Abstract
Background
Lysophosphatidic acid (LPA) is involved in numerous biological processes, including neurodevelopment, chronic inflammation, and immunologic response in the central nervous system. Autotaxin (ATX) is a secreted enzyme that produces LPA from lysophosphatidylcholine (LPC). Previous studies have demonstrated decreased protein levels of ATX in cerebrospinal fluid (CSF) of patients with major depressive disorder (MDD). Based on those studies, the current study investigated the levels of lysophospholipids species including LPA and related metabolic enzymes, in CSF of patients with MDD and schizophrenia (SCZ).
Methods
The levels of lysophospholipids species and related metabolic enzymes were measured with either liquid chromatography-tandem mass spectrometry or enzyme-linked immunosorbent assay. Japanese patients were diagnosed with DSM-IV-TR. CSF was obtained from age- and sex-matched healthy controls (n = 27) and patients with MDD (n = 26) and SCZ (n = 27).
Results
Of all lysophospholipids species, the levels of LPA 22:6 (LPA - docosahexaenoic acid) were significantly lower in patients with MDD and SCZ than in healthy controls. These levels were negatively correlated with several clinical symptomatic scores of MDD, but not those of SCZ. In addition, the levels of LPA 22:6 were significantly correlated with the levels of LPC 22:6 among all 3 groups. On the other hand, the levels of LPA 22:6 were not correlated with ATX activity in patients with MDD and SCZ.
Conclusion
The lower levels of LPA 22:6 in patients with MDD and SCZ suggest an abnormality of LPA 22:6 metabolism. In addition, several depressive symptoms in patients with MDD were significantly associated with the lower levels of LPA 22:6, suggesting an involvement of LPA 22:6 in the pathophysiology of MDD.
Keywords: Cerebrospinal fluid, docosahexaenoic acid, lysophosphatidic acid, major depressive disorder, schizophrenia
Significance Statement.
Lysophosphatidic acid (LPA) is involved in numerous biological processes in the central nervous system. Autotaxin (ATX) is a secreted enzyme that produces LPA from lysophosphatidylcholine (LPC). There are numerous LPA molecular species (LPAs) identified by the length and degree of saturation of the fatty acid moiety. The current study found that the levels of LPA 22:6 (LPA - docosahexaenoic acid [DHA]) were significantly lower in cerebrospinal fluid (CSF) of patients with major depressive disorder (MDD) and schizophrenia (SCZ) and that they were negatively correlated with several clinical symptomatic scores of MDD, but not those of SCZ. In addition, the levels of LPA 22:6 were significantly correlated with the levels of LPC 22:6 among all 3 groups, but the levels of LPA 22:6 were not correlated with ATX activity in patients with both MDD and SCZ. These results suggest involvement of LPA 22:6 in the pathophysiology of MDD.
Introduction
Lysophosphatidic acid (LPA) is involved in numerous biological processes, including neuroprogenitor cell function (Yung et al., 2011), myelination (Anliker et al., 2013), synaptic transmission (Trimbuch et al., 2009), chronic inflammation (Barbayianni et al., 2015), and immunologic response in the CNS (Schilling et al., 2004). There are a large number of molecular species of LPA (LPAs) present in biological fluids identified by the length and degree of saturation of the fatty acid moiety (Baker et al., 2000), and each LPA has a various physiological function in various tissues and organs (Kurano et al., 2015). LPAs exert their effects through at least 6 cognate G protein-coupled receptors (LPA1–LPA6) (Yung et al., 2015) and are degraded by the ecto-activity of a family of the phospholipid phosphatases (PLPPs: PLPP1–3), which hydrolyzes the phosphate head group from LPA to produce monoacylglycerol (MAG) (Benesch et al., 2016).
In some pathways of LPA production, the most significant is the autotaxin (ATX) pathway. In this pathway, ATX cleaves lysophosphatidylcholine (LPC) to form LPA and choline (Umezu-Goto et al., 2002; Perrakis and Moolenaar, 2014; Yang and Chen, 2018). Other minor lysophospholipids, such as lysophosphatidylserine (LPS) and lysophosphatidylethanolamine (LPE), and phosphatidic acid (PA) are also enzymatically metabolized to LPA (Pagès et al., 2001; Ramesh et al., 2018; Yang and Chen, 2018).
Recent studies suggest that LPA may be implicated in a number of neuropsychological functions and neuropsychiatric diseases (Yung et al., 2015). Antidepressants bind to the LPA1 receptor expressed in brain astrocytes (Kajitani et al., 2016; Olianas et al., 2016) and increase the expression of glial cell line–derived neurotropic factor (Kajitani et al., 2016), an important mediator of major depressive disorder (MDD) (Takebayashi et al., 2006; Uchida et al., 2011; Tsybko et al., 2017). Decreased protein levels of ATX were observed in serum and cerebrospinal fluid (CSF) of patients with MDD (Itagaki et al., 2019). LPA1 receptor-deficient mice demonstrate signs of anxious depression (Moreno-Fernández et al., 2017) and schizophrenia (SCZ) (Harrison et al., 2003; Roberts et al., 2005). LPA signaling initiates SCZ-like brain and behavioral changes in a mouse model of prenatal brain hemorrhage (Mirendil et al., 2015). These studies suggest a possible association between LPA and the pathophysiology of major psychiatric disorders such as MDD and SCZ.
Although there were no published studies about blood or brain LPA levels in MDD and SCZ, examining LPA levels in CSF may contribute to the elucidation of the pathophysiology of MDD and SCZ. Recently, the measurement of total LPA levels in CSF with enzyme-linked immunosorbent assays showedthere were no significant differences in the levels of total LPA in CSF among healthy controls (HC) and patients with MDD and SCZ (Gotoh et al., 2019a, 2019b). However, these studies did not examine the levels of each LPAs and related metabolic enzymes, such as ATX and PLPP. Therefore, we measured the levels of molecular LPAs including LPA and related metabolic enzymes in CSF of HC and patients with MDD and SCZ.
Methods and Materials
Participants
Japanese patients were recruited at the National Center of Neurology and Psychiatry (NCNP), Tokyo, Japan, through an announcement on the NCNP website between May 2010 and July 2017. HC were recruited from the community through advertisements in a free local magazine and an announcement on the website of the NCNP. All individuals underwent a structured interview with the Mini-International Neuropsychiatric Interview, Japanese version (Sheehan et al., 1998; Otsubo et al., 2005), administered by trained psychologists or psychiatrists. For patients with either MDD or SCZ, a consensus diagnosis was performed according to DSM-IV criteria (American Psychiatric Association, 2000) based on the Mini-International Neuropsychiatric Interview, additional unstructured interviews, and medical records. Individuals were excluded from this study if they had a history of CNS disease, severe head injury, or substance abuse. From all individuals, a CSF sample was collected via lumbar puncture. Twenty-six patients were diagnosed with MDD and 27 patients were diagnosed with SCZ. Twenty-seven healthy individuals, with no history of past or current mental disorders, were recruited as HC. Antidepressant doses were converted to imipramine (IMI)-equivalent doses (Inada and Inagaki, 2015). Antipsychotic doses were converted to chlorpromazine (CPZ)-equivalent doses (Inada and Inagaki, 2015). This study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of the National Hospital Organization Kure Medical Center and by the ethics committee of the NCNP, Japan. Written informed consent was obtained from all participants.
Assessment of Clinical Symptoms
Clinical symptoms of MDD were assessed with a 21-item version of the Hamilton Rating Scale for Depression (HAMD 21-items). Clinical symptoms of SCZ were assessed with the Positive and Negative Syndrome Scale (PANSS).
Lumbar Puncture
Lumbar puncture was performed according to procedure guidelines at the NCNP (Hattori et al., 2015). Samples were obtained at the NCNP between May 2010 and July 2017. After neurologic examination, each participant received local anesthesia followed by a lumbar puncture at L3–4 or L4–5 using an atraumatic pencil point needle (Uniever 22G, 75 mm, Unisis Corp, Tokyo, Japan). A quantity of 8 mL CSF was collected in a low-protein adsorption tube (PROTEOSAVE SS 15 mL Conicaltube, Sumitomo Bakelite Co., Japan) and immediately chilled on ice. CSF was then centrifuged (4000g × 10 minutes, 4°C), and the supernatant was dispensed into 0.5-mL aliquots in low-protein adsorption tubes (PROTEOSAVE SS 1.5 mL Slimtube, Sumitomo Bakelite Co.) and stored in a deep freezer (−80°C) until use.
Measurement of Lysophospholipids and PA Species Using liquid chromatography-tandem mass spectrometry (LC-MS/MS)
Lysophospholipids and PA species were measured as previously described with minor modification (Okudaira et al., 2014; Kawana et al., 2019). The CSF samples were mixed and sonicated with a ninefold volume of methanol containing internal standard (0.1 µM LPA 17:0, 1 µM LPC 17:0, and 1 µM PA 24:0, final concentration). After centrifugation at 21 500 × g, the resulting supernatant was filtrated. Then, 20 μL of methanol extract was separated using UltiMate 3000 (Thermo Fisher Scientific) equipped with a C18 CAPCELL PAK ACR column (1.5 × 100 mm; Shiseido, Tokyo, Japan) for lysophospholipids or a C8 CAPCELL PAK UG120 column (1.5 × 150 mm; Shiseido) for PA using a gradient of solvent A (5 mM ammonium formate in water, pH 4.0) and solvent B (5 mM ammonium formate in 95% [v/v] acetonitrile, pH 4.0). In this study, lipids were extracted under neutral conditions, and 1-acyl- 2- and 2-acyl-1- lysophospholipids were not separated. Mass spec analysis was performed with a TSQ Quantiva system equipped with a heated electrospray ionization (ESI) interface and operated in the selected reaction monitoring mode. The precursor ions of LPC and PA were [M+H]+ for the positive ion mode, and those of other lysophospholipids were [M-H]− for the negative ion mode. The concentrations of lysophospholipids and PA were calculated from the area ratio to the internal standard: LPA 17:0 (for LPA, LPE, lysophosphatidylglycerol (LPG), lysophosphatidylinositol (LPI), and LPS species), LPC 17:0 (for LPC species), or PA 24:0 (for PA species).
ATX Activity Assay
Lysophospholipase D activity of ATX (ATX activity) in CSF samples was assessed based on the amount of choline released using LPC as the substrate, as described previously (Umezu-Goto et al., 2002).
Measurement of the Levels of PLPPs by Enzyme-Linked Immunosorbent Assay
The levels of PLPP 1, 2, and 3 in CSF were measured with the corresponding human lipid phosphate phosphohydrolase 1, 2, and 3 immunoassays (MyBioSource, Inc., San Diego, CA, USA) according to the manufacturer’s instructions. Quantification was performed with a Multiskan GO Microplate spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). As a pilot study, the levels of PLPP1, 2, and 3 in CSF were measured in 2 HC. Pilot data showed that PLPP1 was detected in the CSF of 2 HC and that PLPP2 and 3 levels were below the detection limit (data not shown). The PLPP1 levels in CSF were measured in an additional 26 patients with MDD, an additional 27 patients with SCZ, and an additional 27 HC.
Statistical Analysis
Data are shown as mean ± SD. Tests for normality were performed using the Shapiro-Wilk test and data were analyzed with nonparametric statistics. Kruskal-Wallis test was used to evaluate significant differences in parameters (clinical and/or laboratory values) among the groups (HC, MDD, and SCZ). Bonferroni test was performed as a post-hoc test. Significant differences in parameters between patients with MDD and SCZ were evaluated with Mann-Whitney U-test. The chi-square test was used for categorical variables. Potential effects of confounding factors were corrected with multivariate linear regression analysis for the levels of lysophospholipids and related substances. As the information of medication was not unavailable in many of patients with MDD and SCZ, IMI and CPZ equivalent doses were excluded as confounding factors. Statistical significance was defined as a 2-tailed P < .05. All statistical analyses were performed with SPSS version 22.0 for Windows (IBM Japan Corporation, Tokyo, Japan).
Results
Clinical and Laboratory Data
Details of participant clinical data are shown in Table 1. Twenty seven HC, 26 patients with MDD, and 27 patients with SCZ were included (40 men and 40 women; mean age, 40.3 ± 8.6 years). Sex and mean age did not significantly differ among the HC, MDD, and SCZ groups. Patients with SCZ had significantly higher BMI compared with patients with MDD. The mean HAMD 21-items score showed mild to moderate depression in patients with MDD. The mean PANSS score showed minimal to mild symptoms in patients with SCZ.
Table 1.
Participant Clinical and Laboratory Data
| HC (n = 27) | MDD (n = 26) | SCZ (n = 27) | P value | |
|---|---|---|---|---|
| Sex, female | 14 (51.9%) | 13 (50.0%) | 13 (48.1%) | .964a |
| Age (y) | 40.4 ± 7.8 | 40.4 ± 8.3 | 40.1 ± 10.0 | .967b |
| BMI (kg/cm2) | 22.3 ± 2.8 | 21.5 ± 4.1 | 25.2 ± 5.3 (n=26) | .019b* |
| HAMD21-items score | 15.9 ± 7.3 | |||
| PANSS total score | 70.8 ± 17.9 | |||
| PANSS positive score | 16.1 ± 6.0 | |||
| PANSS negative score | 19.4 ± 6.0 | |||
| PANSS cognitive or general psychopathology score | 35.3 ± 10.7 | |||
| IMI-equivalent doses (mg/d) | 200.5 ± 151.0 (n = 16) | |||
| CPZ-equivalent doses (mg/d) | 26.8 ± 41.1 (n = 14) | 705.5 ± 611.7 (n = 13) | .002c** |
Abbreviations: BMI, body mass index; CPZ, chlorpromazine; HAMD, Hamilton Rating Score for Depression; HC, healthy controls; IMI, imipramine; MDD, major depressive disorder; PANSS, Positive and Negative Syndrome Scale; SCZ, schizophrenia. *P < .05, **P < .01.
Data shown as either mean ± SD and number or number and percent of total (%).
aComparison between 3 groups by chi-squared test.
bComparison between 3 groups by the Kruskal Wallis test.
cComparison between 2 patients groups by the Mann-Whitney U-test.
Levels of LPAs in CSF of HC, MDD, and SCZ
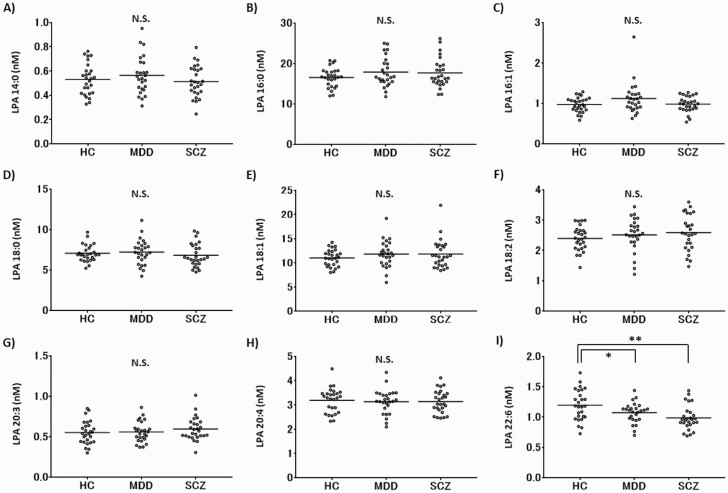
Nine LPAs (14:0, 16:0, 16:1, 18:0, 18:1, 18:2, 20:3, 20:4, and 22:6) were detected in CSF of all participants. The levels of LPA 22:6 in CSF of patients with MDD and SCZ were significantly lower than those of HC (supplementary Table 1; MDD: nonstandardized coefficient B = −0.125, P = .038; SCZ: nonstandardized coefficient B = −0.205, P = .001). On the other hand, there were no significant differences in the levels of other LPAs among all 3 groups (supplementary Table 1; Figure 1). The mean levels of LPA 22:6 adjusted for LPC 22:6 and PLPP1 in CSF of HC and patients with MDD and SCZ were 1.196 ± 0.251, 1.072 ± 0.166, and 0.986 ± 0.199 nM, respectively (Figure 1). There were no significant differences in the levels of LPA total (14:0 + 16:0 + 16:1 + 18:0 + 18:1 + 18:2 + 20:3 + 20:4 +22:6) in CSF among all of three groups (supplementary Table 1; Figure 2A).
Figure 1.
The levels of molecular species of lysophosphatidic acid (LPAs) in cerebrospinal fluid (CSF) of healthy controls (HC) and in patients with major depressive disorder (MDD), and schizophrenia (SCZ). Scatter plot of CSF levels of LPAs in HC and in patients with MDD and SCZ. The horizontal bars represent mean values adjusted for confounding factors for LPAs. N.S., not significant. *P < .05, **P < .01.
Figure 2.
The levels of total lysophosphatidic acid (LPA), autotaxin (ATX) activity, and phospholipid phosphatase1 (PLPP1) and multivariate linear regression analyses for LPA 22:6 in cerebrospinal fluid (CSF) of healthy controls (HC) and in patients with major depressive disorder (MDD) and schizophrenia (SCZ). (A) Scatter plot of CSF levels of total LPA in HC and in patients with MDD and SCZ. The horizontal bars represent mean values adjusted for lysophosphatidylcholine (LPC) total and sex. N.S., not significant. (B) Scatter plot of ATX activity in CSF of HC and in patients with MDD and SCZ. The horizontal bars represent mean values adjusted for age. N.S., not significant. (C) Scatter plot of CSF levels of PLPP1 in HC and in patients with MDD and SCZ. The horizontal bars represent mean values adjusted for age and sex. N.S., not significant. (D) Variables selected among LPC 22:6, ATX activity, PLPP1, age, sex (male), and body mass index by stepwise method. The levels of LPA 22:6 were significantly correlated with the levels of LPC 22:6 among all 3 groups, but the levels of LPA 22:6 were not correlated with ATX activity in patients with MDD and SCZ. *P < .05, **P < .01, ***P < .001.
Levels of LPC and Minor Lysophospholipid Species in CSF of HC, MDD, and SCZ
Eleven LPC species (14:0, 16:0, 16:1, 18:0, 18:1, 18:2, 20:3, 20:4, 20:5, 22:5, and 22:6) were detected in CSF of all participants. There were no significant differences in the levels of all of 11 LPC species in CSF among all 3 groups (supplementary Table 2; supplementary Figure 1). LPE, LPG, LPI, and LPS species were below the detection limit (data not shown).
ATX Activity and Levels of PLPP1 and PA Species in CSF of HC, MDD, and SCZ
ATX activity and the levels of PLPP1 were detected in CSF of all participants. There were no significant differences in ATX activity among all of 3 groups (supplementary Table 3; Figure 2B). Also, there were no significant differences in the level of PLPP1 among all 3 groups (supplementary Table 4; Figure 2C). Although 3 PA species (34:1, 34:2, 36:2) were detected, most were below the limit of detection (data not shown).
Correlation Between Levels of LPA 22:6 and Levels of LPC 22:6 or ATX Activity in CSF of HC, MDD, and SCZ
Correlation coefficients were calculated between the levels of LPA 22:6 and the levels of LPC 22:6 or ATX activity in HC and patients with MDD and SCZ using linear regression analysis (Figure 2D). The levels of LPA 22:6 were significantly correlated with those of LPC 22:6 among all 3 groups. There was a significant positive correlation between the levels of LPA 22:6 and ATX activity in HC (nonstandardized coefficient B = 0.007, P = .036). However, there was no significant correlation between the levels of LPA 22:6 and ATX activity in patients with either MDD or SCZ.
Correlation Between Levels of LPA 22:6 in CSF and Psychiatric Symptom Scores in MDD or SCZ
Correlation coefficients adjusted for LPC 22:6 and PLPP1 were calculated between the levels of LPA 22:6 in CSF and psychiatric symptom scores using linear regression analysis in patients with MDD or SCZ (Figure 3). Although there were no significant correlations between the levels of LPA 22:6 in CSF and total scores of HAMD 21-items in patients with MDD, there were significant negative correlations between the levels of LPA 22:6 in CSF and individual scores of 3 items of HAMD (item 12: loss of appetite, item 16: loss of weight, item 20: paranoid symptoms) in patients with MDD. However, there were no significant correlations between the levels of LPA 22:6 in CSF and either PANSS total score or any individual PANSS score in patients with SCZ.
Figure 3.
Correlation between the levels of lysophosphatidic acid (LPA) 22:6 in cerebrospinal fluid (CSF) and psychiatric symptom scores in patients with major depressive disorder (MDD) or schizophrenia (SCZ). Correlation between the levels of LPA 22:6 in CSF and psychiatric symptom scores in patients with MDD or SCZ. The correlation coefficients were calculated using linear regression analysis and adjusted for lysophosphatidylcholine (LPC) 22:6 and phospholipid phosphatase1 (PLPP1). *P < .05. (1) Correlation between the levels of LPA 22:6 and the Hamilton Rating Scale for Depression (HAMD) scores in patients with MDD. (2) Correlation between the levels of LPA 22:6 and the Positive and Negative Syndrome Scale (PANSS) scores in patients with SCZ.
Discussion
The levels of LPA 22:6 in CSF were specifically and significantly lower in patients with MDD and SCZ than in HC, although there were no significant differences in the levels of total LPA in CSF among all 3 groups. In addition, the levels of LPA 22:6 were negatively correlated with several HAMD item scores in patients with MDD but not correlated with any PANSS individual scores in patients with SCZ. This is the first study, to our knowledge, to show the correlation of a specific molecular LPAs with MDD.
Although the levels of LPA 22:6 were significantly correlated with those of LPC 22:6, a precursor of LPA, the levels of LPA 22:6 were not correlated with ATX activity in patients with MDD and SCZ among all 3 groups. In addition, LPA 22:6 is most likely generated via an ATX-mediated pathway in HC. These suggest that the LPC-ATX-LPA pathway may work abnormally in patients with MDD and SCZ, which may lead to lower levels of LPA 22:6 in patients with MDD and SCZ. In addition, it has been shown that LPC 22:6 is specifically transported across the blood brain barrier via Mfsd2a, a specific transporter of LPC (Lagarde et al., 2001; Nguyen et al., 2014; Sugasini et al., 2017). Mfsd2a-deficient mice exhibit severe signs of psychiatric disturbance such as anxiety (Nguyen et al., 2014). Although these studies of Mfsd2a may suggest the involvement of Mfsd2a in the lower level of LPA 22:6 in patients with MDD and SCZ, there were also no significant differences in the levels of LPC 22:6 among all 3 groups in this study. Therefore, to elucidate the role of LPA 22:6 in the pathophysiology of MDD and SCZ, it is necessary to investigate the abnormal metabolic regulation of LPA 22:6 via the Mfsd2a/LPC 22:6/ATX/LPA 22:6 pathway with not only CSF, but also postmortem brains of patients with MDD and SCZ, in vivo neurochemical brain imaging with magnetic resonance spectroscopy or brains of nonhuman animal models.
In addition to the LPC/ATX pathway, LPA 22:6 is also generated from minor lysophospholipids such as 22:6 LPE (Kurano et al., 2015) and PA (Pagès et al., 2001). However, the levels of minor lysophospholipids, such as 22:6 LPE, and PA in CSF were below the level of detection in all 3 groups. These suggest that the decreased levels of minor lysophospholipids and PA may be involved in the lower level of CSF LPA 22:6 in patients with MDD and SCZ. To confirm it, measurement of minor lysophospholipids and PA with a higher sensitive method such as gas chromatography is needed.
LPA 22:6 represents 1- or 2-acyl LPA-containing docosahexaenoic acid (DHA). DHA, an essential omega 3 fatty acid, is specifically concentrated in nervous tissues, including the brain and retina, and is essential for normal neurological development and function (Sugasini et al., 2017). DHA deficiency is associated with several psychiatric disorders, including depression (McNamara, 2010; Grosso et al., 2016) and SCZ (Sethom et al., 2010). Previous postmortem brain studies indicate the decreased level of DHA in the orbitofrontal cortex of patients with MDD (McNamara et al., 2007a) and SCZ (McNamara et al., 2007b). Supplementation with DHA (Marangell et al., 2003; Mischoulon et al., 2008) or omega-3 polyunsaturated fatty acids containing DHA (Liao et al., 2019) improve depressive symptoms. DHA penetrates to the brain as LPC-DHA (LPC 22:6) via Mfsd2a (Lagarde et al., 2001; Nguyen et al., 2014; Sugasini et al., 2017). LPC-DHA is metabolized to LPA-DHA (LPA 22:6), which is in turn metabolized to MAG-DHA (MAG 22:6) by PLPPs. MAG-DHA could be eventually converted to DHA in the brain (Destaillats et al., 2018). Therefore, the lower levels of LPA 22:6 in patients with MDD and SCZ might lead to the decreasd levels of MAG-DHA, which might be involved in the pathophysiology of MDD and SCZ. In addition, several depressive symptoms in patients with MDD were significantly associated with the lower level of LPA 22:6, suggesting an involvement of LPA 22:6 in the pathophysiology of MDD. In the case of SCZ, the mean PANSS score showed minimal to mild symptoms in patients with SCZ in this study. This may explain why the levels of LPA 22:6 were not correlated with any PANSS individual scores in patients with SCZ. Therefore, the possibility of involvement of LPA 22:6 in the pathophysiology of SCZ cannot be denied. In a future study, it is necessary to investigate SCZ patients with a wider range of severity.
Our previous report showed the decreased protein levels of ATX in CSF of patients with MDD (Itagaki et al., 2019). Contrary to expectations, this study showed that ATX activity in CSF of patients with MDD did not significantly differ from that of HC. As the levels of LPC in plasma are abundant, the protein levels of ATX strongly correlate with ATX activity (Nakamura et al., 2008). As in this past study, a correlation between the protein levels of ATX and ATX activity in CSF was observed under higher levels of LPC and LPA in the pathological situation such as inflammation. However, the protein levels of ATX did not correlate with ATX activity under normal or low levels of LPC and LPA in CSF (Hayakawa et al., 2019). The levels of LPA and LPC in CSF were normal or relatively low, which may explain the discrepancy between our current study and previous study. Therefore, it is important to examine the levels of LPA to see the ATX activity as an LPA-producing enzyme.
This study has several limitations. First, because of the limited CSF volume, it was not possible to measure MAG-DHA, which is useful to confirm the reduction of LPA 22:6. Next, the correlations between the levels of LPA 22:6 and the IMI and CPZ equivalent doses were not significant in this study (data not shown). In addition, a previous study showed that antipsychotics are able to influence the levels of total LPA in CSF (Gotoh et al., 2019b). Therefore, the current study is not able to completely rule out a potential effect of medications on the levels of LPA 22:6 in CSF. To rule it out, medicated and medication-free patients with MDD and SCZ are needed. Finally, the number of participants in each group was rather small. Therefore, we need to replicate this study with a larger sample to confirm repeatability and validity of our findings.
In conclusion, the levels of LPA 22:6 (LPA-DHA) were significantly lower in the CSF of patients with MDD and SCZ than HC, possibly through an abnormality of LPA 22:6 metabolism. In addition, several depressive symptoms in patients with MDD were significantly associated with the lower levels of LPA 22:6 in CSF, suggesting an involvement of LPA 22:6 in the pathophysiology of MDD.
Supplementary Material
Acknowledgments
The collection, storage, and provision of sample and associated data were supported by the National Center of Neurology and Psychiatry (NCNP) biobank. We thank Professor Junko Tanaka and Tomoyuki Akita for advice on statistical analysis and Dr Aldric Hama for editorial assistance.
This work was supported by JSPS KAKENHI (grant nos. 18K15534 to N.K., 18H02756 to M.T., and 18K07620 to M.O.-T.), Japan Agency for Medical Research and Development grants (18dk0307081h0001, 18dm0107100h0003, and 18dk0307062h0003 to H.K.), grants from the Takeda Science Foundation (to N.K), and the LEAP JP20gm0010004h9904 (to J.A.) from the Japan Agency for Medical Research and Development (AMED).
Statement of Interest
Hiroshi Kunugi MD, PhD, has received donations for research from Takeda Pharmaceutical Co. and Daiichi-Sankyo Co. No financial support from pharmaceutical companies was received for this study.
References
- American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders: DSM-IV-TR. 4th ed.Washington, DC: American Psychiatric Association. [Google Scholar]
- Anliker B, Choi JW, Lin ME, Gardell SE, Rivera RR, Kennedy G, Chun J (2013) Lysophosphatidic acid (LPA) and its receptor, LPA1, influence embryonic schwann cell migration, myelination, and cell-to-axon segregation. Glia 61:2009–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DL, Umstot ES, Desiderio DM, Tigyi GJ (2000) Quantitative analysis of lysophosphatidic acid in human blood fractions. Ann N Y Acad Sci 905:267–269. [DOI] [PubMed] [Google Scholar]
- Barbayianni E, Kaffe E, Aidinis V, Kokotos G (2015) Autotaxin, a secreted lysophospholipase D, as a promising therapeutic target in chronic inflammation and cancer. Prog Lipid Res 58:76–96. [DOI] [PubMed] [Google Scholar]
- Benesch MG, Tang X, Venkatraman G, Bekele RT, Brindley DN (2016) Recent advances in targeting the autotaxin-lysophosphatidate-lipid phosphate phosphatase axis in vivo. J Biomed Res 30:272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destaillats F, Oliveira M, Bastic Schmid V, Masserey-Elmelegy I, Giuffrida F, Thakkar SK, Dupuis L, Gosoniu ML, Cruz-Hernandez C (2018) Comparison of the incorporation of DHA in circulatory and neural tissue when provided as Triacylglycerol (TAG), Monoacylglycerol (MAG) or Phospholipids (PL) provides new insight into fatty acid bioavailability. Nutrients 10:620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh L, Yamada M, Hattori K, Sasayama D, Noda T, Yoshida S, Kunugi H, Yamada M (2019a) Lysophosphatidic acid levels in cerebrospinal fluid and plasma samples in patients with major depressive disorder. Heliyon 5:e01699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh L, Yamada M, Hattori K, Sasayama D, Noda T, Yoshida S, Kunugi H, Yamada M (2019b) Levels of lysophosphatidic acid in cerebrospinal fluid and plasma of patients with schizophrenia. Psychiatry Res 273:331–335. [DOI] [PubMed] [Google Scholar]
- Grosso G, Micek A, Marventano S, Castellano S, Mistretta A, Pajak A, Galvano F (2016) Dietary n-3 PUFA, fish consumption and depression: a systematic review and meta-analysis of observational studies. J Affect Disord 205:269–281. [DOI] [PubMed] [Google Scholar]
- Harrison SM, et al. (2003) LPA1 receptor-deficient mice have phenotypic changes observed in psychiatric disease. Mol Cell Neurosci 24:1170–1179. [DOI] [PubMed] [Google Scholar]
- Hattori K, Ota M, Sasayama D, Yoshida S, Matsumura R, Miyakawa T, Yokota Y, Yamaguchi S, Noda T, Teraishi T, Hori H, Higuchi T, Kohsaka S, Goto Y, Kunugi H (2015) Increased cerebrospinal fluid fibrinogen in major depressive disorder. Sci Rep 5:11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K, Kurano M, Ohya J, Oichi T, Kano K, Nishikawa M, Uranbileg B, Kuwajima K, Sumitani M, Tanaka S, Aoki J, Yatomi Y, Chikuda H (2019) Lysophosphatidic acids and their substrate lysophospholipids in cerebrospinal fluid as objective biomarkers for evaluating the severity of lumbar spinal stenosis. Sci Rep 9:9144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada T, Inagaki A (2015) Psychotropic dose equivalence in Japan. Psychiatry Clin Neurosci 69:440–447. [DOI] [PubMed] [Google Scholar]
- Itagaki K, Takebayashi M, Abe H, Shibasaki C, Kajitani N, Okada-Tsuchioka M, Hattori K, Yoshida S, Kunugi H, Yamawaki S (2019) Reduced serum and cerebrospinal fluid levels of autotaxin in major depressive disorder. Int J Neuropsychopharmacol 22:261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajitani N, Miyano K, Okada-Tsuchioka M, Abe H, Itagaki K, Hisaoka-Nakashima K, Morioka N, Uezono Y, Takebayashi M (2016) Identification of lysophosphatidic acid receptor 1 in astroglial cells as a target for glial cell line-derived neurotrophic factor expression induced by antidepressants. J Biol Chem 291:27364–27370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawana H, Kano K, Shindou H, Inoue A, Shimizu T, Aoki J (2019) An accurate and versatile method for determining the acyl group-introducing position of lysophospholipid acyltransferases. Biochim Biophys Acta Mol Cell Biol Lipids 1864:1053–1060. [DOI] [PubMed] [Google Scholar]
- Kurano M, Suzuki A, Inoue A, Tokuhara Y, Kano K, Matsumoto H, Igarashi K, Ohkawa R, Nakamura K, Dohi T, Miyauchi K, Daida H, Tsukamoto K, Ikeda H, Aoki J, Yatomi Y (2015) Possible involvement of minor lysophospholipids in the increase in plasma lysophosphatidic acid in acute coronary syndrome. Arterioscler Thromb Vasc Biol 35:463–470. [DOI] [PubMed] [Google Scholar]
- Lagarde M, Bernoud N, Brossard N, Lemaitre-Delaunay D, Thiès F, Croset M, Lecerf J (2001) Lysophosphatidylcholine as a preferred carrier form of docosahexaenoic acid to the brain. J Mol Neurosci 16:201–204; discussion 215–221. [DOI] [PubMed] [Google Scholar]
- Liao Y, Xie B, Zhang H, He Q, Guo L, Subramaniapillai M, Fan B, Lu C, Mclntyer RS (2019) Efficacy of omega-3 PUFAs in depression: a meta-analysis. Transl Psychiatry 9:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangell LB, Martinez JM, Zboyan HA, Kertz B, Kim HF, Puryear LJ (2003) A double-blind, placebo-controlled study of the omega-3 fatty acid docosahexaenoic acid in the treatment of major depression. Am J Psychiatry 160:996–998. [DOI] [PubMed] [Google Scholar]
- McNamara RK (2010) DHA deficiency and prefrontal cortex neuropathology in recurrent affective disorders. J Nutr 140:864–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Hahn CG, Jandacek R, Rider T, Tso P, Stanford KE, Richtand NM (2007a) Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biol Psychiatry 62:17–24. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Jandacek R, Rider T, Tso P, Hahn CG, Richtand NM, Stanford KE (2007b) Abnormalities in the fatty acid composition of the postmortem orbitofrontal cortex of schizophrenic patients: gender differences and partial normalization with antipsychotic medications. Schizophr Res 91:37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirendil H, Thomas EA, De Loera C, Okada K, Inomata Y, Chun J (2015) LPA signaling initiates schizophrenia-like brain and behavioral changes in a mouse model of prenatal brain hemorrhage. Transl Psychiatry 5:e541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischoulon D, Best-Popescu C, Laposata M, Merens W, Murakami JL, Wu SL, Papakostas GI, Dording CM, Sonawalla SB, Nierenberg AA, Alpert JE, Fava M (2008) A double-blind dose-finding pilot study of docosahexaenoic acid (DHA) for major depressive disorder. Eur Neuropsychopharmacol 18:639–645. [DOI] [PubMed] [Google Scholar]
- Moreno-Fernández RD, Pérez-Martín M, Castilla-Ortega E, Rosell Del Valle C, García-Fernández MI, Chun J, Estivill-Torrús G, Rodríguez de Fonseca F, Santín LJ, Pedraza C (2017) maLPA1-null mice as an endophenotype of anxious depression. Transl Psychiatry 7:e1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Igarashi K, Ide K, Ohkawa R, Okubo S, Yokota H, Masuda A, Oshima N, Takeuchi T, Nangaku M, Okudaira S, Arai H, Ikeda H, Aoki J, Yatomi Y (2008) Validation of an autotaxin enzyme immunoassay in human serum samples and its application to hypoalbuminemia differentiation. Clin Chim Acta 388:51–58. [DOI] [PubMed] [Google Scholar]
- Nguyen LN, Ma D, Shui G, Wong P, Cazenave-Gassiot A, Zhang X, Wenk MR, Goh EL, Silver DL (2014) Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 509:503–506. [DOI] [PubMed] [Google Scholar]
- Okudaira M, Inoue A, Shuto A, Nakanaga K, Kano K, Makide K, Saigusa D, Tomioka Y, Aoki J (2014) Separation and quantification of 2-acyl-1-lysophospholipids and 1-acyl-2-lysophospholipids in biological samples by LC-MS/MS. J Lipid Res 55:2178–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olianas MC, Dedoni S, Onali P (2016) LPA1 mediates antidepressant-induced ERK1/2 signaling and protection from oxidative stress in glial cells. J Pharmacol Exp Ther 359:340–353. [DOI] [PubMed] [Google Scholar]
- Otsubo T, Tanaka K, Koda R, Shinoda J, Sano N, Tanaka S, Aoyama H, Mimura M, Kamijima K (2005) Reliability and validity of Japanese version of the Mini-International Neuropsychiatric Interview. Psychiatry Clin Neurosci 59:517–526. [DOI] [PubMed] [Google Scholar]
- Pagès C, Simon MF, Valet P, Saulnier-Blache JS (2001) Lysophosphatidic acid synthesis and release. Prostaglandins Other Lipid Mediat 64:1–10. [DOI] [PubMed] [Google Scholar]
- Perrakis A, Moolenaar WH (2014) Autotaxin: structure-function and signaling. J Lipid Res 55:1010–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh S, Govindarajulu M, Suppiramaniam V, Moore T, Dhanasekaran M (2018) Autotaxin(-)Lysophosphatidic acid signaling in Alzheimer’s disease. Int J Mol Sci 19:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts C, Winter P, Shilliam CS, Hughes ZA, Langmead C, Maycox PR, Dawson LA (2005) Neurochemical changes in LPA1 receptor deficient mice–a putative model of schizophrenia. Neurochem Res 30:371–377. [DOI] [PubMed] [Google Scholar]
- Schilling T, Stock C, Schwab A, Eder C (2004) Functional importance of Ca2+-activated K+ channels for lysophosphatidic acid-induced microglial migration. Eur J Neurosci 19:1469–1474. [DOI] [PubMed] [Google Scholar]
- Sethom MM, Fares S, Bouaziz N, Melki W, Jemaa R, Feki M, Hechmi Z, Kaabachi N (2010) Polyunsaturated fatty acids deficits are associated with psychotic state and negative symptoms in patients with schizophrenia. Prostaglandins Leukot Essent Fatty Acids 83:131–136. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998) The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59Suppl 20:22–33;quiz 34–57. [PubMed] [Google Scholar]
- Sugasini D, Thomas R, Yalagala PCR, Tai LM, Subbaiah PV (2017) Dietary docosahexaenoic acid (DHA) as lysophosphatidylcholine, but not as free acid, enriches brain DHA and improves memory in adult mice. Sci Rep 7:11263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebayashi M, Hisaoka K, Nishida A, Tsuchioka M, Miyoshi I, Kozuru T, Hikasa S, Okamoto Y, Shinno H, Morinobu S, Yamawaki S (2006) Decreased levels of whole blood glial cell line-derived neurotrophic factor (GDNF) in remitted patients with mood disorders. Int J Neuropsychopharmacol 9:607–612. [DOI] [PubMed] [Google Scholar]
- Trimbuch T, et al. (2009) Synaptic PRG-1 modulates excitatory transmission via lipid phosphate-mediated signaling. Cell 138:1222–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsybko AS, Ilchibaeva TV, Popova NK (2017) Role of glial cell line-derived neurotrophic factor in the pathogenesis and treatment of mood disorders. Rev Neurosci 28:219–233. [DOI] [PubMed] [Google Scholar]
- Uchida S, Hara K, Kobayashi A, Otsuki K, Yamagata H, Hobara T, Suzuki T, Miyata N, Watanabe Y (2011) Epigenetic status of Gdnf in the ventral striatum determines susceptibility and adaptation to daily stressful events. Neuron 69:359–372. [DOI] [PubMed] [Google Scholar]
- Umezu-Goto M, Kishi Y, Taira A, Hama K, Dohmae N, Takio K, Yamori T, Mills GB, Inoue K, Aoki J, Arai H (2002) Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J Cell Biol 158:227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Chen GX (2018) Production of extracellular lysophosphatidic acid in the regulation of adipocyte functions and liver fibrosis. World J Gastroenterol 24:4132–4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung YC, Mutoh T, Lin ME, Noguchi K, Rivera RR, Choi JW, Kingsbury MA, Chun J (2011) Lysophosphatidic acid signaling may initiate fetal hydrocephalus. Sci Transl Med 3:99ra87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung YC, Stoddard NC, Mirendil H, Chun J (2015) Lysophosphatidic acid signaling in the nervous system. Neuron 85:669–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.