Abstract
Background
Seltorexant, a selective antagonist of human orexin-2 receptors, demonstrated antidepressant effects in a previous exploratory study in patients with major depressive disorder (MDD).
Methods
To replicate and extend this observation, a double-blind, adaptive dose-finding study was performed in patients with MDD who had an inadequate response to 1–3 selective serotonin/serotonin-norepinephrine reuptake inhibitors in the current episode. Patients were randomized (2:1:1) to placebo or seltorexant (20 mg or 40 mg) once-daily, administered adjunctively to the antidepressant the patient had been receiving at screening. After an interim analysis (6 weeks post-randomization of 160th patient), newly recruited patients randomly received (3:3:1) placebo or seltorexant 10 mg or 20 mg; the 40-mg dose was no longer assigned. Patients were stratified by baseline Insomnia Severity Index (ISI) scores (ISI ≥ 15 vs < 15). The primary endpoint was change from baseline Montgomery-Åsberg Depression Rating Scale (MADRS) total score at week 6.
Results
Mixed-Model for Repeated Measures analysis showed a greater improvement in MADRS total score in the seltorexant 20-mg group vs placebo at weeks 3 and 6; least-square means difference (90% CI): −4.5 (−6.96; −2.07), P = .003; and −3.1 (−6.13; −0.16), P = .083, respectively. The improvement in MADRS score at week 6 for seltorexant 20 mg was greater in patients with baseline ISI ≥ 15 vs those with ISI < 15; least-square means difference (90% CI) vs placebo: −4.9 (−8.98; −0.80) and −0.7 (−5.16; 3.76), respectively. The most common (≥5%) adverse events with seltorexant were somnolence, headache, and nausea.
Conclusions
A clinically meaningful reduction of depressive symptoms was observed for seltorexant 20 mg. In the subset of patients with sleep disturbance (ISI ≥ 15), a larger treatment difference between seltorexant 20 mg and placebo was observed, warranting further investigation. No new safety signal was identified.
Registration
ClinicalTrials.gov Identifier: NCT03227224
Previous presentation
Poster presented at 58th Annual Meeting of American College of Neuropsychopharmacology (ACNP), December 8–11, 2019, Orlando, FL.
Keywords: Adjunctive therapy, major depressive disorder, orexin-2, seltorexant
Significance Statement.
Seltorexant, a selective human orexin-2 receptor (OX2R) antagonist, previously displayed antidepressant effects in patients with major depressive disorder (MDD) regardless of sleep impairment and improved sleep efficiency in patients with insomnia. The current study was a randomized, placebo-controlled, adaptive dose-finding trial that investigated the efficacy and safety of 3 doses of seltorexant (10, 20, and 40 mg) as adjunctive therapy in patients with MDD who had experienced an inadequate response to current antidepressant therapy. Patients were stratified based on region and baseline sleep disturbance (Insomnia Severity Index [ISI] score ≥ 15 vs < 15). With seltorexant 20 mg, a statistically significant and clinically meaningful reduction of depressive symptoms was observed. In the subpopulation of MDD patients with sleep disturbance (ISI ≥ 15), a larger treatment difference between seltorexant 20 mg and placebo was observed, warranting further investigation. No new safety concerns were observed following seltorexant treatment.
Introduction
Major depressive disorder (MDD) is a common, often recurring, potentially chronic illness that is associated with significant distress and socio-occupational impairment. Episodes of MDD commonly prove difficult to treat, and those who suffer from this illness have an increased risk of suicidal behaviors and substantially reduced longevity (Cuijpers and Smit, 2002; Lopez et al., 2006; Kupferberg et al., 2016). Antidepressant drugs of the selective serotonin reuptake inhibitor (SSRIs) and serotonin and norepinephrine reuptake inhibitor (SNRIs) classes are generally the first-line treatments of MDD (National Collaborating Centre for Mental Health, 2010; Cleare et al., 2015; Kennedy et al., 2016). Despite the availability of numerous treatment options, nearly 50% of patients do not respond to a first course of therapy with a conventional antidepressant and up to one-third do not remit despite multiple sequential courses of therapy (Rush et al., 2006; Bauer et al., 2019). As such, identification of alternate therapies for those who do not respond to conventional antidepressants remains a significant clinical challenge. Among the many strategies evaluated for antidepressant non-responders, switching to antidepressants from a different class, combining antidepressants, or adding atypical antipsychotics as adjunctive treatments to the ineffective antidepressant are the widely used strategies after nonresponse to 1 or 2 trials of conventional antidepressant pharmacotherapy (Al-Harbi, 2012; de Sousa et al., 2015). Among these strategies, adjunctive therapy with atypical antipsychotics (e.g., aripiprazole, brexpiprazole, or quetiapine XR) are approved by health authorities for this indication, although adverse effects and long-term side effects limit or complicate their use (Fleurence et al., 2009; Wright et al., 2013; Thase, 2016). Thus, there remains an unmet need for new treatments with different mechanisms of action that are effective and better tolerated to improve treatment strategies for MDD (Thase, 2017). Combining the standard antidepressant therapy with an augmentation treatment that targets both depressive symptoms and insomnia symptoms of the MDD disorder may offer the advantage of maintaining already achieved antidepressant effects while also allowing for the potential of an additional response to the newly added study treatment in a comprehensive treatment approach. Moreover, while SSRIs may cause insomnia, sleep problems more commonly arise as symptoms of MDD and have a substantial impact on the course and outcome of depression (Gulec et al., 2011).
One promising area for novel therapeutics discovery in mood disorders is illuminated by emerging data characterizing the neurobiology of the orexinergic system. In addition to their role in regulation of sleep/wake cycling, the orexins participate in mediating behavior under conditions of high motivational relevance, such as responding to stress, threat, or reward-related stimuli, possibly through altering arousal and energy balance (P. L. Johnson et al., 2012; Nollet and Leman, 2013; Li et al., 2014; Mahler et al., 2014; Yeoh et al., 2014; Chen et al., 2015). Some aspects of these roles are differentially mediated by orexin 1 vs orexin 2 receptor signaling. For example, orexin 2 receptor signaling specifically plays a role in mediating the arousal from sleep (i.e., wakefulness). Animal models of stress and depression suggested orexin-2 receptor antagonists might have antidepressant-like activity (Fitch et al., 2014). Dual orexin receptor antagonists, suvorexant and lemborexant, have been approved for the treatment of insomnia disorder in the United States, characterized by difficulties with sleep onset and/or sleep maintenance in adults based on the results of pivotal phase 3 studies. However, these agents have not been shown to be effective or approved for MDD (Herring et al., 2019; Rosenberg et al., 2019; Kärppä et al., 2020). Filorexant, also a dual orexin antagonist, was tested as augmentation therapy in patients with MDD and showed no difference in efficacy from placebo (Connor et al., 2017).
Seltorexant, a potent and highly selective antagonist of orexin-2 receptor, is being developed as a treatment for MDD due to its potential role in normalizing sleep (Bonaventure et al., 2015). In exploratory clinical studies, seltorexant displayed antidepressant effects by improving core symptoms of depression in patients with MDD regardless of sleep impairment and improved sleep efficiency in patients with insomnia (Brooks et al., 2019; Recourt et al., 2019). The present study investigated the antidepressant effects, safety, and tolerability of various doses of seltorexant as adjunctive therapy in patients with MDD who had experienced inadequate response to the current antidepressant therapy.
Methods
Patients
Adult men or women (aged 18–70 years [inclusive]) who met Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) diagnostic criteria for MDD (confirmed by the Structured Clinical Interview for DSM-5 Axis I Disorders – Clinical Trials Version) and who had inadequate response to ≥1 but ≤3 antidepressants (defined as showing <50% improvement in depressive symptoms since starting the current antidepressant), administered at an adequate dose and duration in the current episode of depression (assessed by Massachusetts General Hospital Antidepressant Treatment Response Questionnaire) were eligible for study enrolment. Patients had to be receiving monotherapy treatment for depressive symptoms with 1 of the following SSRI/SNRI antidepressants, in any formulation: citalopram, duloxetine, escitalopram, fluvoxamine, fluoxetine, milnacipran, levomilnacipran, paroxetine, sertraline, venlafaxine, desvenlafaxine, vilazodone, or vortioxetine at a stable dose (at or above the minimum therapeutic dose level) for at least 4 weeks and for no greater than 12 months. Additionally, patients were required to have Montgomery-Ǻsberg Depression Rating Scale (MADRS) total score ≥25 at screening and did not demonstrate >20% improvement in MADRS total score from the screening to baseline visit.
Patients were excluded if they had a history or current diagnosis of a psychotic disorder; bipolar disorder; autism spectrum disorder or borderline personality disorder; somatoform disorders; chronic fatigue syndrome or fibromyalgia; significant primary sleep disorder, including obstructive sleep apnea or restless leg syndrome; or parasomnias. In addition, patients who had a current or recent (within the prior 6 months) history of active suicidal ideation with some intent to act, without plan or active suicidal ideation with specific plan and intent, or a history of suicidal behavior within the past year (based on the Columbia Suicide Severity Rating Scale [C-SSRS]) at screening or day 1 were not eligible for enrolment. Patients having a history of moderate or severe substance or alcohol use disorder according to DSM-5 criteria within 6 months before screening or positive test result(s) for alcohol and/or drugs of abuse at screening or at baseline were also excluded. Use of monoamine oxidase inhibitors, antipsychotics, benzodiazepines, hypnotics drugs (eg, zolpidem, zopiclone, zaleplon, eszopiclone, suvorexant, and ramelteon), was prohibited during the study.
Study Design
This randomized, double-blind (DB), parallel-group, placebo-controlled, 6-week adaptive dose finding, multi-center phase 2b study (NCT03227224) was conducted across 81 sites in 7 countries (Bulgaria, Finland, Germany, Japan, the Russian Federation, Ukraine, and the United States) between August 31, 2017 and January 19, 2019. The study consisted of a screening phase (up to 4 weeks), a DB treatment phase (6 weeks), and a post-treatment follow-up phase (2 weeks). Eligible patients were initially randomized (2:1:1) to receive placebo, seltorexant 20 mg or 40 mg once daily. All patients continued to receive their baseline SSRI/SNRI antidepressant (at the same dose, without change, and at approximately the same time as prior to entering the study). An interactive web response system was used for treatment assignment. The randomization was balanced by using randomly permuted blocks and stratified by region (the United States, Europe, and Japan) and by insomnia status (the presence of moderate-severe insomnia symptoms was defined using an Insomnia Severity Index [ISI] clinician’s version score ≥15 at baseline, as distinguished from having no or mild insomnia symptoms, as defined by an ISI clinician’s version score <15 at baseline). Blinded treatment was administered at the study site on day 1 of the DB treatment phase and was self-administered by the patient from day 2 to day 41 at home once daily at bedtime, approximately 3 hours after the last meal. After completion of the DB treatment phase or early withdrawal, patients completed a follow-up visit of 7 to 14 days after the last dose of study medication.
An interim analysis (IA) was pre-specified to occur with a data cutoff at 6 weeks post-randomization of the 160th patient. Based on the Interim Analysis Committee decision, following the IA the newly recruited patients were randomized per protocol to receive placebo or seltorexant 10 mg or seltorexant 20 mg, with an allocation ratio 3:3:1, adapted according to the dose response curve seen at the IA. No additional participants were assigned to the seltorexant 40-mg dose.
This study was conducted in accordance with the ethical principles of the Declaration of Helsinki and in accordance with the International Conference on Harmonization’s Good Clinical Practice guidelines and applicable regulatory requirements. The independent ethics committee and institutional review board reviewed and approved the study protocol and amendment. All patients provided written informed consent prior to study enrolment.
Study Endpoints
Efficacy assessments were performed during the DB treatment phase (supplementary Appendix). The primary endpoint was the change in MADRS total score from baseline to the end of week 6 (day 42). The secondary endpoints included (1) change from baseline MADRS total score at week 6 in patients with baseline ISI score ≥15 vs ISI < 15; (2) proportion of patients achieving response (≥50% improvement from baseline MADRS total score), and remission (MADRS total score of ≤12) at week 6; and (3) change from baseline in MADRS-6 (subscale that focuses on the core symptoms of depression as assessed by 6 items: apparent sadness, reported sadness, inner tension, lassitude, inability to feel, pessimistic thoughts). Exploratory endpoints involved assessment of change in Hamilton Anxiety Rating Scale (HAM-A) total score, and Clinical Global Impression – Severity (CGI-S) score at week 3 (day 22) and week 6; (4) change from baseline in Patient Health Questionnaire 9-Item (PHQ-9), Patient Reported Outcomes Measurement Information System-Sleep Disturbance (PROMIS-SD) and Fatigue (PROMIS-F) Short Form, and Snaith-Hamilton Pleasure Scale (SHAPS) overtime to week 6.
The MADRS and CGI-S were administered by independent, centralized remote raters over the telephone; the HAM-A and ISI were administered by the investigators or designee at the site; and the PHQ-9, PROMIS-SD, PROMIS-F, and SHAPS were completed by the patients.
Safety was recorded until the follow-up visits and was assessed based on treatment-emergent adverse events (TEAEs), clinical laboratory tests, vital sign measurements, physical examinations, and electrocardiogram findings. Suicidal ideations and suicidal behavior were evaluated by means of C-SSRS (Posner et al., 2007). TEAEs of special interest were cataplexy, sleep paralysis, abnormal dreams, and complex sleep-related behaviors.
Statistical Methods
Sample Size Determination—
The sample size was determined based on the primary efficacy endpoint of the study using the Multiple Comparison Procedure-Modeling (MCP-Mod) approach (Pinheiro et al., 2014). The sample size of 280 randomized patients was required to provide an average weighted power of approximately 85% depending on the underlying true dose-response shape, assuming a 1-sided significance level of 0.05, a treatment difference from placebo of 4.5 points in change in MADRS total score, a SD of 10, and a 25% overall dropout rate.
The data cutoff for the unblinded IA was 6 weeks after randomization of 160 patients, which corresponded to approximately 57% of the sample size. This number of patients provided approximately 93% power to detect a dose-response relationship depending on the underlying true dose-response shape, assuming a treatment difference of 4.5 and a SD of 10 when tested by means of the MCP-Mod approach at the 30% significance level (1-sided).
Statistical Analyses—
The primary efficacy endpoint was evaluated at a 1-sided significance level of 0.05 using the MCP-Mod approach to test for dose-response. The candidate set included 4 standardized model profiles: “linear” (no parameters), “Emax” (ED50 = 1.053), “exponential” (δ = 18.20), and “sigEmax” (ED50 = 14.14, h = 8.496). The MCP-Mod approach was applied towards the estimates obtained from the mixed model for repeated measures (MMRM) analysis, which included region, time, treatment, baseline insomnia status, and treatment-by-time interaction as factors and baseline MADRS total score as a covariate. In conjunction with the MCP-Mod analysis, the pairwise comparison between seltorexant doses and placebo was performed using the appropriate contrasts directly from the MMRM analysis using estimates at the end of week 6. Other than MCP-Mod (which included corrections for 4 model profiles tested), no multiplicity adjustment was performed and the 90% confidence interval (CI) for the difference in least squares (LS) means and P value was calculated based on the contrast test statistic for each seltorexant dose level. Secondary endpoints, except change in baseline CGI-S and ISI total scores, response, and remission, were analyzed using the same MMRM as for the primary efficacy endpoint, with the corresponding baseline as a covariate. Patients with missing MADRS at a given time point were imputed as non-responders and non-remitters. Changes in CGI-S and ISI total score were analyzed using an ANCOVA model (for CGI-S, ANCOVA on ranks). Efficacy analyses were based on the full analysis set (defined as all patients who were randomized and received ≥1 dose of study drug), and safety analyses were based on the full safety analysis set (which was the same as the full analysis set).
Results
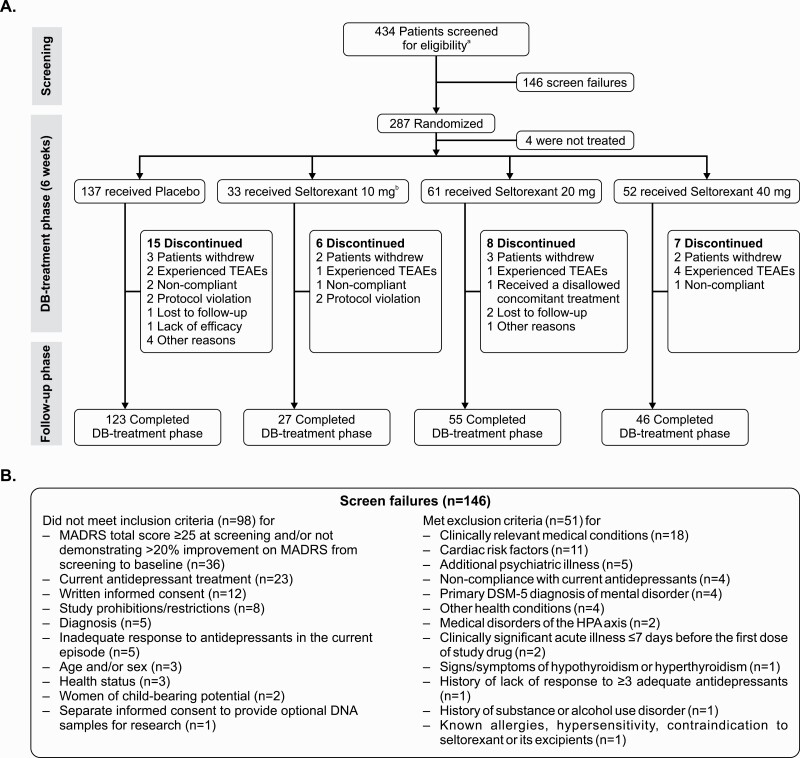
A total of 434 patients with MDD were screened, of which 287 patients were randomized to receive seltorexant 10 mg (n = 33), 20 mg (n = 63), 40 mg (n = 53), or placebo (n = 138) in the double-blind treatment phase; 4 of 287 patients who were randomized did not receive any study treatment. A total of 146 patients were screen failures; 24.7% (36 of 146) were attributable to having a MADRS total score <25 at screening and/or having an improvement of >20% on their MADRS total score from the screening to baseline visit. A total of 251 of the 283 (87.5%) patients who received study treatment completed the 6-week DB treatment phase and 36 (12.5%) patients discontinued the study. Discontinuation rates at the end of DB phase for placebo (11%) were similar to those of the groups that received seltorexant (14%). The most common reason for discontinuation was withdrawal of consent (n = 10 [3.5%]), followed by AE (n = 8 [2.8%] patients) (Figure 1).
Figure 1.
Study design and patient disposition (A) and screen failures (B). aOne patient was screened but was neither randomized nor screen failed and was not included in any of the analysis sets. bInitial randomization ratio was 2:1:1 (placebo, seltorexant 20 mg, and 40 mg). A pre-planned interim analysis was conducted with the data cutoff at 6 weeks after randomizing 160 patients in the study; after the interim analysis, the seltorexant 10-mg dose was added to randomization and the seltorexant 40-mg dose was dropped from randomization. The allocation ratio after the interim analysis was adjusted to 3:3:1 (placebo, seltorexant 10 mg, and 20 mg).
Of the 283 patients treated, a slight majority were women (53.7%; 7.8% were women of child-bearing potential), and more than three-quarters identified themselves as White (78.8%). The mean (SD) age of patients was 49.1 (12.34) years (Table 1). The mean (SD) baseline MADRS total score was 33.7 (5.40) and CGI-S score was 4.8 (0.72), mean duration of the current episode of depression was 20.4 (12.19) weeks, and mean baseline ISI score was 16.1 (5.67) (per interactive web response system ISI ≥ 15, 57.6%, and ISI < 15, 42.4%) (Table 1). Most patients had only 1 antidepressant treatment of sufficient dose (based on MGH-ATRQ) and duration (≥4 weeks) during this episode (84.5%), and the majority (68.9%) were currently taking an SSRI. The majority of patients had experienced ≥3 major depressive episodes (Table 1)
Table 1.
Demographics and Baseline Characteristics (Full Analysis Set)
| Characteristics | Placebo (n = 137) | Seltorexant | Total (n = 283) | ||
|---|---|---|---|---|---|
| 10 mg (n = 33) | 20 mg (n = 61) | 40 mg (n = 52) | |||
| Age, y | 49.6 (11.71) |
49.4 (15.31) |
48.5 (13.56) |
48.3 (10.52) |
49.1 (12.34) |
| Sex, women | 74 (54.0%) |
20 (60.6%) |
30 (49.2%) |
28 (53.8%) |
152 (53.7%) |
| Age when diagnosed with MDD, y | 38.8 (13.11) |
43.0 (16.03) |
38.9 (14.21) |
38.2 (11.41) |
39.2 (13.43) |
| Duration of current depressive episode, wk | 19.1 (12.27) |
25.7 (13.79) |
20.1 (11.02) |
21.2 (11.56) |
20.4 (12.19) |
| MADRS total score | 33.7 (5.67) |
31.7 (4.31) |
34.0 (5.03) |
34.7 (5.51) |
33.7 (5.40) |
| CGI-S score | 4.8 (0.77) |
4.6 (0.61) |
4.8 (0.67) |
4.9 (0.69) |
4.8 (0.72) |
| Current antidepressant type | |||||
| SSRI | 100 (73.0%) |
21 (63.6%) |
46 (75.4%) |
28 (53.8%) |
195 (68.9%) |
| SNRI | 37 (27.0%) |
12 (36.4%) |
15 (24.6%) |
24 (46.2%) |
88 (31.1%) |
| Baseline ISI (per IWRS) | |||||
| ≥15 | 81 (59.1%) |
5 (15.2%) |
38 (62.3%) |
39 (75.0%) |
163 (57.6%) |
| <15 | 56 (40.9%) |
28 (84.8%) |
23 (37.7%) |
13 (25.0%) |
120 (42.4%) |
| No. of major depressive episodes in lifetime, including current episode | 136 | 33 | 61 | 52 | 282 |
| 1 | 19 (14.0%) |
9 (27.3%) |
7 (11.5%) |
6 (11.5%) |
41 (14.5%) |
| 2 | 17 (12.5%) |
8 (24.2%) |
18 (29.5%) |
12 (23.1%) |
55 (19.5%) |
| ≥3 | 100 (73.5%) |
16 (48.5%) |
36 (59.0%) |
34 (65.4%) |
186 (66.0%) |
Abbreviations: CGI-S, Clinical Global Impression – Severity; ISI, Insomnia Severity Index; IWRS, interactive web response system; MADRS, Montgomery-Asberg Depression Rating Scale; MDD, major depressive disorder; SNRI, serotonin-norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor. All values are expressed as mean (SD) or n (%).
Efficacy
Primary Endpoint—
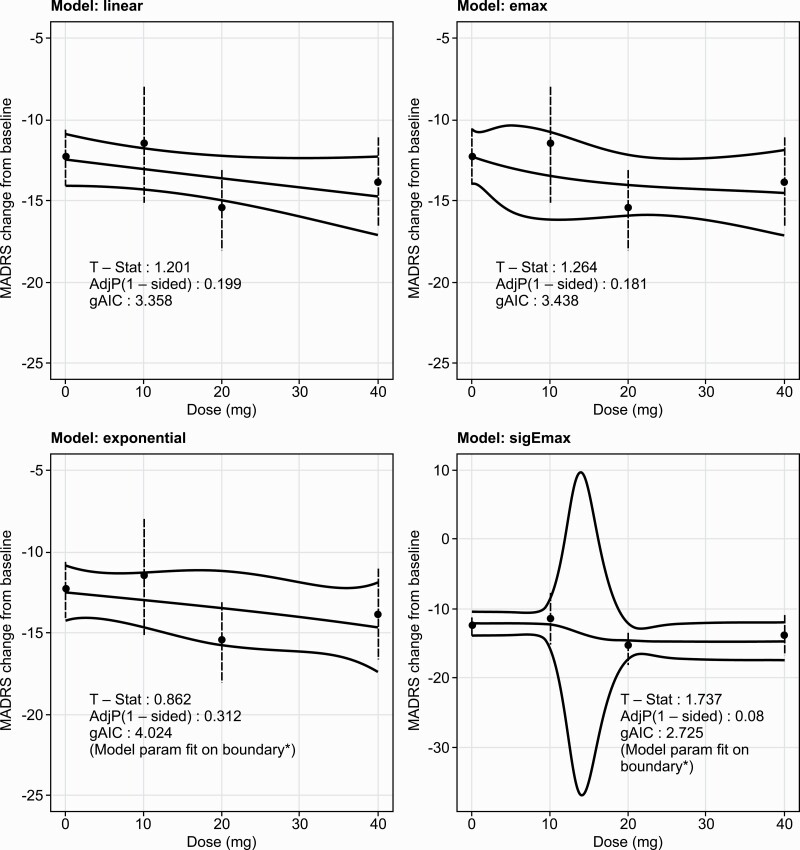
Based on MCP-Mod analysis, after applying corrections for multiple testing there was no significant dose-response relationship associated with pre-specified models in change from baseline MADRS total score at week 6. The sigmoid Emax model showed the best fit of the 4 pre-specified models, with multiplicity-adjusted, 1-sided P = .08 (pre-specified significance threshold 1-sided α = .05) (Figure 2).
Figure 2.
Change in MADRS total score from baseline to week 6: MCP-Mod test results with model estimated treatment effects and MMRM treatment effects and 90% CI (full analysis set). Note: solid points and dashed lines represent estimates and 90% CI from MMRM with change from baseline as the response variable and the fixed effect model terms for treatment (placebo and seltorexant dose groups), time, region, baseline insomnia status, and time-by-treatment and baseline value as a covariate. Negative change in score indicates improvement. *Caution is needed for interpreting the model fitted values and confidence curves for Exponential and sigEmax models, for which some model parameters were fit on boundaries. Abbreviations: MADRS, Montgomery-Asberg Depression Rating Scale; MMRM, mixed model for repeated measures; Selt, seltorexant.
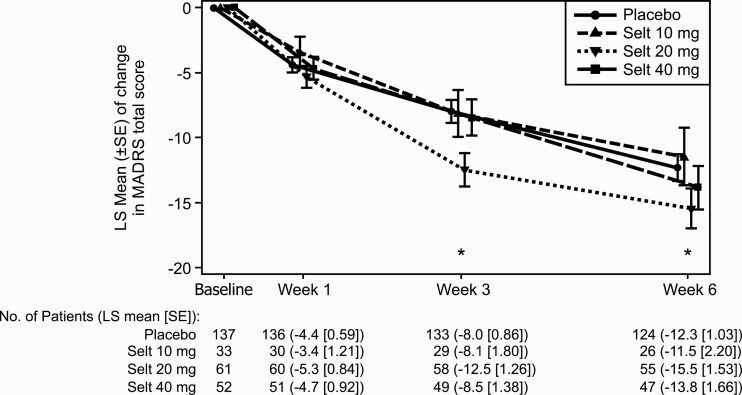
Based on MMRM analysis, the greatest improvement in MADRS total score vs placebo was observed in the seltorexant 20-mg group at weeks 3 and 6 with LS mean difference (90% CI): −4.5 (−6.96; −2.07), P = .003; and −3.1 (−6.13; −0.16), P = .083, respectively. This difference was greater in the 20-mg dose than in the seltorexant 40 mg (LS mean difference [90% CI], −0.5 [−3.12; 2.09] and −1.5 [−4.70; 1.63], respectively). The seltorexant 10-mg treatment group showed a change from baseline in MADRS total score that did not differ significantly from placebo at any timepoint (Figure 3).
Figure 3.
LS mean change in MADRS over time (observed case MMRM; Full analysis set). *Seltorexant 20 mg vs placebo 2-sided P < .10. Abbreviations: LS, least squares; MADRS, Montgomery-Asberg Depression Rating Scale; MMRM, mixed model for repeated measures; SE, Standard error; Selt, seltorexant.
Secondary Endpoints—
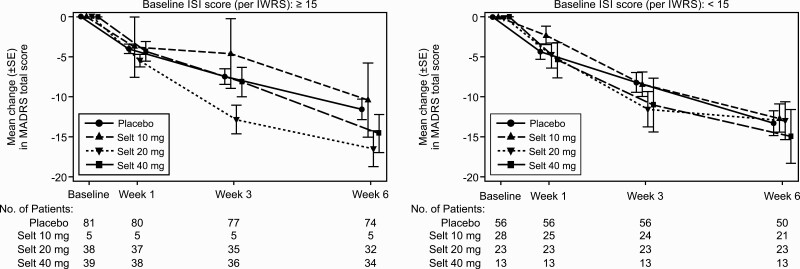
In the seltorexant 20-mg group, the improvement in MADRS score at week 6 was greater in patients with baseline ISI ≥ 15 than with baseline ISI < 15, with LS mean difference (90% CI) vs placebo of −4.9 (−8.98, −0.80) and −0.7 (−5.16, 3.76), respectively. In the seltorexant 40-mg group, there was a similar change in MADRS total score at week 6 vs with placebo in both ISI subgroups (Figure 4).
Figure 4.
Mean change in MADRS over time by baseline ISI total score (per IWRS) (observed case; Full analysis set). Abbreviations: ISI, Insomnia Severity Index; IWRS, interactive web response system; MADRS, Montgomery-Asberg Depression Rating Scale; Selt, seltorexant.
At week 6, response rates (≥50% reduction from baseline in MADRS total score) for the seltorexant 10-mg, 20-mg, and 40-mg dose groups were 24.2%, 41.0%, and 38.5%, respectively, vs 28.5% for the placebo group. Similarly, remission rates (based on MADRS total score at study endpoint of ≤12) for the seltorexant 10-mg, 20-mg, and 40-mg groups were 15.2%, 29.5%, and 26.9%, respectively, vs 19.0% for the placebo group.
Based on an MMRM analysis of the change in MADRS-6 total score from baseline, seltorexant 20 mg and 40 mg showed greater improvement compared with placebo at weeks 3 and 6 (Table 2). In addition, improvement was also observed in the subgroup of patients with an ISI ≥ 15, with the seltorexant 20-mg group showing a greater change than the 40-mg group at weeks 3 and 6. The LS mean difference [90% CI] for seltorexant 20 mg vs placebo at weeks 3 and 6 was −4.0 (−6.19; −1.75) and −3.7 (−6.57; −0.89), respectively, and for seltorexant 40 mg vs placebo was −0.6 (−2.80; 1.58) and −1.1 (−3.92; 1.68), respectively.
Table 2.
Secondary Endpoints (Full Analysis Set)
| Secondary endpoints | Placebo (n = 137) |
Seltorexant | ||
|---|---|---|---|---|
| 10 mg (n = 33) |
20 mg (n = 61) |
40 mg (n = 52) |
||
| Responsea at wk 6, n (%) | 39 (28.5%) | 8 (24.2%) | 25 (41.0%) | 20 (38.5%) |
| Remissionb at wk 6, n (%) | 26 (19.0%) | 5 (15.2%) | 18 (29.5%) | 14 (26.9%) |
| MADRS-6 | ||||
| LS mean difference (90% CI) | ||||
| Wk 3 | 0.4 (−1.91; 2.67) | −3.1 (−4.87; −1.41) | −0.8 (−2.59; 1.09) | |
| Wk 6 | 0.7 (−2.04; 3.54) | −1.8 (−3.93; 0.26) | −1.0 (−3.18; 1.27) | |
| MADRS-6 (baseline ISI ≥15) | ||||
| LS mean difference (90% CI) | ||||
| Week 3c | 0.6 (−4.50; 5.61) | −4.0 (−6.19; −1.75) | −0.6 (−2.80; 1.58) | |
| Week 6d | −0.4 (−6.73; 5.98) | −3.7 (−6.57; −0.89) | −1.1 (−3.92; 1.68) | |
| ISI total score at wk 6 | ||||
| LS mean difference (90% CI) | −0.5 (−2.63; 1.60) | −2.3 (−3.90; −0.67) | −1.9 (−3.64; −0.24) | |
| HAM-A total score at wk 6 | ||||
| LS mean difference (90% CI) | −1.9 (−4.42; 0.60) | −1.3 (−3.22; 0.56) | −1.1 (−3.10; 0.95) | |
| Patient-reported outcomes | ||||
| PHQ-9 at wk 6 | ||||
| LS mean difference (90% CI) | −0.5 (−2.56; 1.63) | −1.2 (−2.80; 0.36) | −0.0 (−1.73; 1.67) | |
| Remissione at wk 6 | 17 (12.4%) | 4 (12.1%) | 16 (26.2%) | 13 (25.0%) |
| Responsef at wk 6 | 56 (40.9%) | 12 (36.4%) | 30 (49.2%) | 22 (42.3%) |
| SHAPS | ||||
| LS mean difference (90% CI) | ||||
| Wk 3 | 0.4 (−0.89; 1.65) | −1.2 (−2.14; −0.22) | 0.3 (−0.70; 1.36) | |
| Wk 6 | 0.7 (−0.80; 2.12) | −0.7 (−1.84; 0.35) | 0.1 (−1.05; 1.30) | |
| PROMIS-SD T-score | ||||
| LS mean difference (90% CI) | ||||
| Wk 3 | −1.1 (−4.00; 1.84) | −4.3 (−6.49; −2.08) | −5.3 (−7.68; −2.96) | |
| Wk 6 | −1.0 (−4.17; 2.21) | −4.4 (−6.82; −2.04) | −5.3 (−7.83; −2.70) | |
| PROMIS-F T-score | ||||
| LS mean difference (90% CI) | ||||
| Wk 3 | −0.6 (−3.32; 2.19) | −2.9 (−4.98; −0.80) | −0.3 (−2.56; 1.92) | |
| Wk 6 | −0.8 (−4.16; 2.65) | −2.0 (−4.57; 0.58) | −1.0 (−3.78; 1.74) |
Abbreviations: HAM-A, Hamilton Anxiety Rating Scale; ISI, Insomnia Severity Index; LS, least squares; MADRS, Montgomery-Asberg Depression Rating Scale; PHQ, Patient Health Questionnaire; PROMIS-F, Patient Reported Outcome Measurement Information System- Fatigue; PROMIS-SD, Patient Reported Outcome Measurement Information System-Sleep Disturbance; SHAPS, Snaith-Hamilton Pleasure Scale.
a Defined as ≥50% improvement from baseline MADRS total score.
b Defined as MADRS total score ≤12.
c Placebo, n = 77; seltorexant 10 mg, n = 5; 20 mg, n = 35; 40 mg, n = 36.
d Placebo, n = 74; seltorexant 10 mg, n = 5; 20 mg, n = 32; 40 mg, n = 34.
e Defined as a PHQ-9 total score <5.
f Defined as ≥50% improvement in PHQ-9 total score from baseline.
Negative change in score indicates improvement.
A clinically meaningful improvement (decrease) in ISI score was observed at week 6 in both the seltorexant 20-mg and 40-mg groups compared with placebo; the LS mean difference (90% CI) in change in ISI vs placebo was −2.3 (−3.90; −0.67) for seltorexant 20 mg and −1.9 (−3.64; −0.24) for 40 mg. There was no apparent difference in improvement in anxiety symptoms based on the HAM-A total score change from baseline to week 6 between any seltorexant treatment group compared with placebo by observed case MMRM analysis. A greater percentage of patients had a CGI-S score corresponding to “normal” at week 6 with seltorexant 20 mg (18.3%) than with 40 mg (7.7%), 10 mg (3.3%), or placebo (7.4%).
The PHQ-9 remission (defined by a score <5 at study endpoint) rates for the seltorexant 20-mg and 40-mg groups were 26.2% and 25.0%, respectively, vs 12.4% for the placebo group. The percentage of responders (based on a ≥50% improvement in PHQ-9 total score from baseline) in the seltorexant 20-mg and 40-mg groups vs placebo was 49.2% and 42.3% vs 40.9%, respectively (Table 2).
The difference in baseline disease severity in the stratum with ISI < 15 vs that with ISI ≥ 15 is shown in supplementary Table 1, indicating a higher baseline MADRS score in the ISI ≥ 15 in part from the higher score on the sleep item. There was a numerically greater improvement from baseline at week 6 in the MADRS total score compared with placebo for patients treated with SNRIs than SSRIs for all seltorexant dose groups (supplementary Table 2). This was, however, in part due to a greater placebo response on the MADRS total score for patients taking SSRIs than those taking SNRIs. Due to the difference in response to placebo between patients with SSRIs and SNRIs, the effect of seltorexant vs placebo as augmentation with an SNRI vs SSRI should be interpreted with caution.
Based on MMRM analysis, improvement in sleep disturbance as measured using the change in PROMIS-SD T-score from baseline was greater for patients in the seltorexant 40-mg treatment group vs placebo with an LS mean difference (90% CI) of −5.3 (−7.68; −2.96) at week 3 and −5.3 (−7.83; −2.70) at week 6. Similar improvement in PROMIS-SD T-score from baseline was noted with seltorexant 20 mg vs placebo at weeks 3 and 6 with LS mean difference (90% CI) of −4.3 (−6.49; -2.08) and −4.4 (−6.82; −2.04), respectively (Table 2; supplementary Figure 1A). The improvement in PROMIS-F T-score was numerically greater for seltorexant 20 mg and 40 mg vs placebo at weeks 3 and 6 (Table 2; supplementary Figure 1B). No clinically meaningful improvement in SHAPS scores was observed, as the difference in LS mean (90% CI) from placebo with seltorexant 20 mg was −1.2 (−2.14; 0.22) at week 3 and −0.7 (−1.84; 0.35) at week 6 (Table 2).
Safety
Overall, 55/146 (37.7%) patients in seltorexant groups (10 mg: 11/33 [33.3%], 20 mg: 25/61 [41.0%] and 40 mg: 19/52 [36.5%]) and 56/137 (40.9%) patients in the placebo group experienced at least 1 TEAE (Table 3). The most common TEAEs (≥5% of patients in the seltorexant groups vs placebo) were headache (9 [6.2%] vs 9 [6.6%]), somnolence (9 [6.2%] vs 7 [5.1%]), and nausea (8 [5.5%] vs 4 [2.9%]). Overall, somnolence-related adverse events were reported in 7 (5.1%) patients in the placebo group, 12 (8.2%) patients in seltorexant treatment arms, of which 7 (11.5%) were patients from the seltorexant 20-mg treatment arm. TEAEs leading to discontinuation in the seltorexant groups were insomnia (2.1%), sleep paralysis (1.4%), irritability, nausea, vomiting, and increased alanine aminotransferase and aspartate aminotransferase (0.7% each). Most TEAEs were mild or moderate in severity. No deaths or serious TEAEs were reported in any of the seltorexant treatment groups. One patient in the placebo group reported a serious TEAE of polycythemia vera. The TEAEs of special interest reported in seltorexant treatment groups vs placebo were abnormal dreams (2.7% vs 0.7%), sleep paralysis (1.4% vs 0.7%), and nightmare (1.4% vs 0). However, there was no clear evidence of a dose effect on the reporting of TEAEs of special interest between the seltorexant 10-mg, 20-mg, and 40-mg doses (Table 3). There was no clinically relevant increase in suicidal ideation across the treatment groups, and no case of suicidal behavior was reported based on either the report of such a serious adverse event or the change in C-SSRS score.
Table 3.
Treatment-Emergent Adverse Events Experienced by at Least 5% of Patients in Any Treatment Group (Safety Analysis Set)
| Characteristics, n (%) | Placebo (n = 137) | Seltorexant | |||
|---|---|---|---|---|---|
| 10 mg (n = 33) | 20 mg (n = 61) | 40 mg (n = 52) | Total (n = 146) | ||
| Patients with ≥1 TEAE | 56 (40.9%) | 11 (33.3%) | 25 (41.0%) | 19 (36.5%) | 55 (37.7%) |
| Discontinuations due to TEAEs | 2 (1.5%) | 1 (3.0%) | 1 (1.6%) | 4 (7.7%) | 6 (4.1%) |
| Most common TEAEs (≥5% of patients in any group) | |||||
| Headache | 9 (6.6%) | 2 (6.1%) | 1 (1.6%) | 6 (11.5%) | 9 (6.2%) |
| Somnolence | 7 (5.1%) | 1 (3.0%) | 6 (9.8%) | 2 (3.8%) | 9 (6.2%) |
| Nausea | 4 (2.9%) | 1 (3.0%) | 4 (6.6%) | 3 (5.8%) | 8 (5.5%) |
| Diarrhea | 7 (5.1%) | 0 | 0 | 1 (1.9%) | 1 (0.7%) |
| Insomnia | 1 (0.7%) | 2 (6.1%) | 1 (1.6%) | 2 (3.8%) | 5 (3.4%) |
| TEAEs of special interest | 3 (2.2%) | 1 (3.0%) | 2 (3.3%) | 3 (5.8%) | 6 (4.1%) |
| Abnormal dreams | 1 (0.7%) | 0 | 2 (3.3%) | 2 (3.8%) | 4 (2.7%) |
| Nightmare | 0 | 0 | 1 (1.6%) | 1 (1.9%) | 2 (1.4%) |
| Sleep paralysis | 1 (0.7%) | 1 (3.0%) | 0 | 1 (1.9%) | 2 (1.4%) |
| Parasomnia | 1 (0.7%) | 0 | 0 | 0 | 0 |
Abbreviations: TEAE, treatment-emergent adverse event.
Discussion
In this phase 2b, adaptive dose-finding study, a clinically meaningful reduction of depressive symptoms based on MMRM analysis across up to 6 weeks of double-blind treatment was observed with the seltorexant 20-mg dose compared with placebo (added adjunctively to the oral antidepressant that patients had been receiving prior to randomization). In the subgroup of MDD patients with significant insomnia symptoms (ISI ≥ 15), a larger treatment difference was observed between seltorexant 20 mg and placebo. For response and remission rates, both seltorexant 20 mg and 40 mg showed similar improvements, which were numerically greater than that of placebo.
The use of MCP-Mod for powering and analyses was selected because it allows the dose-response evaluation to be conducted in a smaller number of patients than with pairwise comparisons between individual seltorexant doses and placebo, before taking into account the multiplicity adjustment. The study did not achieve significance in its primary analysis of MCP Mod because the pre-specified models did not fit the actual observed dose-response relationship characterized by better outcomes with the seltorexant 20-mg dose than the 40-mg dose. Nevertheless, it is noteworthy that the sigEmax model approached significance, indicating a potential plateauing of effects at the seltorexant 20-mg and 40-mg doses; this prespecified model profile provided the closest fit to the actual results, in which 20 mg showed clearly greater efficacy than 10 mg (which showed no benefit) and a trend toward greater efficacy than 40 mg (which showed modest evidence of benefit) on the mean change in MADRS scores.
Based on MMRM analysis, as determined by the change in MADRS total score, antidepressant efficacy favored the seltorexant 20-mg dose, but not the 10-mg and 40-mg doses, even though the groups that received the 20- and 40-mg doses both showed improvement in insomnia symptoms and on the dichotomous categorical variables of response and remission. The efficacy benefits observed in this study support the findings of an earlier phase 1 study conducted in patients with MDD (Recourt et al., 2019), where the group randomized to receive seltorexant 20 mg showed greater improvement than the group randomized to receive placebo in the core symptoms of depression, as assessed by HDRS-6 and HDRS “adjusted” by removal of the sleep-related items. Similarly, in the current study, the improvement in depressive symptoms was demonstrated using both the total MADRS (which includes only 1 sleep-related item) and MADRS-6 subscale scores.
Sleep disturbance in patients with MDD is associated with a larger number of depressive episodes, more persistent illness course, and poorer clinical outcomes (Murphy and Peterson, 2015). In this study, randomization was stratified based on patients with (ISI ≥ 15) vs without significant insomnia symptoms (ISI < 15). The findings indicate that seltorexant 20 mg was more effective in patients with insomnia symptoms. In addition, analyses of MADRS-6 suggest that the greater improvement observed in the MDD subgroup with ISI ≥ 15 signifies improvement in core depression symptoms and not just improvement in insomnia symptoms.
Patients treated with the seltorexant 20-mg and 40-mg doses were more likely to manifest a clinical response, defined as ≥50% improvement in MADRS scores than patients who received placebo. Similarly, the remission rate was higher for the seltorexant 20-mg and 40-mg doses vs the placebo or seltorexant 10-mg groups. Further, improvements in patient-reported outcomes such as PHQ-9, PROMIS-SD, and PROMIS-F supported the efficacy benefits of seltorexant 20 mg. Of note, based on the PROMIS-SD, an early improvement in sleep symptoms was observed in the seltorexant 40-mg dose arm, but this study arm also showed a numerically lower improvement in depressive symptoms compared with the 20-mg dose arm.
Overall, no effect on primary and secondary efficacy measures was observed for seltorexant 10 mg compared with placebo. Potential confounding factors were that the number of patients receiving the seltorexant 10-mg dose was relatively low (n = 31) compared with the sample sizes in which other doses were tested (seltorexant 20 mg, n = 61; seltorexant 40 mg, n = 52), and the proportion of patients in the seltorexant 10-mg group with baseline ISI < 15 (85%) was higher than for the 20-mg (38%) and 40-mg (25%) groups.
From a clinical and physiological perspective, it is possible that the 20-mg dose may be the most effective dose for augmenting the antidepressant effects of SSRI/SNRI treatments in MDD even though both the 20-mg and 40-mg doses improve sleep. It is possible that a therapeutic window of seltorexant is characterized by an inverted U-shaped or curvilinear dose-response relationship, which previously has been observed in the evaluation of other antidepressant drugs, such as ketamine, esketamine, and nortriptyline (Asberg et al., 1971; Kragh-Sørensen et al., 1973; Davis et al., 1978; Fava et al., 2020). In considering the dose-response relationship for seltorexant, the potential neurobiological basis for a curvilinear relationship remains unclear but is under exploration. The differential effects of the 20- and 40-mg doses will be explored in other studies. Also, the use of effects of seltorexant with SSRIs or SNRIs will be explored in future studies.
Of note, seltorexant, a selective orexin 2 antagonist, demonstrated efficacy as adjunctive treatment for MDD while the non-selective orexin antagonist, filorexant, showed no difference in efficacy from placebo (Connor et al., 2017). This may be due to differential effects of selective orexin antagonism on stress response and REM sleep seen in animal models (Dugovic et al., 2009; Yun et al., 2017)
There were no new safety concerns observed with the use of seltorexant, and the findings were consistent with previous seltorexant studies (De Boer et al., 2018; Brooks et al., 2019; Recourt et al., 2019). Notably, the overall tolerability was favorable relative to that observed with atypical antipsychotics, which comprise the only pharmacotherapeutic agents currently approved as adjunctive treatments for MDD (Nelson and Papakostas, 2009; Wright et al., 2013). Daytime sleepiness, which is common in MDD, was not specifically measured but only captured as part of the medical history if present at the beginning of the study or as an adverse event. Although the seltorexant 20-mg group showed a slightly higher incidence of somnolence (Table 3), the events were mostly mild or moderate in intensity.
One of the main limitations of this study is the relatively small number of patients evaluated in the dose/stratification groups. In addition, at the time of IA, the number of patients with an ISI score ≥15 was nearing the sample limit as pre-specified in the protocol. Therefore, when the seltorexant 10-mg dose was introduced and the seltorexant 40-mg dose was dropped from randomization following the IA (as part of the adaptive design), the dose groups were not balanced based on ISI score. Based on ongoing preclinical evaluation in childbearing age, women of child-bearing potential were excluded from further enrolment and hence <10% of patients in this study were women of child-bearing potential. Finally, the generalizability of data is limited by the applied eligibility criteria (Zimmerman et al., 2019).
In conclusion, a clinically meaningful reduction of depressive symptoms was observed in the seltorexant 20-mg-dose arm. In the subpopulation of MDD with sleep disturbance (ISI ≥15), a larger treatment difference between seltorexant 20 mg and placebo was observed, which warrants investigation in future studies. No new safety signal was observed following seltorexant treatment over the dose range (10, 20, and 40 mg) and treatment duration tested. Seltorexant with its novel mechanism of action holds the potential to produce antidepressant effects with a favorable safety profile, as an adjunctive therapy in patients manifesting MDD with sleep disturbance and who have experienced an inadequate response to the current antidepressant therapy. Phase 3 studies are ongoing to assess the efficacy and safety of seltorexant 20 mg as adjunctive therapy to antidepressants in adult and elderly patients with MDD with insomnia symptoms who have responded inadequately to antidepressant therapy (NCT04532749, NCT04533529, and NCT04513912).
Supplementary Material
Acknowledgments
The authors thank all patients for their participation in this study and acknowledge the collaboration and commitment of the investigators and their staff. Writing assistance was provided by Ramji Narayanan, M Pharm, ISMPP CMPP (SIRO Clinpharm Pvt. Ltd.) funded by Janssen Global Services and additional editorial support for this manuscript was provided by Ellen Baum, PhD (Janssen Global Services, LLC).
The study was funded by Janssen Research & Development, LLC.
Statement of Interest
Adam Savitz, Ewa Wajs, Yun Zhang, Haiyan Xu, Mila Etropolski, and Wayne Drevets are employees of Janssen Research & Development and hold company stocks. Dr Michael Thase has been a consultant/advisor for Acadia, Inc., Akili, Inc., Alkermes PLC, Allergan, Inc., Axsome, BioHaven, Inc., Clexio Pharma, Gerson Lehrman Group, Inc., H. Lundbeck A/S, Jazz Pharmaceuticals, Johnson & Johnson (Janssen), Merck & Company, Inc., Otsuka Pharmaceutical Company, Ltd., Pfizer, Inc., Sage Pharmaceuticals, Seelos Pharmaceuticals, Sunovion Pharmaceuticals, Inc., Takeda Pharmaceutical Company, Ltd; received grant support from Acadia, Inc., Allergan, Inc., AssureRx Health, Axsome Therapeutics Inc., BioHaven, Inc., Intracellular, Inc., Johnson & Johnson, Otsuka Pharmaceutical Company, Ltd., Patient-Centered Outcomes Research Institute (PCORI), Takeda Pharmaceutical Company, Ltd; received royalties from American Psychiatric Foundation, Guilford Publications, Herald House, Kluver-Wolters, W.W. Norton & Company, Inc.
References
- Al-Harbi KS (2012) Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Prefer Adherence 6:369–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asberg M, Crönholm B, Sjöqvist F, Tuck D (1971) Relationship between plasma level and therapeutic effect of nortriptyline. Br Med J 3:331–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer M, Hefting N, Lindsten A, Josiassen MK, Hobart M (2019) A randomised, placebo-controlled 24-week study evaluating adjunctive brexpiprazole in patients with major depressive disorder. Acta Neuropsychiatr 31:27–35. [DOI] [PubMed] [Google Scholar]
- Bonaventure P, Shelton J, Yun S, Nepomuceno D, Sutton S, Aluisio L, Fraser I, Lord B, Shoblock J, Welty N, Chaplan SR, Aguilar Z, Halter R, Ndifor A, Koudriakova T, Rizzolio M, Letavic M, Carruthers NI, Lovenberg T, Dugovic C (2015) Characterization of JNJ-42847922, a selective orexin-2 receptor antagonist, as a clinical candidate for the treatment of insomnia. J Pharmacol Exp Ther 354:471–482. [DOI] [PubMed] [Google Scholar]
- Brooks S, Jacobs GE, de Boer P, Kent JM, Van Nueten L, van Amerongen G, Zuiker R, Kezic I, Luthringer R, van der Ark P, van Gerven JM, Drevets W (2019) The selective orexin-2 receptor antagonist seltorexant improves sleep: an exploratory double-blind, placebo controlled, crossover study in antidepressant-treated major depressive disorder patients with persistent insomnia. J Psychopharmacol 33:202–209. [DOI] [PubMed] [Google Scholar]
- Chen Q, de Lecea L, Hu Z, Gao D (2015) The hypocretin/orexin system: an increasingly important role in neuropsychiatry. Med Res Rev 35:152–197. [DOI] [PubMed] [Google Scholar]
- Cleare A, Pariante CM, Young AH, Anderson IM, Christmas D, Cowen PJ, Dickens C, Ferrier IN, Geddes J, Gilbody S, Haddad PM, Katona C, Lewis G, Malizia A, McAllister-Williams RH, Ramchandani P, Scott J, Taylor D, Uher R; Members of the Consensus Meeting (2015) Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2008 British Association for Psychopharmacology guidelines. J Psychopharmacol 29:459–525. [DOI] [PubMed] [Google Scholar]
- Connor KM, Ceesay P, Hutzelmann J, Snavely D, Krystal AD, Trivedi MH, Thase M, Lines C, Herring WJ, Michelson D (2017) Phase II proof-of-concept trial of the orexin receptor antagonist filorexant (MK-6096) in patients with major depressive disorder. Int J Neuropsychopharmacol 20:613–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuijpers P, Smit F (2002) Excess mortality in depression: a meta-analysis of community studies. J Affect Disord 72:227–236. [DOI] [PubMed] [Google Scholar]
- Davis JM, Erickson S, Dekirmenjian H (1978) Plasma levels of antipsychotic drugs and clinical response. In: Psychopharmacology: a generation of progress (Lipton MA, DiMascio A, Killam KF, eds), pp 906. New York, NY: Raven Press. [Google Scholar]
- De Boer P, Drevets WC, Rofael H, van der Ark P, Kent JM, Kezic I, Parapatics S, Dorffner G, van Gerven J, Beneš H, Keicher C, Jahn H, Seiden DJ, Luthringer R (2018) A randomized phase 2 study to evaluate the orexin-2 receptor antagonist seltorexant in individuals with insomnia without psychiatric comorbidity. J Psychopharmacol 32:668–677. [DOI] [PubMed] [Google Scholar]
- de Sousa RT, Zanetti MV, Brunoni AR, Machado-Vieira R (2015) Challenging treatment-resistant major depressive disorder: a roadmap for improved therapeutics. Curr Neuropharmacol 13:616–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugovic C, Shelton JE, Aluisio LE, Fraser IC, Jiang X, Sutton SW, Bonaventure P, Yun S, Li X, Lord B, Dvorak CA, Carruthers NI, Lovenberg TW (2009) Blockade of orexin-1 receptors attenuates orexin-2 receptor antagonism-induced sleep promotion in the rat. J Pharmacol Exp Ther 330:142–151. [DOI] [PubMed] [Google Scholar]
- Fava M, Freeman MP, Flynn M, Judge H, Hoeppner BB, Cusin C, Ionescu DF, Mathew SJ, Chang LC, Iosifescu DV, Murrough J, Debattista C, Schatzberg AF, Trivedi MH, Jha MK, Sanacora G, Wilkinson ST, Papakostas GI (2020) Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol Psychiatry 25:1592–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch TE, Benvenga MJ, Jesudason CD, Zink C, Vandergriff AB, Menezes MM, Schober DA, Rorick-Kehn LM (2014) LSN2424100: a novel, potent orexin-2 receptor antagonist with selectivity over orexin-1 receptors and activity in an animal model predictive of antidepressant-like efficacy. Front Neurosci 8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleurence R, Williamson R, Jing Y, Kim E, Tran QV, Pikalov AS, Thase ME (2009) A systematic review of augmentation strategies for patients with major depressive disorder. Psychopharmacol Bull 42:57–90. [PubMed] [Google Scholar]
- Gulec M, Selvi Y, Boysan M, Aydin A, Besiroglu L, Agargun MY (2011) Ongoing or re-emerging subjective insomnia symptoms after full/partial remission or recovery of major depressive disorder mainly with the selective serotonin reuptake inhibitors and risk of relapse or recurrence: a 52-week follow-up study. J Affect Disord 134:257–265. [DOI] [PubMed] [Google Scholar]
- Herring WJ, Connor KM, Snyder E, Snavely DB, Morin CM, Lines C, Michelson D (2019) Effects of suvorexant on the Insomnia Severity Index in patients with insomnia: analysis of pooled phase 3 data. Sleep Med 56:219–223. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Molosh A, Fitz SD, Truitt WA, Shekhar A (2012) Orexin, stress, and anxiety/panic states. Prog Brain Res 198:133–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kärppä M, Yardley J, Pinner K, Filippov G, Zammit G, Moline M, Perdomo C, Inoue Y, Ishikawa K, Kubota N (2020) Long-term efficacy and tolerability of lemborexant compared with placebo in adults with insomnia disorder: results from the phase 3 randomized clinical trial SUNRISE 2. Sleep 43:zsaa123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SH, Lam RW, McIntyre RS, Tourjman SV, Bhat V, Blier P, Hasnain M, Jollant F, Levitt AJ, MacQueen GM, McInerney SJ, McIntosh D, Milev RV, Müller DJ, Parikh SV, Pearson NL, Ravindran AV, Uher R; CANMAT Depression Work Group (2016) Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 3. Pharmacological treatments. Can J Psychiatry 61:540–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragh-Sørensen P, Asberg M, Eggert-Hansen C (1973) Plasma-nortriptyline levels in endogenous depression. Lancet 1:113–115. [DOI] [PubMed] [Google Scholar]
- Kupferberg A, Bicks L, Hasler G (2016) Social functioning in major depressive disorder. Neurosci Biobehav Rev 69:313–332. [DOI] [PubMed] [Google Scholar]
- Li J, Hu Z, de Lecea L (2014) The hypocretins/orexins: integrators of multiple physiological functions. Br J Pharmacol 171:332–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ (2006) Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 367:1747–1757. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Moorman DE, Smith RJ, James MH, Aston-Jones G (2014) Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nat Neurosci 17:1298–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MJ, Peterson MJ (2015) Sleep disturbances in depression. Sleep Med Clin 10:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Collaborating Centre for Mental Health (2010) National Institute f or Health and Clinical Excellence: Guidance. In: Depression: the treatment and management of depression in adults (Updated Edition), pp 1–707. Leicester, UK: The British Psychological Society & The Royal College of Psychiatrists. [Google Scholar]
- Nelson JC, Papakostas GI (2009) Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatry 166:980–991. [DOI] [PubMed] [Google Scholar]
- Nollet M, Leman S (2013) Role of orexin in the pathophysiology of depression: potential for pharmacological intervention. CNS Drugs 27:411–422. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bornkamp B, Glimm E, Bretz F (2014) Model-based dose finding under model uncertainty using general parametric models. Stat Med 33:1646–1661. [DOI] [PubMed] [Google Scholar]
- Posner K, Oquendo MA, Gould M, Stanley B, Davies M (2007) Columbia Classification Algorithm of Suicide Assessment (C-CASA): classification of suicidal events in the FDA’s pediatric suicidal risk analysis of antidepressants. Am J Psychiatry 164:1035–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recourt K, de Boer P, Zuiker R, Luthringer R, Kent J, van der Ark P, Van Hove I, van Gerven J, Jacobs G, van Nueten L, Drevets W (2019) The selective orexin-2 antagonist seltorexant (JNJ-42847922/MIN-202) shows antidepressant and sleep-promoting effects in patients with major depressive disorder. Transl Psychiatry 9:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R, Murphy P, Zammit G, Mayleben D, Kumar D, Dhadda S, Filippov G, LoPresti A, Moline M (2019) Comparison of lemborexant with placebo and zolpidem tartrate extended release for the treatment of older adults with insomnia disorder: a phase 3 randomized clinical trial. JAMA Netw Open 2:e1918254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M (2006) Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 163:1905–1917. [DOI] [PubMed] [Google Scholar]
- Thase ME (2016) Adverse effects of second-generation antipsychotics as adjuncts to antidepressants: are the risks worth the benefits? Psychiatr Clin North Am 39:477–486. [DOI] [PubMed] [Google Scholar]
- Thase ME (2017) New medications for treatment-resistant depression: a brief review of recent developments. CNS Spectr 22:39–48. [DOI] [PubMed] [Google Scholar]
- Wright BM, Eiland EH 3rd, Lorenz R (2013) Augmentation with atypical antipsychotics for depression: a review of evidence-based support from the medical literature. Pharmacotherapy 33:344–359. [DOI] [PubMed] [Google Scholar]
- Yeoh JW, Campbell EJ, James MH, Graham BA, Dayas CV (2014) Orexin antagonists for neuropsychiatric disease: progress and potential pitfalls. Front Neurosci 8:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun S, Wennerholm M, Shelton JE, Bonaventure P, Letavic MA, Shireman BT, Lovenberg TW, Dugovic C (2017) Selective inhibition of orexin-2 receptors prevents stress-induced ACTH release in mice. Front Behav Neurosci 11:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman M, Balling C, Chelminski I, Dalrymple K (2019) Have treatment studies of depression become even less generalizable? Applying the inclusion and exclusion criteria in placebo-controlled antidepressant efficacy trials published over 20 years to a clinical sample. Psychother Psychosom 88:165–170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.