Abstract
Actinomycin-D and vincristine are cytotoxic drugs commonly used to treat cancers in children. This prospective study assessed pharmacokinetic variability and toxicity of these drugs in children.
Blood samples were collected in 158 patients. Actinomycin-D or vincristine concentrations were quantified using high performance liquid chromatography-tandem mass spectrometry. Pharmacokinetic parameters were estimated using non-compartmental methods. Target toxicities were collected prospectively.
Actinomycin-D pharmacokinetics (n=52 patients) were highly variable. The median (coefficient of variation, CV%) area under the concentration-time curve (AUC) was 332 ng/ml•hr (110%); Clearance was 4.6 L/hr/m2 (90%); half-life was 25 hours (60%). No patient met the defined criteria for myelosuppression. In multivariate analysis, none of the demographic nor pharmacokinetic parameters were predictors of acute hepatotoxicity.
Vincristine pharmacokinetics (n=132 patients) demonstrated substantial variability. The median (CV%) AUC was 78ng/ml•hr (98%); clearance was 17.2L/hr/m2 (67%); half-life was 14.6 hours (73%). In multivariate analysis, the effect of increasing age for a given BSA was an increase in neuropathy while the effect of increasing BSA for a given age was a decrease in neuropathy.
Conclusion
Pharmacokinetics of both drugs were highly variable. For actinomycin-D, there was no correlation between demographic or pharmacokinetic parameters and target toxicities. For vincristine, the correlations of age and BSA and neuropathy are confounded by the correlation between age and BSA in children and the ability to ascertain neuropathy in infants. Variability may be attributed to dose reductions and capped doses for both drugs. Investigation of BSA-based dosing in young children is warranted to decrease variability of exposure.
Keywords: Vincristine, Actinomycin-D, Pharmacokinetics, Childhood Cancer
Introduction
Combination chemotherapy is a cornerstone of curative therapy for children with cancer. The most commonly used drug, vincristine has potent cytotoxicity in many cancers in children. Vincristine is not myelosuppressive; toxicity includes neuropathy that can manifest as ptosis, vocal cord paralysis, jaw pain, foot-drop, paresthesia, constipation and ileus. Acute sensory and motor neuropathies are cumulative. Actinomycin-D is a component of curative therapy for children with Wilms tumor, rhabdomyosarcoma, or other solid tumors. It is myelosuppressive and has been associated with hepatotoxicity including sinusoidal obstructive syndrome. Despite decades of use, dosing of these drugs in infants and children is empiric. Historically, infants have received reduced dose and a capped maximum dose has been employed to mitigate excessive toxicity in older patients, indicating an incomplete understanding of dosing and the relationship of drug dose, exposure and toxicity.
Age related differences in toxicity of chemotherapy have been reported.1-3 Actinomycin-D and vincristine related adverse events have been summarized for Children’s Oncology Group (COG) trials for children and adolescents with rhabdomyosarcoma (4 trials, 1588 patients) and Wilms tumor (2 trials, 2979 patients). Investigators report that actinomycin-D related hepatotoxicity was more frequent in patients < 1 year old and the frequency of vincristine neuropathy increased with age.4 To determine if differences in drug exposure contribute to differences in toxicity, the COG conducted a prospective evaluation of the toxicity and pharmacokinetics of actinomycin-D and vincristine in children (ADVL06B1, NCT00674193).
Material and Methods
Patients
Patients <17 years old treated with actinomycin-D or vincristine were eligible. Patients were enrolled to one of four age cohorts: <1 year; ≥1 to <3 years; ≥3 to <12 years; and ≥12 to <17 years. Patients were permitted to enroll during any treatment cycle that contained actinomycin-D or vincristine. Repeat pharmacokinetic sampling and administration of additional anti-cancer drugs were permitted. COG therapeutic protocol enrollment was not required. Dosing of actinomycin-D and vincristine was defined by a clinical trial or prescribed as a standard of care (Supplemental Table 1).
The study was approved by the NCI Pediatric Central Institutional Review Board (IRB) or local site IRBs. Consent and assent were obtained according to institutional guidelines. Children from age 7 through 12 years received a Youth Information Sheet describing study aims and procedures.
Pharmacokinetics
Blood samples were obtained from an indwelling central venous catheter or separate peripheral catheter. Catheter clearance of drug was performed as previously described.5,6 In brief, using a three-way stopcock and separate syringes for waste and sample collection, samples were obtained following a three-draw-flush procedure by which previous data supported catheter clearance of both actinomycin-D and vincristine to between less than 0.2% and undetectable levels of initial infusion concentrations. A limited sampling strategy was used. Five (2.5 mL) blood samples (maximum 12.5 mL/patient) were obtained according to a randomly assigned schedule: Schedule 1:5 minutes, 10 minutes; 2-3, 12-48, and 48-168 hours post-infusion; Schedule 2: 5 minutes, 0.75-1.5, 5-6, 12-48, and 48-168 hours post-infusion. Two sample schedules of varying time points were selected in order to permit a robust collection of pharmacokinetic time points across subjects, without needing all subjects to submit to all time points; the time points selected were based upon pharmacokinetic model assumptions previously described.7,8
If both drugs were administered, actinomycin-D was administered prior to vincristine and the sampling time was relative to the end of the vincristine infusion. A second episode of pharmacokinetic sampling using the same schedule was requested but not required. Samples were collected in sodium heparin, plasma was separated by centrifugation within 6 hours of collection and frozen. Concentrations of actinomycin-D and vincristine were quantified using a liquid chromatography tandem mass spectrometry assay.7 The lower limit of quantification for actinomycin-D and vincristine was 0.5 ng/mL.
Pharmacokinetic parameters were analyzed by noncompartmental methods using Phoenix WinNonlin 8.1 (Certara Corporation, Princeton, NJ). The terminal elimination rate constants (kz) were determined by linear least-squares regression with the last 3 points in the terminal phase. Area under the concentration-time curve (AUClast) was determined using the linear trapezoidal rule from time zero to the time of the last detectable sample (Clast). Total AUC (AUC∞) was calculated by adding the value of Clast/kz to AUClast. The adequacy of the sampling duration was assessed by the percent of AUC that was extrapolated (% AUCextrap=extrapolated AUC (Clast/kz)/total AUC (AUClast + Clast/kz) x100). Clearance (CL) was calculated as dose divided by AUC∞. The apparent volume of distribution (Vz), based on the terminal elimination phase, was calculated as dose/(kz•AUC∞).
Toxicity
Toxicities were prospectively collected and graded according to the National Cancer Institute Common Toxicity Criteria (CTC v3, prior to 10/1/10) or Common Terminology Criteria for Adverse Events (CTCAE version 4.03). There were no changes in definitions of target toxicities between these versions. For actinomycin-D the target toxicities were hepatotoxicity and myelosuppression. Specifically grade≥3 hepatoxicity [elevated alanine aminotransferase, aspartate aminotransferase, gamma glutamyl transferase (GGT) or sinusoidal obstructive syndrome] and grade≥3 decrease in absolute neutrophil count or platelet count (myelosuppression) related to actinomycin-D were reported weekly for 21 days following sampling. For vincristine the target toxicity was CTC/CTCAE grade≥2 neuropathy collected weekly for one month and monthly for 6 months from the time of sampling.
Statistical Analysis
For each drug, a probit model was fit to the pharmacokinetic parameters from the first episode of sampling (Dose, Dose per Body Surface Area (BSA), Half-Life, AUC, Vz, Vz/BSA, Vss, Vss/BSA, Clearance, Clearance/BSA) and demographics variables (age, sex, race, ethnicity, BSA) as predictor variables and toxicity of interest as a binary outcome variable. Each target toxicity was analyzed separately. To be included in the model, the target toxicity had to be new onset or an increase in grade over baseline must have occurred after sampling. Each pharmacokinetic parameter and demographic variable was analyzed with a univariate logistic regression model. P-value ≤0.05 was considered significant. Multivariate analysis was then performed using the backward selection (SAS 9.4 Proc Logistic) including all demographic and pharmacokinetic parameters in the starting model.
The second analyses compared pharmacokinetic parameters of each sampling episode. A two-sided paired t-test using the difference of the log-transformed pharmacokinetic parameters was performed to detect a change between the first and second episodes of sampling. The correlation between parameter differences and the number of days between episodes was assessed using the Pearson’s chi-squared test (SAS 9.4 Proc Corr). P-value ≤0.05 was considered significant.
Results
Patients
One hundred fifty-eight patients were enrolled on the study. Complete data from at least one episode of pharmacokinetic sampling was available from 143 patients; 49 patients had both actinomycin-D and vincristine measured. Fifty-three participated in sampling for actinomycin-D (n=4 patients <1 year; n=13 patients ≥1 and <3 years; n=28 patients ≥3 and <12 years; n=8 patients ≥12 and <17 years old). One hundred thirty-two patients participated in pharmacokinetic sampling for vincristine (n=9 <1 year; n=36 ≥1 and <3 years; n=62 ≥3 and <12 years; n=25 ≥12 and <17 years). In addition, 28 patients had a second episode of pharmacokinetic sampling between 18 and 244 days after initial sampling. Diagnoses included leukemia/lymphoma (n=47), rhabdomyosarcoma (n=43), Wilms/other renal tumors (n=20), primary brain tumor (n=14), Ewing sarcoma (n=10), retinoblastoma (n=8) or other (n=16). Thirty-nine percent of patients (61/158) were enrolled on a COG treatment protocol, the other 61% received standard treatment that included actinomycin-D or vincristine. For patients enrolled on COG protocols, prescribed actinomycin-D or vincristine doses are listed in Supplemental Table 1.
Actinomycin-D
Actinomycin-D pharmacokinetics were characterized for 52 of 57 patients (Table 1). Five patients were omitted due to missing data. The median values for the extrapolated AUC were ≤17% of the total AUC for each age group, consistent with adequacy of the sample collection schedules (Supplementary Table 2). A statistically significant difference was observed for Vz (p=0.04), but not for AUC0-∞ (p=0.82) or CL (p=0.80). The median (coefficient of variation, CV%) AUCinf was 332 ng/ml•hr (110%); Clearance was 4.6 L/hr/m2 (90%); half-life was 25 hours (60%).
Table 1:
Pharmacokinetic Parameters Median (Range); CV%
| Actinomycin-D | Vincristine | |||||||
|---|---|---|---|---|---|---|---|---|
| Group | Group 1: < 1 year (n=4) |
Group 2: ≥ 1 to < 3 years (n=13) |
Group 3: ≥ 3 to < 12 years (n=28) |
Group 4: ≥ 12 to < 17 years (n=7) |
Group 1: < 1 year (n=9) |
Group 2: ≥ 1 to < 3 years (n=36) |
Group 3: ≥ 3 to < 12 years (n=62) |
Group 4: ≥ 12 to < 17 years (n=25) |
| Age (years) | 0.85 (0.46-0.86); 25% | 1.5 (1.0 - 2.8); 32% | 6.0 (3.1 - 11.9); 40% | 15.0 (12.2 - 16.7); 9% | 0.69 (0.21 - 0.96); 37% | 2.12 (1.00 - 2.80); 25% | 6.72 (3.05 - 11.9); 37% | 15.3 (12.1 - 16.8); 8% |
| Weight (kg) | 8.40 (6.30 - 8.80); 13% | 11.3 (7.9 - 15.5); 16% | 20.9 (12.4 - 68.0); 52% | 53.8 (38.2 – 131); 47% | 7.9 (6.3 - 9.9); 16% | 12.3 (7.9 - 17.8); 16% | 22.9 (12.4 - 68.0); 43% | 59.2 (32.7 - 111); 34% |
| BSA (m2) | 0.41 (0.32 - 0.42); 10% | 0.51 (0.40 - 0.66); 13% | 0.82 (0.56 - 1.76); 32% | 1.56 (1.23 - 2.45); 22% | 0.40 (0.32 - 0.45); 11% | 0.55 (0.40 - 0.68); 12% | 0.88 (0.56 - 1.76); 27% | 1.67 (1.17 - 2.36); 19% |
| Dose (mg) | 0.20 (0.17 - 0.36); 34% | 0.50 (0.20 - 0.90); 29% | 0.90 (0.56 - 2.50); 45% | 2.20 (1.50 - 2.50); 17% | 0.3 (0.1 – 0.5); 44% | 0.70 (0.20 – 1.10); 30% | 1.22 (0.50 – 2.00); 28% | 2.00 (1.00 – 2.80); 17% |
| Dose (mg/kg) | 0.03 (0.02 - 0.03); 34% | 0.04 (0.02 - 0.06); 22% | 0.05 (0.02 – 0.06); 17% | 0.04 (0.01 – 0.05); 31% | 0.05 (0.01 – 0.05); 39% | 0.05 (0.02 – 0.11); 28% | 0.06 (0.03 – 0.08); 17% | 0.03 (0.02 – 0.06); 30% |
| Dose (mg/m2) | 0.50 (0.49 - 0.87); 28% | 1.01 (0.48 - 1.51); 22% | 1.14 (0.54 - 1.60); 18% | 1.45 (0.61 - 1.55); 23% | 0.92 (0.25 – 1.11); 40% | 1.23 (0.45 – 2.21); 27% | 1.49 (0.72 – 2.01); 13% | 1.20 (0.66 – 1.54); 20% |
| Half-life (h) | 23.6 (8.8 - 30.6); 36% | 21.5 (8.6 - 80.1); 71% | 24.8 (4.9 - 86.3); 56% | 32.6 (24.4 - 40.3); 20% | 17.3 (4.7 - 20.1); 37% | 12.4 (4.0 - 38.8); 58% | 14.5 (3.4 - 85.9); 82% | 16.0 (6.2 - 30.8); 38% |
| CL (L/hr/m2) | 3.3 (1.9 - 7.9); 61% | 4.9 (0.5 - 17.4); 81% | 4.6 (0.6 - 35.2); 105% | 5.3 (2.9 - 7.8); 29% | 13.7 (5.0 - 25.3); 43% | 14.9 (2.3 - 78.9); 80% | 16.7 (1.6 - 45.8); 57% | 18.3 (5.0 - 73.3); 67% |
| AUC0-∞ (ng/mL•h) | 153 (64 - 322); 54% | 201 (33.1 – 1934); 130% | 266 (34 – 1961); 101% | 241 (134 – 498); 43% | 43.9 (26.2 - 180); 72% | 95.8 (8.1 - 510); 84% | 84.8 (33.7 - 935); 106% | 62.8 (20.8 - 191); 58% |
CV= coefficient of variation; BSA= body surface area; CL= plasma clearance; AUC0-∞= area under the concentration time curve extrapolated to infinity
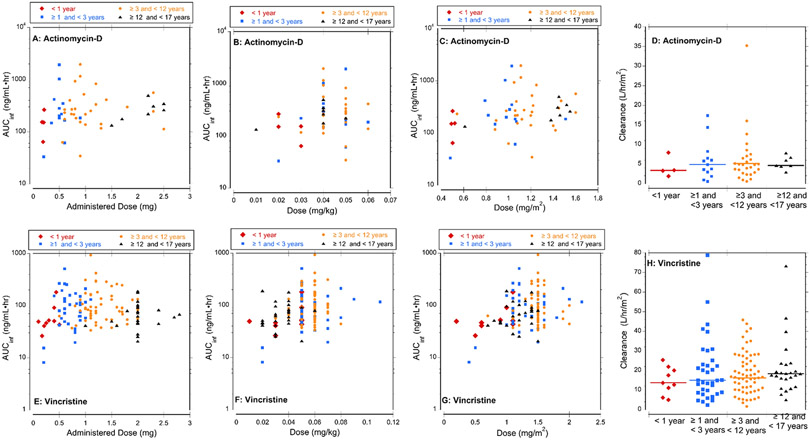
When analyzed by age group, children in the oldest group displayed less variability (%CV) in half-life, AUC and clearance; variability was high in children <1 year old. The BSA-normalized dose was more than 2-fold higher in children ≥1 year old compared to the youngest children and increased with age group [35%; range (0.98-1.35 mg/m2)]. Consistent with the difference in dose, the median AUC for the youngest group was approximately 50% lower than the oldest groups. Actinomycin-D dose did not appear to be related to AUC and clearance corrected for BSA did not appear to be related to age (Figure 1).
Figure 1: Exposure (AUCinf) and Clearance of Actinomycin-D and Vincristine.
(A) Actual actinomycin-D dose (mg) administered to each patient versus exposure. (B) Actinomycin-D weigh-based dose (mg/kg) versus exposure. (C) Actinomycin-D BSA-based dose (mg/m2) versus exposure. (D) Actinomycin-D clearance for each age cohort, lines indicate median clearance. (E) Actual vincristine dose (mg) administered to each patient versus exposure (F) Vincristine weigh-based dose (mg/kg) versus exposure. (G) Vincristine BSA-based dose (mg/m2) versus exposure. (H) Vincristine clearance in each age cohort, lines indicate median clearance.
For patients (n=9) with two episodes of sampling, the volume of distribution (Vz and Vz/BSA) showed statistically significant changes between sampling episodes. (Supplemental Table 2). No other pharmacokinetic parameters had significant changes between sampling episodes. Changes in pharmacokinetic parameters was not correlated with the number of days between sampling episodes.
Target toxicity was defined as greater than baseline because participants in this study were receiving multi-agent, multimodality disease therapy and were permitted to enroll at any time during therapy. No patients who participated in actinomycin-D pharmacokinetic sampling experienced myelosuppression with grade greater than baseline. Two patients who participated in actinomycin-D pharmacokinetic sampling experienced a liver toxicity which was greater than baseline. There were 4 unique AEs experienced by these two patients, grade 3 alanine aminotransferase increase, grade 3 GGT increase, grade 4 alanine aminotransferase increase, and grade 4 aspartate aminotransferase increase. No participant experienced sinusoidal obstructive syndrome during the study.
None of the demographic or pharmacokinetic parameters were significant predictors for hepatotoxicity in univariate nor multivariate analysis.
Vincristine
Vincristine pharmacokinetics were characterized for 132 of 144 patients. Eleven patients were omitted from analysis due to missing data (n=7) or inability to calculate kz (n=5). The median values for the extrapolated AUC were ≤9% of the total AUC for each age group, consistent with adequacy of the sample collection schedules (Supplementary Table 3). A statistically significant difference was not observed for Vz (p=0.70), AUC0-∞ (p=0.11) or CL (p=0.16). Substantial variability was observed in all parameters. The median (CV%) AUC was 78 ng/ml•hr (98%); Clearance was 17.2L/hr/m2 (67%); half-life was 14.6 hours (73%).
Age had a minimal effect on variability of vincristine pharmacokinetics. Compared to children <1 year old, the BSA-adjusted dose was 60%, 79% and 44% higher Groups 2, 3 and 4, respectively. Consistent with the differences in dose, the median AUC was lowest for Group 1 and highest in Group 2 and 3. Clearance did not appear to be related to age (Figure 1).
For patients (n = 17) with two episodes of sampling , statistically significant changes were observed in Vz (Ratio = 1.16 (95% CI 1.03,2.53); P=0.04) and clearance (Ratio =1.54 (1.03,2.31); P=0.04). However, when corrected for BSA the changes were marginally significant Vz/BSA (Ratio =1.56 (0.99–2.46); P=0.05) and CL/BSA (Ratio =1.50 (0.99–2.25); P=0.05) (Supplemental Table 3). Changes were not correlated with the number of days between sampling episodes.
For patients (n=73) who participated in vincristine pharmacokinetic sampling and documented the therapy cycle in which sampling was performed, there was no difference in the timing of pharmacokinetic sampling for patients with or without neuropathy. For patients (n=26) with neuropathy sampling was performed at median (range) cycle 3 (1-15); for patients (n=47) without neuropathy, sampling was performed during cycle 2.5 (1-15) (P=0.77). Thus, cumulative neurotoxicity did not appear to impact our results. Using the criteria greater than baseline, there were four patients who experienced grade 3 peripheral motor neuropathy and four patients who experienced grade 3 peripheral sensory neuropathy; all others had grade 2 neuropathy. In univariate analysis, none of the demographic or pharmacokinetic parameters were significant predictors for neuropathy. In the multivariate analysis, age ( P=0.02) and BSA (P=0.02) were selected for the final model. Because age and BSA are highly correlated in children, the effect of increasing age for a given BSA was an increase in toxicity (OR=1.49 (95% CI 1.08–2.06) per year increase in age) while the effect of increasing BSA for a given age was a decrease in toxicity (OR=0.62 (0.42–0.93) per 0.1 m2 increase in BSA). No other demographic and none of the pharmacokinetic parameters were significant predictors of neuropathy in the multivariate model.
Discussion
Similar to other studies, including studies with population pharmacokinetic modeling in children8,9, we demonstrated that the pharmacokinetics of actinomycin-D and vincristine are highly variable. Our data demonstrate that the dose independent parameter, clearance corrected for BSA of these drugs does not differ by age. In addition, empiric dosing strategies including dose reductions for infants, capped maximum doses, and weight-based dosing of vincristine for infants influence exposure and confound conclusions regarding physiology as a contributing factor to exposure or toxicity.
Consistent with the empiric actinomycin-D dose reduction by 50% for children <1 year old, dose dependent pharmacokinetic parameters including AUC are generally lower. The clearance appears slightly lower in infants <1 year compared to older children, however, small sample size and high variability result in overlap in clearance range across all age groups. Due to empiric dose reductions for infants and the capped dose, the administered doses of actinomycin-D ranged from 0.01- 0.05 mg/kg across all ages; participant weight ranged from 6.3-131 kg. The impact of dose reductions and a capped maximum dose on actinomycin-D exposure and variability with weight-based and BSA-based dosing is demonstrated in Figure 1A-C. In patients , <1 year old, the administered dose and BSA-based dose are consistently lower that other age groups. However, due to dose capping the youngest patients do not consistently receive the lowest weight-base dose. Conventions for actinomycin-D dosing differ in the US and Europe. In the US, dosing is weight-based (mg/kg) and in Europe, dosing is BSA-based (mg/m2). In our study, children ≥3 and <12 years old received the highest weight-based dose (0.05 mg/kg) while adolescents >12 years received the highest BSA-based dose (1.45 mg/m2). Based on our definitions of myelosuppression and low frequency of hepatotoxicity, no relationship between toxicity and dose, exposure or demographics was identified. No participant experienced sinusoidal obstructive syndrome during the study.
Vincristine pharmacokinetics were highly variable but similar to previously published results.10-13 Studies evaluating correlations of vincristine related neuropathy have been reviewed by van de Velde et al.3 In our multivariate analysis using backward selection, increased age for a given BSA correlated with increased occurrence of neuropathy, while increased BSA for a given age was associated with decreased occurrence of neuropathy. The ability of older children to report neuropathy and the limitation of CTCAE grading based on activities of daily living may have introduced an age-related reporting bias for neuropathy. Constipation was not specifically collected as a target toxicity. The frequency of neuropathy decreased as BSA increased for a given age which may have resulted from using a maximum vincristine dose (2 mg). The administered vincristine dose and exposure are generally lower in children < 1 year old (Figure 1E). However, exposure for weight-based and BSA-based doses of vincristine in children < 1 year is not consistently lower than other age groups (Figure 1F,G). In addition, the vincristine clearance is not different among the age cohorts, indicating that vincristine exposure may be more uniform across age groups if BSA-based dosing is used.
Empiric dose reductions for infants and capped doses in adolescents for both actinomycin-D and vincristine contributed to the variability observed in dose dependent parameters. Minor age-related differences in actinomycin-D and vincristine clearance may exist, however, may not be clinically relevant. In addition, recommendations for dose reductions based on age and weight contribute to variability. In this study, the clinical dosing practice and protocol treatment guidelines were not standardized. For clinical trials incorporating actinomycin-D, two different dosing guidelines were used; for vincristine 8 different guidelines were used (Supplemental Table 1). An analysis of COG phase 2 and 3 clinical trials collated the empiric dosing recommendations for infants and children, identified 11 different milestones (age, weight, BSA or combinations of these) and 8 different dosing recommendations for infants receiving cytotoxic therapy. This resulted in dramatic fluctuations in dose. For example, transitions from weight-based to BSA-based dosing at a prespecified age (1 or 3 years) or weight (10 or 12 kg) can lead to an increase in the dose of vincristine 2.5-fold when a child has a small change in weight or a birthday. 14
Many factors contribute to variability in toxicity experienced by patients. This and other studies assessing the relationship of actinomycin-D or vincristine exposure and toxicity have been hindered by interpatient variability in pharmacokinetic parameters, limited patient populations due to the complexities of pharmacokinetic studies in infants and children, categorical toxicity grading that is not specific to children, and rarity of life-threatening toxicities such as sinusoid obstructive syndrome. Interpatient variability in exposure will limit the ability to assess the impact of pharmacogenomics or assessment of pharmacokinetic interactions when standard agents are combined with investigational agents and impact the assumptions of population pharmacokinetic modeling. The design of pharmacokinetic studies of standard chemotherapy should be re-assessed. Caution is necessary when population pharmacokinetic modeling in children incorporates highly correlated variables such as age and body size. Based on our observation that clearance of both actinomycin-D and vincristine is not dramatically altered by age, standardization of dosing is warranted.
Supplementary Material
Grant Support:
This work was supported by an NCTN Operations Center Grant (U10CA180866) and NCTN Statistics and Data Center Grant (U10CA180899) and the St Baldrick’s Foundation.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Gupta AA, Anderson JR, Pappo AS, et al. Patterns of chemotherapy-induced toxicities in younger children and adolescents with rhabdomyosarcoma: a report from the Children's Oncology Group Soft Tissue Sarcoma Committee. Cancer 2012;118:1130–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paioli A, Luksch R, Fagioli F, et al. Chemotherapy-related toxicity in patients with non-metastatic Ewing sarcoma: influence of sex and age. J Chemother 2014;26:49–56. [DOI] [PubMed] [Google Scholar]
- 3.van de Velde ME, Kaspers GL, Abbink FCH, Wilhelm AJ, Ket JCF, van den Berg MH. Vincristine-induced peripheral neuropathy in children with cancer: A systematic review. Crit Rev Oncol Hematol 2017;114:114–30. [DOI] [PubMed] [Google Scholar]
- 4.Langholz B, Skolnik JM, Barrett JS, et al. Dactinomycin and vincristine toxicity in the treatment of childhood cancer: a retrospective study from the Children's Oncology Group. Pediatr Blood Cancer 2011;57:252–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skolnik JM, Zhang AY, Barrett JS, Adamson PC. Approaches to clear residual chemotherapeutics from indwelling catheters in children with cancer. Ther Drug Monit 2010;32:741–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards AY, Skolnik JM, Dombrowsky E, Patel D, Barrett JS. Modeling and simulation approaches to evaluate pharmacokinetic sampling contamination from central venous catheters in pediatric pharmacokinetic studies of actinomycin-D: a report from the children's oncology group. Cancer Chemother Pharmacol 2012;70:83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skolnik JM, Barrett JS, Shi H, Adamson PC. A liquid chromatography-tandem mass spectrometry method for the simultaneous quantification of actinomycin-D and vincristine in children with cancer. Cancer Chemother Pharmacol 2006;57:458–64. [DOI] [PubMed] [Google Scholar]
- 8.Mondick JT, Gibiansky L, Gastonguay MR, et al. Population pharmacokinetic investigation of actinomycin-D in children and young adults. J Clin Pharmacol 2008;48:35–42. [DOI] [PubMed] [Google Scholar]
- 9.Hill CR, Cole M, Errington J, Malik G, Boddy AV, Veal GJ. Characterisation of the clinical pharmacokinetics of actinomycin D and the influence of ABCB1 pharmacogenetic variation on actinomycin D disposition in children with cancer. Clin Pharmacokinet 2014;53:741–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crom WR, de Graaf SS, Synold T, et al. Pharmacokinetics of vincristine in children and adolescents with acute lymphocytic leukemia. J Pediatr 1994;125:642–9. [DOI] [PubMed] [Google Scholar]
- 11.Kellie SJ, Koopmans P, Earl J, et al. Increasing the dosage of vincristine: a clinical and pharmacokinetic study of continuous-infusion vincristine in children with central nervous system tumors. Cancer 2004;100:2637–43. [DOI] [PubMed] [Google Scholar]
- 12.Plasschaert SL, Groninger E, Boezen M, et al. Influence of functional polymorphisms of the MDR1 gene on vincristine pharmacokinetics in childhood acute lymphoblastic leukemia. Clin Pharmacol Ther 2004;76:220–9. [DOI] [PubMed] [Google Scholar]
- 13.Moore AS, Norris R, Price G, et al. Vincristine pharmacodynamics and pharmacogenetics in children with cancer: a limited-sampling, population modelling approach. J Paediatr Child Health 2011;47:875–82. [DOI] [PubMed] [Google Scholar]
- 14.Balis FM, Womer RB, Berg S, et al. Dosing anticancer drugs in infants: Current approach and recommendations from the Children's Oncology Group's Chemotherapy Standardization Task Force. Pediatr Blood Cancer 2017;64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.