Abstract
The significance of dairy in human health and nutrition is gaining significant momentum as consumers continue to desire wholesome, nutritious foods to fulfill their health and wellness needs. Bovine milk not only consists of all the essential nutrients required for growth and development, it also provides a broad range of bioactive components that play an important role in managing human homeostasis and immune function. In recent years, milk bioactives, including α-lactalbumin, lactoferrin, glycomacropeptide, milk fat globule membrane, and milk oligosaccharides, have been intensively studied because of their unique bioactivity and functionality. Challenges for the application of these bioactive components in food and pharmaceutical formulations are associated with their isolation and purification on an industrial scale and also with their physical and chemical instability during processing, storage, and digestion. These challenges can be overcome by advanced separation techniques and sophisticated nano- or micro-encapsulation technologies. Current knowledge about the chemistry, separation, and encapsulation technology of major bioactives derived from bovine milk and their application in the food industry is reviewed here.
Keywords: bioactive components, bovine milk, encapsulation, α-lactalbumin, lactoferrin, glycomacropeptide, milk fat globule membrane, milk oligosaccharides, separation
INTRODUCTION
The significance of dairy products in human health and nutrition has been extensively studied and is gaining significant momentum as consumers continue to desire wholesome, nutritious foods to fulfill their health and wellness needs. Moreover, as the COVID-19 pandemic raged across the globe, there has been a renewed focus on foods that promote human nutrition and well-being. To that effect, bovine milk (BM) plays an important role as a wholesome food that has well-documented nutritional and health benefits throughout a person’s life span. The health and nutrition benefits of consuming BM include promoting growth, boosting the immune function, reducing blood pressure, preventing gastrointestinal infections, and improving physical performance.1 A recent review explored the role of BM in regulating human homeostasis during the COVID-19 pandemic.2 According to the database from Centers for Disease Control and Prevention, mortality rates of infant s and young patients was lower than those of adults during the COVID-19 outbreak.3 Milk, in the form of infant formula and as the main food of infants, plays a role in managing human homeostasis as it relates to immune function, particularly fighting disease, because milk not only provides essential nutrients but is also an important source of natural bioactive components. Milk bioactives are specific constituents found in small quantities in milk. They provide health benefits beyond basic nutritional values.4 Milk bioactives come from milk proteins, lipids, and carbohydrates, such as bioactive peptides, α-lactalbumin (α-La), immunoglobulins, lactoferrin (LF), growth factors, glycomacropeptide (GMP), milk fat globule membrane (MFGM), and milk oligosaccharides (OSs).5
To date, significant advances have been made in the science, technology, and applications of these BM bioactives. Separation technologies to fractionate dairy ingredients such as casein and whey were adopted by the dairy industry in the early 1970s, and were further extended to separate milk bioactives in the industry.6 Chromatographic and membrane separation techniques have been adopted by industry to isolate some of those milk bioactives from milk or whey on a large scale. Challenges for the application of these bioactive components in foods and pharmaceuticals are associated with their physical and chemical instability during processing, storage, and digestion. These challenges can be overcome by encapsulation technology, which encloses the bioactive compound in matrix materials using an appropriate formulation and structural design.
In this article, we review the current knowledge about the chemistry, separation and purification, and encapsulation technologies of major bioactive components derived from BM and their application in the food industry. Emphasis has been given to α-La, LF, GMP, MFGM, and milk OSs, which have been the subjects of concentrated research in recent decades because of their unique bioactivity and functionality.
COMPOSITION AND BIOACTIVE COMPONENTS OF MILK
Milk is a major source of essential nutrients required for growth and development. In this review, unless specified, only BM is discussed. The major components of milk are lactose, lipid, protein, minerals, and water. The composition of milk differs between species, individuals, the stage of lactation, and other external factors. Table 1 shows the average composition of major compounds in bovine colostrum and milk.
Table 1.
Major composition and bioactive components in bovine colostrum and bovine milk5
| Concentration (g/L) |
Molecular weight (Da) | ||
|---|---|---|---|
| Component | Colostrum | Milk | |
| Lipids | 40–60 | 35–42 | |
| Protein | 250 | 33 | |
| Caseins | 26 | 28 | 19 000–25 000 |
| Whey | 30–200 | 5 | |
| β-Lactoglobulin | 8.0 | 3.3 | 184 000 |
| α-Lactalbumin | 3.0 | 1.2 | 142 000 |
| Immunoglobulins | 20–150 | 0.5–1.0 | 15 000 |
| Lactoferrin | 1.5 | 0.1 | 80 000 |
| Growth factors | 50 μg to –40 mg/L | <1 μg to 2 mg/L | 6400–30 000 |
| Lactose | 30–40 | 46 | |
| Oligosaccharides | 0.7–1.2 | 0.1–0.2 | |
| Minerals | 7–10 | 7 | |
Milk contains approximately 3%–6% lipids (30–60 g/L). The majority of lipids in milk are fat globules surrounded by a membrane of polar lipids and proteins—the MFGM. Triglycerides are the main molecule within the core of the fat globule, comprising 96%–98% of the total weights.5 Other milk fats include free fatty acids, cholesterol, diglycerides, monoglycerides, and phospholipids. The major carbohydrate in milk is lactose (specifically β-d-galactopyranosyl-(1,4)-d-glucopyranose),8 which provides energy for infant mammals and is responsible for the characteristically sweet flavor of milk. Milk contains 30–36 g/L of total protein. Milk proteins are a high-quality source of dietary proteins for humans. Two major classes of protein are present in milk: caseins and whey protein. Caseins in milk compose ∼ 80% of milk protein. Caseins can further be divided into α, β, and κ fractions, which have various structures and hydrophobic and hydrophilic properties. The caseins in milk exist in a unique, highly hydrated, spherical aggregated complex known as the casein micelle. Casein micelles range from 30 to 600 nm in diameter.8 In a majority of cheeses, casein micelles associate with each other, a phenomenon driven by the action of rennet on κ-casein. Whey proteins are fractionated into the whey during cheese manufacturing. Industrial caseins are produced by the isoelectric precipitation at the isoelectric point (pI; ie, the pH at which proteins have a zero net charge and minimum solubility) of caseins (∼4.6).8 Whey proteins, representing 20% of total milk protein, exist in the nonmicellar, aqueous phase of milk. Whey fractions mainly include β-lactoglobulin (β-Lg), α-La, LF, immunoglobulins, bovine serum albumin, and various enzymes. Whey proteins have a more ordered spherical structure than do caseins. Whey proteins have disulfide linkages and denature on the application of heat. In addition to macronutrients, milk also contains citrate, phosphate, and chloride salts of H+, K+, Na+, Mg2+, and Ca2+. Calcium exists in milk in both soluble and colloidal forms. In the colloidal form, it is mainly associated with the caseins.
Milk not only contains several bioactive components in its original form but also generates functional peptides during protein digestion and/or fractionation.9Table 1 lists the major bioactive components in BM and colostrum, their concentrations, and molecular weight (MW). Many of the bioactive components, such as α-La, immunoglobulins, and LF are present in higher concentrations in the colostrum than in the mature BM, suggesting a significant contribution to newborn nutrition. BM proteins are the predominant source of bioactives in milk and have been well investigated. BM fat and carbohydrates also assist in carrying out important physiological functions.
α-Lactalbumin
Mature BM contains α-La at a concentration of 1.0–1.5 g/L, representing ∼ 3.5% of total protein and ∼ 17% of the whey proteins.9 α-La is the predominant whey protein in human milk, composing ∼ 22% of total protein and ∼ 36% of the whey proteins.9 α-La is rich in essential amino acids, in particular, tryptophan and cysteine. α-La participates in catalyzing the final step of lactose biosynthesis. Peptides hydrolyzed from α-La have several biological functions, including immune modulation and antimicrobial, antiviral, antihypertensive, opioid, and antioxidative activities.9
α-La is a single-chain, calcium-binding globular protein and consists of 123 amino acids, 4 disulfide bonds, and no free thiols.10 It is relatively small, with a MW of approximately 14 kDa. The pI of α-La is between pH 4.2 and 4.6. α-La has a high water solubility and can be dissolved in chloride salt solutions. The presence or absence of calcium could influence its melting temperature and thus influence its thermal stability during processing.11 Calcium depletion induces structural changes of α-La to form a so-called molten globule state,10 which influences the bioactivity and purification processes of the protein. α-La demonstrates a unique property to interact with hydrophobic compounds such as peptides, lipids, and fatty acids.12 These properties influence the purification processes of α-La and influence the functional properties of foods in which α-La is used as an ingreident.
Lactoferrin
LF is a multifunctional protein in the transferrin family. It can be found in many biological fluids such as milk, saliva, and seminal fluid.13 Human colostrum contains the highest amount of LF (5 g/L) and mature human milk contains 2–3 g/L of LF, whereas bovine colostrum contains approximately 0.8–1.5 g/L LF and mature BM contains only 0.1–0.5 g/L.14 Biological functions of LF include binding and transporting iron, promoting iron absorption in the human body, promoting cell growth, and detoxifying free radicals. LF also presents antibacterial, anti-inflammatory, and anticarcinogenic properties.13,15,16
LF is a single polypeptide chain, globular glycoprotein with 17 disulfide bonds.13 LF was reported to contain 691 amino acids in human milk and 696 amino acids in BM.14 LF has a relatively large MW (∼ 80 kDa).13 It has 2 homologous lobes (N- and C-lobes), each composed of 2 domains. A short, 3-turn helix links the 2 lobes and regulates iron binding and release by opening and closing these domains.14 Depending on the iron saturation, LF could be classified as apo-LF (< 15% iron), natural LF (15% to ∼20% iron), and holo-LF (> 20% iron). A remarkable structural feature of LF is that it has a positively charged surface, which facilitates binding with various anionic biopolymers. The pI of LF is within the 8.0–9.0 pH range. Therefore, LF has a positive charge at pH < 8.0, which is the pH range of almost all food products. It is soluble in water at nearly every pH range except for its pI. The binding affinity between iron and LF depends on pH, because of changes in its tertiary structure. It is reported that LF starts to release iron at pH 5.0–6.5; at pH 2.0, > 90% of iron is released.17 The iron saturation and environmental pH also affect the thermal stability of LF, which we discuss later in the article.
Glycomacropeptide
GMP, also called caseinomacropeptide, is a C-terminal glycopeptide (f 106–169) from κ-casein.8 GMP partitions into the whey during the cheese production process. GMP is the third richest whey protein in cheese whey, after β-Lg and α-La, constituting 12%–25% of the total whey protein.18 In recent decades, interest in studying the biological activities of GMP has grown. Authors of a recent review documented the positive impacts of GMP on human health, including antibacterial, prebiotic, remineralizing, antitumor, and immune-modulatory activities, and modulation of the digestion process and metabolism.19
GMP has a MW of ∼ 8 kDa, thus making it a rather small molecule. GMP is abundant in branched-chain amino acids, but it does not contain aromatic amino acids (ie, phenylalanine [Phe], tryptophan, and tyrosine) or cysteine.20 Carbohydrates make up 50%–60% of GMP and include galactose, N-acetyl-galactosamine, and N-neuraminic acid. The pI of GMP is approximately 4–5. GMP is reported to be water soluble and thermally stable.21 This peptide is hydrophilic and negatively charged even at low pH values. GMP also demonstrates good emulsifying capacity and gelation properties; thus, it can be applied as an innovative functional food ingredient.21
Milk fat globule membrane
BM lipids are mainly present as globules varying from 0.1 to 15 μm in diameter, surrounded by a thin film (10–20 nm) that is the MFGM.22 Although BM contains only 2 g/L MFGM, this component helps ensure the fat globule is stable in the milk aqueous phase and protects the fat globule from being degraded by enzymes. The MFGM has highly complex structures. It consists of ∼ 60% proteins, mainly glycosylated, which account for the 1%–2% protein in total milk.22 More than 500 proteins have been identified in MFGM, including integral, peripheral, and loosely attached proteins, such as enzymes and immunoglobulins.23 MFGM contains 40% lipids, mainly polar lipids (PLs), such as phospholipids and sphingolipids. Neutral lipids are also found, such as triglycerides, diglycerides, monoglycerides, and cholesterol.5 The protein and lipid compositions of MFGM vary depending on the specific isolation, processing, and analysis techniques and the cow’s breed.
The protein fractions of MFGM have demonstrated important biological properties for human health. These proteins have shown potential as anticancer, antibacterial, and antiadhesive agents, and have a role in improving immune function and preventing coronary heart disease.24 The bioactivity of the lipid fractions (mainly PLs) in MFGM has also been well documented, including preventing cholesterol-induced steatosis, preventing pathogenic infection, and maintaining gut function.25
Milk oligosaccharides
Besides lactose, oligosaccharides (OSs) are also important carbohydrates in milk. Human milk contains a high amount of OSs in human colostrum (25 g/L) and mature human milk (14 g/L). Bovine milk has an OS concentration of 0.7–1.2 g/L in bovine colostrum and 100 mg/L in mature BM.7 The milk OS structure contains either lactose or N-acetyl-lactosamine at their reducing end and have monosaccharide residues with the nonreducing galactose.26 Recently, BM was reported to possess BM oligosaccharides (BMOs) that are structurally similar to human milk oligosaccharides (HMOs), which contain branched OSs with N-acetylneuraminic acid and fucose.27 This structural similarity implies that BMO may also show biological functions similar to those of HMO. Because of their abundance in human milk, research on OS was mainly concentrated on the HMO and its proven biological benefits. Recently, this research has extended to investigate the similar structures and bioactivities of BMO. Although BM is low in BMO concentration, the enormous production of whey permeate (a byproduct of whey protein isolate manufacture) in the food industry provides a potential source for extracting large quantities of BMOs, which are relatively underused so far.28
SEPARATION AND PURIFICATION TECHNOLOGIES OF MILK BIOACTIVES
Isolation and purification of bioactive, health-enhancing BM proteins in recent years have emerged as a new valuable sector for the dairy industry. Particularly, whey, as a byproduct in the production of cheese, contains numerous bioactive compounds, as noted in previous sections of this review. Considering the large amount of production of cheese worldwide, millions of tons of whey is produced globally.29 However, so far, only approximately half the whey generated is further used,29 which indicates that better solutions are still needed to separate and use the whey to produce more value-added products. Numerous separation technologies are available, such as membrane separation, centrifugation, chromatography, and solvent extraction.
Here, we will introduce those mainly used in the dairy industry. Their principles and applications are briefly introduced, and the different separation approaches that are applied to producing specific bioactive compounds are extensively reviewed.
Overview of separations techniques in the dairy industry
Membrane separation processes.
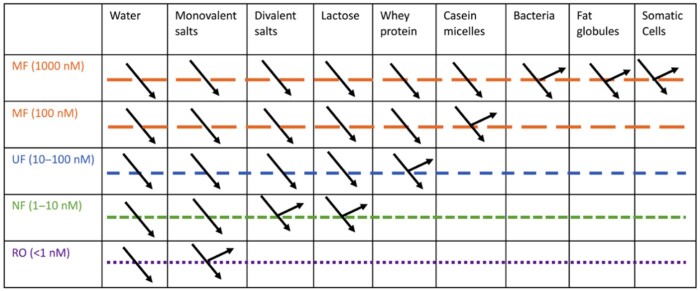
Membrane separation processes (MSPs) refer to a fractionation process that can separate constituents through a membrane that serves as a semipermeable barrier and selectively restricts or allows the passage of certain molecules.29 Compared with conventional concentration and fractionation techniques such as evaporation and distillation, MSPs consume less energy, are easy to integrate with other separation processes, and are easily scalable. MSPs also are relatively clean processes because they use milder processing conditions (namely, temperature and shear) and are free of additives and solvents. As illustrated in Figure 1, there are 4 types of MSPs, based on their MW cutoff and differential pressure applied as driving forces in the filtration process: 1) microfiltration (MF), (2) ultrafiltration (UF) (3) nanofiltration (NF), and (4) reverse osmosis (RO), in order of ascending pressure.30 Generally, the higher the pressure applied, the smaller the particles that can be retained by the membrane.
Figure 1.
Scheme of the main membrane processed applied in the separation of milk components.30 MF, microfiltration; NF, nanofiltration; RO, reverse osmosis; UF, ultrafiltration. (Reproduced with permission from Elsevier Ltd.)
MF is mainly applied in the dairy industry for removing bacteria and fat and is also used as a pretreatment before the UF process.29 The pore size of MF is 0.2–2 μm; MW cutoff is > 200 kDa, and the applied pressure is < 200 kPa.31 MF can be applied to the removal of microorganisms in skim milk to reduce the microbial load of incoming milk. MF is an effective way to bleach whey, by removing the norbixin (used as a coloring agent in cheese), and can be an alternative method to chemical agents such as hydrogen peroxide and benzoyl peroxide.32 Recently, MF has been used to separate caseins and whey proteins from skim milk to produce specialized dairy protein fractions. A recent review provided a detailed introduction to the MF of casein and whey proteins in milk.30 To fractionate micellar casein (50–500 nm) and whey protein (3–6 nm), the pore size of MF membranes used is approximately 100–140 nm for ceramic membranes and 100–500 nm for spiral-wound membranes.33,34 Heating skim milk may influence the fractionation of caseins and whey proteins using MF. Heat-induced aggregation of whey proteins is the likely cause.30
UF has been commonly applied for the removal of lactose, minerals, and water and to retain macromolecules and colloids, such as whey protein.35 The pore size of UF is ∼0.01 μm, the MW cutoff is 1–200 kDa, and the applied pressure is < 1000 kPa.31 Diafiltration (DF) is commonly used in combination with the UF process. It is a mode of operation that involves the addition of a solvent such as water to promote washes in the concentrated fractions.29 The combination of UF and DF could improve the process performance and also improve the purification of the concentrates. Studies have shown that combining UF and DF enabled removal of ash (minerals) from protein concentrates and produced greater protein purification.36
NF has a membrane sizing from 1 to10 nm, which rejects almost all milk components except for water and monovalent salts. The MW cutoff of NF is 300–100 Da and the applied pressure is 1.5–3.0 MPa.31 NF separates ions on the basis of diffusion and charge. Reverse osmosis has a membrane with the smallest size (< 1 nm) and MW cutoff (∼100Da) under pressure (3.0–3.5 MPa) in which only water can pass through; therefore, reverse osmosis is regarded a concentration technique.31
Chromatographic separation.
Gel filtration is also known as size-exclusion chromatography. The stationary phase of gel filtration is made of hydrated, sponge-like materials with pores with molecular dimensions and sizes equivalent to molecular sieves.37Figure 2 demonstrates the principle and process of gel filtration in separating molecules of different sizes. When an aqueous feed solution passes through the gel filtration column, molecules larger than the gel pores move fast; smaller molecules move slowly through the column. This technique is relatively simple: Dilute buffer solutions serve as eluents, separation occurs under mild conditions, and the gels require no regeneration. Gel filtration is mainly applied to the isolation of proteins and other water-soluble molecules. Sephadex (Cytiva) is the trade name of a series of cross-linked dextran gels that differ in the degree of cross-linking. The gels provide unique swelling properties and pore sizes; thus, they can be applied in the separation of different-sized proteins and peptides.
Figure 2.
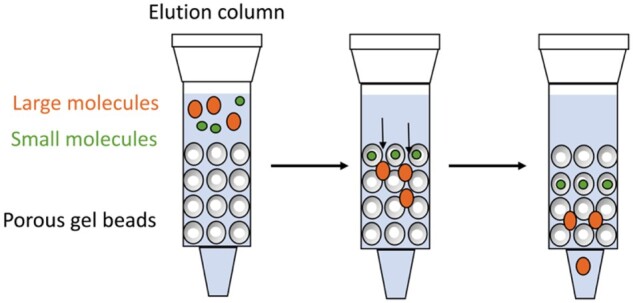
Scheme of gel filtration applied in the separation of molecules of different sizes.37 (Reproduced with permission from Elsevier Ltd.)
Ion-exchange chromatography (IEC) uses resins to separate proteins on the basis of their surface charges. IEC involves the electrostatic bonds and interactions between ionized groups on the surface of the resin and the opposite charges on the proteins, followed by a selective release of these bonds by changing the concentration or pH of the eluent.37 Anion-exchange chromatography is referred to the column containing a resin with positive charges that can interact with negatively charged solutes. Cation-exchange chromatography uses a column that is made of a negatively charged resin that attracts positively charged solutes. Synthetic exchange resins are very useful for the separation of amino acids and peptides. Figure 3 shows the general process of IEC to separate differently charged proteins. In low-salt concentrations, proteins with an opposite charge will be retained in the column. At higher salt concentrations, the eluting buffer will compete with the resin for proteins; thus, the bound proteins can be eluted out.37 In other cases, proteins below the pI and with positive charges will bind to negatively charged resins, then a buffer with pH higher than that of the pI is applied to the column. At this pH, the bound protein will become negatively charged and elute from the column. IEC is one of the most common techniques for the fractionation and isolation of proteins, peptides, and enzymes.
Figure 3.
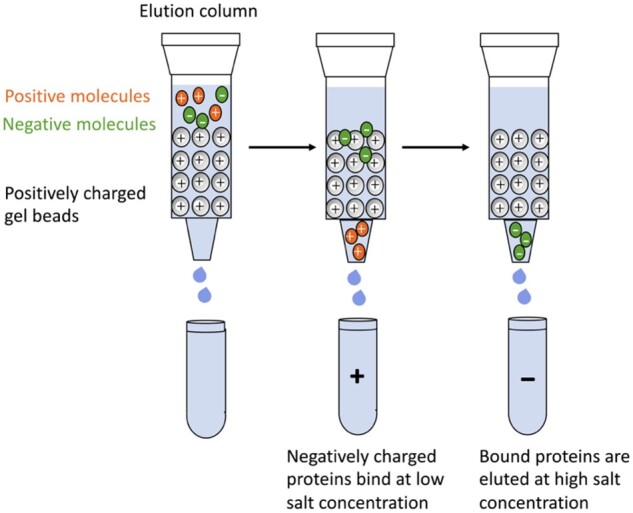
Scheme of ion-exchange chromatography applied in the separation of molecules of different charges. 37 (Reproduced with permission from Elsevier Ltd.)
Other separation methods.
Someother separation methods can be used in series with MSPs and chromatography techniques. Membrane distillation is a non-isothermal treatment that generates temperature and water vapor–pressure differences across the membranes. Membrane distillation enhances the flow and retention rate of milk components such as protein.29 Electrodialysis is based on electromigration through semipermeable membranes due to a potential gradient.38 This operation is commonly applied in the dairy industry for the removal of ions, particularly for the development of infant formula.39 Electrodialysis has also been used to remove lactate ions from acidic whey before the drying process.40 Phase separation is a process that introduces heat, salts, organic solvents, or polyelectrolytes that selectively induce precipitation of whey proteins. The advantages of this approach include large capacity, low cost, high efficiency, and relatively simple operation.41 However, there are some disadvantages; for example, heat treatment leads to denaturation and aggregation of proteins. The process of selective precipitation usually uses chemical reagents such as acetone, hydrochloric acids, or ammonium sulfate.42 It is unclear if the use of these reagents changes the properties of the proteins, so they need to be applied with care.
Currently, the most efficient and commonly used techniques for whey protein separation and purification are membrane and chromatography separation (including size exclusion and ion exchange). MSPs are volume dependent, which means the separation capacity and cost are correlated to the feeding volume instead of the mass of the final product.38 Moreover, membrane separation is likely to have the issues of chemical or biofouling, slow flux rate, reduced mechanical strength, and chemical and thermal instability.29 High cross-flow velocity has been applied to reduce the accumulation of foulant and the occurrence of biofouling.10 Compared with membrane filtration, chromatographic separation is highly selective and efficient to separate bioactive peptides; however, this approach is time consuming, expensive, and may result in possible changes or even elimination of the bioactivities in the final products.
Apart from these techniques, precipitation, centrifugation, drying, and enzyme hydrolysis are commonly combined with the aforementioned separation processes to improve the isolation and purification efficiency of the target products.29,31 Industrial operations typically combine techniques to separate a series of target bioactive compounds. Table 2 summarizes different separation and purifications techniques that have been applied to the production of milk bioactives and also notes the commercialization potentials of these methods, based on the references.
Table 2.
Different separation techniques used in the production of milk bioactives and their commercial potential
| Milk bioactive and source | Isolation/separation technique | Reference | Commercial potentiala |
|---|---|---|---|
| α-La | |||
| Whey | Isoelectric/thermal precipitation and solubilization | Bramaud et al (1997)43; | Pilot scale |
| Tolkach et al (2006)44 | |||
| Whey | Thermal precipitation + MF+UF | Toro-Sierra et al (2013)45 | Pilot scale |
| Whey protein concentrate | Thermal precipitation+ continuous centrifugation | Haller and Kulozik (2020)46 | Commercially feasible |
| Whey | UF/MF with DF | Cheang and Zydney (2003)47 | Industrial scale-up is feasible |
| Whey | MF with enzymatic hydrolysis | Cheison et al, (2011)49; Konrad and Kleinschmidt (2008)50; Lisak et al (2013)51 | Pilot scale |
| Whey protein concentrate | Anion-exchange chromatography and UF | Geng et al (2015)52 | Commercially available |
| Bovine milk | Gel filtration + anion exchange chromatography | Neyestani et al (2003)53 | Small and medium scale |
| Whey | 2-step ion-exchange chromatography | Ye et al (2000)54 | Pilot scale |
| Bovine milk | Salt out/+ anion-exchange chromatography | Mao et al (2017)55 | Pilot scale |
| Cow milk/whey | Cationic-exchange expanded-bed chromatography | Conrado et al (2005)56 | Commercially feasible |
| Skim milk | HHP + UF | Touhami et al (2021)57 | Laboratory scale |
| LF | |||
| Bovine milk/whey | Cation exchange chromatography/+ MSP | Liang et al (2011)58; Lu et al (2007)59 | Commercially available |
| Whey | Cation exchange chromatography with modified stationary phases | Hirsch et al(2020)60; Matijašić et al (2020)61; Teepakorn et al (2015)62 | Industrial scale-up is feasible |
| Whey | Electrodialysis with MSP | Brisson et al (2007)63; Ndiaye et al (2010)64; Wang et al (2020)65. | Laboratory scale |
| Whey | Metal/dye/magnetic affinity chromatography | Baieli et al (2014)66; L. Chen et al (2007)67; Lönnerdal et al (1977)68 | Laboratory scale |
| Whey | Hydrophobic interaction chromatography | Santos et al (2011)69 | Laboratory scale |
| Whey | Cationic exchange expanded bed chromatography/+UF | Maciel et al (2020)70 | Laboratory scale |
| GMP | |||
| Whey | Anion exchange chromatography | Nakano et al (2018)71 and (2020)72 | Large-scale production |
| Whey | DF+ anion exchange chromatography + UF | Davis et al (2004)73; Etzel et al (2011)74 | Commercially available |
| Whey | Gel filtration | Takuo et al (2002)75 | Laboratory scale |
| Whey | Affinity chromatography | Baieli et al (2017)76 | Laboratory scale |
| Whey | Hydrophobic interaction chromatography | Silva-Hernandez et al (2002)77 | Laboratory scale |
| Milk | Deproteinization with TCA | Nakano et al (2002)75 | Laboratory scale |
| Whey | Thermal treatment and ethanol precipitation | Berrocal and Neeser (1993)78; Rojas and Torres (2013)79 | Industrial scale up is feasible |
| Whey | Cellulose acetate electrophoresis | Nakano et al (2009)80 | Laboratory scale |
| MFGM | |||
| Bovine milk | Fat remover + cream wash +centrifugation+ UF/MF | Hansen et al (2020)81; Le et al (2009)82; Ye et al (2004)83 | Laboratory application |
| Butter milk | MF/+DF (several circles) | Fuller et al (2013)84; Morin et al (2007) 85 | Pilot-scale |
| Butter serum | MF | Rombaut et al (2006)86 | Laboratory scale |
| Whey | UF | Rombaut et al (2007)87 | Laboratory scale |
| Butter milk | Acid/rennet coagulation + DF/MF | Holzmüller et al (2016)88 | Pilot scale is feasible |
| BMO | |||
| Whey | Enzymatic digestion of lactose + (graphitized carbon-) SPE | Robinson et al (2018)89; Ward (2009)90 | Laboratory scale only |
| Whey | Enzymatic digestion of lactose + NF/+ DF | Altmann et al (2015)91; Cohen et al (2017)92 | Scale-up experiment |
| Whey | Integrated method: Lactose hydrolysis + fermentation + NF | De Moura Bell et al (2018)93 | Pilot scale |
Based on statements in the referenced articles or in patents.
Abbreviations: α-La, α-lactalbumin; BMO, bovine milk oligosaccharide; DF, diafiltration; GMP, glycomacropeptide; HHP, high hydrostatic pressure; LF, lactoferrin; MF, microfiltration; MFGM, milk fat globule membrane; MSP, membrane separation process; NF, nanofiltration; SPE, solid-phase extraction; TCA, UF, ultrafiltration.
Separation and purification of major milk bioactives
α-La can be enriched from cheese whey using a membrane separation method combined with a pH thermal treatment process. UF membranes have been used to remove impurities such as BSA and immunoglobulins to concentrate α-La, with 90% recovery.48 DF processes can be also be performed to improve the purification of α-La in the permeate. Usually, a two membrane cascade filtration process (MF and UF) is applied to improve the purification of α-La; however, this approach still lacks good selectivity for the separation of α-La and β-Lg because of their similar MWs.94 An improved method was developed by Konrad and Kleinschmidt,50 who combined the enzymatic treatment with membrane filtration to isolate native α-La. UF was initially applied to concentrate whey proteins from sweet whey by the use of membranes with 100 kDa and 150 kDa MW cutoffs. By tryptic hydrolysis of the permeate, all the β-Lg fraction was hydrolyzed, while α-La was retained with only one remaining impurity, BSA. Then a second UF and DF process was performed using a 10 kDa membrane to recover α-La. The purity was as high as 93%. This approach is easy to scale up and can produce native α-La without generating lots of waste products. However, the major challenge is the accurate termination of the tryptic hydrolysis to prevent the further hydrolysis of α-La after the completion of β-Lg digestion. Recently, Touhami et al57 developed a method combining high hydrostatic pressure and UF to fractionate α-La and β-Lg from skim BM by generating a large β-Lg complex under high hydrostatic pressure conditions.57 High hydrostatic pressure is nonthermal processing that applies ultra-high pressure (> 50 MPa) instead of high temperature to inactivate enzymes and microorganisms of food products.95,96 It is commonly applied in the food industry for the preservation and structural modification of natural products with minimal effects on thermally sensitive bioactive components like polyphenols.97–100
A more sophisticated means of separating α-La from whey is chromatographic separation, including gel filtration and IEC. This method is usually combined with other pretreatment processes to extract protein fractions from BM or whey. Neyestani et al53 have isolated α-La, β-Lg, and BSA from BM using the combined approaches of precipitation, gel filtration, and IEC.53 β-Lg was removed by being retained in an anion-exchange column while α-La and BSA were co-eluted out. Then α-La was isolated from BSA through Sephadex G-50 gel filtration. This approach yielded α-La with high purity and antigenicity. Two-step IEC has been applied to isolate α-La from cheese whey.54 The first step uses a strong cationic exchange resin that can co-elute LF and lactoperoxidase. The second step involves a strong anion exchange resin by which α-La is isolated in a 0.13 M NaCl solution at pH 8.0, while the β-Lg was isolated at a higher NaCl concentration at pH 6.8. Adopting a similar approach, Mao et al55 developed a 1-step approach to quickly fractionate α-La and β-Lg from BM with a purity of 84.85% and 94.91%, respectively.55 α-La and β-Lg first were salted out from milk after a pH adjustment, and then they were further separated by anion-exchange chromatography.
Isolation of α-La could also be carried out using phase separation, particularly by precipitating β-Lg or α-La aggregates by modifying environment or process conditions such as heat treatment, the addition of acids, or the use of restricted protein solubility at pI range.43,44 This method is inexpensive and simple to perform; however, the purity of α-La is low and subsequent purification procedures are necessary.
LF could be isolated from colostrum and cheese whey using chromatography and membrane separation techniques. Because LF is heat sensitive, pasteurized milk is not suitable for LF purification. Various chromatographic methods have been investigated to isolate LF, such as cation exchange,58,59 metal/dye/magnetic affinity,66–68 and hydrophobic interactions.69 Cation-exchange chromatography is the most commonly used technique for the isolation of LF, which has been commercially industrialized. LF is a positively charged protein in whey, so it can be attracted by negatively charged resins and then washed out by high-salt solutions. Then the LF should be desalted, concentrated by a membrane-filtration process, and further freeze-dried or spray-dried into powders with high purity (> 90%).101 The effect of pH on the LF selectivity has been studied and it has been determined that pH 5 and pH 10 are the optimal conditions for absorbing and desorbing LF, respectively.102 Other alternative stationary phases have been developed to test the feasibility of isolating LF, such as mixed matrix,60 monolithic columns, and chromatographic membranes.62 LF was isolated from sweet whey in a recent study using MF and UF followed by expanded-bed chromatography based on cationic exchange.70 This integrated technique yielded LF with a purity of 92.7% and recovery of 87.0%.
The MSP was also used to isolate LF from BM and whey. However, MSP is not effective in separating other proteins in whey that have similar sizes to LF, such as BSA and lactoperoxidase.102,103 Therefore, different strategies have been studied to address this protein separation problem. For example, charged membranes and electrically enhanced cross-flow MF have been used.63 This approach used electrical fields to affect protein transmission and could improve the separation efficiency compared with conventional pressure-driven membrane filtration. Electrodialysis with UF membrane has been used to isolate LF from model and whey solutions. However, the selectivity between β-Lg and LF was low, due to the simultaneous migration of proteins across the membrane in the process.64 Recently, Wang et al65 developed an electrodialysis with filtration membrane process that is used with polyvinyl alcohol membranes. This method could separate LF and BSA on the basis of the large aggregation sizes of LF (MWs of ∼300 kDa) compared with those of BSA (MWs of ∼66 kDa). However this method was only tested in low-volume samples (40 mL). Scale-up is still needed to test if the method could be used in the industrial production of LF.
GMP has been separated from cheese whey by various technologies, such as thermal treatment, complexation, UF, gel filtration, affinity chromatography, and IEC.18 Feeney et al18 conducted a detailed review of the purification technology of GMP. IEC is considered one of the most effective techniques used for GMP separation, considering that GMP has a pI (∼pH 4) lower than most other whey proteins. Therefore, GMP has a negative charge at a low pH, whereas the other whey proteins have a positive charge. GMP lacks aromatic acid Phe, thus the concentration of Phe is usually used to indicate the purity of GMP. Sialic acids, a carbohydrate moiety of GMP, are the groups with the most important biological functions in GMP; thus, they are usually used to indicate the isolation efficiency of GMP. Tanimoto et al104 have used rennet-coagulated cheese whey for the large-scale production of GMP by IEC, presenting a low Phe level (0.9%). Outinen et al105 have used a strongly basic anion exchange resin to isolate the GMP from cheese whey with a purity of 70%–80%. Nakano et al71 have isolated GMP from bovine whey with a low level of aromatic amino acids through protein precipitation combined with anion-exchange chromatography; however, this resin is not food grade. Recently, the same group developed a food-grade anion-exchange resin to isolate GMP from the soluble whey fraction.72 The production of GMP included a 78% recovery of sialic acids, with few contaminating amino acids.
GMP can be isolated by deproteinization with trichloroacetic acid because GMP is soluble in 8% trichloroacetic acid solution, whereas other sweet whey proteins are precipitated.75 Cellulose acetate electrophoresis was also used to separate GMP from sweet whey, due to the difference of pI between GMP and other proteins.80 However, these methods are usually used to isolate GMP for laboratory use, which is not suitable for large-scale food production.
MFGM and its fractions can be isolated from raw milk before utilizing the other milk components. A requirement is to isolate the MFGM fractions with the fewest possible effects on the non-MFGM components. Although various procedures have been used to isolate MFGM from milk, generally they follow these 4 major steps: 1) fat separation, 2) cream washing, 3) release of MFGM from globules, and 4) the collection of MFGM material.23 Although some pilot-scale tests have been done to obtain MFGM from raw BM by using MF before or after pasteurization,81 the isolation of MFGM from BMs so far is only for laboratory applications and research purposes, rather than industrial manufacturing.
In the dairy industry, MFGM is typically extracted from dairy byproducts, including buttermilk from butter production,84 butter serum from the production of anhydrous milk fat,86 or whey proteins from cheese production87 by MSPs. The first method is the application of cross-flow membrane filtration, including UF and MF. By regulating the filtration conditions such as temperature, pH, pore sizes, and membrane materials, the amount and flow rate of components across the membrane can be adjusted.87 Lactose and whey proteins can be isolated easily because they are relatively small. However, the challenge of this approach lies in the similar sizes of casein micelles (0.05–0.5 μm)33 and MFGM fragments (0.1–3.0 μm).106 DF usually is combined with MF to facilitate the separation efficiency and the purity of the retentate. By repeating the DF steps several times, the casein retained with the MFGM fractions is decreased from 30% to 6%.107 However, the increase in DF steps would increase the loss of MFGM materials. Another approach is to remove caseins through coagulation before introducing the solutions for MF to remove the whey proteins. The addition of an acidulant, such as lactic acid bacteria (fermentation products reduce the pH) or rennet treatment, has been used as a coagulation agent.88 However, coagulation may also induce the coagulation of MFGM protein. Compared with the addition of an acidic agent, rennet coagulation was reported to be a more efficient method to coagulate casein micelles, while also increasing the yield of MFGM proteins by forming a weaker gel structure.88 Holzmüller and Kulozik108 provided a critical review on the major technical difficulties in isolating MFGM in industrial settings. They pointed out that there is no universally accepted or standard procedure to isolate MFGM, and it is not possible to obtain pure MFGM in raw milk without any losses. Currently, DF with MF of buttermilk or whey is the most suitable approach to obtain MFGM materials.
As mentioned in the previous section, MFGM contains 40% lipids, and the majority are PLs, including phospholipids and sphingolipids. Unlike MFGM protein fractions, which mainly demonstrated health effects through in vitro and in vivo study, many PLs components have been clinically commercialized, because of their therapeutical properties such as the defense against pathogenic bacteria, prevention of colon carcinogenesis and neurocognitive disease, and lowering plasma cholesterol levels.109 Therefore, the purification of PLs from milk or MFGM is desirable. Different approaches have been studied to purify PLs from milk and milk byproducts. Boyd et al110 used alcohol fractionation on whey powder and obtained an alcohol-insoluble fraction with 84% phosphatidylethanolamine. Supercritical fluid extraction is a common method to extract lipids from foods and has been adopted in the dairy industry to isolate PLs.111 It has been used with UF and, subsequently, DF to produce MFGM fractions that were particularly rich in PLs. Using supercritical fluid extraction combined with near-critical dimethyl ether, Catchpole et al112 produced a final product containing 70% PLs with few neutral lipids and proteins. It was preferable to apply supercritical fluid extraction and dimethyl ether to powder samples rather than liquid products to avoid the denaturation of proteins. The efficiency of PL extraction increases as the lactose content of the powder decreases. For liquid samples, such as buttermilk and whey, solid-phase extraction is usually applied to fractionate PLs. Different sorbents have been studied for PL purification, including normal phase (silica and amino columns) and reversed phase (C8 and C18 columns), with the former generally providing higher-purity PLs.
The production, particularly the biosynthesis, of HMO has received lots of interest due to its significant health benefits. However, synthesized HMO is expensive, requires complex synthetic processes, and may not fully represent the diversity of OS structures from natural HMO. Recently the isolation of BMO has gained much more attention, motivated by the fact that BMO contains several identical OGs with HMOs, such as the OGs 3-sialyl-lactose and 6-sialyl-lactose, suggesting that BMO could have a similar biological effect as HMO for infants. Currently, isolation of BMO from milk on a laboratory scale has been realized by the following procedures: Lipids are first separated from milk using centrifugation, proteins are precipitated, peptides are removed by solid-phase extraction, and then salts are removed by graphitized carbon chromatography.7,90
The major challenge for the commercialization of BMOs as an ingredient is to separate it on an industrial scale. Especially considering the complex structures of BMO and their similarities in carbohydrate structures, the selection of isolation techniques that can provide high selectivity and efficiency at lower costs and minimal use of solvents is challenging. Lactose removal is essential for the production of high-purity BMO, because it is the most abundant carbohydrate in milk or milk whey.7 Lactose can be removed using MSPs or IEC. Alternatively, lactase hydrolysis is the most commonly used method to remove lactose, because of its simplicity and low cost. Lactose is digested first into glucose and galactose with lactase, then BMO is separated from glucose and galactose, using different separation techniques.113
The industrial isolation of BMO from whey has been studied using the combination of lactose hydrolysis and MSPs. Particularly, the use of different UFs and NFs was reported to provide good recovery of BMO from BM. Altmann et al91 performed the UF process on an industrial scale (1000 L of milk) with the 300 Da spiral wound in lactose hydrolyzed, skimmed, and ultra-filtrated milk permeate. The results showed a recovery of 70%–97% of 3 major BMOs (3-sialyl-lactose, 6-sialyl-lactose, and N-acetylgalactosa-minyl lactose). More recently, de Moura Bell et al93 developed an integrated process to enhance both the yield and purity of BMO from BM whey permeate at pilot scale. They used this approach to investigate the optimal conditions for lactose hydrolysis, monosaccharides fermentation by yeast, and BMO recovery by NF and finally enabled high recovery (95%) with high purity (> 90%).
ENCAPSULATION TECHNOLOGIES TO IMPROVE THE STABILITY OF MILK BIOACTIVES
Major stability challenges of milk bioactives
In the preparation of food and pharmaceutical products, various unit operations, such as thermal and shear treatments, and biomechanical processing are applied that can influence the structure and properties of milk bioactive components. Moreover, many of these bioactive components are unstable, being prone to denaturation, oxidation, or enzymatic degradation induced by exposure to heat, light, oxygen, or other environmental factors during processing, storage, or consumption, which eventually decreases their bioactivity.
Thermal treatment is an essential process in the manufacture of nearly all food products. Thermal treatment affects the functionality of milk proteins most significantly compared with other food processing treatments. Different chemical or physical alterations could occur in milk protein during thermal processing, including denaturation, glycation, β-elimination, and isopeptide bond formation, which largely affect the bioactivity of these compounds. A typical pasteurization condition varies from 63°C to 78°C, depending on the treatment time (15 s to 30 min). The thermal denaturation temperature of milk proteins varies from 60°C to 90°C. The proteins’ thermal stability can also be influenced by environmental conditions. In the absence of calcium, the denaturation temperature of α-La is as low as 43°C, whereas the denaturation temperature is 68°C in the presence of saturated calcium. Similarly, the thermal stability of LF depends on pH and the presence of iron. The thermal denaturation temperature of apo-LF (with lowest iron content [< 10%]) is ∼ 70°C, whereas for the holo-LF (with high iron content [> 20%]), it is ∼ 90°C. During thermal processing, chemical reactions may occur between the lysine residues of milk proteins or peptides with reducing sugars, which are called Maillard or nonenzymatic browning reactions. This reaction will affect the sensory properties of final products, reduce the nutritional values, and decrease the bioavailability of lysine. Intramolecular reactions may occur during thermal treatment among several reactive amino acid residues in milk proteins or peptides including lysine, tryptophan, threonine, and glutamine. These reactions further form intra- or interchain cross-links, which deteriorate the protein digestibility and bioavailability or even introduce toxicity.114 Although carbohydrates and lipids are relatively more stable than milk proteins, thermal treatment could also break down the MFGM structure, influence its composition, and further influence its functional and colloidal properties.115
pH modification during isolation of bioactive components (ie, acid or alkaline hydrolysis, precipitation, or solubilization) or during the product formulation procedures can also induce different chemical changes of milk bioactive compounds. For example, alkali racemization of amino acids can impact protein digestibility as the consequence of formed cross-linkage.116 Bioactive components can also be degraded or oxidized during transportation and shelf-life storage, because of the temperature change and the exposure to light and oxygen.117 MFGM undergoes lipid oxidation along with structural and functional changes during storage.118 The phospholipids of the MFGM, which contains 40%–60% unsaturated fatty acids, are susceptible to oxidation and could develop oxidized flavor defects.119
Apart from processing and storage stability, digestion stability (ie, bioavailability or digestibility) is vital to ensure the effective use of these milk bioactive compounds contributing to human health benefits. Many nutrients need to pass through salivary and gastric digestion before being digested into small molecules and absorbed by the intestinal tract for further use in the body. For example, the health efficacy of LF is based on its integrated protein structure conformation; therefore, the gastric hydrolysis of LF would result in an undesirable loss of its positive biological properties.120
Overview of the encapsulation method
As discussed in the previous section, many milk bioactive compounds are sensitive to environmental changes induced by heat, light, oxygen, pH, and enzymes. To improve their chemical and physical stability, enhance their bioavailability, and enable their incorporation into various formulations, micro- or nanoencapsulation strategies have been used. Micro- or nanoencapsulation, which originated in the pharmaceutical industry, creates a physical barrier or coat to protect the bioactive compounds. This technology has been applied to mask off-tastes and flavors and off-colors, to prevent the degradation of bioactive components, and to provide a controlled release of target components.121–123 Many bioactive compounds have been successfully encapsulated, such as vitamins, antioxidants, fatty acids, and peptides.124–126 The encapsulation materials could be natural or synthetic polymers, and they should be generally recognized as safe for human health.127 A growing interest is focused on the use of food-derived materials or natural ingredients rather than synthetic chemicals. These materials include carbohydrates, gums, lipids, and proteins.128
Microencapsulation can be developed through physical, chemical, or physicochemical processes. The physical methods include spray drying, spray chilling, freeze-drying, fluid bed coating, extrusion, among others.128 Chemical methods involve molecular inclusion and interfacial polymerization. The physical-chemical method created a microcapsule or microsphere shell through coacervation, phase separation, or liposome entrapment. The formed microcapsules generally have a particle size ranging from 1 to 1000 μm and have various structures (Figure 4). Nanoencapsulation involves a more complex process than microencapsulation. It produces capsules in nano sizes mainly through emulsification, coacervation, nanoprecipitation, inclusion complexation, and emulsification-solvent evaporation.129 There are many reviews introducing the encapsulation techniques.128,130 Here, we provide a general overview of encapsulation methods and their application to milk bioactive components (Table 3).
Figure 4.
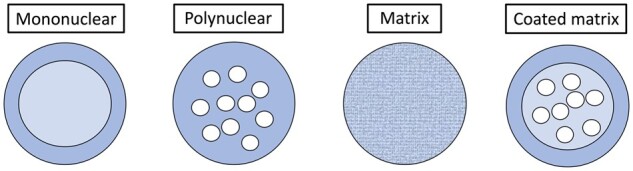
Morphology types of microcapsules.131 (Reproduced with permission from Springer with slight modifications)
Table 3.
Overview of common encapsulation processes and their application on milk bioactives131 (Reproduced with permission from Springer with slight modifications)
| Technology | Process steps | Morphology | Load (%) | Particle size (μm) | Milk bioactive |
|---|---|---|---|---|---|
| Spray-drying |
|
Matrix | 5–50 | 10–400 | LF132 |
| Fluid bed coating |
|
Mononuclear (or reservoir) | 5–50 | 5–5000 | |
| Spray-chilling/cooling |
|
Matrix | 10–20 | 20–200 | |
| Extrusion-dropping |
|
Matrix | 20–50 | 200–5000 | LF120 |
| Emulsification |
|
Matrix | 1–100 | 0.2–4000 | α-La133 |
| Multiple emulsions (micro-/nano-) |
|
Mononuclear (or reservoir) | 1–90 | 0.2–5000 | GMP134; LF135 |
| Coacervation |
|
Mononuclear | 40–90 | 10–800 | LF136–138; GMP139 |
| (or reservoir) | |||||
| Inclusion complexation |
|
Molecular inclusion | 5–15 | 0.001–0.01 | |
| Liposomes (micro or nano) |
|
Various | 5–50 | 10–1000 | LF140,141; GMP142 |
Abbreviations: α-La, α-lactalbumin; GMP, glycomacropeptide; LF, lactoferrin.
Spray drying.
Spray drying is commonly applied in the industry to microencapsulate bioactive components via the formation and stabilization of polynuclear capsules. The liquid-based solutions or emulsions consisting of bioactive materials are sprayed into a drying chamber for moisture evaporation. Depending on the operating conditions, concentrations, and materials applied, the microcapsules formed range in size from 10 to 400 μm.131 Spray drying is a relatively simple procedure and can be applied to a wide range of coating materials. However, it has some limitations. For example, the high temperature during spray drying may affect the properties of heat-sensitive molecules.128 Also, this process is based primarily on water-based dispersions; therefore, the coating materials should be ultra water soluble. If they are to be included, hydrophobic core materials first should be dissolved in an oil phase and then emulsified in an aqueous medium.
Spray chilling.
The spray chilling process, also called spray cooling, atomizes liquid-based ingredients consisting of bioactive core materials into solid particles ranging in size from 20 to 200 μm, through cold air or liquid nitrogen. In comparison, the spray drying process uses hot air. The operating temperature is 45°C–122°C for spray cooling and 32°C–42°C for spray chilling.143 The coating materials are usually lipophilic materials with a high melting temperature (eg, fatty acids). Frozen liquids and thermally unstable molecules, such as ω-3 fatty acids and probiotics, can be encapsulated through the spray chilling process. This process is low cost; however, the encapsulation efficiency is quite low and the coating materials are easily located at the surface.144 These problems may be solved by subsequently coating using other techniques.
Fluid bed coating.
Fluid bed coating is particularly suitable for the encapsulation of solid particles. The solid particles are suspended in the air and then coated by a spray of coating liquids. Fluid bed coating can coat particles using different types of shell materials, such as hydrocolloids, proteins, enteric coating, complex formulations, and yeast cell extract.145 This method is efficient for creating a uniform coating layer on solid particles, generating reservoir-type microspheres. There are 3 spray modes (ie, top, bottom, and tangential spray), depending on the location of the nozzle. The drops and particles range from 0.01μm to 0.04 μm and 100 μm to millimeters, respectively, in a fluid bed.146 This method provides a secondary coating of target molecules. Also, it can be used in combination with spray drying methods to dry and coat particles at lower temperatures.
Extrusion process.
The extrusion process produces encapsulates by driving the liquid materials, consisting of target bioactive core molecules, into the hardening bath via a porthole.128 The smaller the hole, the smaller the size of the formed capsules. Typically, extrusion dripping or spring extrusion is used to form alginate beads by extruding the alginate solution with bioactive molecules into a calcium chloride solution.147 Extrusion has been widely used for encapsulating unstable volatile flavors in a glossy carbohydrate matrix. By using this approach, unstable flavor compounds can be sustained in the glossy matrix with less oxidation to extend shelf life.148
Emulsification.
Emulsification is a process of dispersing 2 or more immiscible liquids into semi-stable mixtures called emulsions. Microemulsions have a droplet size ranging from 1μm to 500 μm, whereas nanoemulsions have a particle size between 20 nm and 1000 nm.128,129 Emulsions can be divided into water-in-oil or oil-in-water emulsions and water-in-oil-in-water or oil-in-water-in-oil double emulsions.128 They are effective systems for delivering active molecules that are hydrophilic or hydrophobic. The emulsions can be applied either in liquid form or be spray-dried or freeze-dried into powder form, which yields a versatile product that can be incorporated into different types of nutritional products. Emulsification is easy to scale up and is applicable for various coating materials. The disadvantage of emulsification is that the process involves additional procedures, including emulsification and separation of oil. Particularly, nanoemulsions, as nonequilibrium systems, cannot be formed spontaneously. The production of nanoemulsions usually involves high-energy inputs by mechanical devices or must be driven by chemical potentials. Generally, the formation of microemulsions or nanoemulsions requires the input of high-energy emulsification equipment, such as high-shear stirring, high-speed or high-pressure homogenization, and ultrasonication.129
Coacervation.
Coacervation is a phase-separation process from initial mixture solutions. It is carried out in 3 major steps. First is the formation of a mixture solution of core molecules and coating materials, and the solvent phase separation induced by the adjustment of solution parameters, such as temperature, pH, or ionic strength. The solution is separated into 2 liquid phases: One is a polymer-rich phase (coacervate) and the other comprises solutions consisting of the same ratio but less polymers than the coacervate. The second step is the deposition of the polymer-rich liquid phase by adjusting the temperature or adding cross-linkers. The third step is the solidification of coating layers, using freeze-drying or evaporation. Coacervation is regarded as a true microencapsulation technique, because the core bioactive can be completely entrapped by the coating materials.143 Complex coacervation involves 2 oppositely charged polymers—usually positively charged proteins and anionic biopolymers such as gelatin and gum arabic, respectively. They form complex coacervates (ie, capsules) when the two opposite charges are neutralized. Complex coacervation has been widely studied using different coating materials for the microencapsulation of bioactive compounds such as vitamins, flavor oils, and fatty acids. Some commonly used coating systems include gelatin/gum acacia, carrageenan/chitosan, gelatin/carboxymethylcellulose, and guar gum/dextran.145
Molecular inclusion.
Molecular inclusion is an encapsulation technique that takes place at a molecular level; the other encapsulation methods described previously are at the polymer level.143 Cyclodextrin generally is used as the encapsulating medium. Cyclodextrin is produced through the partial hydrolysis of starch by enzymes produced from selected microorganisms. It is made up of cyclic dextrin containing 7 α-, 7 β-, or 8 γ-glucose monomers. The external groups of cyclodextrin, which are the polar hydroxyl groups of glucose, are hydrophilic, and the internal groups, which are hydrogen and glycosidic oxygen molecules, are hydrophobic. Because of the hydrophobic property of the cavity, cyclodextrin can interact noncovalently with specific molecules such as flavor substances and protein hydrolysates with suitable size, shape, and hydrophobicity.149,150
Encapsulation of milk bioactive compounds
α-La is relatively heat stable compared with BSA and immunoglobulins, because it has no free SH groups. However, irreversible denaturation of α-La during thermal processing was also observed, especially when coexisting with other milk proteins such as β-Lg.151,152 Water-in-oil microemulsions have been studied as nanoreactors to produce whey protein nanoparticles that were rich in α-La.133 The small (5–100 nm) particles formed transparent dispersions and reduced the aggregation of protein particles. Compared with native whey protein, the microcapsules did not gel after being treated at 80°C for 20 minutes at neutral pH.
LF is susceptible to thermal denaturation and gastric digestion. Besides iron saturation and PEGylation approaches, microencapsulation is the most commonly accepted method to protect LF.14 LF has been encapsulated in the sodium alginate and has shown enhanced thermal stability and higher retention during digestion.120 A double-layer capsule consisting of BSA and tannic acids demonstrated >76% of the protein efficiency of LF during gastric digestion and a complete release of LF in the intestinal phase after the shell was degraded.153 Nanoencapsulation of LF via multiple water-in-oil-in-water emulsions was also studied. These emulsions encompassed the full stabilization of the 3-dimensional structures of LF with improved thermal and long-term storage stability of LF over a 2-week storage test.135 Liposomes were also applied to protect LF using soy-, rapeseed-, and MFGM-derived phospholipids.140,141 However, the inclusion of a large amount of lipids influenced liposome storage stability, due to lipid oxidation, especially at room temperature, compared with refrigeration conditions.141 Among all the encapsulation methods, complex coacervation of LF using various biopolymers was studied most intensively, because of the advantages of complex coacervation, including high encapsulation efficiency, simple operation, and easy scale-up. Because LF is a positively charged protein at a wide pH range, it can easily form a coacervate complex with many anionic polysaccharides, such as gum arabic and pectin, as well as negatively charged proteins, such as caseins and β-Lg.132,154,155 However, most of these studies investigated the optimal conditions for the formation of coacervates, and fewer examined its impact on the thermal stability and the functionality of LF for the final application, which deserves more investigation.
GMP is stabilized via the entrapment with water-in-oil-in-water multiple micro- or nanoemulsions.134 The encapsulation was not only intended to protect the peptides from thermal degradation but also to help to mask bitter flavors, which are usually generated from a high content of peptides. Recently, there is an increased interest in using liposomes to deliver and encapsulate whey and other food peptides. Compared with other encapsulation methods, liposomes are particularly efficient in encapsulating peptides, considering they contain polar, nonpolar, and amphiphilic regions within the particles.142 However, liposome formation usually involves complicated processes and use of large amounts of lipids, requiring a high economic investment. Thus, its application is mainly in pharmaceutical products, rarely in food industries.
APPLICATION OF MILK BIOACTIVES
Production and market report
Dairy ingredients are highly valued in providing not only functional and organoleptic functions but also nutritional and clinical needs for foods, nutraceuticals, and pharmaceuticals. Fractionation and marketing of milk bioactives is now an emerging business in the dairy industry and other specialized bioindustries. Some milk bioactives have been commercialized and consumed either in daily healthy diets or as special healthcare products. On the basis of function, the milk bioactive compounds market has been segmented into antihypertensive, antithrombotic, immunomodulating, anti-stress, antimicrobial, antiviral, antitumor, and transport facilitator (ie, calcium, iron, and other trace elements). The global dairy-ingredient market is predicted to expand at an annual growth rate of 4.1% from 2020 to 2027 to reach $71.5 billion by 2027, according to Meticulous Research, a global market study company.156 The top 10 milk ingredients producers, as of this writing, are Arla Foods (Denmark), Fonterra (New Zealand), Royal Friesland Campina (the Netherlands), Lactalis Group (France), Dairy Farmers of America (United States), Saputo Inc. (Canada), Glanbia (Ireland), Sodiaal International (France), Agropur Cooperative (Canada), and Yili (China). Table 4 lists some examples of commercial products using milk bioactives for different health claims.
Table 4.
Examples of commercial products containing milk bioactives
| Brand name | Type of product | Functional bioactive | Health/function claim | Reference/Manufacturer |
|---|---|---|---|---|
| Vivinal® Alpha | Ingredients | α-La rich whey protein hydrolysate | Aids relaxation and sleep | Korhonen and Phihlanto (2007)157; Domo Ingredients (BDI), the Netherlands, |
| BioPRO® Alpha9000 | Whey protein isolate | α-La protein isolate | High in tryptophan, leucine, and cysteine for fibromyalgia and chronic pain syndromes treatment, sleep quality improvement, and mood control | Agropur, USA |
| NutriPRO™ Alpha | Whey protein isolate | α-La protein isolate | Improve sleep quality and gastrointestinal and immune function for infants | Milk Specialties Global, USA |
| Praventin | Food supplement/capsule | LF-enriched whey protein hydrolysate | Helps reduce acne for skin care | Korhonen and Phihlanto (2007)157; DMV International, the Netherlands |
| Bioferrin® | Whey protein isolate | LF content >95% | Iron supplement | Glanbia Nutritionals, Ireland |
| BioPURE-GMP | Whey protein isolate | GMP (κ-casein (f 106–169) | Prevent dental caries, blood clotting, infection by viruses and bacteria | Agropur, USA |
| BioPRO® GMP9000 | Whey protein isolate | >95% pure GMP | Stimulate satiety-simulating factors; tooth remineralization and dental plaque reduction | Agropur, USA |
| Lacprodan® MFGM-10 | Milk fat ingredients | MFGM with a unique protein and lipid profile | Supports brain development for infants; reduces incidence and severity of diarrhea episodes; supports intestinal maturation and a healthy microbiota, and so forth | Arla Food Ingredients, Denmark |
| SureStart® MFGM lipids | Milk lipids ingredients | Complex lipids from MFGM | Support brain development and cognition in infants | NZMP, New Zealand |
| Milk Phospholipids 70 | Milk lipids ingredients | Complex lipids from MFGM | Supports mood and cognitive performance under stress for adults | NZMP, New Zealand |
| Goodstart® | Infant formula with milk ingredients | Modeled 2'-FL HMO | “Prebiotics that improve good bacteria in tummies” | Gerber, USA |
| Gentle Pro | ||||
| Similac Pro-Advance® | Infant formula with milk ingredients | Modeled 2'-FL HMO | Prebiotics that help strengthen baby’s immune system | Abbott, USA |
Abbreviations: α-La, α-lactalbumin; FL-HMO, fucosyllactose human milk oligosaccharide; GMP, glycomacropeptide; LF, lactoferrin; MFGM, milk fat globule membrane.
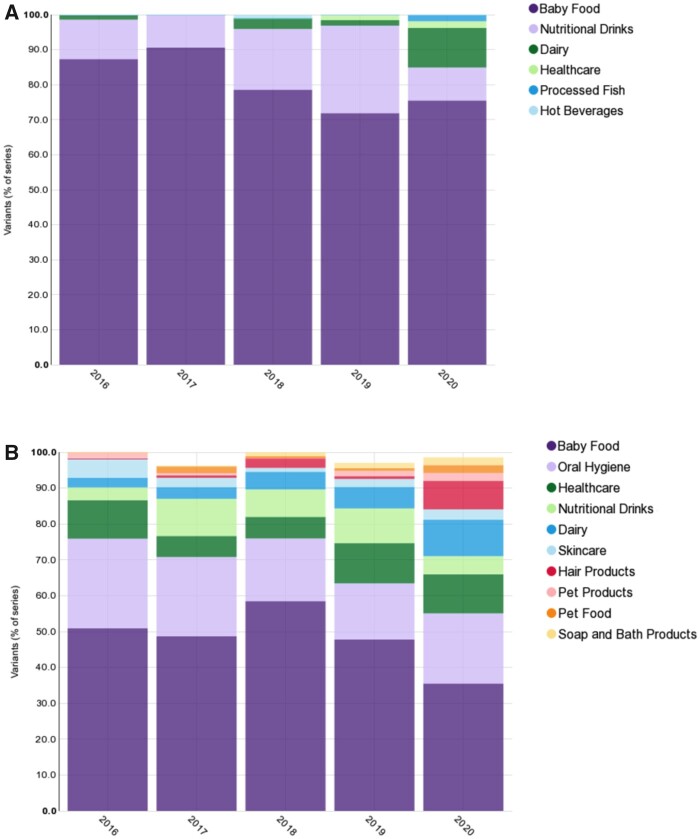
According to Mintel Data, which collects the label information of fast-moving consumer goods globally, products enriched with milk bioactives have gradually increased during the past 5 years. Figure 5 shows the proportions of major categories of α-La– and LF-enriched products in the past 5 years, based on information from Mintel Data.158 α-La is mainly used as an ingredient in baby foods, comprising >50% of α-La–enriched products. The second-largest group of α-La–enriched products is nutritional drinks and beverages, which are mainly whey protein isolate–based products. Dairy products surged to be the third-largest group of α-La–enriched products, with its proportion increasing from 1.4% to 11.3% from 2016 to 2020 (Figure 5A). α-La also is an ingredient in meals, baked goods, shakes, and healthcare products. Compared with α-La, LF has been applied in fast-moving consumer goods more diversely, including in baby foods, oral products, healthcare products, nutritional drinks, skin care products, dairy products, hair products, and pet products, in descending order of their proportions. Particularly, there has been a noticeable increase in its application in dairy products (2.7% to 10.1%), hair products (0.1% to 8.0%), and pet products (1.8% to 4.4%) from 2016 to 2020 (Figure 5B). GMPs were mainly found in nutritional and sports drinks, baby foods, and phenylketonuria diets. In the past, the use of MFGM was limited to infant foods. Recently, a product containing MFGM and targeted to pregnant women was introduced by Capac International (New Zealand).
Figure 5.
Category proportions of (A) α-lactalbumin–enriched and (B) lactoferrin-enriched products from 2016 to 2020. Based on Mintel Data158
Specific application of milk bioactives in foods and pharmaceuticals
The health benefits of α-La have been linked with the following groups: 1) intact whole protein, 2) peptides from the partial hydrolysis of α-La, and 3) amino acids from fully hydrolyzed α-La.11 Bovine α-La is structurally similar to human α-La; thus, the supplementation of bovine α-La can help make BM a closer match to human milk for infants. The α-La hydrolyzed peptides have shown various health benefits, such as antihypertensive activity, antiviral activity, antimicrobial activity, and growth-promoting and opioid activity.11 Thus, various bovine α-La hydrolysates also gained high interest in the sector of functional foods targeting these specific health benefits. The nutritional aspects of amino acids derived from α-La have gained a great emphasis, because α-La is particularly rich in essential amino acids such as tryptophan and cysteine, which play a crucial role in brain function and oxidation reduction, respectively. New potential applications of α-La in cancer treatment and to promote immune function have gained interests due to findings of antitumor activities of human α-La made lethal to tumor cells and its equivalent complex from bovine α-La.10
α-La is also a great candidate for various product formulations, because of its unique physical properties such as being free of off-flavor and having desirable water solubility and thermal stability. Recently, α-La has been shown to self-assemble to form long, uniform tubular strands that were extremely stable under pasteurization and autoclaving, and had outstanding stability in the presence of organic solvents.159 The outstanding stability of α-La nanotubes enables them to be applied in different formulations under processing conditions. They can be used as stabilizers,160 viscosifying agents161 and gelling agents in food systems.162 α-La nanotubes have also been used as vehicles for targeted or controlled delivery of drugs, nutraceuticals, chemicals, and genes.163
LF is a multifunctional protein that helps provide both nutritional and medicinal values. LF has been used as an iron fortification source for people such as infants for promoting body growth, athletes for compensating for the loss of iron during exercise, and pregnant women for preventing iron-deficiency anemia.14 The supplementation of LF in infant formulas also showed benefits such as modulation of immune function, antipathogenic effects, and better absorption of other nutrients, such as calcium.164 Current commercial food application of LF includes yogurts, skim milk, drinks, baby foods, infant formulas, and pet foods.14,165 Bovine LF is commercially produced, mainly by cation-exchange chromatography systems, by food companies. Some pioneering producers of LF include Oleofina Company (Belgium), MILEI GmbH (Germany), Morinaga Milk Industry (Japan), and DMV International (the Netherlands).165 LF has also been incorporated in human skin care (eg, lotions creams, moisturizing gels, face washes) and oral health products (eg, mouthwashes, toothpaste, chewing gums) contributing to hygiene, moistening, and antioxidation in the skin and mouth.166 Some vaccines and antimicrobial drugs have also used LF as the main agent because of its antimicrobial and bacteriostatic effects. The application of LF as a drug is expected to prevent bacteria- and virus-induced diseases such as hepatitis and human immunodeficiency viruses.167,168 A recent supplemental intervention study was performed with a cohort of 75 patients with COVID-19 and 256 family members who were in contact with patients. Researchers reported that the liposomal LF product can potentially prevent and treat COVID-19 infection.169
GMPs, either in their intact or hydrolyzed form, have been used in therapeutic foods and dietary supplements because of their different health benefits. GMP is a good source of branched-chain amino acids and is low in methionine, making it suitable to be included in the diets of patients with hepatic diseases.170 GMP is also a good protein source in cases of phenylketonuria, because it has no Phe in its amino acid composition.171 Moreover, the sialic acids of GMP play an important role in cell membrane function and normal brain development.20 GMP was reported to have an effect on promoting satiety (ie, the feeling of fullness), thus the dietary implementation of GMP can be used for food intake regulation and weight management.172 In addition, GMP has been incorporated in oral care products because it has anticariogenic and remineralization properties.173 GMP showed good emulsifying stability toward pH variation with a maximum at alkaline conditions, which means that GMP can be applied to foods that undergo pH changes during processing.174 GMP also showed good foaming properties, especially for the nonglycosylated GMP and synergistic mixtures of GMP with other biopolymers.175 GMP also has been used in food systems such as fermented milk to promote gelation and increase elasticity.176
The potential application of MFGM can be divided into nutritional and technological aspects, due to its physiological benefits and physicochemical properties, respectively. The first major application is to incorporate MFGM into infant formulas to promote the cognitive development of infants, because there is a much lower intake of MFGM by formula-fed infants than breastfed ones.25 Several phospholipid and glycoprotein components in MFGM play an important role in regulating neural and cognitive function. MFGM is also used for the prevention of several diseases and conditions, including cardiovascular diseases, inflammation, bacterial infection, cognitive decline, and muscle loss.177 Dairy-derived MFGM fractions can be divided into 2 categories: phospholipids extracts and the MFGM-enriched ingredients. Phospholipid extracts are used in cosmetics and oral care products; the main MFGM fraction is used for nutritional applications. Besides being health promoting and used as components in personal care products, MFGM is also considered a good emulsifying material due to its amphiphilic properties. Therefore, MFGM-based ingredients can be used in emulsion-based products such as cream, salad dressings, and yogurt.22 MFGM-enriched ingredients have been exploited and patented for use in food, drink, or food supplements that have been shown to improve intestinal health and cognitive performance.178 Overall, there have been many attempts to prepare MFGM-enriched materials for their use in food or pharmacy.
The interest in the isolation and application of BMOs originated from the health effects of HMOs and their structural similarities compared with other sources of OS in commercial prebiotics. So far, >50 OS structures have been recognized in BMO. BMO was reported to promote the growth of Bifidobacterium longum subspecies infantis in the infant intestine to the same extent as HMO, and has been reported to have various biological functions, such as improving brain development and alleviating metabolic abnormalities.26,27 Thus, BMO can be used as an alternative OS to HMO for promoting human health. Considering the large amount of whey production globally as a dairy byproduct, the scaleup isolation and production of BMO are possible. The main difference between HMO and BMO is that HMO contains predominantly neutral (fucosylated) OS, whereas BMO contains primarily sialylated OS.179 It is possible to produce functional mimics of HMO by enzymatic glycosylation of BMO.180 Fucosylated OS as a food ingredient originated from BMO is available in marketed infant formulas.181,182
Regulation and safety concerns
Food that has dairy bioactive ingredient additives can be identified by regulatory agencies as a novel or nontraditional food. Such foods are likely to be required to undergo a risk-based assessment before they can be commercialized and marketed. Being derived from BM, the milk bioactive components covered in this review (ie, α-La, LF, GMP, MFGM, and BMO) have been approved by the US Food and Drug Administration as food ingredients that have been generally recognized as safe,183 as well as confirmed by European food safety authorities as a dietary supplement in food products.184,185 In the European Union, α-La has been on the market before 1997, when the Novel Food Regulation was enacted; therefore, α-La does not need safety approval for marketing in the member states of the European Union. The requirement for usage limitations, food labeling, and health claims are regulated by authorities depending on the final applications and consumers. For example, the US Food and Drug Administration approved the use of bovine LF in spray products to decrease microbial pollution of raw beef carcasses at no more than 2% by weight.186
Special attention should be paid to the usage limitation of these bioactive components, especially in infant formulas. The US Food and Drug Administration and the Codex Alimentarius Commission have required that the protein-to-energy ratio of infant formulas should be no less than 1.8 g/100 kcal based on BM content.187 To meet the amino acid requirements of the neonate, the protein content of infant formulas is usually 13–15 g/L for BM-based formulas, which is higher than that of human milk (9–11 g/L).188 However, the higher protein content of infant formulas has been suggested to induce metabolic burden on tissues, such as the liver and kidneys, in infants.189 It was reported that infants fed infant formula with a higher protein content gained weight more quickly than breast-fed infants.190
In June 2020, the US Food and Drug Administration updated the Nutrition Fact Label requirements on food packages. The agency required the new label to be based on updated science and nutrition research, and input from the public. The new label requirement would help consumers more easily recognize the health benefits of various milk bioactive components that are widely used in food products. On the other hand, generally, almost all milk proteins and derivatives may cause allergic reactions, which should be claimed on the labels. Although no direct evidence has shown the allergenicity of these milk bioactives, some immunoglobulin proteins may be retained along with, for example, the isolated protein and MFGM, demonstrating allergenicity.
CONCLUSION
The use of individual milk bioactives has increased in recent years because of their unique properties and health benefits. The COVID-19 pandemic has increased the public’s perception of the important role of functional milk bioactives for human nutrition and immune function.2,191 Recently, there has been a rapid increase in knowledge about the fundamental mechanism of biological action, isolation and purification technology, and production formulation strategies of major milk proteins, bioactive peptides, lipids, and OGs. Membrane filtration, chromatography, and their combinations are major techniques that have been used on an industrial or semi-industrial scale for the separation and purification of milk bioactives from colostrum, milk, and whey. BMO is the exception; its extraction is still in the experimental stage. Moreover, the type and conditions of the separation technique can affect the biological activity of milk bioactives. Industrial thermal processing and the outer environment can further influence the stability of these bioactives, thereby altering their properties and changing the biological activity. Modern, sophisticated micro or nanoencapsulation technology offers potential solutions for protecting sensitive milk bioactive components, promoting in vitro bioavailability and improving sensory properties. Therefore, seeking new isolation and formulation technologies that maximally retain the structural integrity and native properties of these bioactive components is essential for ensuring they can fully preserve and deliver biological function for desirable human health.
Acknowledgments
Funding: The authors acknowledge funding support from the National Dairy Council (Rosemont, Illinois, USA).
Declaration of interest. The authors declare no conflict of interest.
References
- 1. Haug A, Høstmark AT, Harstad OM.. Bovine milk in human nutrition – a review. Lipids Health Dis. 2007;6:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ren G, Cheng G, Wang J.. Understanding the role of milk in regulating human homeostasis in the context of the COVID-19 global pandemic. Trends Food Sci Technol. 2021;107:157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Risk for COVID-19 infection, hospitalization, and death by age group. 2020. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-age.html. Accessed May 6, 2021.
- 4. Santos DI, Saraiva JMA, Vicente AA, et al. Methods for determining bioavailability and bioaccessibility of bioactive compounds and nutrients. In: Innovative Thermal and Non-Thermal Processing, Bioaccessibility and Bioavailability of Nutrients and Bioactive Compounds. Sawston, Cambridge: Woodhead Publishing: Woodhead Publishing Series in Food Science, Technology and Nutrition; 2019:23–54. doi:10.1016/B978-0-12-814174-8.00002-0 [Google Scholar]
- 5. Korhonen HJ. Bioactive milk proteins, peptides and lipids and other functional components derived from milk and bovine colostrum. In: Saarela M, ed. Functional Foods. 2nd ed. Sawston, Cambridge: Woodhead Publishing: Woodhead Publishing Series in Food Science, Technology and Nutrition; 2011:471–511. doi:10.1533/9780857092557.3.471 [Google Scholar]
- 6. Pouliot Y. Membrane processes in dairy technology—from a simple idea to worldwide panacea. Int Dairy J. 2008;18:735–740. [Google Scholar]
- 7. Dallas DC, Meyrand M, Barile D.. Production and bioactivity of bovine milk oligosaccharides. In: Food Oligosaccharides. Hoboken, NJ: John Wiley & Sons, Ltd; 2014:21–34. doi:10.1002/9781118817360.ch2 [Google Scholar]
- 8. Majid S. Bioactive components in milk and dairy products [Bachelor Thesis]. Sweish University of Agricultural Sciences; 2016. Available at: http://urn.kb.se/resolve?urn=urn:nbn:se:slu:epsilon-s-5960. Accessed March 15, 2020.
- 9. Layman DK, Lönnerdal B, Fernstrom JD.. Applications for α-lactalbumin in human nutrition. Nutr Rev. 2018;76:444–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kamau SM, Cheison SC, Chen W, et al. Alpha-lactalbumin: its production technologies and bioactive peptides. Compr Rev Food Sci Food Saf. 2010;9:197–212. [Google Scholar]
- 11. Chatterton DEW, Smithers G, Roupas P, et al. Bioactivity of β-lactoglobulin and α-lactalbumin—technological implications for processing. Int Dairy J. 2006;16:1229–1240. [Google Scholar]
- 12. Barbana C, Pérez MD, Sánchez L, et al. Interaction of bovine α-lactalbumin with fatty acids as determined by partition equilibrium and fluorescence spectroscopy. Int Dairy J. 2006;16:):18–25. [Google Scholar]
- 13. Lönnerdal B, Iyer S.. Lactoferrin: molecular structure and biological function. Annu Rev Nutr. 1995;15:93–110. [DOI] [PubMed] [Google Scholar]
- 14. Wang B, Timilsena YP, Blanch E, et al. Lactoferrin: structure, function, denaturation and digestion. Crit Rev Food Sci Nutr. 2019;59:580–596. [DOI] [PubMed] [Google Scholar]
- 15. King JCJ, Cummings GE, Guo N, et al. A double-blind, placebo-controlled, pilot study of bovine lactoferrin supplementation in bottle-fed infants. J Pediatr Gastroenterol Nutr. 2007;44:245–251. [DOI] [PubMed] [Google Scholar]
- 16. Wang A, Duncan SE, Lesser GJ, et al. Effect of lactoferrin on taste and smell abnormalities induced by chemotherapy: a proteome analysis. Food Funct. 2018;9:4948–4958. [DOI] [PubMed] [Google Scholar]
- 17. Rastogi N, Singh A, Singh PK, et al. Structure of iron saturated C-lobe of bovine lactoferrin at pH 6.8 indicates a weakening of iron coordination. Proteins Struct Funct Bioinforma. 2016;84:591–599. [DOI] [PubMed] [Google Scholar]
- 18. Feeney S, Joshi L, Hickey RM.. Biological roles and production technologies associated with bovine glycomacropeptide. In: Maria Hayes, ed. Novel Proteins for Food, Pharmaceuticals and Agriculture. Hoboken, NJ: John Wiley & Sons, Ltd; 2018:1–28. doi:10.1002/9781119385332.ch1 [Google Scholar]
- 19. Córdova-Dávalos LE, Jiménez M, Salinas E.. Glycomacropeptide bioactivity and health: a review highlighting action mechanisms and signaling pathways. Nutrients. 2019;11:598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thomä-Worringer C, Sørensen J, López-Fandiño R.. Health effects and technological features of caseinomacropeptide. Int Dairy J. 2006;16:1324–1333. [Google Scholar]
- 21. Neelima Sharma R, Rajput YS, Mann B.. Chemical and functional properties of glycomacropeptide (GMP) and its role in the detection of cheese whey adulteration in milk: a review. Dairy Sci Technol. 2013;93:21–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Le TT, Van Camp J, Dewettinck K.. Chapter 12. Milk fat globule membrane material: isolation techniques, health-beneficial properties, and potential applications. In: Atta-ur-Rahman, ed. Studies in Natural Products Chemistry, Vol 41. Amsterdam, Netherlands: Elsevier; 2014:347–382. doi:10.1016/B978-0-444-63294-4.00012-7. [Google Scholar]
- 23. Dewettinck K, Rombaut R, Thienpont N, et al. Nutritional and technological aspects of milk fat globule membrane material. Int Dairy J. 2008;18:436–457. [Google Scholar]
- 24. Fontecha J, Brink L, Wu S, et al. Sources, production, and clinical treatments of milk fat globule membrane for infant nutrition and well-being. Nutrients. 2020;12:1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Timby N, Domellöf M, Lönnerdal B, et al. Supplementation of infant formula with bovine milk fat globule membranes. Adv Nutr. 2017;8:351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Robinson RC. Structures and metabolic properties of bovine milk oligosaccharides and their potential in the development of novel therapeutics. Front Nutr. 2019;6:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zivkovic AM, Barile D.. Bovine milk as a source of functional oligosaccharides for improving human health. Adv Nutr. 2011;2:284–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Barile D, Tao N, Lebrilla CB, et al. Permeate from cheese whey ultrafiltration is a source of milk oligosaccharides. Int Dairy J. 2009;19:524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Argenta A, Scheer A.. Membrane separation processes applied to whey: a review. Food Rev Int. 2019;36:1–30. [Google Scholar]
- 30. Carter BG, Cheng N, Kapoor R, et al. Invited review: microfiltration-derived casein and whey proteins from milk. J Dairy Sci. 2021;104:2465–2479. [DOI] [PubMed] [Google Scholar]
- 31. Sgarbieri VC, et al. Advancements in obtaining and utilizing bovine milk proteins in foods and nutrition. In: Barbosa-Cánovas GV, María Pastore G, Candoğan K, eds. Global Food Security and Wellness. New York City, NY: Springer; 2017:141–174. [Google Scholar]
- 32. Qiu Y, Smith TJ, Foegeding EA, et al. The effect of microfiltration on color, flavor, and functionality of 80% whey protein concentrate. J Dairy Sci. 2015;98:5862–5873. [DOI] [PubMed] [Google Scholar]
- 33. Mercier-Bouchard D, Benoit S, Doyen A, et al. Process efficiency of casein separation from milk using polymeric spiral-wound microfiltration membranes. J Dairy Sci. 2017;100:8838–8848. [DOI] [PubMed] [Google Scholar]
- 34. Hernández A, Harte FM.. Isolation of caseins from whey proteins by microfiltration modifying the mineral balance in skim milk. J Dairy Sci. 2009;92:5357–5362. [DOI] [PubMed] [Google Scholar]
- 35. Cheryan M. Ultrafiltration and Microfiltration Handbook. Boca Raton, FL: CRC Press; 1998. doi:10.1201/9781482278743. [Google Scholar]
- 36. Baldasso C, Barros TC, Tessaro IC.. Concentration and purification of whey proteins by ultrafiltration. Desalination. 2011;278:381–386. [Google Scholar]
- 37. Shen C-H. Chapter 8. Quantification and analysis of proteins. In: Shen C-H, ed. Diagnostic Molecular Biology. Cambridge, MA: Academic Press; 2019:187–214. doi:10.1016/B978-0-12-802823-0.00008-0. [Google Scholar]
- 38. Chen Q, Zhao L, Yao L, et al. Technological Approaches for Novel Applications in Dairy Processing. London, UK: IntechOpen; 2018. doi:10.5772/intechopen.76320.
- 39. Andrés LJ, Riera FA, Alvarez R.. Skimmed milk demineralization by electrodialysis: conventional versus selective membranes. J Food Eng. 1995;26:57–66. [Google Scholar]
- 40. Chen GQ, Eschbach FII, Weeks M, et al. Removal of lactic acid from acid whey using electrodialysis. Sep Purif Technol. 2016;158:230–237. [Google Scholar]
- 41. Xu Y, Mazzawi M, Chen K, et al. Protein purification by polyelectrolyte coacervation: influence of protein charge anisotropy on selectivity. Biomacromolecules. 2011;12:1512–1522. [DOI] [PubMed] [Google Scholar]
- 42. Ganju S, Gogate PR.. A review on approaches for efficient recovery of whey proteins from dairy industry effluents. J Food Eng. 2017;215:84–96. [Google Scholar]
- 43. Bramaud C, Aimar P, Daufin G.. Optimisation of a whey protein fractionation process based on the selective precipitation of α-lactalbumin. Lait. 1997;77:411–423. [Google Scholar]
- 44. Tolkach A, Steinle S, Kulozik U.. Optimization of thermal pretreatment conditions for the separation of native α-lactalbumin from whey protein concentrates by means of selective denaturation of β-lactoglobulin. J Food Sci. 2006;70:E557–E566. [Google Scholar]
- 45. Toro-Sierra J, Tolkach A, Kulozik U.. Fractionation of α-lactalbumin and β-lactoglobulin from whey protein isolate using selective thermal aggregation, an optimized membrane separation procedure and resolubilization techniques at pilot plant scale. Food Bioprocess Technol. 2013;6:1032–1043. [Google Scholar]
- 46. Haller N, Kulozik U.. Continuous centrifugal separation of selectively precipitated α-lactalbumin. Int Dairy J. 2020;101:104566. [Google Scholar]
- 47. Cheang B, , Zydney AL.. Separation of alpha-lactalbumin and beta-lactoglobulin using membrane ultrafiltration. Biotechnol Bioeng. 2003;83:201–209. 9 [DOI] [PubMed] [Google Scholar]
- 48. Muller A, Daufin G, Chaufer B.. Ultrafiltration modes of operation for the separation of α-lactalbumin from acid casein whey. J Membr Sci. 1999;153:9–21. [Google Scholar]
- 49. Cheison SC, Leeb E, Toro-Sierra J, et al. Influence of hydrolysis temperature and pH on the selective hydrolysis of whey proteins by trypsin and potential recovery of native alpha-lactalbumin. Int Dairy J. 2011;21:166–171. [Google Scholar]
- 50. Konrad G, Kleinschmidt T.. A new method for isolation of native α-lactalbumin from sweet whey. Int Dairy J. 2008;18:47–54. [Google Scholar]
- 51. Lisak K, Toro-Sierra J, Kulozik U, et al. Chymotrypsin selectively digests β-lactoglobulin in whey protein isolate away from enzyme optimal conditions: potential for native α-lactalbumin purification. J Dairy Res. 2013;80:14–20. [DOI] [PubMed] [Google Scholar]
- 52. Geng XL, Tolkach A, Otte J, et al. Pilot-scale purification of α-lactalbumin from enriched whey protein concentrate by anion-exchange chromatography and ultrafiltration. Dairy Sci & Technol. 2015;95:353–368. [Google Scholar]
- 53. Neyestani TR, Djalali M, Pezeshki M.. Isolation of α-lactalbumin, β-lactoglobulin, and bovine serum albumin from cow’s milk using gel filtration and anion-exchange chromatography including evaluation of their antigenicity. Protein Expr Purif. 2003;29:202–208. [DOI] [PubMed] [Google Scholar]
- 54. Ye X, Yoshida S, Ng TB.. Isolation of lactoperoxidase, lactoferrin, α-lactalbumin, β-lactoglobulin B and β-lactoglobulin A from bovine rennet whey using ion exchange chromatography. Int J Biochem Cell Biol. 2000;32:1143–1150. [DOI] [PubMed] [Google Scholar]
- 55. Mao X, Zhang G-F, Li C, et al. One-step method for the isolation of α-lactalbumin and β-lactoglobulin from cow’s milk while preserving their antigenicity. Int J Food Prop. 2017;20:792–800. [Google Scholar]
- 56. Conrado LS, Veredas V, Nóbrega ES, et al. Concentration of alpha-lactalbumin from cow milk whey through expanded bed adsorption using a hydrophobic resin. Braz J Chem Eng. 2005;22:501–509. [Google Scholar]
- 57. Touhami S, Chamberland J, Perreault V, et al. Coupling high hydrostatic pressure and ultrafiltration for fractionation of alpha-lactalbumin from skim milk. Sep Sci Technol. 2021;56:1102–1111. [Google Scholar]
- 58. Liang Y, Wang X, Wu M, et al. Simultaneous isolation of lactoferrin and lactoperoxidase from bovine colostrum by SPEC 70 SLS cation exchange resin. Int J Environ Res Public Health. 2011;8:3764–3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lu RR, Xu SY, Wang Z, et al. Isolation of lactoferrin from bovine colostrum by ultrafiltration coupled with strong cation exchange chromatography on a production scale. J Membr Sci. 2007;297:152–161. [Google Scholar]
- 60. Hirsch DB, Martínez Álvarez LM, Urtasun N, et al. Lactoferrin purification and whey protein isolate recovery from cheese whey using chitosan mini-spheres. Int Dairy J. 2020;109:104764. [Google Scholar]
- 61. Matijašić BB, Oberčkal J, Mohar Lorbeg P, et al. Characterisation of lactoferrin isolated from acid whey using pilot-scale monolithic ion-exchange chromatography. Processes. 2020;8:804. [Google Scholar]
- 62. Teepakorn C, Fiaty K, Charcosset C.. Optimization of lactoferrin and bovine serum albumin separation using ion-exchange membrane chromatography. Sep Purif Technol. 2015;151:292–302. [Google Scholar]
- 63. Brisson G, Britten M, Pouliot Y.. Electrically-enhanced crossflow microfiltration for separation of lactoferrin from whey protein mixtures. J Membr Sci. 2007;297:206–216. [Google Scholar]
- 64. Ndiaye N, Pouliot Y, Saucier L, et al. Electroseparation of bovine lactoferrin from model and whey solutions. Sep Purif Technol. 2010;74:93–99. [Google Scholar]
- 65. Wang Q, Chen GQ, Kentish SE.. Isolation of lactoferrin and immunoglobulins from dairy whey by an electrodialysis with filtration membrane process. Sep Purif Technol. 2020;233:115987. [Google Scholar]
- 66. Baieli MF, Urtasun N, Miranda MV, et al. Isolation of lactoferrin from whey by dye-affinity chromatography with yellow HE-4R attached to chitosan mini-spheres. Int Dairy J. 2014;39:53–59. [Google Scholar]
- 67. Chen L, Guo C, Guan Y, et al. Isolation of lactoferrin from acid whey by magnetic affinity separation. Sep Purif Technol. 2007;56:168–174. [Google Scholar]
- 68. Lönnerdal B, Carlsson J, Porath J.. Isolation of lactoferrin from human milk by metal-chelate affinity chromatography. FEBS Lett. 1977;75:89–92. [DOI] [PubMed] [Google Scholar]
- 69. Santos MJ, Teixeira JA, Rodrigues LR.. Fractionation and recovery of whey proteins by hydrophobic interaction chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:475–479. [DOI] [PubMed] [Google Scholar]
- 70. Maciel KS, Santos LS, Bonomo RCF, et al. Purification of lactoferrin from sweet whey using ultrafiltration followed by expanded bed chromatography. Sep Purif Technol. 2020;251:117324. [Google Scholar]
- 71. Nakano T, Ozimek L, Betti M.. Separation of bovine κ-casein glycomacropeptide from sweet whey protein products with undetectable level of phenylalanine by protein precipitation followed by anion exchange chromatography. J Dairy Res. 2018;85:110–113. [DOI] [PubMed] [Google Scholar]
- 72. Nakano T, Betti M.. Isolation of κ-casein glycomacropeptide from bovine whey fraction using food grade anion exchange resin and chitin as an adsorbent. J Dairy Res. 2020;87:127–133. [DOI] [PubMed] [Google Scholar]
- 73. Davis ME, Ming F, Yang M, et al. Isolation of glycoproteins from bovine milk. 2004. https://patents.google.com/patent/US6800739B2/en?q=glycomacropeptide&assignee=Agropur&oq=Agropur+glycomacropeptide. Accessed April 22, 2021.
- 74. Etzel MR, Helm TR, Vyas HK. Methods involving whey protein isolates. 2011. https://patents.google.com/patent/US8071152B2/en. Accessed April 22, 2021.
- 75. Nakano T, Silva‐Hernandez ER, Ikawa N, et al. Purification of κ-casien glycomacropeptide from sweet whey with undetectable level of phenylalanine. Biotechnol Prog. 2002;18:409–412. [DOI] [PubMed] [Google Scholar]
- 76. Baieli MF, Urtasun N, Martinez MJ, et al. Affinity chromatography matrices for depletion and purification of casein glycomacropeptide from bovine whey. Biotechnol Prog. 2017;33:171–180. [DOI] [PubMed] [Google Scholar]
- 77. Silva-Hernandez ER, Nakano T, Ozimek L.. Isolation and analysis of κ-casein glycomacropeptide from goat sweet whey. J Agric Food Chem. 2002;50:2034–2038. [DOI] [PubMed] [Google Scholar]
- 78. Berrocal R, Neeser J-R. Production of kappa-caseino-glycomacropeptide. 1993. https://patents.google.com/patent/US5216129A/en?q=glycomacropeptide&assignee=Agropur&oq=Agropur+glycomacropeptide. Accessed April 22, 2021.
- 79. Rojas E, Torres G.. Isolation and recovery of glycomacropeptide from milk whey by means of thermal treatment. Food Sci Technol. 2013;33:14–20. [Google Scholar]
- 80. Nakano T, Watanabe T, Ikawa N, et al. Cellulose acetate electrophoresis of whey proteins and glycomacropeptide. Milchwissenschaft. 2009;64:291–295. [Google Scholar]
- 81. Hansen SF, Hogan SA, Tobin J, et al. Microfiltration of raw milk for production of high-purity milk fat globule membrane material. J Food Eng. 2020;276:109887. [Google Scholar]
- 82. Le TT, Van Camp J, Rombaut R, et al. Effect of washing conditions on the recovery of milk fat globule membrane proteins during the isolation of milk fat globule membrane from milk. J Dairy Sci. 2009;92:3592–3603. [DOI] [PubMed] [Google Scholar]
- 83. Ye A, Singh H, Taylor MW, et al. Interactions of whey proteins with milk fat globule membrane proteins during heat treatment of whole milk. Lait. 2004;84:269–283. [Google Scholar]
- 84. Fuller KL, Kuhlenschmidt TB, Kuhlenschmidt MS, et al. Milk fat globule membrane isolated from buttermilk or whey cream and their lipid components inhibit infectivity of rotavirus in vitro. J Dairy Sci. 2013;96:3488–3497. [DOI] [PubMed] [Google Scholar]
- 85. Morin P, Britten M, Jiménez-Flores R, et al. Microfiltration of buttermilk and washed cream buttermilk for concentration of milk fat globule membrane components. J Dairy Sci. 2007;90:2132–2140. [DOI] [PubMed] [Google Scholar]
- 86. Rombaut R, Dejonckheere V, Dewettinck K.. Microfiltration of butter serum upon casein micelle destabilization. J Dairy Sci. 2006;89:1915–1925. [DOI] [PubMed] [Google Scholar]
- 87. Rombaut R, Dejonckheere V, Dewettinck K.. Filtration of milk fat globule membrane fragments from acid buttermilk cheese whey. J Dairy Sci. 2007;90:1662–1673. [DOI] [PubMed] [Google Scholar]
- 88. Holzmüller W, Gmach O, Griebel A, et al. Casein precipitation by acid and rennet coagulation of buttermilk: impact of pH and temperature on the isolation of milk fat globule membrane proteins. Int Dairy J. 2016;63:115–123. [Google Scholar]
- 89. Robinson RC, Colet E, Tian T, et al. An improved method for the purification of milk oligosaccharides by graphitised carbon-solid phase extraction. Int Dairy J. 2018;80:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ward RE. Isolation of milk oligosaccharides using solid-phase extraction. Open Glycosci. 2009;2:9–15. [Google Scholar]
- 91. Altmann K, Wutkowski A, Kämpfer S, et al. Comparison of the efficiency of different NF membranes for the enrichment of milk oligosaccharides from bovine milk. Eur Food Res Technol. 2015;241:803–815. [Google Scholar]
- 92. Cohen JL, Barile D, Liu Y, et al. Role of pH in the recovery of bovine milk oligosaccharides from colostrum whey permeate by nanofiltration. Int Dairy J. 2017;66:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. de Moura Bell JMLN, Cohen JL, de Aquino LFMC, et al. An integrated bioprocess to recover bovine milk oligosaccharides from colostrum whey permeate. J Food Eng. 2018;216:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bottomley RC. Process for obtaining concentrates having a high α-lactalbumin content from whey. 1991. https://patents.google.com/patent/US5008376A/en. Accessed April 1, 2021.
- 95. Lin T, O'Keefe S, Duncan S, et al. Manipulation of the dry bean (Phaseolus vulgaris L.) matrix by hydrothermal and high-pressure treatments: impact on in vitro bile salt-binding ability. Food Chem. 2019;310:125699. [DOI] [PubMed] [Google Scholar]
- 96. Zou H, Lin T, Bi X, et al. Comparison of high hydrostatic pressure, high-pressure carbon dioxide and high-temperature short-time processing on quality of mulberry juice. Food Bioprocess Technol. 2016;9:217–231. [Google Scholar]
- 97. Lin T, O'Keefe S, Duncan S, et al. Retention of primary bile salts by dry beans (Phaseolus vulgaris L.) during in vitro digestion: role of bean components and effect of food processing. Food Res Int. 2020;137:109337. [DOI] [PubMed] [Google Scholar]
- 98. Lin T, Fernández-Fraguas C.. Effect of thermal and high-pressure processing on the thermo-rheological and functional properties of common bean (Phaseolus vulgaris L.) flours. LWT. 2020;127:109325. [Google Scholar]
- 99. Xu Z, Lin T, Wang Y, et al. Quality assurance in pepper and orange juice blend treated by high pressure processing and high temperature short time. Innov Food Sci Emerg Technol. 2015;31:28–36. [Google Scholar]
- 100. Wang S, Lin T, Man G, et al. Effects of anti-browning combinations of ascorbic acid, citric acid, nitrogen and carbon dioxide on the quality of banana smoothies. Food Bioprocess Technol. 2014;7:161–173. [Google Scholar]
- 101. Tomita M, Wakabayashi H, Yamauchi K, et al. Bovine lactoferrin and lactoferricin derived from milk: production and applications. Biochem Cell Biol. 2002;80(1):109–112. doi:10.1139/o01-230. [DOI] [PubMed] [Google Scholar]
- 102. Almécija MC, Ibáñez R, Guadix A, et al. Influence of pH in the recovery of lactoferrin from whey with ceramic membranes. Desalination. 2006;200:475–476. [Google Scholar]
- 103. Nyström M, Aimar P, Luque S, et al. Fractionation of model proteins using their physiochemical properties. Colloids Surf Physicochem Eng Asp. 1998;138:185–205. [Google Scholar]
- 104. Tanimoto M, Kawasaki Y, Dosako S, et al. Large-scale preparation of κ-casein glycomacropeptide from rennet casein whey. Biosci Biotechnol Biochem. 1992;56:140–141. [DOI] [PubMed] [Google Scholar]
- 105. Outinen M, Tossavainen O, Syväoja E-L, et al. Chromatographic isolation of k-casein macropeptide from cheese whey with a strong basic anion exchange resin. 1995. https://jukuri.luke.fi/handle/10024/469227. Accessed April 3, 2021.
- 106. Morin P, Jiménez-Flores R, Pouliot Y.. Effect of processing on the composition and microstructure of buttermilk and its milk fat globule membranes. Int Dairy J. 2007;17:1179–1187. [Google Scholar]
- 107. Corredig M, Roesch RR, Dalgleish DG.. Production of a novel ingredient from buttermilk. J Dairy Sci. 2003;86:2744–2750. [DOI] [PubMed] [Google Scholar]
- 108. Holzmüller W, Kulozik U.. Technical difficulties and future challenges in isolating membrane material from milk fat globules in industrial settings – a critical review. Int Dairy J. 2016;61:51–66. [Google Scholar]
- 109. Verardo V, Gómez-Caravaca AM, Arráez-Román D, et al. Recent advances in phospholipids from colostrum, milk and dairy by-products. Int J Mol Sci. 2017;18:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Boyd LC, Drye NC, Hansen AP.. Isolation and characterization of whey phospholipids. J Dairy Sci. 1999;82:2550–2557. [DOI] [PubMed] [Google Scholar]
- 111. Costa MR, Elias-Argote XE, Jiménez-Flores R, et al. Use of ultrafiltration and supercritical fluid extraction to obtain a whey buttermilk powder enriched in milk fat globule membrane phospholipids. Int Dairy J. 2010;20:598–602. [Google Scholar]
- 112. Catchpole OJ, Tallon SJ, Grey JB, et al. Extraction of lipids from a specialist dairy stream. J Supercrit Fluids. 2008;45:314–321. [Google Scholar]
- 113. Sarney DB, Hale C, Frankel G, et al. A novel approach to the recovery of biologically active oligosaccharides from milk using a combination of enzymatic treatment and nanofiltration. Biotechnol Bioeng. 2000;69:461–467. [DOI] [PubMed] [Google Scholar]
- 114. Nongonierma AB, FitzGerald RJ.. Milk proteins as a source of tryptophan-containing bioactive peptides. Food Funct. 2015;6:2115–2127. [DOI] [PubMed] [Google Scholar]
- 115. Atehli D, Wang J, Yu J, et al. Effects of mono- and diglycerides of fatty acids on the milk fat globule membrane after heat treatment. Int J Dairy Technol. 2020;73:667–673. [Google Scholar]
- 116. Hayashi R, Kameda I.. Racemization of amino acid residues during alkali-treatment of protein and its adverse effect on pepsin digestibility. Agric Biol Chem. 1980;44:891–895. [Google Scholar]
- 117. Wang A, Duncan SE, Whalley NW, et al. Interaction effect of LED color temperatures and light-protective additive packaging on photo-oxidation in milk displayed in retail dairy case. Food Chem. 2020;323:126699. [DOI] [PubMed] [Google Scholar]
- 118. Bouzas J, Kamarei AR, Karel M.. Storage stability of milkfat globule membrane. J Food Processing Preservation. 1985;9:11–24. [Google Scholar]
- 119. Wijayanti HB, Bansal N, Deeth HC.. Stability of whey proteins during thermal processing: a review. Comprehen Rev Food Sci Food Saf. 2014;13:1235–1251. [Google Scholar]
- 120. Bokkhim H, Bansal N, Grøndahl L, et al. In-vitro digestion of different forms of bovine lactoferrin encapsulated in alginate micro-gel particles. Food Hydrocoll. 2016;52:231–242. [Google Scholar]
- 121. Gong C, Lee MC, Godec M, et al. Ultrasonic encapsulation of cinnamon flavor to impart heat stability for baking applications. Food Hydrocoll. 2020;99:105316. [Google Scholar]
- 122. Zhang Z, Cho S, Dadmohammadi Y, et al. Improvement of the storage stability of C-phycocyanin in beverages by high-pressure processing. Food Hydrocoll. 2021;110:106055. [Google Scholar]
- 123. Tan C, Pajoumshariati S, Arshadi M, et al. A simple route to renewable high internal phase emulsions (HIPEs) strengthened by successive cross-linking and electrostatics of polysaccharides. Chem Commun (Camb). 2019;55:1225–1228. [DOI] [PubMed] [Google Scholar]
- 124. Yan B, Davachi SM, Ravanfar R, et al. Improvement of vitamin C stability in vitamin gummies by encapsulation in casein gel. Food Hydrocoll. 2021;113:106414. [Google Scholar]
- 125. Lee MC, Tan C, Abbaspourrad A.. Combination of internal structuring and external coating in an oleogel-based delivery system for fish oil stabilization. Food Chem. 2019;277:213–221. [DOI] [PubMed] [Google Scholar]
- 126. Jannasari N, Fathi M, Moshtaghian SJ, et al. Microencapsulation of vitamin D using gelatin and cress seed mucilage: production, characterization and in vivo study. Int J Biol Macromol. 2019;129:972–979. [DOI] [PubMed] [Google Scholar]
- 127. Nazzaro F, Orlando P, Fratianni F, et al. Microencapsulation in food science and biotechnology. Curr Opin Biotechnol. 2012;23:182–186. [DOI] [PubMed] [Google Scholar]
- 128. Tolve R, Galgano F, Caruso MC, et al. Encapsulation of health-promoting ingredients: applications in foodstuffs. Int J Food Sci Nutr. 2016;67:888–918. [DOI] [PubMed] [Google Scholar]
- 129. Ezhilarasi PN, Karthik P, Chhanwal N, et al. Nanoencapsulation techniques for food bioactive components: a review. Food Bioprocess Technol. 2013;6:628–647. [Google Scholar]
- 130. Ye Q, Georges N, Selomulya C.. Microencapsulation of active ingredients in functional foods: from research stage to commercial food products. Trends Food Sci Technol. 2018;78:167–179. [Google Scholar]
- 131. Zuidam NJ, Shimoni E.. Overview of microencapsulates for use in food products or processes and methods to make them. In: Zuidam NJ, Nedovic V, eds. Encapsulation Technologies for Active Food Ingredients and Food Processing. New York City, NY: Springer; 2010:3–29. doi:10.1007/978-1-4419-1008-0_2. [Google Scholar]
- 132. Wang B, Blanch E, Barrow CJ, et al. Preparation and study of digestion behavior of lactoferrin-sodium alginate complex coacervates. J Funct Foods. 2017;37:97–106. [Google Scholar]
- 133. Zhang W, Zhong Q.. Microemulsions as nanoreactors to produce whey protein nanoparticles with enhanced heat stability by thermal pretreatment. Food Chem. 2010;119:1318–1325. [DOI] [PubMed] [Google Scholar]
- 134. Moutinho C, Teixeira JA, Balcão VM. Structural and Functional Stabilization of Glycomacropeptide via Encapsulation within Multiple Emulsions. Presented at: Microbiotec’11; 2011; Braga, Portugal. [Google Scholar]
- 135. Balcão VM, Costa CI, Matos CM, et al. Nanoencapsulation of bovine lactoferrin for food and biopharmaceutical applications. Food Hydrocoll. 2013;32:425–431. [Google Scholar]
- 136. Bastos LPH, de Carvalho CWP, Garcia-Rojas EE.. Formation and characterization of the complex coacervates obtained between lactoferrin and sodium alginate. Int J Biol Macromol. 2018;120:332–338. [DOI] [PubMed] [Google Scholar]
- 137. da S. Gulão E, de Souza CJF, da Silva FAS, et al. Complex coacervates obtained from lactoferrin and gum arabic: formation and characterization. Food Res Int. 2014;65:367–374. [Google Scholar]
- 138. Bengoechea C, Jones OG, Guerrero A, et al. Formation and characterization of lactoferrin/pectin electrostatic complexes: impact of composition, pH and thermal treatment. Food Hydrocoll. 2011;25:1227–1232. [Google Scholar]
- 139. Mendanha DV, Molina Ortiz SE, Favaro-Trindade CS, et al. Microencapsulation of casein hydrolysate by complex coacervation with SPI/pectin. Food Res Int. 2009;42:1099–1104. [Google Scholar]
- 140. Liu W, Ye A, Liu W, et al. Stability during in vitro digestion of lactoferrin-loaded liposomes prepared from milk fat globule membrane-derived phospholipids. J Dairy Sci. 2013;96:2061–2070. [DOI] [PubMed] [Google Scholar]
- 141. Vergara D, Shene C.. Encapsulation of lactoferrin into rapeseed phospholipids based liposomes: optimization and physicochemical characterization. J Food Eng. 2019;262:29–38. [Google Scholar]
- 142. Mohan A, McClements DJ, Udenigwe CC.. Encapsulation of bioactive whey peptides in soy lecithin-derived nanoliposomes: influence of peptide molecular weight. Food Chem. 2016;213:143–148. [DOI] [PubMed] [Google Scholar]
- 143. Poshadri A, Aparna K.. Microencapsulation technology: a review. J Res Angrau. 2010;38:86–102. [Google Scholar]
- 144. Okuro PK, Junior M, de FE, Favaro-Trindade CS.. Technological challenges for spray chilling encapsulation of functional food ingredients. Food Technol Biotechnol. 2013;51:171–182. [Google Scholar]
- 145. Gouin S. Microencapsulation: industrial appraisal of existing technologies and trends. Trends Food Sci Technol. 2004;15:330–347. [Google Scholar]
- 146. Guignon B, Duquenoy A, Dumoulin ED.. Fluid bed encapsulation of particles: principles and practice. Dry Technol. 2002;20:419–447. [Google Scholar]
- 147. Ai F, Yan J, Ruan H, et al. Preparation and characterization of hydroxyapatite macrobeads based on pneumatic extrusion dripping. Ceram Int. 2019;45:16399–16404. [Google Scholar]
- 148. Zasypkin D, Porzio M.. Glass encapsulation of flavours with chemically modified starch blends. J Microencapsul. 2004;21:385–397. [DOI] [PubMed] [Google Scholar]
- 149. Rukluarh S, Kanjanapongkul K, Panchan N. Effect of inclusion conditions on characteristics of spray dried whey protein hydrolysate/γ-cyclodextrin complexes. 2019.
- 150. Xiao Z, Hou W, Kang Y, et al. Encapsulation and sustained release properties of watermelon flavor and its characteristic aroma compounds from γ-cyclodextrin inclusion complexes. Food Hydrocoll. 2019;97:105202. [Google Scholar]
- 151. Hong Y-H, Creamer LK.. Changed protein structures of bovine β-lactoglobulin B and α-lactalbumin as a consequence of heat treatment. Int Dairy J. 2002;12:345–359. [Google Scholar]
- 152. McGuffey MK, Epting KL, Kelly RM, et al. Denaturation and aggregation of three α-lactalbumin preparations at neutral pH. J Agric Food Chem. 2005;53:3182–3190. [DOI] [PubMed] [Google Scholar]
- 153. Kilic E, Novoselova MV, Lim SH, et al. Formulation for oral delivery of lactoferrin based on bovine serum albumin and tannic acid multilayer microcapsules. Sci Rep. 2017;7:44159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Anema SG, (Kees) de Kruif CG.. Phase separation and composition of coacervates of lactoferrin and caseins. Food Hydrocoll. 2016;52:670–677. [Google Scholar]
- 155. Niu Z, Loveday SM, Barbe V, et al. Protection of native lactoferrin under gastric conditions through complexation with pectin and chitosan. Food Hydrocoll. 2019;93:120–130. [Google Scholar]
- 156.Meticulous Market Research. Dairy Ingredients Market Worth $71.5 billion by 2027 - Exclusive Report by Meticulous Research. GlobeNewswire News Room. 2020. https://www.globenewswire.com/news-release/2020/06/10/2046096/0/en/Dairy-Ingredients-Market-Worth-71-5-billion-by-2027-Exclusive-Report-by-Meticulous-Research.html. Accessed May 3, 2021.
- 157. Korhonen H, Pihlanto A.. Technological options for the production of health-promoting proteins and peptides derived from milk and colostrum. Curr Pharm Des. 2007;13:829–843. [DOI] [PubMed] [Google Scholar]
- 158.Mintel Group Ltd. Mintel GNPD - Global New Products Database: CPG and FMC. Mintel. Available at: https://www.mintel.com/global-new-products-database. Accessed November 11, 2021. [Google Scholar]
- 159. Graveland-Bikker JF, de Kruif CG.. Unique milk protein based nanotubes: food and nanotechnology meet. Trends Food Sci Technol. 2006;17:196–203. [Google Scholar]
- 160. Liu B, Liu B, Wang R, et al. α-Lactalbumin self-assembled nanoparticles with various morphologies, stiffnesses, and sizes as pickering stabilizers for oil-in-water emulsions and delivery of curcumin. J Agric Food Chem. 2021;69:2485–2492. [DOI] [PubMed] [Google Scholar]
- 161. Katouzian I, Jafari SM.. Nanotubes of α-lactalbumin for encapsulation of food ingredients. In: Jafari SM, ed. Biopolymer Nanostructures for Food Encapsulation Purposes. Nanoencapsulation in the Food Industry. Cambridge, MA: Academic Press; 2019:101–124. [Google Scholar]
- 162. Geng XL, Bjerrum MJ, Arleth L, et al. Formation of nanotubes and gels at a broad pH range upon partial hydrolysis of bovine α-lactalbumin. Int Dairy J. 2016;52:72–81. [Google Scholar]
- 163. Sozer N, Kokini JL.. Nanotechnology and its applications in the food sector. Trends Biotechnol. 2009;27:82–89. [DOI] [PubMed] [Google Scholar]
- 164. Legrand D. Overview of lactoferrin as a natural immune modulator. J Pediatr. 2016;173:S10–S15. [DOI] [PubMed] [Google Scholar]
- 165. Tomita M, Wakabayashi H, Shin K, et al. Twenty-five years of research on bovine lactoferrin applications. Biochimie. 2009;91:52–57. [DOI] [PubMed] [Google Scholar]
- 166. Shin K, Yaegaki K, Murata T, et al. Effects of a composition containing lactoferrin and lactoperoxidase on oral malodor and salivary bacteria: a randomized, double-blind, crossover, placebo-controlled clinical trial. Clin Oral Investig. 2011;15:485–493. [DOI] [PubMed] [Google Scholar]
- 167. Kaito M, Iwasa M, Fujita N, et al. Effect of lactoferrin in patients with chronic hepatitis C: combination therapy with interferon and ribavirin. J Gastroenterol Hepatol. 2007;22:1894–1897. [DOI] [PubMed] [Google Scholar]
- 168. Wang WY, Wong JH, Ip DTM, et al. Bovine lactoferrampin, human lactoferricin, and lactoferrin 1-11 inhibit nuclear translocation of HIV integrase. Appl Biochem Biotechnol. 2016;179:1202–1212. [DOI] [PubMed] [Google Scholar]
- 169. Serrano G, Kochergina I, Albors A, et al. Liposomal lactoferrin as potential preventative and cure for COVID-19. Int J Res Health Sci. 2020;8:08–15. [Google Scholar]
- 170. Song J, Gao J, Du M, et al. Casein glycomacropeptide hydrolysates ameliorate hepatic insulin resistance of C57BL/6J mice challenged with high-fat diet. J Funct Foods. 2018;45:190–198. [Google Scholar]
- 171. Zaki OK, El-Wakeel L, Ebeid Y, et al. The use of glycomacropeptide in dietary management of phenylketonuria. J Nutr Metab. 2016;2016:2453027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Bergia RE III, Hudson JL, Campbell WW.. Effect of whey protein supplementation on body composition changes in women: a systematic review and meta-analysis. Nutr Rev. 2018;76:539–551. [DOI] [PubMed] [Google Scholar]
- 173. Zhang YP, Gaffar A. Fluoride free dental remineralization. 2001. https://patents.google.com/patent/US6207138B1/en. Accessed April 10, 2021.
- 174. Martín-Diana AB, Frías J, Fontecha FJ. Emulsifying properties of whey protein concentrate and caseinomacropeptide of cow, ewe and goat. 2005. https://digital.csic.es/handle/10261/121597. Accessed April 10, 2021.
- 175. Morales R, Martinez MJ, Pilosof AMR.. Synergistic effect of casein glycomacropeptide on sodium caseinate foaming properties. Colloids Surf B Biointerfaces. 2017;159:501–508. [DOI] [PubMed] [Google Scholar]
- 176. Morales R, Martinez MJ, Pilosof AMR.. Dynamics of gelation, textural and microstructural properties of gelatin gels in the presence of casein glycomacropeptide. Food Res Int. 2016;84:102–107. [Google Scholar]
- 177. Rosqvist F, Smedman A, Lindmark-Månsson H, et al. Potential role of milk fat globule membrane in modulating plasma lipoproteins, gene expression, and cholesterol metabolism in humans: a randomized study. Am J Clin Nutr. 2015;102:20–30. [DOI] [PubMed] [Google Scholar]
- 178. Banavara D, Alvey JD, Gonzalez JM, Nutritional compositions containing structured fat globules and uses thereof. 2019. Accessed April 11, 2021. https://patents.google.com/patent/US10455854B2/en
- 179. Bode L, Contractor N, Barile D, et al. Overcoming the limited availability of human milk oligosaccharides: challenges and opportunities for research and application. Nutr Rev. 2016;74:635–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Weinborn V, Li Y, Shah IM, et al. Production of functional mimics of human milk oligosaccharides by enzymatic glycosylation of bovine milk oligosaccharides. Int Dairy J. 2020;102:104583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gerber® Good Start® GentlePro Powder Infant Formula. Gerber.com. https://www.gerber.com/gerber-good-start-gentle-powder-formula. Accessed April 11, 2021.
- 182.Similac Pro-Advance® Infant Formula, with 2′-FL HMO for Immune Support, Non-GMO, Baby Formula, Powder, 1.45 lb. https://www.similac.com/products/baby-formula/pro-advance-powder/1-45lb.html. Accessed April 11, 2021.
- 183.US Food and Drug Administration. The inventory of GRAS notices. https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=GRASNotices. Accessed April 11, 2021
- 184.European Food Safety Authority. Opinion of the Scientific Panel on dietetic products, nutrition and allergies [NDA] related to the safety and suitability for particular nutritional use by infants of formula based on whey protein partial hydrolysates with a protein content of at least 1.9 g protein/100 kcal. EFSA J. 2005;3:280. [Google Scholar]
- 185.European Food Safety Authority. Scientific Opinion on bovine lactoferrin. EFSA J. 2012;10:2811. [Google Scholar]
- 186. Taylor S, Brock J, Kruger C, et al. Safety determination for the use of bovine milk-derived lactoferrin as a component of an antimicrobial beef carcass spray. Regul Toxicol Pharmacol. 2004;39:12–24. [DOI] [PubMed] [Google Scholar]
- 187. Koletzko B, Shamir R.. Standards for infant formula milk. BMJ. 2006;332:621–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Rudloff S, Kunz C.. Protein and nonprotein nitrogen components in human milk, bovine milk, and infant formula: quantitative and qualitative aspects in infant nutrition. J Pediatr Gastroenterol Nutr. 1997;24:328–344. [DOI] [PubMed] [Google Scholar]
- 189. Räihä NC, Axelsson IE.. Protein nutrition during infancy. An update. Pediatr Clin North Am. 1995;42:745–764. [DOI] [PubMed] [Google Scholar]
- 190. Koletzko B, von Kries R, Closa R, et al. ; European Childhood Obesity Trial Study Group. Lower protein in infant formula is associated with lower weight up to age 2 y: a randomized clinical trial. Am J Clin Nutr. 2009;89:1836–1845. [DOI] [PubMed] [Google Scholar]
- 191. Chang R, , NgTB, , Sun W-Z.. Lactoferrin as potential preventative and adjunct treatment for COVID-19. Int J Antimicrob Agents. 2020;56:106118. [DOI] [PMC free article] [PubMed] [Google Scholar]