O2 or HOCl can be used as an antibiofilm agent to avoid oxidative damage to the host tissue. Therefore, it is advantageous to design systems that can continuously deliver biocides to wound beds while maintaining concentrations below cytotoxic levels.
Electroceutical wound treatment is an emerging approach in which biocides such as H2O 2 or HOCl are generated in situ via electrochemical reactions. In previous reports, our group designed electrochemical scaffolds (e-scaffolds) that deliver controlled amounts of H2O2 or HOCl. We demonstrated the activity of H2O2- and HOCl-producing e-scaffolds against mono- and polymicrobial biofilms grown in liquid media (Kiamco et al., 2019; Raval et al., 2020; Sultana et al., 2015). While useful for demonstrating antimicrobial efficacy in laboratory settings, the e-scaffold design is not suitable for animal experiments and ultimately clinical settings. First, the system was immersed in a liquid medium to maintain an electrolytic connection between the e-scaffold electrodes and facilitate the mass transport of generated biocides to biofilms. Second, a benchtop potentiostat was used to supply power and control the potential of the electrodes. In this communication, we report the development of a novel H2O2-generating electrochemical bandage (e-bandage) controlled by a wearable potentiostat developed to treat wound infections. The H2O2-generating e-bandage was designed to be applied in a similar manner to the application of adhesive bandages on wounds. First, we developed and validated a small, lightweight, and battery-operated potentiostat that supplies power and controls the H2O2-generating e-bandage for a period of at least 4 days. The wearable potentiostat is designed such that size and weight are small enough for use in small experimental animal wound infection models. The potentiostat is cylindrical in shape with 1.5 cm diameter and 1 cm height and weighed 1.7 g, including battery weight. Second, we demonstrated the use of a xanthan gum-based hydrogel to maintain electrolytic conductivity between e-bandage electrodes and facilitate mass transport of generated H2O 2 to biofilms. This enables the use of the e-bandage to treat biofilms on surfaces with minimum moisture. The developed e-bandage was evaluated in an in vitro agar biofilm model designed to emulate an infected wound bed, where biofilms were grown on polycarbonate membranes uptake nutrients from agar plates (Stoffel et al., 2020). The e-bandage was applied atop the biofilm and secured to the agar surface using a Tegaderm™ film, similar to what might be envisioned with the application of an antimicrobial dressing on a wound bed.
The working principle of the H 2O2-generating e-bandage presented in this study is detailed in our previous publication (Sultana et al., 2015). The e-bandage continuously generates H2O2 on the surface of polarized carbon fabric (Equation (1)). H2O2 can be generated as a result of partial reduction of dissolved oxygen when the electrode potential is controlled below the formal potential for 2-electron oxygen reduction reaction (0.085 VAg/AgCl) (Bard & Faulkner, 2001; Sultana et al., 2015). In previous work, applied potential of −0.6 VAg/AgCl was used to produce a low concentration of H2O2 that reduced biofilms without observable damage to the underlying mammalian tissue (Sultana et al., 2015).
| (1) |
The wearable potentiostat design and verification of its operation using a resistive dummy cell are detailed in Supplementary Information S1 and S2. To evaluate the operation of the H2O2-generating e-bandage under relevant conditions, we characterized the e-bandage operation using an in vitro agar membrane model (without a biofilm present). The e-bandage was tested to assess (1) ability to control the working electrode (WE) potential, (2) WE current—and thus H2O2 generation rate, (3) current consumption during e-bandage operation, and (4) duration for which the e-bandage can be operated using a battery. Figure 1a shows WE and counter electrode (CE) potentials over 100 h of operation. Before connecting the potentiostat battery, the open circuit potentials were 0.154 ± 0.001 V and 0.195 ± 0.001 V for WE and CE, respectively. After connecting the battery, the WE potential rapidly moved toward the set value of −0.685 V relative to the quasi reference electrode (QRE) (−0.6 VAg/AgCl), reaching below −0.6 V within 15 s, and below −0.65 V within 715 s (Figure 1b), and thereafter remaining constant at −0.686 ± 0.001 V for 100 h of operation (Figure 1a). By comparison, the CE potential increased to a maximum value of 1.412 V after 14.8 min, thereafter gradually decreasing until it reached a stable value of 1.257 ± 0.006 V between 40 and 100 h (Figure 1a). We speculate that this transient response is due to the large discharge current associated with changing the WE potential, which could not be immediately supplied by the battery. WE current started at −962.9 μA, but decayed to below −400 μA within 48.9 min, thereafter reaching a stable value of −171.2 ± 11.5 μA between 20 and 100 h (Figure 1c). This current corresponds to H2O2 generation rate of 3.19 μmol/h, assuming all current goes to oxygen reduction to H2O2. The total current consumed to operate the potentiostat is shown in Figure 1c. By subtracting the WE current, we deduced that 99.9 ± 2.4 µA was required to operate the potentiostat circuit (Figure 1d). Overall, the data demonstrate that the potentiostat adequately controls the WE potential and that the H2O2–generating e-bandage can be operated continuously for over 4 days without battery change in the agar membrane model.
FIGURE 1.
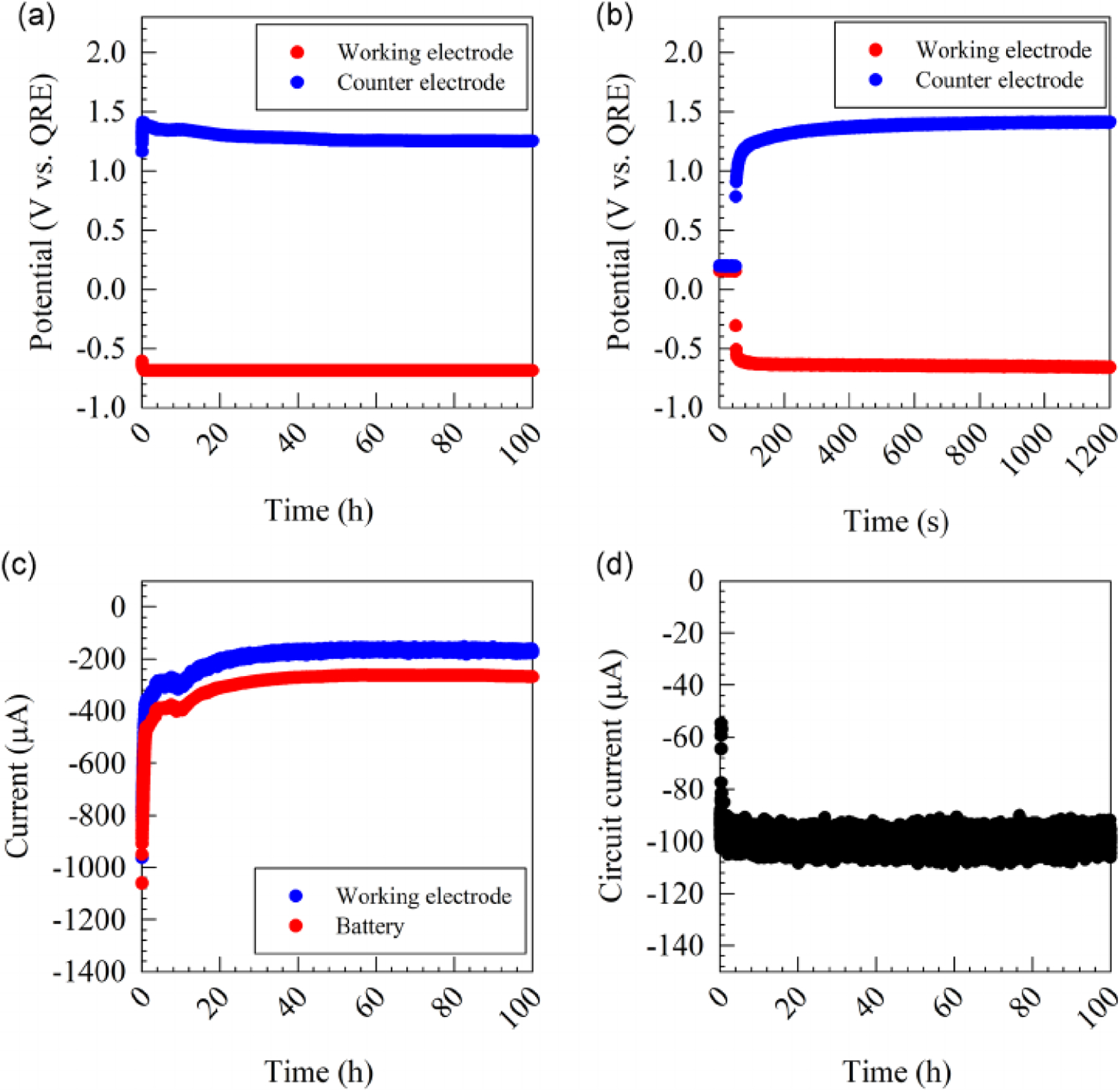
Representative data showing electrochemical characteristics of the e-bandage in an in vitro agar membrane biofilm model. Working and counter electrode potentials for 100 h (a) and 1200 s (b) of operation. (c) Current delivered through the working electrode and total current consumed through the system. (d) Current required to operate the potentiostat circuit [Color figure can be viewed at wileyonlinelibrary.com]
We used H2O 2 microelectrodes to verify the generation of H2O2 near the WE surface. Figure 2a shows H2O2 concentration 50 μm from the WE while the WE potential is swept from 0.4 VAg/AgCl to −0.8 VAg/AgCl; H2O2 is generated at WE potentials below −0.2 VAg/AgCl. Depth-concentration profiles were recorded to show H2O2 generation while the WE was polarized at −0.6 VAg/AgCl. An increase in H2O2 concentration over time was observed at 50 μm from WE surface, with concentrations of 29.3, 210, and 325 μM measured after 30, 60, and 90 min of polarization, respectively. Concentration decreased with increasing distance from WE surface as the generated H2O2 diffused into the hydrogel; 18.1, 152, and 294 μM were measured 400 μm away from the WE surface after 30, 60, and 90 min of polarization, respectively. In comparison, H2O2 was not detected when the WE was not polarized. While useful in showing H 2O2 generation, we acknowledge that H2O2 concentration would likely be lower in the treatment model due to continuous H2O2 consumption in biofilms and in wound tissue. Furthermore, the change in H2O2-generating e-bandage orientation for microelectrode experiments allowed a higher rate of oxygen transport to the WE surface and hence increased H2O2 generation. Collectively, the data confirm the continuous generation of H2O2 on the WE surface of polarized e-bandages, which would subsequently be transported through the hydrogel to biofilms, if present, or to the wound bed.
FIGURE 2.
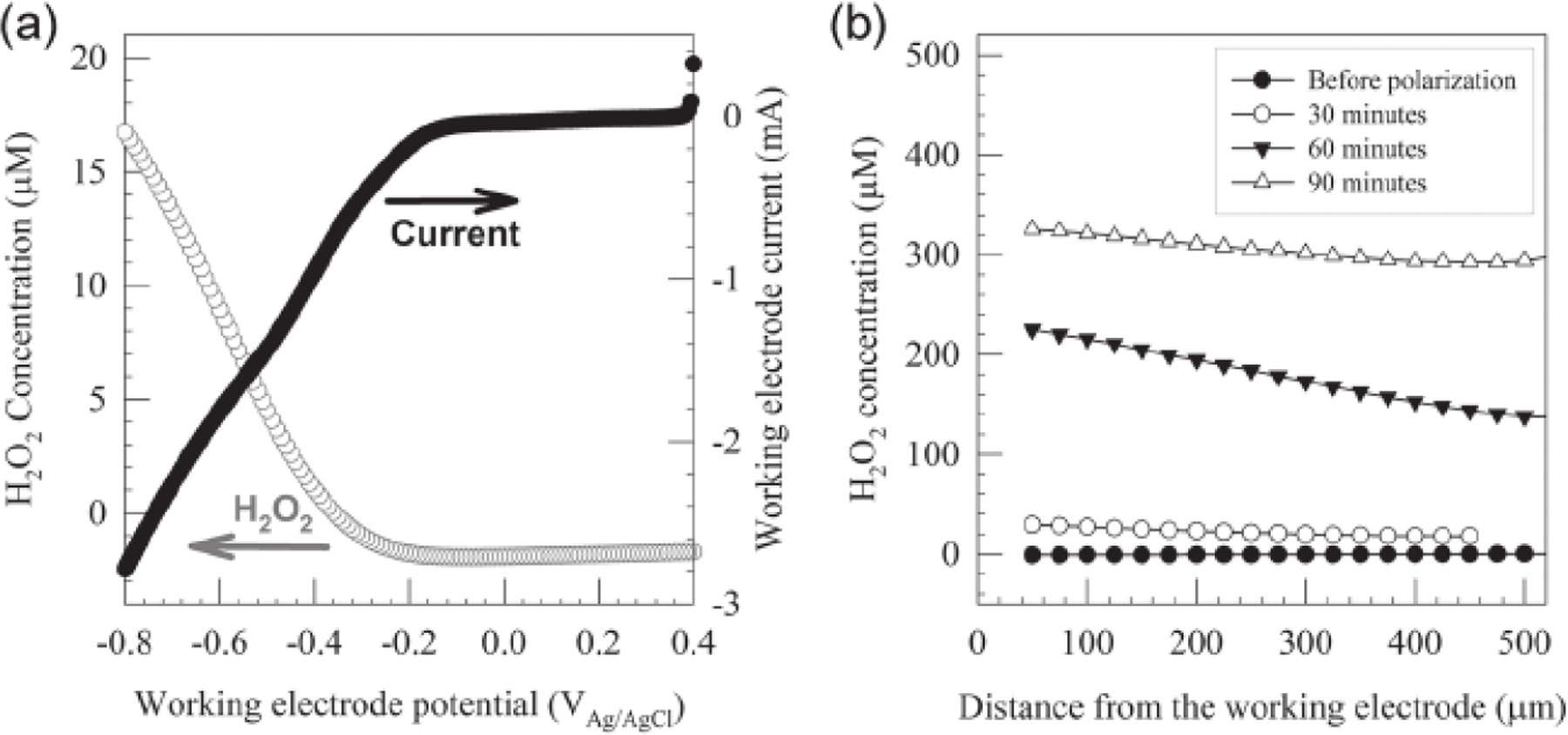
(a) H2O2 concentration at a 50 μm distance from the working electrode surface during a linear sweep voltammetry experiment, where the potential was swept from 0.4 VAg/AgCl to −0.8 V Ag/AgCl at a scan rate of 0.01 V/s. (b) Depth profiles of H2O2 concentration before polarization and after 30, 60, and 90 min of polarization at −0.6 VAg/AgCl. The x-axis shows the distance from the working electrode surface
After verifying the operation of H2 O2 -generating e-bandage and wearable potentiostat, we tested its activity against Acinetobacter baumannii biofilms grown on polycarbonate membranes for 24 h, starting quantities were 9.54 ± 0.16 log10 colony-forming units (CFU/cm2). Biofilms showed a time-dependent response to H2O2-generating e-bandage treatment. Nonpolarized controls excluded anti-biofilm activity due to exposure to the hydrogel or e-bandage material (Figure 3). Treating biofilms for 12 h resulted in a 2.42 log10(CFU/cm2) reduction of viable cells (7.32 ± 1.70 vs. 9.73 ± 0.09 log10[CFU/cm2]) in treated and control biofilms, respectively; p = 0.0079). A 5.68 log10(CFU/cm2) reduction in cell viability was observed after 24 h of treatment (4.10 ± 12.64 vs. 9.78 ± 0.08 log10[CFU/cm2]) in treated and control biofilms, respectively; p = 0.0091). Treating biofilms for 48 h reduced viable bacteria below the limit of detection of the quantitative and broth cultures (i.e., below 0.71 log10[CFU/cm2]); p = 0.0043). We did not observe a reduction in cell viability in control experiments, which suggests that WE polarization and the resultant H2O2 generation were responsible for reduced cell viability.
FIGURE 3.
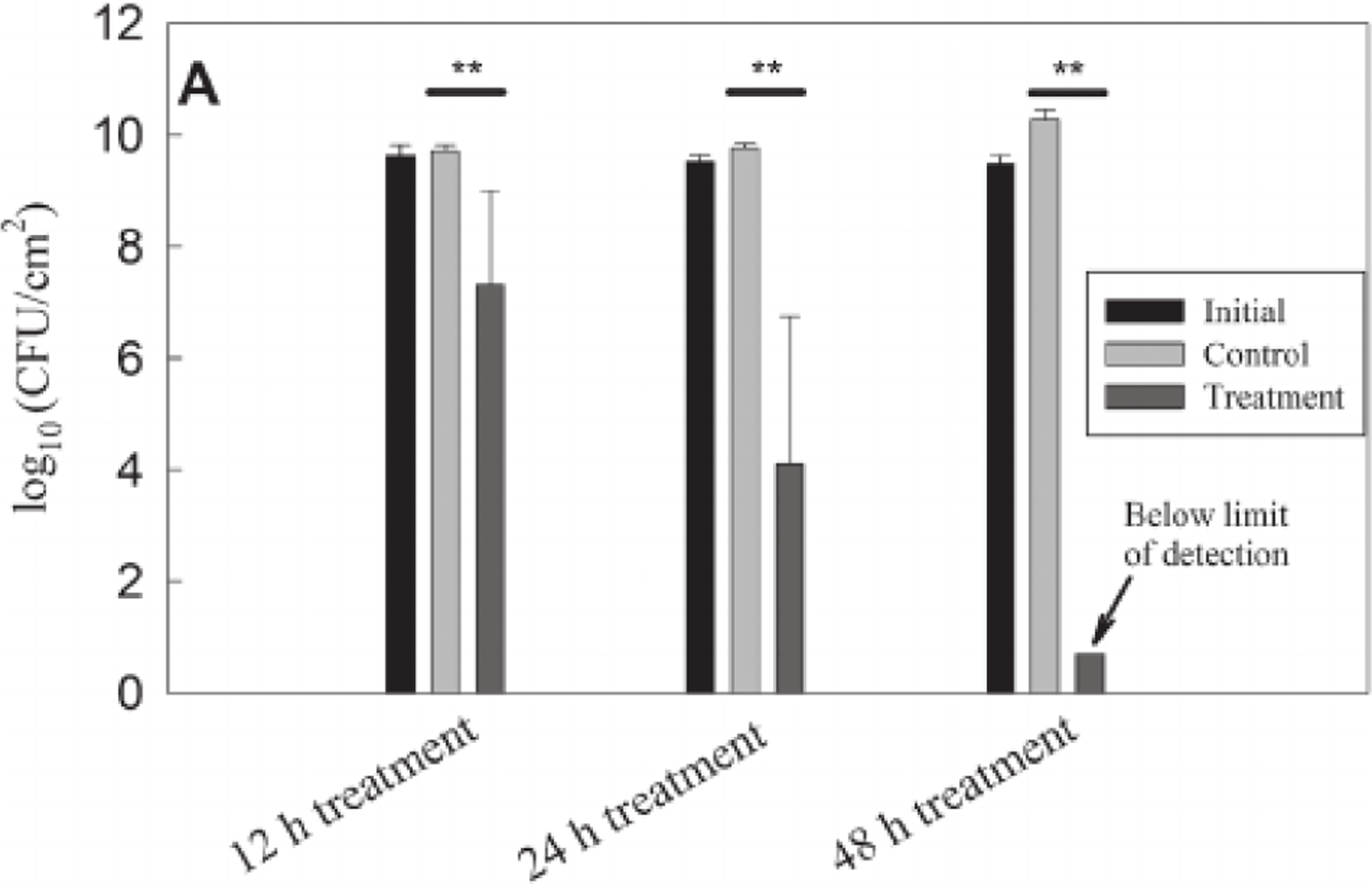
Colony forming unit counts of in vitro Acinetobacter baumannii biofilms after e-bandage treatment for 12, 24, and 48 h compared with initial and untreated controls. Data are represented as means and standard deviations of at least three independent biological replicates. Statistical analysis was performed using two-sided Wilcoxon rank-sum test with the corresponding control group (**p ≤ 0.01)
Our previous work demonstrated the activity of electrochemical generation of biocides against in vitro biofilms. However, all experiments were performed in liquid media, and electrodes were controlled using a benchtop potentiostat (Kiamco et al., 2019; Sultana et al., 2015). This limited the relevance of the developed system to the treatment of wound infections in animal experiments and clinical settings. Furthermore, the number of experiments was limited due to the cost of benchtop potentiostats and the size of the experimental setup. In this communication, we introduced the e-bandage as a new design that is directly translatable to relevant test environments. Biofilms were grown on a membrane placed on an agar plate, simulating a wound bed. The liquid electrolyte was replaced by a hydrogel. This allowed the e-bandage to be applied to in vitro biofilms in a similar fashion to the application of regular bandages to wounds. The hydrogel maintained an electrolytic connection between the e-bandage electrodes without drying for at least 4 days, enabling the potential development of an antimicrobial dressing that is active for a clinically relevant duration. Finally, the H2O2-generating e-bandage was controlled by an inexpensive and lightweight battery-operated potentiostat, costing $5.05 per unit at the time of writing this manuscript. The development of a battery-operated wearable potentiostat allowed the H2O2-generating e-bandage to operate as a standalone device that can be used for in vitro as well as in vivo experiments.
The results reported in this study demonstrate the activity of the H2O2-generating e-bandage against in vitro A. baumannii biofilms, suggesting it could be developed as a potential treatment for wound infections. This study represents a first step in the development of an antimicrobial wound bandage. Future studies will investigate the H2O2-generating e-bandage’s activity against a range of microorganisms that cause wound infection in in vitro and in in vivo models. Moreover, the system could be further advanced by integrating wireless communication units to enable wireless control, and remote monitoring of e-bandages applied to wound surfaces.
2 |. EXPERIMENTAL METHODS
2.1 |. Electrochemical bandage
The e-bandage is a three-electrode system consisting of 1.77 cm 2 carbon fabric (Panex 30 PW-06, Zoltek Companies Inc.) WE and CE and silver/silver chloride (Ag/AgCl) wire as a QRE (Figure 4). The electrodes are separated using a cotton cloth separator and are held together using silicone adhesive applied lightly on the outer edge. Nylon sew snaps (Dritz, Spartanburg, SC, item #85) are used to press titanium wires (TEMCo, Amazon.com, catalog #RW0524) onto the conductive fabric, establishing an electrical connection. The connection resistance between the electrodes and wires is less than 2 Ω.
FIGURE 4.
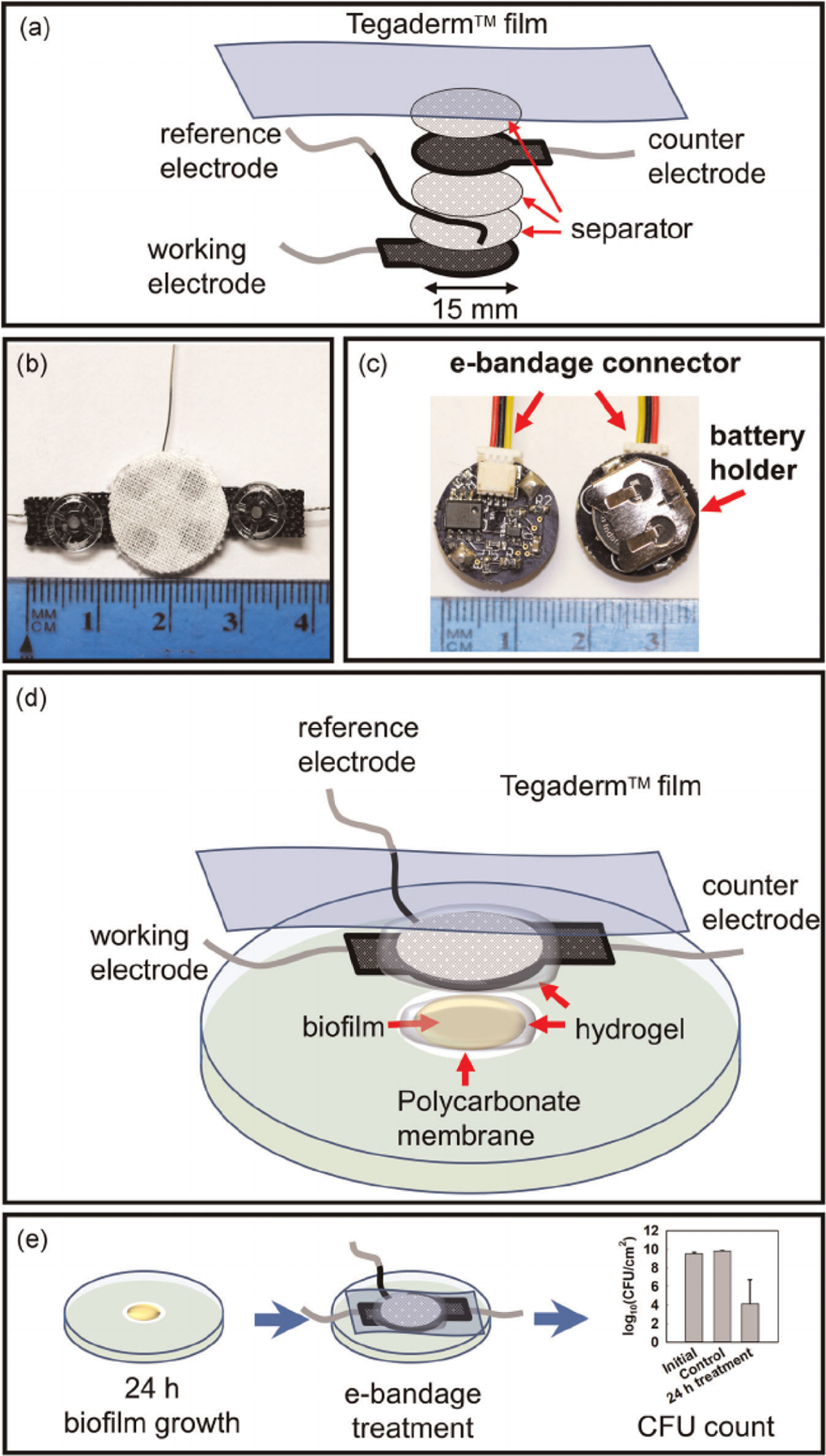
(a) Components of the e-bandage. Photographs of (b) the e-bandage, and (c) the wearable potentiostat controlling the e-bandage. (d) Components of the in vitro membrane biofilm model. Biofilms grown on polycarbonate membranes on agar plates were treated with e-bandages. (e) Procedure and sample results from the evaluation of e-bandage activity against in vitro biofilms [Color figure can be viewed at wileyonlinelibrary.com]
2.2 |. In vitro agar membrane biofilm model
A single colony of A. baumannii ATCC BAA-1605 from an overnight agar plate was placed in 3 ml of tryptic soy broth (TSB) incubated on a shaker (150 rpm) at 37°C overnight. A 2.5-μl aliquot of diluted culture (OD600= 0.5) was used to inoculate a 13 mm polycarbonate membrane (WHA10417401, Sigma-Aldrich) resting on a tryptic soy agar (TSA) plates. Membranes were sterilized before inoculation by UV exposure for 15 min per side. Plates were inverted and incubated at 37°C for 24 h to allow biofilm growth.
Autoclaved e-bandages were submerged in phosphate buffer saline (PBS) solution to ensure initial hydration of the electrodes and the separators. A hydrogel made by dissolving 1.8% w/v xanthan gum in PBS was used as the electrolyte and to maintain the moisture level of the e-bandage during the course of the experiment. 100 μl of hydrogel was placed directly atop of membrane biofilms, with another 100 μl of hydrogel injected between the separator layers of the e-bandage. The e-bandage was then placed atop the biofilm, with the WE facing the biofilm. 100 μl of hydrogel was placed atop of the e-bandage to ensure proper hydration of CE. A 5 × 4 cm piece of sterile Tegaderm™ transparent film (16002, 3 M) was used to tape and cover the e-bandage.
During treatment, the WE was polarized at −0.685 V relative to QRE using the wearable potentiostat for 12, 24, and 48 h. The design and verification of the wearable potentiostat are detailed in Supplementary Information (S1 and S2). Polarization potential was selected based on previous work (Sultana et al., 2015). After treatment, e-bandages were removed and rinsed separately with 5 ml PBS to remove attached cells. Membrane biofilms were vortexed for 5 min in the e-bandage rinsing solution to detach bacterial cells. The cell suspension was centrifuged at a relative centrifugal force of 4200 g for 8 min, the supernatant was discarded, and 1 ml of fresh PBS was added (repeated 3× to remove H2 O2). The sample was then re-suspended and CFUs were quantified on TSA plates using drop-plate count method. 100 μl of the re-suspended solution was added to 5 ml of sterile TSB and incubated overnight at 37°C to check for potential bacterial growth. The limit of detection is 1.87 log10 (CFU/cm2) and 0.71 log10(CFU/cm2) for the drop plate and broth culture methods, respectively.
Microelectrodes were used to measure H2 O2 concentration according to published protocols (Atci et al., 2016). The e-bandage was operated with WE side facing up and with 600 μl hydrogel added atop the e-bandage to provide electrolytic conductivity. Example profiles are reported to show continuous H2O2 generation in polarized e-bandages.
2.3 |. Statistical analysis
Data were averaged and displayed as means ± standard errors of the means for at least three biological replicates. A two-sided Wilcoxon rank-sum test was performed to determine if H2O 2 generation reduced biofilm viability (polarized e-bandage vs. non-polarized control); p values below 0.05 were considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number R01 AI091594 and by an Amazon Catalyst award (Amazon.com, Inc.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding information
National Institute of Health, Grant/Award Number: R01 AI091594; Amazon Catalyst
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Atci E, Babauta JT, & Beyenal H (2016). A hydrogen peroxide microelectrode to use in bioelectrochemical systems. Sensors and Actuators B: Chemical, 226, 429–435. 10.1016/j.snb.2015.12.004 [DOI] [Google Scholar]
- Bard AJ, & Faulkner LR (2001). Fundamentals and applications. Electrochemical Methods, 2(482), 580–632. [Google Scholar]
- Dowd SE, Delton Hanson J, Rees E, Wolcott RD, Zischau AM, Sun Y, White J, Smith DM, Kennedy J, & Jones CE (2011). Survey of fungi and yeast in polymicrobial infections in chronic wounds. Journal of Wound Care, 20(1), 40–47. 10.12968/jowc.2011.20.1.40 [DOI] [PubMed] [Google Scholar]
- Hampton MB, Kettle AJ, & Winterbourn CC (1998). Inside the neutrophil phagosome: Oxidants, myeloperoxidase, and bacterial killing. Blood, 92(9), 3007–3017. 10.1182/blood.V92.9.3007 [DOI] [PubMed] [Google Scholar]
- Han G, & Ceilley R (2017). Chronic wound healing: A review of current management and treatments. Advances in Therapy, 34(3), 599–610. 10.1007/s12325-017-0478-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiamco MM, Zmuda HM, Mohamed A, Call DR, Raval YS, Patel R, & Beyenal H (2019). Hypochlorous-acid-generating electrochemical scaffold for treatment of wound biofilms. Scientific Reports, 9(1), 2683. 10.1038/s41598-019-38968-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah TF, & O’Toole GA (2001). Mechanisms of biofilm resistance to antimicrobial agents. Trends in Microbiology, 9(1), 34–39. 10.1016/s0966-842x(00)01913-2 [DOI] [PubMed] [Google Scholar]
- Metcalf DG, & Bowler PG (2013). Biofilm delays wound healing: A review of the evidence. Burns & Trauma, 1(1), 5–12. 10.4103/2321-3868.113329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen D, Joshi-Datar A, Lepine F, Bauerle E, Olakanmi O, Beer K, Mckay G, Siehnel R, Schafhauser J, Wang Y, Britigan BE, & Singh PK (2011). Active starvation responses mediate antibiotic tolerance in biofilms and nutrient-limited bacteria. Science, 334(6058), 982–986. 10.1126/science.1211037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raval YS, Mohamed A, Song J, Greenwood-Quaintance KE, Beyenal H, & Patel R (2020). Hydrogen peroxide-generating electrochemical scaffold activity against trispecies biofilms. Antimicrobial Agents and Chemotherapy, 64(4), e02332–19. 10.1128/AAC.02332-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, & Longaker MT (2009). Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair and Regeneration, 17(6), 763–771. 10.1111/j.1524-475X.2009.00543.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel JJ, Kohler Riedi PL, & Hadj Romdhane B (2020). A multimodel regime for evaluating effectiveness of antimicrobial wound care products in microbial biofilms. Wound Repair and Regeneration, 28(4), 438–447. 10.1111/wrr.12806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana ST, Atci E, Babauta JT, Falghoush AM, Snekvik KR, Call DR, & Beyenal H (2015). Electrochemical scaffold generates localized, low concentration of hydrogen peroxide that inhibits bacterial pathogens and biofilms. Scientific Reports, 5(1), 14908. 10.1038/srep14908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Bassiri M, Najafi R, Najafi K, Yang J, Khosrovi B, & Robson MC (2007). Hypochlorous acid as a potential wound care agent: Part I. Stabilized hypochlorous acid: A component of the inorganic armamentarium of innate immunity. Journal of Burns and Wounds, 6, e5. [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Usui ML, Lippman SI, James GA, Stewart PS, Fleckman P, & Olerud JE (2013). Biofilms and inflammation in chronic wounds. Advances in Wound Care, 2(7), 389–399. 10.1089/wound.2012.0381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Wang Q, Lu S, & Niu Y (2017). Hydrogen peroxide: A potential wound therapeutic target? Medical Principles and Practice, 26(4), 301–308. 10.1159/000475501 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


