Abstract
Dairy foods are a heterogeneous group of products that vary in physical state and structure; profile and amounts of essential nutrients, bioactive ingredients, and other constituents; the extent of alteration of these constituents by processing, whether they are fermented or aged; and addition of constituents during manufacture. The complexity of the dairy matrix is associated with a heterogeneous impact on health outcomes from increased, decreased, or neutral effects for specific dairy products and specific health outcomes. Researchers must become more nuanced and systematic in their study of the role of dairy products in health to develop meaningful dietary recommendations. This review of the evidence for the dairy matrix and health points out the dearth of randomized controlled trials and of mechanistic insights. The variable effects of dairy-product consumption on health suggest possibilities for personalized nutrition advice.
Keywords: appetite regulation, chronic disease, dairy matrix, milk fat globule membrane, nutrient profiling
WHAT IS THE DAIRY MATRIX?
The dairy matrix is not only the composition of nutrients, bioactive constituents, and other compounds present in milk and other dairy products but also how they are packaged and compartmentalized. It reflects the processing that the product undergoes, including changes in physical state of the product, altered endogenous constituents, and addition of inert and live chemicals or microorganisms. Dairy products include fluid milk that may have been pasteurized, homogenized, microfiltered, condensed, evaporated, or converted into powdered solids. Milk can be processed into yogurt, a semisolid gel, or cheese with a wide range of moisture content (soft to hard cheeses).
Do these processing steps and alterations in form, microstructure, and composition influence health? A group of 18 experts assembled in September 2016 to address that question. The results of that discussion were published.1 The group discussed ways in which the dairy matrix might influence digestion, nutrient absorption, appetite regulation, physiological functions, and disease risks. The purpose of this review is to update and further consider how the dairy matrix may influence health and identify research gaps. The traditional reductionist approach is reviewed to contrast the benefits of approaching health benefits through the lens of the whole dairy matrix. The ways the dairy matrix is influenced by processing is described. Then, what is known about the influence of form and matrix of dairy products on appetite and health is reviewed. Because this is a rather new research approach, gaps in our knowledge and priority research areas are identified.
TRADITIONAL REDUCTIONIST APPROACH
The traditional approach to evaluating nutritional value of foods is to assess the nutrient and bioactive composition and evaluate the contribution of a single nutrient or bioactive constituent to health. The recommended 3 servings of milk or equivalent daily, the amount recommended by the Dietary Guidelines Advisory Committee (DGAC)2 for most Americans (ie, those older than 2 years and requiring at least 1600 Kcal/d of energy) provide the entire requirement for calcium, 99% for phosphorus, > 50% of the protein, 33% of riboflavin, 42% of potassium for women, 25% of magnesium, 86% of vitamin D, and a good contribution of recommended vitamin A, zinc, iodine, and other essential nutrients. A comparison of selected dairy products for key nutrients by serving is given in Table 1. The DGAC recommended 3 servings of low-fat dairy or equivalent daily largely for calcium and potassium contributions, because it is difficult to meet recommended intakes for these minerals without dairy-product consumption. Fortified foods or supplements are required to meet calcium requirements without dairy. Americans typically do not consume sufficient fruits and vegetables to meet potassium recommendations without dairy, and fortification and supplementation with potassium salts pose challenges. Various milk, yogurt, and cheese products are all good sources of bioavailable calcium.3 Milk and yogurts are good sources of potassium, but not cheese. Milks fortified with vitamin D are a good source of that vitamin, but fortification of other dairy products with vitamin D is variable. Only the milks are a good source of iodine, and milk is the major source of this mineral in the diet.
Table 1.
Contents of selected nutrients and bioactive components in a serving of selected dairy products
| Characteristic | USDA Food Name |
|||||||
|---|---|---|---|---|---|---|---|---|
| Whole milk | Skim milk | Butter milk | Whipping cream | Yogurt, plain, low fat | Cheese, cheddar | Cheese, cottage | Butter | |
| FDC ID no. | 746,782 | 1085 | 1230 | 1053 | 1117 | 1009 | 1015 | 1145 |
| Serving size, g | 249 | 246 | 245 | 30 | 245 | 28 | 110 | 14 |
| Energy, kcal | 152 | 84 | 152 | 101 | 154 | 115 | 90 | 100 |
| Protein, g | 8.0 | 8.4 | 7.9 | 0.85 | 13 | 6.5 | 12 | 0.12 |
| Total fat, g | 8.0 | 0.2 | 8 | 10.8 | 3.8 | 9.5 | 2.5 | 11.4 |
| SFA, g | 4.6 | 0.12 | 4.7 | 6.9 | 2.5 | 5.4 | 21.4 | 7 |
| MUFA, g | 1.7 | 0.04 | 2.0 | 2.7 | 1.0 | 2.1 | 0.5 | 3.3 |
| PUFA, g | 0.27 | 0.02 | 0.49 | 0.47 | 0.1 | 0.33 | 0.08 | 0.42 |
| Carbohydrates, g | 11.3 | 12.1 | 12 | 0.85 | 17.2 | 0.68 | 5 | 0 |
| Lactose, g | 12 | 12.4 | 9 | 0 | 4 | 0.04 | 4.4 | 0 |
| Cholesterol, g | 30 | 7.4 | 27 | 34 | 15 | 28 | 13 | 30 |
| Calcium, mg | 306 | 324 | 282 | 20 | 448 | 198 | 114 | 2 |
| Phosphorus, mg | 257 | 268 | 208 | 17 | 353 | 129 | 163 | 2.7 |
| Sodium, mg | 95 | 101 | 257 | 8 | 172 | 183 | 353 | 1.4 |
| Magnesium, mg | 30 | 31 | 25 | 2 | 42 | 7.5 | 10 | 2.7 |
| Potassium, mg | 374 | 411 | 331 | 28 | 573 | 22 | 132 | 0.01 |
| Zinc, mg | 1 | 1 | 0.9 | 0.07 | 2.2 | 1 | 1.5 | 0 |
| Iodine, µg | 94 | 88 | 257 | – | – | – | – | 0 |
| Thiamin, mg | 0.14 | 0.14 | 0.12 | 0 | 011 | 0 | 0.02 | 0 |
| Riboflavin, mg | 0.34 | 0.32 | 0.42 | 0.06 | 0.52 | 0.12 | 0.26 | 0 |
| Vitamin A, RAE | 80 | 157 | 115 | 122 | 125 | 89 | 76 | 0 |
| Vitamin D, IU | 96 | 108 | 127 | 19 | 2.5 | – | – | 0 |
| Milk fat globule membrane, mg | 84 | 36 | 180 | 60 | 37 | 330 | – | <1 |
| Fermented | No | No | No | No | Yes | Yes | Yes | No/Yes |
| Physical state | Liquid | Liquid | Liquid | Liquid or G/L | Gel | Hard solid | Soft solid | O/W |
Abbreviations: FDC, FoodData Central; G, gas; ID, identification; L, liquid; MUFA, monounsaturated fatty acid; O, oil; PUFA, polyunsaturated fatty acid; RAE, retinol activity equivalent; S, solid; SFA, saturated fatty acid; USDA, US Department of Agriculture; W, water.
The type of dairy products preferred depends on the individual, ingredients in chosen recipes or foods, cost, availability, and desirable nutrient profiles. One deciding factor for many consumers is the lactose content. Consumers with perceived or diagnosed lactose intolerance or maldigestion often seek dairy products with low lactose content or avoid dairy products altogether to avoid gastrointestinal discomfort. The range of lactose content in various dairy products is broad (Table 1). Addition of lactase (lactase-phlorizin hydrolase) by the manufacturer or consumer or processing to remove lactose are approaches used to create lactose-free or reduced-lactose versions of most dairy products.4 Another constituent of dairy products that consumers often prioritize in selection of dairy product is the fat content of the diet. Whole milk contains 3 .25% milk fat (Table 1), but the fat can be removed to yield various levels of fat. The DGAC recommends low-fat or fat-free dairy products,2 partly to reduce energy intake and partly to minimize intake of saturated fatty acids for protection against cardiovascular disease. Dairy provides 13% of saturated fats, primarily as part of mixed dishes, in the American diet.2 There is controversy over whether it is the saturated fatty acid content per se that is associated with risk of cardiovascular disease or the replacement of dairy fat with polyunsaturated fat from plant sources that is responsible for the observed cardiovascular benefits in clinical trials.5,6 Nevertheless, current dietary guidelines recommend < 10% of kilocalories come from saturated fats.2 Another factor in the decision about which dairy products to consume is the addition of added sugars to flavor dairy products. Dairy products contribute 4% of added sugar in the United States. Attempts have been made to score foods for nutrient contributions. One popular nutrient profiling system, the Nutrient-Rich Food Index score rates quality of 9 nutrients to encourage (protein, fiber, vitamin A, vitamin C, vitamin E, calcium, iron, potassium, and magnesium) and 3 constituents to limit (saturated fats, sugar, and sodium), according to the DGAC. Using the Nutrient-Rich Food Index, version 6.3, scores decreased as fat level and added sugars increased (Table 2).7 To more fully account for the nutrient density of whole foods encouraged by the DGAC2 beyond the 12 components considered in the Nutrient-Rich Food Index, a hybrid scoring system was proposed that considers both nutrient density and whole foods.7 Scores are higher for dairy products using this hybrid system. For comparison, white bread scores 15 compared with whole-wheat bread, which scores 72, using the proposed hybrid nutrient density scoring system.
Table 2.
Nutritional ratings of selected dairy products by the NRF Index, version 6.3, and proposed Hybrid Nutrient Density Score7
| Product | NRF Index score | Hybrid Nutrient Density Score |
|---|---|---|
| Calcium-fortified skim milk | 73 | 112 |
| 2% Milk | 25 | 52 |
| 1% Chocolate milk | 22 | 45 |
| Whole milk, plain yogurt | 7 | 29 |
| Low-fat, fruit-flavored yogurt | 7 | 21 |
Abbreviation: NRF, Nutrient-Rich Food.
Drewnowski8 evaluated dairy products on the 4 domains of sustainable diets: energy and nutrient density, affordability, cultural and societal value, and environmental footprint. Dairy products (namely, milk, yogurt, and cheese) rated favorably in all domains: they are nutrient dense relative to the energy they contribute; they provide calcium at the lowest cost of any food; and they are appealing. The environmental impact depends on the type of dairy food. Greenhouse gas emissions for milk, yogurt, and white cheese are comparable to those of starchy tubers and grains but lower per 100 g than for hard, semihard, or soft cheeses, emissions of which are comparable to meat.9 Modeling the tradeoff between nutrients contributed and environmental impact shows the challenge in meeting nutrient needs without dairy cows if the cows were removed from the environment in attempt to reduce greenhouse gas emissions.10 Using linear programming to identify food combinations to replace dairy in the National Health and Nutrition Examination Survey 2011–2014, an unfeasible amount of alternative foods was needed to provide the essential nutrients contributed by dairy products.11 This also led to increased energy intake and cost.
The DGAC2 emphasis on food patterns and the proposed hybrid nutrient density and food-group profiling system7 are steps toward appreciating the value of the whole food beyond the traditional approach of a single or a cluster of nutrients. Yet there may be additional value in exploring the whole matrix, with its vast array of microconstituents, for its influences on health.
TRANSFORMING THE DAIRY MATRIX THROUGH PROCESSING
Dairy products are derived from fluid milk. There are undoubtedly matrix differences due to the source of milk, but this review focuses only on bovine milk.
Raw, fluid whole milk is an oil-in-water emulsion, a colloidal suspension of casein micelles, and a solution of minerals, whey proteins, and sugars dissolved in the aqueous phase. The large fat droplets are stabilized by the milk fat globule membrane (MFGM). This complex structure of proteins, polar lipids (phospholipids and sphingolipids), neutral lipids, and enzymes account for 2%–6% of the total mass of fat globules and 1%–4% of total milk proteins.12 MFGMs are produced in the epithelial cells of mammary glands. The trilayer structure consists of an outer layer of polar lipids derived from the endoplasmic reticulum, a middle protein layer, and an inner layer of polar lipids. Fat globules range in particle size, with smaller droplets having greater surface area, which may influence biological functions like immunomodulatory capacity.13 Sphingolipids and their metabolites13 have antiproliferative activity and glycosphingolipids have immune activity. Less is known about the whole MFGM and its anti-inflammatory properties. Exploiting MFGM as a byproduct of dairy manufacturing also has potential to influence health. MFGM particles can be used as a delivery system for liposomes and bioactives such as curcumin,14 epigallocatechin gallate, or β-carotene.15
Most milk is pasteurized (via low temperature for a long time; high temperature for a short time; or ultraheat treatment) to destroy pathogens and homogenized. Homogenization subdivides fat globules to stabilize the lipid phase. These processes alter the rates of protein hydrolysis and lipid release during digestion.16
Separation of the cream to make skimmed milk concentrates the milk fat globules and, for whipping cream, is then adjusted to about 40% fat and pasteurized. During churning of cream in the manufacture of butter, the aqueous phase is released as buttermilk, which contains most of the MFGM. The concentrated polar lipids in buttermilk are mostly from disrupted fragments of MFGM. The solid reverts from an oil-in-water emulsion to a water-in-oil emulsion as butter. Skim milk can be spray dried to make nonfat, dry milk powder.
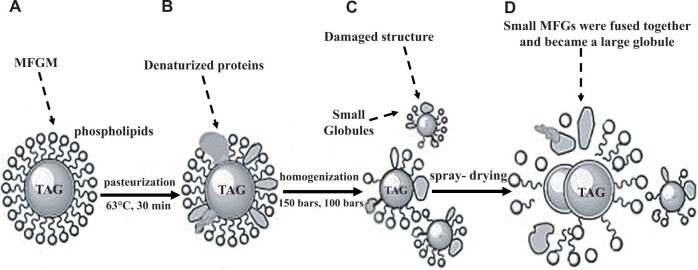
Changes in the MFGM during processing can be extensive. High-fat products, except for butter, are rich in MFGM. Yao et al17 studied the changes in MFGM through basic processing steps by confocal Raman microscopy (Figure 1). Some denaturation of proteins in the MFGM occurs during pasteurization, and the MFGM is further damaged during homogenization. During homogenization, some MFGM leaves the surface of the fat globules and is replaced by casein and whey proteins. This enables fat globules to be digested more rapidly. Even more extensive damage to MFGM occurs during spray drying. The health impacts of these changes are largely unknown. However, the composition of MFGM in dairy products is similar to that in the milk from which they are derived.18
Figure 1.
Structure changes in milk fat globules (MFGs) and milk fat globule membrane MFGM during manufacture of powdered milk. A) Globules in raw bovine milk. B) Globules in pasteurized bovine milk. Some denatured proteins in the membrane were visible. C) Globules in pasteurized and homogenized bovine milk. The MFGM structure was damaged and the MFGs were split into small globules. D) Globules in spray-dried bovine milk. Some small MFGs were fused together and they became a large globule. Reproduced with permission from Yao et al.17 Tag, triacylglycerol.
Yogurt is made by adding starter culture containing Streptococcus salivarius subsp. thermophilus and Lactobacillus delbrueckii subsp. bulgaricus to homogenized and pasteurized milk. Fermentation of the milk occurs as bacteria in the starter culture produce lactic acid from hydrolysis of lactose. If fermentation occurs in retail pots, a set yogurt is produced. If the gel is disrupted by agitation, stirred yogurt is produced. Either type results in a gel as the aqueous phase becomes trapped in a casein network as the decrease in pH from the lactic acid produced during fermentation approaches the isoelastic point of casein (pH 4.6).19
During the making of cheese, milk is acidified to solubilize colloidal calcium phosphate within the casein micelles, and intramicellar interactions loosen. If the pH reaches 5.0, casein is dissociated from the micelle; the extent of dissociation is temperature dependent.20 In the manufacture of fresh, fermented milk products such as yogurt and cottage cheese, milk is heated to denature the whey proteins, which then associate with the casein micelles. In the manufacture of some cottage cheeses and firmer cheeses, rennet (proteases that clot milk) is added to curdle the milk. The enzyme removes the negatively charged macropeptide ends from kappa casein located on the outside of casein micelles, which, in the native state, function to keep the casein micelles suspended through electrostatic repulsion. Once the negatively charged peptide fragments are moved, the micellar structure is destabilized, and a solid curd is formed. Much of the calcium is lost when whey is expressed from the casein curds. Calcium salts may be added to cheese during manufacturing. Various cheeses are fermented with different bacterial strains.
Fermented products like yogurt and cheese contain bacteria that can produce short-chain fatty acids and bioactive peptides. The impact on health may vary with the type of culture introduced in manufacturing, the available substrate in the product and co-ingested foods, and the profile of metabolites produced.
INFLUENCE OF FORM ON APPETITE
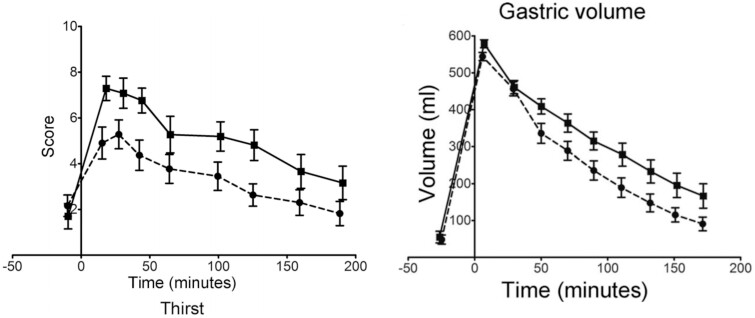
The physical state of dairy products can influence appetite and, subsequently, the amount of food consumed overall. In a study using a crossover design to compare isocaloric meals of grated cheese and yogurt with a liquid emulsion of equal mass, the semisolid meal prolonged satiety and delayed gastric emptying compared with the liquid meal (Figure 2).21 The study authors concluded that enhanced gastric retention was responsible for decreased appetite mediated by increased viscosity in the stomach and perhaps intestinal nutrient signaling, and could not be explained by plasma cholecystokinin secretion. This hormone aids in secretion of bile and enzymes that facilitate digestion. Slower digestion rates that occur from eating foods with structure result in more satiation.
Figure 2.
Comparison of isocaloric semisolid (cheese and yogurt) and lipid emulsion meals on A) visual Analog Scale (VAS) score of fullness and B) gastric volume (mean + standard error of the mean). Reprinted from Mackie et al21 with permission.
More recently, Vien et al.22 reported effects of the dairy matrix on appetite, glycemic and insulin responses, and food intake. Surprisingly, they found that the dairy-matrix effect depended on age and sex of the consumer. Men and women in the age categories 20–30 years and 60–67 years consumed test meals of whole milk, skim milk, Greek yogurt, and cheddar cheese in a randomized order, crossover design. Appetite suppression in older adults showed greater suppression by solid and semisolid products (ie, cheese and yogurt > skim milk > whole milk). Only yogurt suppressed appetite in younger adults. Furthermore, appetite suppression was greater in women than men. That sex, age, and dairy matrix interact to influence appetite and food intake is another example that precision nutrition is a more nuanced approach to promoting health and risk reduction of chronic disease.
INFLUENCE OF MFGM ON PLASMA LIPIDS
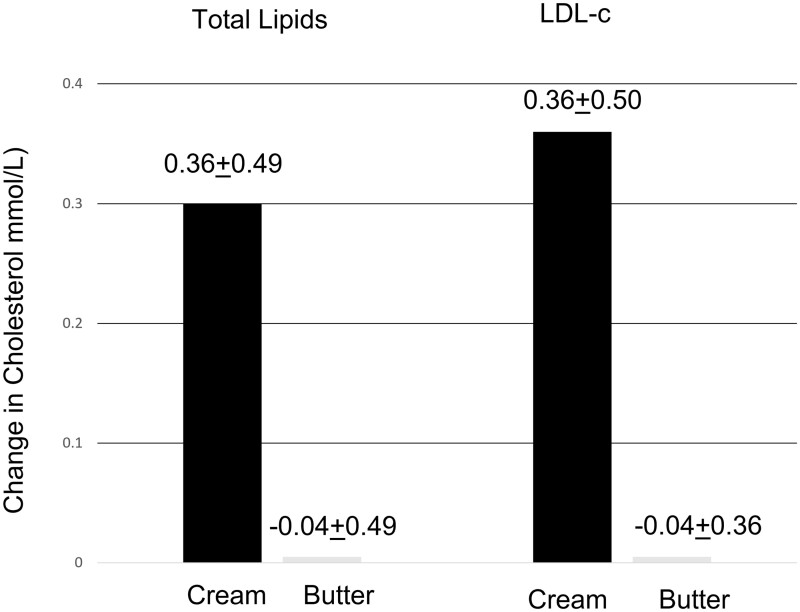
Total fat or saturated fatty acid content typically has been the criterion used to assess risk of cardiovascular disease, through impact on serum lipids. Yet a closer examination of the dairy matrix suggests that the relationship is more complex. To evaluate the role of the MFGM on serum lipids, Rosqvist et al23 compared isocaloric diets with the same total fat content of 40 g/day that differed in MFGM content for 8 weeks on plasma lipids in 45 overweight men and women, using a parallel-arm design. The high MFGM diet used whipping cream as a source of MFGM and the low MFGM diet used butter to provide the fat. Plasma total cholesterol and plasma low-density lipoprotein cholesterol, a major risk factor for cardiovascular disease, exhibited the expected increases with fat added at 40 g/day as butter (Figure 3). But when the fat was enclosed by MFGM, the lipoprotein profile was unchanged. Genes associated with lipid metabolism were altered in parallel with changes in lipid content. However, there were no differences between the 2 diets in serum triglyceride levels, which were little altered from baseline. Thus, caution should be used in consuming high-fat dairy products such as whipping cream.
Figure 3.
Comparison of whipping cream (high milk fat globule membrane [MFGM]) and butter (low MFGM) effects on serum total cholesterol (P = 0.024) and of low-density lipoprotein cholesterol (LDL-c; P = 0.024) in diet containing 40 g/d fat in 46 overweight men and women. Data from Rosqvist et al.23
The plasma cholesterol-lowering effect of MFGM-rich dairy may be due to the cholesterol absorption-lowering influence of sphingolipids in the MFGM. In a mouse study, sphingomylin supplementation of a high-fat diet decreased cholesterol absorption by 30% and liver accumulation by 40%.24 Total lipids and triglycerides were also reduced in livers of the mice by similar magnitudes. High-MFGM products such as cheese increase fecal fat excretion (lower absorption) compared with butter.1 This suggests a protection not obvious with whipping cream on serum triglycerides in the human study.23 The protection may be more for hepatic steatosis than cardiovascular disease.
DAIRY MATRIX AND HEALTH
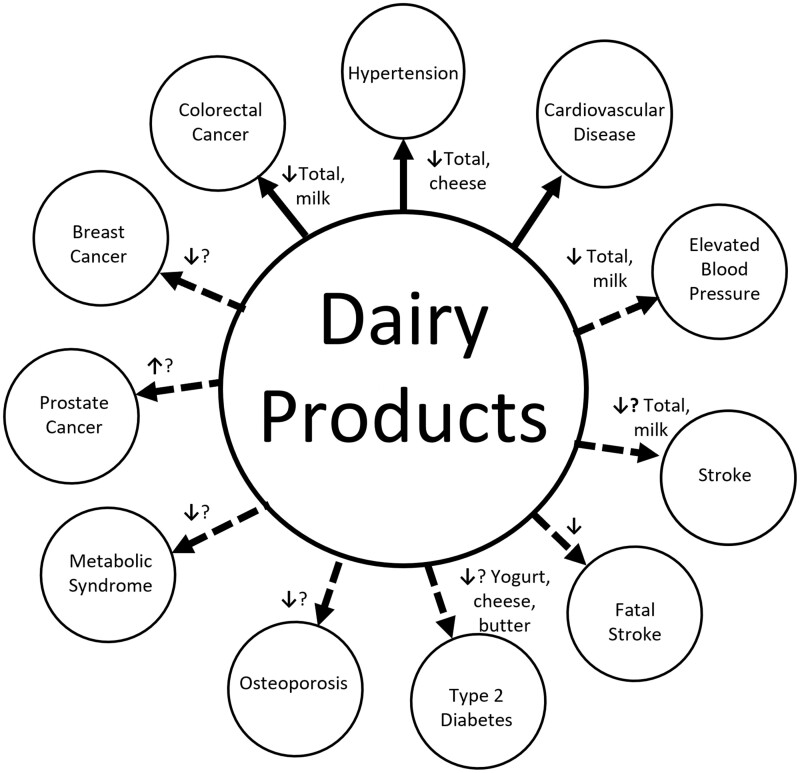
A review of meta-analyses on the association between dairy product consumption and health outcomes was undertaken.25 An illustrative summary of the strength of the reported associations is provided in Figure 4.
Figure 4.
Strength of associations between dairy product consumption and various health outcomes. Solid lines indicate the strongest associations. The direction of the small arrows indicate the relationship is either negative or positive. A question mark indicates uncertainty. Based on Godos et al.25
The strongest and most convincing evidence for a benefit of dairy consumption was a consistent negative relationship with colorectal cancer and hypertension. The authors concluded there was probable evidence of decreased risk of cardiovascular disease, elevated blood pressure, and fatal stroke for total dairy consumption. There was possible decreased risk of breast cancer, metabolic syndrome, stroke, and type 2 diabetes, and a possible increased risk of Parkinson’s disease and prostate cancer with dairy consumption.
Few studies have directly compared dairy against a control matched for the major nutrient profile or compared with various dairy products for their role on health outcomes, which could be used to evaluate matrix effects. This review focuses on studies that give insights on the effect of the dairy matrix.
Cardiovascular and other cardiometabolic diseases
Cardiovascular disease is responsible for the majority of deaths worldwide, and hypertension is the primary risk factor for cardiovascular disease. In their umbrella review, Godos et al25 found total dairy and cheese consumption was associated with a lower risk of cardiovascular disease and stroke. The relationship is not as simple as saturated fatty acid content or even total fat, because butter consumption was not significantly associated with cardiovascular disease, coronary heart disease, or stroke, despite its high content of saturated fatty acids26 and its ability to increase serum low-density lipoprotein cholesterol compared with cheese and milk.27 Nor is the association simply through reduction of elevated blood pressure and hypertension incidence, which was most strongly related to milk consumption25 and low-fat dairy products.28 The Prospective Urban Rural Epidemiology (PURE) study was a large prospective study of 136 384 individuals from 21 countries on 5 continents who were followed for 9 years. The researchers found milk and yogurt consumption were strongly inversely associated with all-cause mortality and coronary vascular disease.29
Intermediate biomarkers of cardiometabolic diseases include lipid metabolism biomarkers, insulin-like growth factor (IGF) signaling, and chronic inflammation. An important biomarker of cardiovascular disease risk is low-density lipoprotein cholesterol. A meta-analysis of intervention studies showed the benefit of cheese over butter in reducing low-density lipoprotein cholesterol.30 Cheese differs from butter in that it contains more MFGMs and more bacterial cultures are added. Both are solids. A more comprehensive comparison of dairy product ingestion on intermediate biomarkers of cardiometabolic diseases was recently undertaken with a large cohort.31 Total and individual dairy-product consumption (milk, cheese, yogurt, butter, and low-fat varieties of these) were related to twenty biomarkers in the Women’s Health Initiative from 35 352 postmenopausal women aged 50 to 79 years at 40 US centers. The percent difference between the highest and lowest quintiles of each dairy category for selected biomarkers is reported in Table 3.31 Overall, dairy-product consumption, except butter, was associated with favorable lipid profiles (eg, lower triglyceride levels and higher high-density lipoprotein cholesterol) and inflammatory biomarkers that would be associated with a lower risk of cardiometabolic disease. The relative magnitude of the association of dairy products with lower triglycerides on a per-serving basis was yogurt > butter > total dairy = full-fat dairy = total cheese = full-fat cheese > milk = low-fat dairy = low-fat cheese. The relative magnitude of the association with higher high-density lipoprotein cholesterol was yogurt = butter > total dairy = total cheese = full-fat dairy = full-fat cheese. Milk was associated with lower high-density lipoprotein cholesterol levels. The order of effects was not fully explained by content of fat, MFGM, calcium, or vitamin D; the physical state; or fermentation of the products. Nor did the order of biomarker effects parallel those related to lowering cardiovascular disease risk.25 Thus, lipid metabolism biomarkers are not the sole underlying factor explaining the association of dairy with cardiovascular disease.
Table 3.
Percent difference between the highest and lowest quintiles among 35 352 postmenopausal women in the Women’s Health Initiative31
| Biomarker | Dietary category, % difference (P value) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total dairy | Low-fat dairy | Full-fat dairy | Total cheese | Low-fat cheese | Full-fat cheese | Total milk | Total yogurt | Total butter | |
|
Triglycerides, mg/dL |
−1.9 (0.03) | 2.7 (0.94) | −4.1 (0.002) | −3.1 (0.01) | 2.8 (0.70) | −4.0 (0.01) | 1.0 (0.94) | −3.9 (0.0003) | −5.5 (0.0000) |
|
LDL-c, mg/dL |
0.8 (0.70) | −0.1 (0.65) | 1.4 (0.52) | 1.7 (0.52) | 0.3 (0.94) | 1.4 (0.54) | −1.6 (0.11) | −1.7 (0.03) | 1.9 (0.02) |
|
HDL-c, mg/dL |
1.6 (0.02) | −0.2 (0.90) | 2.2 (0.03) | 2.2 (0.02) | 0.3 (0.47) | 2.2 (0.03) | −1.4 (0.03) | 1.3 (0.03) | 3.2 (0.0000) |
|
Glucose, mg/dL |
−1.4 (0.0003) | −2.0 (0.0000) | −0.7 (0.06) | −0.6 (0.02) | −0.4 (0.29) | −0.8 (0.03) | 0.06 (0.94) | −1.4 (0.0000) | 0.2 (0.42) |
|
Insulin, μIU/mL |
−4.8 (0.0000) | −8.5 (0.000) | −0.1 (0.07) | −2.3 (0.02) | −4.5 (0.0000) | −0.4 (0.09) | 0.7 (0.94) | −7.6 (0.0000) | 3.9 (0.003) |
|
IGF-1 ng/mL |
3.1 (0.56) | 6.0 (0.15) | −2.0 (0.94) | −0.02 (0.76) | 2.5 (0.72) | −3.1 (0.84) | 5,7 (0.02) | 4.1 (0.94) | −5.9 (0.11) |
|
CRP mg/L |
−8.5 (0.0000) | −5.8 (0.002) | −3.0 (0.003) | −5.0 (0.002) | 1.1 (0.89) | −3.4 (0.003) | 4.6 (0.67) | 2.2 (0.0000) | 1.7 (0.38) |
|
IL-6 pg/mL |
−10.0 (0.0000) | −13.0 (0.0000) | −2.9 (0.03) | −5.2 (0.01) | −7.0 (0.09) | −3.0 (0.02) | 2.5 (0.99) | −6.9 (0.01) | 3.6 (0.38) |
Abbreviations: CRP,C-reactive protein; HDL-c,high-density lipoprotein cholesterol; IGF,insulin-like growth factor; IL,interleukin; LDL-c,low-density lipoprotein cholesterol.
Of the 8 biomarkers measured related to IGF signaling, the 2 most associated with dairy were glucose and insulin. The order of dairy products on a per-serving basis associated with decreased glucose and insulin levels fell in the following approximate order considering magnitude and P value: yogurt > low-fat dairy > total dairy > total cheese > full-fat cheese = full-fat dairy > total milk = low-fat cheese > butter. Butter consumption was actually associated with a significant increase in insulin level. In addition, milk consumption was associated with higher IGF-1 levels; low-fat cheese consumption was associated with higher IGF binding protein 1; and total yogurt and low-fat dairy consumption was associated with lower free IGF-1 level.
The largest associations of the 8 inflammatory markers measured with dairy products were for C-reactive protein and the cytokine interleukin-6. The decreasing order of association of specific dairy products and C-reactive protein was yogurt > total dairy > low-fat dairy > full-fat dairy = total cheese = full-fat cheese > low-fat cheese = milk = butter (the association of dairy consumption and C-reactive protein was not statistically significant). The decreasing order of association of specific dairy products and interleukin-6 was yogurt = low-fat dairy > total dairy > full-fat dairy = full-fat cheese > total cheese > low-fat cheese > milk = butter (the association of dairy consumption and C-reactive protein was not statistically significant). Thus, fermented products had the most favorable association with IGF signaling and inflammatory markers compared with butter. Fat content was not a controlling factor.
The results of the Shi et al31 study call into question the sensitivity of biomarkers for IGF signaling and chronic inflammation for predicting cardiovascular risk in response to diet, compared with blood pressure and blood lipids. In support of their findings, observational studies also reported inverse associations between fermented dairy products such as yogurt and low-fat cheese on fasting glucose and glycated hemoglobin.32 In contrast, an 8-week, crossover RCT of 3–4 servings/d of dairy (MedDairy), compared with a low-fat control diet, in 41 men and women aged ≥45 years who were at risk for cardiovascular disease showed the dairy-rich diet led to significant changes in markers of cardiovascular risk, including lower systolic and diastolic blood pressure, increased high-density lipoprotein cholesterol and lower triglyceride levels, but no changes in C-reactive protein, plasma glucose, or serum insulin.33 An RCT is a stronger design than observational studies, and this study should have been of sufficient duration to eliminate the usual design limitations.
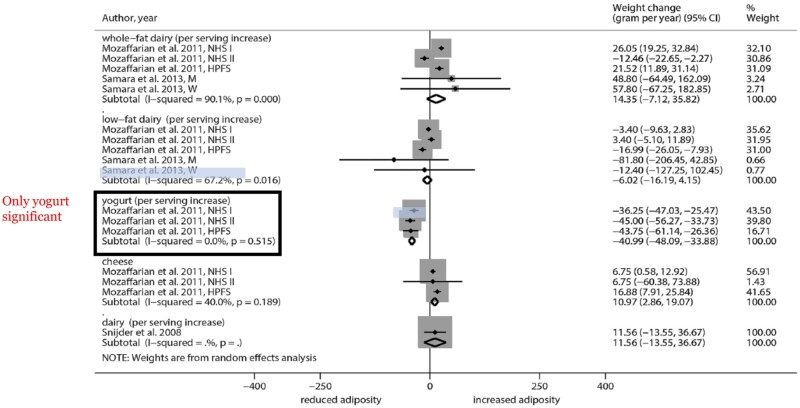
The mechanism for the benefits of fermented dairy products on circulating biomarkers is not known. The probiotic action of Bifidobacterium and Lactobacillus may be responsible through production of short-chain fatty acids, which have been favorably associated with body mass index and blood glucose levels.31 In the large European Prospective Investigation into Cancer and Nutrition (EPIC)–Norfolk study, only fermented dairy (ie, yogurt and low-fat cheese) consumption was associated with lower increased body weight and bone mineral density.34 Similarly, only yogurt consumption was associated with reduced adiposity in a meta-analysis (Figure 5).35
Figure 5.
Forest plot of associations between changes in body weight (grams per year) and dairy consumption in cohort studies of adults. Reprinted with permission from Schwingshackl et al.35
Type 2 diabetes
Type 2 diabetes is characterized by elevated glucose levels and many complications. Because it is the most preventable form of diabetes, priority should be given to lifestyle choices for prevention rather than medical treatments to treat the disease after diagnosis. Incidence of diabetes is rising at alarming rates, especially in non-Western countries. A meta-analysis of observational studies found total and low-fat dairy consumption at 200 g/day was associated with a 3%–4% lower risk of diabetes.36 Yogurt consumption had the most striking inverse association with diabetes, with up to 15% lower risk. No associations were found for cheese or milk separately. In partial support of these findings, the cardiometabolic disease risk factors in the Women’s Health Initiative cohort largely reflected the benefit of yogurt and cheese (fermented dairy), regardless of fat content, with butter.31 The large, multicountry PURE study,37 published after the meta-analysis of Soedamah-Muthu and de Goede,36 showed higher intakes of whole-fat, but not low-fat, dairy products were associated with lower prevalence of metabolic syndrome, hypertension, and diabetes.
The relationships reported between dairy consumption and diabetes are from observational studies. Soedamah-Muthu and de Goede36 cautioned that residual confounding is of concern because of the known association of milk and yogurt intake with other healthy behaviors. Thus, additional research is needed that uses causal designs (ie, RCTs), but it is difficult to have the duration needed for interventions to affect disease outcomes. Mendelian randomization studies in which lactose-persistent genes are used to genetically predict milk intake have been used to more causally relate dairy consumption and hypertension,28 but no associations were found with genetically predicted milk intake and diabetes38 or coronary heart disease.39 This approach cannot distinguish among milk, cheese, and yogurt consumption. However, it reduces confounding and reverse causation, because the randomization occurs with the assortment of alleles for the lactose-persistent gene at birth. Caution should be used in interpretation, however, in that gene variants may predict lactase persistence, but it does not mean individuals choose to consume dairy.
A 12-week RCT conducted with 72 men and women with metabolic syndrome who were randomly assigned to intake of lower dairy, low-fat dairy, or full-fat dairy including milk, yogurt, and cheese found no changes in glucose tolerance, but both dairy diets did increase insulin sensitivity.40 It is possible that the patients with metabolic syndrome were no longer sensitive to a diet intervention, and diet behaviors must be modified before metabolic syndrome develops. Little could be inferred about a matrix effect beyond fat level from this study that combined dairy products.
If the observational studies are correct that the greatest association of dairy products with reduced cardiometabolic risk factors and reduced diabetes risk is for fermented dairy products, then calcium, magnesium, vitamin D, or proteins may not be the mediating constituents. The effect of constituents in the dairy matrix requires more study. In the large Danish Diet, Cancer and Health Cohort, substitution modeling showed that substituting whole-fat yogurt for milk decreased 10-year risk of type 2 diabetes, and substituting skim milk for reduced-fat milk increased the risk, but all predicted changes were < 1%.41 In an 8-week RCT of parallel groups randomized to a high MFGM diet with whipping cream or a low MFGM diet with butter in overweight men and women, there was no support for a role of MFGM in modulating insulin sensitivity using indirect indicators.23
Dairy and cancer
The World Cancer Research Fund/American Institute for Cancer Research42 expert panel concluded there is strong evidence that consumption of dairy products helps protect against colorectal cancer, as reinforced subsequently by the umbrella review of Godos et al.25 However, the World Cancer Research Fund/American Institute for Cancer Research panel gave no recommendations for dairy products, because of the possible small increased risk for prostate cancer. Thus, there is heterogeneity in association of dairy with different types of cancer and there is likely heterogeneity due to the type of dairy product consumed, but there is inadequate evidence to draw many conclusions at this time.
Colorectal cancer is the cancer most influenced by diet, and therefore, the most preventable. Protection against cancer may be related to calcium and vitamin D in dairy products. Intracellular calcium influences cell growth and apoptosis of cells and unabsorbed calcium that reaches the lower gut can bind bile acids and fatty acids, which protects colon cells.25 Moreover, 1,25-dihydroxycholecalciferol regulates signaling pathways that influence proliferation, apoptosis, differentiation, inflammation, invasion, angiogenesis, and metastasis. Fermented dairy products can produce short-chain fatty acids, which are protective of the colon. One large study from the Nurses’ Health Study and Health Professionals Follow-Up Study found no associations of dairy and colorectal cancer-specific death, but low-fat dairy consumption was associated with lower overall mortality in contrast to high-fat dairy consumption.43
Dairy and bone
Dairy provides a package of essential nutrients needed for bone development and maintenance, including calcium, vitamin D, potassium, protein, magnesium, and phosphorus. A National Osteoporosis Foundation position paper described 19 systematic reviews of studies conducted between 2000 and 2016 on lifestyle factors that influence development of peak bone mass.44 Evidence for dairy consumption received a B grade on the basis of 3 RCTs and 1 observational study. An animal-model study demonstrated a matrix effect beyond adequate nutrients in a control diet without dairy.45 Growing female rats given a diet with nonfat dry-milk powder as the source of calcium until adulthood had stronger and bigger bones with better microarchitecture than rats fed a diet with CaCO3 as the source of calcium that met all nutrient requirements. Furthermore, rats fed nonfat dry-milk powder during growth retained an advantage in bone properties over rats fed CaCO3 after both groups were switched to low-calcium diets with CaCO3 as the source of calcium. The benefit of dairy to bone could have been due to bioactive constituents in the nonfat dry-milk powder but was not due to enhanced calcium absorption. Similarly, a short-term balance study in adolescent boys and girls showed no difference in calcium retention between dairy and CaCO3 as the source of calcium.3
Few studies have compared dairy products on bone measures. Calcium absorption from milk, cheddar cheese, processed cheese, yogurt, and a cheese analog made from milk intrinsically labeled with a calcium stable isotope was not different among the products in healthy white women.46 Thus, neither lactose content nor fermentation affected calcium bioavailability.
A few observational studies have compared various dairy products on bone outcomes. Sahni et al.47 evaluated the effect of various dairy products on 4-year changes in bone mineral density in the Framingham Study Original Cohort. Dairy had a benefit in participants using vitamin D supplements, but not in nonusers. Milk plus yogurt plus cheese were protective against bone loss at the trochanter, but not the femoral neck or spine. Milk and fluid dairy benefits to BMD were also significant at the trochanter. In a systematic review and meta-analysis of various dairy products and hip fracture, Bian et al.48 showed consumption of yogurt and cheese (fermented products), but not total dairy products and cream, was associated with lower risk of hip fracture in cohort studies. The evidence for milk was insufficient. Probiotics added to milk, as in fermented dairy products, can accelerate the healing process after fracture as well as increase bone mineral density and ameliorate bone loss.49
CONCLUSIONS
The influence of the dairy matrix on health is gaining interest. The evidence to date suggests that solid forms of dairy products have longer gut residence times and suppress appetite more than liquid forms. Products rich in MFGM reduce cholesterol absorption. The strongest evidence for protecting against disease risk is for protection against colorectal cancer by total dairy and milk consumption and for protection against cardiovascular disease by total dairy and cheese consumption. Yogurt consumption is associated with protection against cardiometabolic disease and especially type 2 diabetes. There exists little understanding of mechanisms involved. With fermented dairy products, the capacity for producing short-chain fatty acids can confer colonic health.
Combining all dairy products into 1 category is likely to give misleading associations with disease risk. Evidence from comparisons among dairy products is largely based on observational studies. Intervention studies that can assign causality can compare various dairy matrices for biomarkers of disease, such as lipid profiles, glucose metabolism, blood pressure, IGF-signaling pathways, inflammation markers, body weight and body mass index, bone mineral density, and calcium retention. However, intervention studies of sufficient duration to compare dairy products for disease outcomes are impractical. A clever alternative design using randomized Mendelian gene variants has been applied, but this approach cannot distinguish among dairy-product consumption in a more nuanced way.
The evidence from observational studies and short-term RCTs has largely only compared milk and hard cheese (and sometimes yogurt) with butter. There are so many aspects of the dairy matrix that require investigation, including physical state, bioactive constituents such as MFGM (content and alterations due to processing), fermentation, and interactions among constituents. Processing can alter the concentration of constituents, and additional constituents can be added during manufacture to produce an enormous array of products.
A systematic approach to understanding the role of the dairy matrix on health is needed. The following are some of the research questions to be addressed :
What is the effect of dairy matrix and form (physical state) on disease risk?
What is the impact of the dairy matrix on gut microbiome composition and function?
What is the influence of the dairy matrix on gastric emptying, appetite, and food consumption?
What are subgroup and regional differences on dairy matrix effects on health?
Human nutrition research must be conducted with the highest rigor to produce a strong evidence base for making dietary recommendations. Best practices for conducting human nutrition trials have been published and should be adopted.50–54 No RCTs have been conducted comparing dairy products for some outcomes such as cancer and effects on bone. Because different products have different protective effects for different diseases, a variety of products could be recommended. Alternatively, personalized nutrition recommendations could be adopted for individuals diagnosed with risk factors or who have a family history of a particular disease. The diversity of dairy-product preferences and availability must also be considered. For example, Americans eat more sweet yogurts than do Europeans and, in the United States, cheese is more often eaten on pizza. Fluid milk is rarely consumed in some regions. Fermented milk products are consumed in some regions and not others. Recommendations for specific groups by lifestyle, condition, genetic background, dietary patterns, and so forth will require more evidence than currently exists.
Acknowledgements
Author contributions. C.W. drafter of the manuscript and was responsible for revising the manuscript.
Funding. This paper was sponsored by the National Dairy Council (NDC). The NDC had no role in conception, design, performance, or approval of this paper.
Declaration of interests. C.W. has received grants, contracts, honoraria, and consulting fees from numerous food and beverage companies and other commercial and nonprofit entities with interests in mineral bioavailability and function. She is an ex officio trustee of International Life Sciences Institute, a member of the science board of the US Food and Drug Administration, and a member of the California Prune Board, the California Walnut Board, and the Promoting Better Health scientific advisory panel.
References
- 1. Thorning TK, Bertram HC, Bonjour JP, et al. Whole dairy matrix or single nutrients in assessment of health effects: current evidence and knowledge gaps. Am J Clin Nutr. 2017;105:1033–1045. [DOI] [PubMed] [Google Scholar]
- 2.Dietary Guidelines Advisory Committee. Scientific Report of the 2020 Dietary Guidelines Advisory Report to the Secretary of Agriculture and the Secretary of Health and Human Services. Washington, DC: US Department of Agriculture, Agriculture Research Services; 2020. [Google Scholar]
- 3. Nickel KP, Martin BR, Smith DL, et al. Calcium bioavailability from bovine milk and dairy products in premenopausal women using intrinsic and extrinsic labeling techniques. J Nutr. 1996;126:1406–1411. [DOI] [PubMed] [Google Scholar]
- 4. Jansson-Knodell CL, Krajicek EJ, Savaiano DA, et al. Lactose intolerance: a concise review to skim the surface. Mayo Clin Proc. 2020;95:1499–1505. [DOI] [PubMed] [Google Scholar]
- 5. Yu E, Hu FB.. Dairy products, dairy fatty acids, and the prevention of cardiometabolic disease: a review of recent evidence. Curr Atheroscler Rep 2018;20:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Astrup A, Magkos F, Bier DM, et al. Saturated fats and health: a reassessment and proposal for food-based recommendations. J Am Coll Cardiol. 2020;76:844–857. [DOI] [PubMed] [Google Scholar]
- 7. Drewnowski A, Dwyer J, King JC, et al. A proposed nutrient density score that includes food groups and nutrients to better align with dietary guidance. Nutr Rev. 2019;77:404–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Drewnowski A. Measures and metrics of sustainable diets with a focus on milk, yogurt, and dairy products. Nutr Rev. 2018;76:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Drewnowski A, Rehm CD, Martin A, et al. Energy and nutrient density of foods in relation to their carbon footprint. Am J Clin Nutr. 2015;101:184–191. [DOI] [PubMed] [Google Scholar]
- 10. Liebe DL, Hall MB, White RR.. Contributions of dairy products to environmental impacts and nutritional supplies from United States Agriculture. J Dairy Sci. 2020;103:10867–10881. [DOI] [PubMed] [Google Scholar]
- 11. Cifelli C, Auestad N, Fulgoni VL.. Replacing the nutrients in dairy foods with non-dairy foods will increase the cost, calories, and require large amounts of foods. In: NHANES 2011-2014. Public Health Nutrition; 2020:1–12. doi:10.1017/S1368980020001937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vanderghem C, Bodson P, Danthine S, et al. Milkfat globule membrane and buttermilks: from composition to valorization. Biotechnol Agron Soc Environ 2010;14:485–500. [Google Scholar]
- 13. Arranz E, Corredig M.. Invited review: milk phospholipid vesicles, their colloidal properties, and potential and delivery vehicles for bioactive molecules. J Dairy Sci. 2017;100:4213–4222. [DOI] [PubMed] [Google Scholar]
- 14. Jin H-H, Lu Q, Jiang J-G.. Curcumin liposomes prepared with milk fat globule membrane phospholipids and soybean lecithin. J Dairy Sci. 2016;99:1780–1790. [DOI] [PubMed] [Google Scholar]
- 15. Li Y, Arranz E, Guri A, et al. Mucus interactions with liposomes encapusulating bioactives: interfacial tensiometry and cellular uptake on Caco-2 and cocultures of Caco-2/HT29-MTX. Food Res Int. 2017;92:128–137. [DOI] [PubMed] [Google Scholar]
- 16. Ye A, Cui J, Dalgleish D, et al. Effect of homogenization and heat treatment on the behavior of protein and fat globules during gastric digestion of milk. J Dairy Sci. 2017;100:36–47. [DOI] [PubMed] [Google Scholar]
- 17. Yao Y, Zhao G, Yan Y, et al. Milk fat globules by confocal Raman microscopy: differences in human, bovine and caprine milk. Food Res Int. 2016;80:61–69. [Google Scholar]
- 18. Fontecha J, Brink L, Wu S, et al. Sources, production, and clinical treatments of milk fat globule membrane for infant nutrition and well being. Nutrients. 2020;12:1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee WJ, Lucey JA.. Formation and physical properties of yogurt. Asian Australas J Anim Sci. 2010;23:1127–1136. [Google Scholar]
- 20. Singh H, Roberts MS, Munro PA, et al. Acid-induced dissociation of casein; micelles in milk: effects of heat treatment. J Dairy Sci. 1996;79:1340–1346. [Google Scholar]
- 21. Mackie AR, Rafiee H, Malcolm P, et al. Specific food structures suppress appetite through reduce gastric emptying rate. Am J Physiol Gastrointest Liver Physiol. 2013;304:G1038–G1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vien S, Fard S, El Khoury D, et al. Age and sex interact to determine the effects of commonly consumed dairy products on post-meal glycemia, satiety, and later meal food intakes in adults. 2021;151:2161–2174. [DOI] [PubMed] [Google Scholar]
- 23. Rosqvist F, Smedman A, Lindmark-Månsson H, et al. Potential role of milk fat globule membrane in modulating plasma lipoproteins, gene expression, and cholesterol metabolism in humans: a randomized study. Am J Clin Nutr. 2015;102:20–30. [DOI] [PubMed] [Google Scholar]
- 24. Chung RWS, Kamili A, Tandy S, et al. Dietary sphingomyelin lowers hepatic lipid levels and inhibits intestinal cholesterol absorption in high-fat-fed mice. PLoS One. 2013;8:e55949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Godos J, Tieri M, Ghelfi F, et al. Dairy foods and health: an umbrella review of observational studies. Int J Food Sci Nutr. 2020;71:138–151. [DOI] [PubMed] [Google Scholar]
- 26. Pimpin L, Wu JHY, Haskelberg H, et al. Is butter back? A systematic review and meta-analysis of butter consumption and risk of cardiovascular disease, diabetes, and total mortality. PLoS One. 2016;11:e0158118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tholstrup T, Hoy CE, Andersen LN, et al. Does fat in milk, butter and cheese affect blood lipids and cholesterol differently? J Am Coll Nutr. 2004;23:169–176. [DOI] [PubMed] [Google Scholar]
- 28. Soedamah-Muthu SS, Verberne LD, Ding EL, et al. Dairy consumption and incidence of hypertension: a dose-response meta-analysis of prospective cohort studies. Hypertension. 2012;60:1131–1137. [DOI] [PubMed] [Google Scholar]
- 29. Dehghan M, Mente A, Rangarajan S, et al. Association of dairy intake with cardiovascular and mortality in 21 countries from five continents (PURE): a prospective cohort study. Lancet 2018;10161:P2288–2297. [DOI] [PubMed] [Google Scholar]
- 30. de Goede J, Geleijnse JM, Ding E, et al. Effect of cheese consumption on blood lipids: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. 2015;73:259–275. [DOI] [PubMed] [Google Scholar]
- 31. Shi N, Olivo-Marston S, Jin Q, et al. Associations of dairy intake with circulating biomarkers of inflammation, insulin response, and dyslipidemia among postmenopausal women. J Acad Nutr Diet 2021;S2212–S2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Drehmer M, Pereira MA, Schmidt MI, et al. Associations of dairy intake with glycemia and insulinemia, independent of obesity, in Brazilian adults: the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Am J Clin Nutr. 2015;101:775–782. [DOI] [PubMed] [Google Scholar]
- 33. Wade A, Davis CR, Dyer KA, et al. A Mediterranean diet supplemented with dairy foods improves markers of cardiovascular risk. Results from the MedDairy randomized controlled trial. Am J Clin Nutr. 2018;108:1166–1182. [DOI] [PubMed] [Google Scholar]
- 34. Trichia E, Luben R, Khaw K-T, et al. The associations of longitudinal changes in consumption of total and types of dairy products and markers of metabolic risk and adiposity: findings from the European Investigation into Cancer and Nutrition (EPIC)-Norfolk study, United Kingdom. Am J Clin Nutr. 2020;111:1018–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schwingshackl L, Hoffman G, Schwedhelm C, et al. Consumption of dairy products in relation to changes in anthropometric variables in adult populations: a systematic review and meta-analysis of cohort studies. PLoS One. 2016;11:e0157461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Soedamah-Muthu SS, de Goede J.. Dairy consumption and cardiometabolic diseases: systematic review and updated meta-analysis of prospective cohort studies. Curr Nutr Rep. 2018;7:171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bhavadkarini B, Dehghan M, Mente A, et al. Association of dairy consumption with metabolic syndrome, hypertension and diabetes in 147,812 individuals from 21 countries. BMJ Open Diab Res Care 2020;8:300026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bergholdt HK, Nordestgaard BG, Ellervik C.. Milk intake is not associated with low risk of diabetes or overweight-obesity: a Mendelian randomization study in 97,811 Danish individuals. Am J Clin Nutr. 2015;102:487–496. [DOI] [PubMed] [Google Scholar]
- 39. Yang Q, Lin SL, Au Yeung SL, et al. Genetically predicted milk consumption and bone health, ischemic heart disease, and type 2 diabetes: a Mendelian randomization study. Eur J Clin Nutr. 2017;71:1008–1012. [DOI] [PubMed] [Google Scholar]
- 40. Schmidt KA, Cromer G, Burhans MS, et al. The impacts of diets rich in low-fat or full-fat dairy on glucose tolerance and its determinants: a randomized controlled trial. Am J Clin Nutr. 2021;113:534–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ibsen DB, Overvad K, Laursen ASD, et al. Changes in intake of dairy product subgroups and risk of type 2 diabetes: modeling specified food substitutions in the Danish Diet, Cancer and Health Cohort. Eur J Nutr. 2021;60:3449–3459. [DOI] [PubMed] [Google Scholar]
- 42.World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective. Continuous Update Project Expert Report 2018. World Cancer Research Fund International; 2019. Available at: https://www.wcrf.org/wp-content/uploads/2021/02/Summary-of-Third-Expert-Report-2018.pdf. Accessed April 25, 2021.
- 43. Liu X, Yang W, Wu K, et al. Postdiagnostic dairy products intake and colorectal cancer survival in U.S. males and females. Am J Clin Nutr. 2021;113:1636–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weaver CM, Gordon CM, Janz KF, et al. The National Osteoporosis Foundation's position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporos Int. 2016;27:1281–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Weaver CM, Janle E, Martin B, et al. Dairy versus calcium carbonate in promoting peak bone mass and bone maintenance during subsequent calcium deficiency. J Bone Miner Res. 2009;24:1411–1419. [DOI] [PubMed] [Google Scholar]
- 46. Weaver CM, Campbell WW, Teegarden D, et al. Calcium, dairy products, and energy balance in overweight adolescents: a controlled trial. Am J Clin Nutr. 2011;94:1163–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sahni S, Mangano KM, Kiel DP, et al. Dairy intake is protective against bone loss in older vitamin D supplement users: the Framingham Study. J Nutr. 2017;147:645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bian S, Hu J, Zhang K, et al. Dairy product consumption and risk of hip fracture: a systematic review and meta-analysis. BMC Public Health. 2018;18:165–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lei M, Hua L-M, Wang D-W.. The effect of probiotic treatment on elderly patients with distal radius fracture: a prospective double-blind, placebo-controlled randomized clinical trial. Benef Microbes. 2016;7:631–637. [DOI] [PubMed] [Google Scholar]
- 50. Weaver CM, Lichtenstein AH, Kris-Etherton PM.. Perspective: U.S. documentation and regulation of human nutrition randomized controlled trials. Adv Nutr 2020;1:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lichtenstein AH, Petersen K, Barger K, et al. Perspective: design and conduct of human nutrition randomized controlled trials. Adv Nutr. 2021;12:4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Weaver CM, Fukagawa NK, Liska D, et al. Perspective: US documentation and regulation of human nutrition randomized controlled trials. Adv Nutr. 2021;12:21–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Maki KC, Miller JW, McCabe GP, et al. Perspective: laboratory considerations and clinical data management for human nutrition randomized controlled trials: guidance for ensuring quality and integrity. Adv Nutr. 2021;12:46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Petersen KS, Kris-Etherton PM, McCabe GP, et al. Perspective: planning and conducting statistical analyses for human nutrition randomized, controlled trials: ensuring data quality and integrity. Adv Nutr 2021. doi: 10.1093/advances/nmab045. [DOI] [PMC free article] [PubMed] [Google Scholar]