ABSTRACT
Objective
To evaluate the different components of the resistance of the respiratory system, respiratory muscle strength and to investigate the occurrence of expiratory flow limitation (EFL) in patients with morbid obesity (MO) when seated.
Methods
The sample was composed of MO (BMI≥40 kg/m2) and non-obese individuals (NO) with a BMI between 18 and 30 kg/m2. The protocol consisted of the anthropometric assessment and the following measures of respiratory function: spirometry, maximal inspiratory and expiratory pressures (MIP and MEP, respectively) and impulse oscillometry. The group comparison was performed using T-test for unpaired samples. The correlations were evaluated by the Pearson test with a significance level of 5%.
Results
Fifty MO (age 40±10.4 years, 1.64±0.09 m, 138.8±33.6 kg and 50.7±8.9 kg/m2), and 30 NO (age 37.6±11.5 years, 1.67±0.09 m, 65.2±10.3 kg and 23.2±22 kg/m2) were evaluated. The MO showed higher values of total, peripheral, airways, tissue and central resistance when compared to the NO. No patient showed EFL. The waist circumference was associated with spirometric variables, MIP, and MEP. The waist-to-hip ratio was correlated to respiratory mechanics and spirometric variables, MIP, and MEP.
Conclusion
Morbidly obese patients with no obstructive spirometric pattern show increased total, airway, peripheral, and tissue respiratory system resistance when compared to nonobese. These individuals, however, do not present with expiratory flow limitation and reduced respiratory muscles strength.
Keywords: Respiratory mechanics, Obesity, Impulse oscillometry, Pulmonary resistance, Respiratory system impedance
RESUMO
Objetivo
avaliar os diferentes componentes da resistência do sistema respiratório e a força muscular respiratória, bem como investigar a ocorrência de limitação de fluxo expiratório (LFE) de pacientes obesos mórbidos (OM) na posição sentada.
Métodos
a amostra foi composta de OM (IMC ≥ 40 kg/m2) e de indivíduos não obesos (NO) com IMC entre 18 e 30 kg/m2. O protocolo foi composto de: avaliação antropométrica e da função respiratória (espirometria, pressões inspiratória (PIM) e expiratória máximas (PEM) e oscilometria de impulso). Na comparação entre os grupos, foi utilizado o teste T para amostras não pareadas. As correlações foram avaliadas pelo teste de Pearson, e o nível de significância foi de 5%.
Resultados
Foram avaliados 50 OM (idade 40,0 ± 10,4 anos, 1,64 ± 0,09 m, 138,8 ± 33,6 kg e 50,7 ± 8,9 kg/m2), além de 30 NO (idade 37,6 ± 11,5 anos, 1,67 ± 0,09 m, 65,2 ± 10,3 kg e 23,2 ± 22 kg/m2). Os OM apresentaram maiores valores de resistência total, central, de vias aéreas, tecidual e periférica quando comparados aos NO. Nenhum paciente apresentou LFE. A circunferência abdominal se associou com variáveis espirométricas PIM e PEM. A relação cintura-quadril se correlacionou com variáveis de mecânica respiratória, além das espirométricas PIM e PEM.
Conclusões
pacientes com obesidade mórbida e sem padrão espirométrico obstrutivo apresentam aumento nas resistências total, de vias aéreas, periférica e tecidual do sistema respiratório quando comparados a não obesos. Esses indivíduos, entretanto, não apresentam limitação de fluxo expiratório e redução da força muscular respiratória.
Descritores: Mecânica respiratória, Obesidade, Oscilometria de impulso, Resistência pulmonar, Impedância do sistema respiratório
INTRODUCTION
Obesity is considered a public health problem around the world, presenting an important growth in the last decade.(1) Obesity is a multifactorial condition that can be related to nutritional alteration, genetic, psychological and socioeconomic factors, and sedentary lifestyle.(2) Obesity is classified by the body mass index (BMI), with intervals between 30 and 34.9 kg/m2 considered as obesity class I; 35 to 39.9 kg/m2 considered as obesity class II; and ≥ 40 kg/m2 considered as obesity class III also called as morbid obesity.(3,4) With the increased prevalence of obese people with BMI>50 kg/m2, it was necessary to broaden this classification, considering intervals between 50 and 60 kg/m2 as super obese, and > 60 kg/m2 as super-super obese.(5)
The repercussion of obesity on the respiratory function is associated mainly with the restrictive alteration caused by the excess of adipose tissue.(6,7) The increase of fat mass in the thorax and abdomen can shift the elastic point of balance between the chest and lungs, reducing the functional residual capacity (FRC). This low volume of relaxation of the respiratory system (RS) favors the shift of the pressure-volume curve for its least complacent region. In addition, the reduction of the functional residual capacity is associated with the reduction of the caliber of the airway, resulting in increased resistance.(8,9)
Several methods may be used to study respiratory mechanics in individuals breathing spontaneously; however, the impulse oscillometry (IOS), which is one of the applications of the forced oscillation technique, is featured for not depending on effort, not requiring special maneuvers and for offering resistance values for central, peripheral and tissue of the respiratory system, as well as airways resistance.(10,11) Recently, Albuquerque e cols.(6) used IOS to evaluate the respiratory mechanics of patients with morbid obesity and observed an increase in the RS peripheral resistance in 5Hz. However, the authors did not investigate the mean resistances (associated to the airways resistance) and tissue resistance, as well as the occurrence or not of expiratory flow limitation in obese patients, and the respiratory muscular strength. Thus, this study aimed to evaluate the different components of the respiratory system resistance and respiratory muscular strength in patients with morbid obesity and to investigate the occurrence of expiratory flow limitation (EFL) in a seated position.
METHODS
Sample characteristics
This is a cross-sectional study using a sample composed by patients with morbid obesity (BMI ≥40 kg/m2) from the Program of Bariatric Surgery from the Hospital Universitário Clementino Fraga Filho da Universidade Federal do Rio de Janeiro (PROCIBA / HUCFF-UFRJ), and a group of non-obese individuals, paired by age and gender, with BMI between 18 and 30 kg/m2. All participants were volunteers and signed an informed consent form, which was approved by the Institution’s Research Ethics Committee, according to Resolution 466/12 of the Brazilian National Health Council.
The following exclusion criteria were adopted: a history of pulmonary or cardiac disease, history of smoking, neurological and musculoskeletal diseases, inability to perform the proposed tests and obstructive spirometric pattern (FEV1/FVC ≤ 70%) for both groups.
Study protocol
The study protocol was composed by an anthropometric evaluation of the body composition and respiratory function using spirometry, static respiratory pressures, and impulse oscillometry. All tests were performed in the Respiratory Physiology Laboratory of the Instituto de Biofísica Carlos Chagas Filho da Universidade Federal do Rio de Janeiro (IBCCF-UFRJ).
Anthropometric assessment
The anthropometric assessment obtained data for body mass measures, height, BMI, waist circumference (WC), hip circumference (HC) and waist-to-hip ratio (WHR). The height was verified using a stadiometer (Cardiomed, WCS-Wood, Curitiba/PR, Brazil). The WC was measured in the orthostatic position with upright posture, no clothes or shoes, in the mean point of distance between the lower costal margin and the anterior iliac crest. The HC was measured by taking the larger diameter of the gluteal region, passing over the greater trochanters of the femur, using a metallic tape measure (Sanny® SN-4010, São Paulo, Brazil), with 2m of extension and 0.1 cm precision. Finally, the WHR was calculated by dividing the waist circumference in centimeters by the hip circumference also in centimeters, according to the WHO instructions.(12)
Spirometry
Spirometry was performed according to the American Thoracic Society (13) and Sociedade Brasileira de Pneumologia(14) recommendations, using the computerized spirometer and its components, Lilly pneumotachograph (Erich Jaeger, Hoechberg, Germany) and flow and pressure transducers (Sensym SLP004D, Honeywell Sensing and Control, Golden Valley, MN, USA), following the manufacturer’s calibration instructions. The predicted values for forced vital capacity (FVC), forced expiratory volume in the first second (VEF1) and expiratory peak flow (PF) were calculated according to the equations of Pereira e cols.(14) In addition, the maximal voluntary ventilation (MVV)(15) was verified using the same equipment. For this variable, the predicted values were calculated according with the Brazilian reference equations described by Neder et al.(16)
Maximal Respiratory Pressures
The evaluation of the respiratory muscle strength was performed by measuring the maximal inspiratory and expiratory pressures (MIP and MEP, respectively), according to the ATS/ERS(16) recommendations. An analogical manovaccuometer (M120 – Comercial Médica, São Paulo/SP, Brazil) was used, with a 2 mm orifice on the mouthpiece to dissipate the pressure generated by the muscles of the face and oropharynx. A minimum of three acceptable measurements and a maximum of five were performed. The criteria of acceptability and reproducibility included maneuvers that did not differ in more than 10% of the highest value among themselves. An interval of one minute and thirty seconds among each verification was established. For the measurement of MIP, the individuals were instructed to take a deep breath from the RV in the manovaccuometer’s mouthpiece and maintain the pressure for at least 2 seconds. For the measurement of the MEP, the participants were instructed to take a deep breath until total lung capacity (TLC), take a forced exhalation through the device and maintain the pressure for at least 2 seconds. The predicted values were calculated according to the Brazilian reference equations described by Neder et al.(17)
Impulse oscillometry
The evaluation of the respiratory mechanics was performed with an impulse oscillometer (Erich Jaeger, Hoechberg, German) and its components. After the equipment calibration, the participants remained in a seated position, with the head in a neutral position, manual support on the cheeks and nostrils occluded by a nasal clip. Five sequences of 40 seconds of respiratory signals were collected. Signals of at least 15 seconds, no artifacts, and with at least 80% of the frequency range showing a coherence function equal or higher than 0.9 were adopted as acceptability criteria. The following variables were measured: resistance in 5Hz (R5), resistance in 20 Hz (R20), inspiratory reactance in 5 Hz (X5ins), expiratory reactance in 5 Hz (X5exp), mean reactance in 5 Hz (X5), resonance frequency (f0) and integral of reactance between 5 Hz and f0 (AX). The last 3 parameters may reflect the shift of the frequency curve vs. reactance to the right, which is usually associated with increased peripheral resistance or respiratory system elastance.(10) In addition to the parameters directly provided by the equipment, the extrapolated resistance at 0 Hz (R0), peripheral resistance (PR=R5-R20), mean resistance (Rm), tissue resistance (TR=R5-Rm) and the derivate of the resistance over frequency (dR/dF), which is also associated with peripheral resistance, were also calculated.(11,12)
Statistical analysis
The results were presented as mean ± standard deviation (SD) or proportions (%). Since the data presented normal distribution (Kolmogorov-Smirnov), the comparison among the obtained results by the morbid obese and non-obese was performed using T-test for unpaired samples. The correlations were evaluated via the Pearson correlation test with a 5% significance level. The software SigmaStat 3.1 (Jandel Scientific, San Rafael, CA, USA) was used for all analyses.
RESULTS
The study recruited 107 subjects as shown in figure 1. After the application of the exclusion criteria, 50 morbid obese, 25 obese with BMI = 40-44.9 kg/m2, 19 obese with BMI = 50–59.9 kg/m2 and 6 obese with BMI ≥ 60 kg/m2 and 30 non-obese remained. The anthropometric and demographic data of the individuals are described in table 1.
Figure 1. Flowchart for selection of the patients included in the study. FAS: Antiphospholipid Antibody Syndrome; FEV1/FVC: forced expiratory volume in the first second-forced vital capacity ratio; BMI: body mass index.
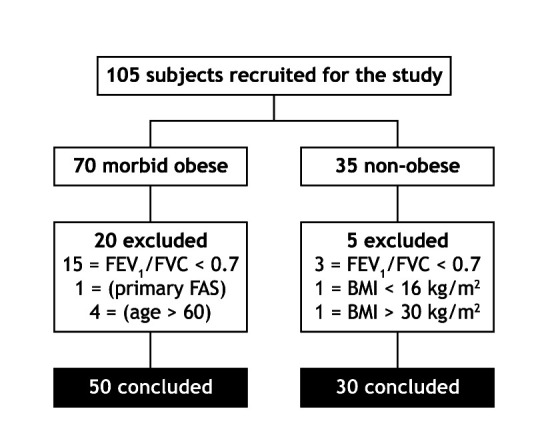
Table 1. Anthropometric and demographic characteristics of the sample components.
| Variables |
Morbid obese
(n=50) |
Non-obese
(n=30) |
P |
|---|---|---|---|
| Age (years) | 40.0±10.4 | 37.6±11.5 | 0.2947 |
| Female gender % (n) | 79 (39) | 70 (21) | 0.4103 |
| Height (m) | 1.64±0.09 | 1.67±0.09 | 0.3004 |
| Body mass (kg) | 138.8±33.6 | 65.2±10.3 | < 0.0001 |
| BMI (kg/m2) | 50.7±8.9 | 23.2±2.2 | < 0.0001 |
| WC (cm) | 136.3±18.8 | 80.5±9.9 | < 0.0001 |
| HC (cm) | 143.4±17.5 | 97.5±5.9 | < 0.0001 |
| WHR | 0.95±0.09 | 0.84±0.08 | < 0.0001 |
BMI: body mass index; WHR: waist-to-hip ratio; WC: waist circumference; HC: hip circumference.
When analyzing the spirometric data obtained from morbid obese and non-obese subjects, significant differences were observed regarding the absolute values of some variables; however, no significant differences were observed among the variables regarding the percentage of the predicted values. The values of maximal respiratory pressures, MIP and MEP, did not showed significant differences among the groups (neither in absolute nor in percentage of the predicted), table 2.
Table 2. Spirometric variables, maximal respiratory pressures and respiratory mechanics in morbid obese and non-obese.
| Variables |
Morbid obese
(n=50) |
Non-obese
(n=30) |
P |
|---|---|---|---|
| Spirometry | |||
| FVC (L) | 3.5±0.7 | 4.0±0.8 | 0.0275 |
| FVC (% pred) | 78.7±6.9 | 100.9±10.6 | 0.4198 |
| FEV1 (L) | 2.8±0.6 | 3.2±0.6 | 0.0157 |
| FEV1 (% pred) | 80.5±7.6 | 97.4±8.0 | 0.0978 |
| FEV1/FVC (L) | 80.4±6.6 | 82.6±5.8 | 0.5384 |
| PF (L/s) | 7.0±1.9 | 7.8±2.0 | 0.0582 |
| PF (% pred) | 83.4±20.3 | 86.6±13.3 | 0.5750 |
| MVV (L) | 114.2±26.1 | 126.6±24.2 | 0.2435 |
| MVV (% pred) | 89.2±23.4 | 89.9±15.6 | 0.3236 |
| Maximal respiratory pressures | |||
| MIP (cmH2O) | 102.0±23.5 | 116.5±22.5 | 0.5862 |
| MIP (% pred) | 100.2±31.5 | 121.7±25.5 | 0.0572 |
| MEP (cmH2O) | 107.5±21.2 | 122.7±24.4 | 0.3084 |
| MEP (% pred) | 107.8±30.5 | 102.0±11.3 | 0.2359 |
| Respiratory mechanics | |||
| R0 (kPa/l/s) | 0.6±0.2 | 0.4±0.1 | 0.0001 |
| R5 (kPa/l/s) | 0.5±0.1 | 0.1±0.1 | 0.0001 |
| R20 (kPa/l/s) | 0.38±0.16 | 0.28±0.08 | 0.0010 |
| Rm (kPa/l/s) | 0.50±0.18 | 0.33±0.09 | 0.0001 |
| PR (kPa/l/s) | 0.18±0.12 | 0.064±0.043 | 0.0027 |
| TR (kPa/l/s) | 0.03±0.02 | 0.01±0.01 | 0.0002 |
| f0 (Hz) | 20.9±4.5 | 13.7±3.5 | 0.0001 |
| AX (kPa/l*Hz) | 1.6±1.3 | 0.4±0.31 | <0.0001 |
| dR/dF | 0.021±0.012 | -0.01±0.001 | <0.0001 |
| X5 (kPa/l/s) | 0.20±0.10 | 0.09±0.02 | 0.0007 |
| X5ins (kPa/l/s) | -0.19±0.08 | 0.12±0.09 | 0.0013 |
| X5exp (kPa/l/s) | -0.20±0.12 | 0.10±0.04 | 0.0007 |
| ∆X5 (kPa/l/s) | 0.07±0.12 | 0.03±0.02 | 0.0739 |
FVC: forced vital capacity; FEV1: forced expiratory volume in the first second; PF: expiratory peak flow; MVV: maximal voluntary ventilation; MIP: maximal inspiratory pressure; MEP: maximal expiratory pressure. R0: resistance extrapolated for 0Hz; R5: resistance in 5Hz; R20: resistance in 20Hz; PR: peripheral resistance (R5-R20); Rm: mean resistance; TR: tissue resistance; f0: resonance frequency; AX: reactance integral between 5Hz and resonance frequency; dR/dF: dependence of respiratory system resistance on frequency; X5ins: inspiratory reactance in 5Hz; X5exp: expiratory reactance in 5Hz; ∆X5: difference between inspiratory and expiratory reactance. Values represented as mean ± standard deviation.
As for the results of respiratory mechanics (Table 2), the morbid obese had higher values of total (R0 and R5), central (R20), airways (Rm), tissue (TR) and peripheral resistance (dR/dF and PR) when compared with non-obese. The differences observed in the AX and X5 values suggested an increased resistance or elastance of the respiratory system in the group of morbid obese. No patient showed EFL.
The WC of obese individuals was not correlated with respiratory mechanics variables; however, it was correlated with the following spirometry variables: FVC (%), FEV1 (%), PF (L/s) and MVV (L), MIP (%) and MEP (%), as shown in table 3. The non-obese subjects showed a correlation of WC with the respiratory mechanics variable R20, and spirometry variables FVC (L), FEV1/FVC, PF (L) and MVV (% and L).
Table 3. Correlation of the variables of respiratory mechanics, spirometry and static respiratory pressures with the waist circumference.
| Variables |
Morbid obese
(n=50) |
Non-obese
(n=30) |
|||
|---|---|---|---|---|---|
| r | P | r | P | ||
| R0 (kPa/l/s) | 0.0959 | 0.5072 | 0.1961 | 0.2990 | |
| Rm (kPa/l/s) | 0.1278 | 0.3763 | 0.2431 | 0.1956 | |
| TR (kPa/l/s) | 0.0464 | 0.7487 | 0.2274 | 0.2268 | |
| R5 (kPa/l/s) | 0.0526 | 0.7163 | 0.1976 | 0.2953 | |
| R20 (kPa/l/s) | 0.2080 | 0.1472 | 0.3933 | 0.0316 | |
| X5 (kPa/l/s) | 0.0364 | 0.8016 | 0.2273 | 0.2270 | |
| f0 (Hz) | -0.1918 | 0.1821 | 0.3093 | 0.0963 | |
| AX (kPa/l*Hz) | -0.0932 | 0.5196 | 0.0444 | 0.8156 | |
| X5 ins (kPa/l/s) | 0.1523 | 0.2910 | 0.1521 | 0.4225 | |
| X5 exp (kPa/l/s) | 0.0953 | 0.5101 | 0.1181 | 0.5342 | |
| ∆X5 (kPa/l/s) | -0.0777 | 0.5917 | -0.0314 | 0.8689 | |
| dR/dF | 0.0519 | 0.7204 | 0.0263 | 0.8900 | |
| FVC (L) | -0.1056 | 0.4656 | -0.4564 | 0.0112 | |
| FVC (% pred) | -0.4257 | 0.0021 | -0.1484 | 0.4339 | |
| FEV1 (L) | -0.1164 | 0.4206 | -0.3559 | 0.0536 | |
| FEV1 (% pred) | -0.3671 | 0.0087 | -0.0108 | 0.9545 | |
| FEV1/FVC (L) | -0.0650 | 0.6536 | -0.4240 | 0.0195 | |
| PF (L/s) | -0.3633 | 0.0095 | -0.5788 | 0.0008 | |
| PF (% pred) | -0.2031 | 0.1573 | -0.3334 | 0.0718 | |
| MVV (L) | -0.2788 | 0.0499 | -0.4633 | 0.0099 | |
| MVV (% pred) | -0.0065 | 0.9637 | -0.3712 | 0.0434 | |
| MIP (cmH2O) | -0.2311 | 0.1063 | -0.4446 | 0.0138 | |
| MIP (% pred) | -0.3758 | 0.0072 | -0.1731 | 0.3603 | |
| MEP (cmH2O) | -0.0545 | 0.7067 | -0.2068 | 0.2730 | |
| MEP (% pred) | -0.3878 | 0.0054 | -0.1667 | 0.3787 | |
FVC: forced vital capacity; FEV1: forced expiratory volume in the first second; PF: expiratory peak flow; MVV: maximal voluntary ventilation; MIP: maximal inspiratory pressure; MEP: maximal expiratory pressure. R0: resistance extrapolated for 0Hz; R5: resistance in 5Hz; R20: resistance in 20Hz; Rm: mean resistance; TR: tissue resistance; f0: resonance frequency; AX: reactance integral between 5Hz and resonance frequency; dR/dF: dependence of respiratory system resistance on frequency; X5ins: inspiratory reactance in 5Hz; X5exp: expiratory reactance in 5Hz; ∆X5: difference between inspiratory and expiratory reactance.
There were no associations between the hip circumference and the variables of respiratory mechanics, maximal static respiratory pressures and spirometry for morbid obese and non-obese groups.
There were correlations between WHR of morbid obese and the following respiratory variables: AX, f0, PF (%), MIP (%) and MEP (%). For non-obese individuals, WHR was correlated with R20, X5ins, delta X5, FVC (L), PF (L), MVV (L) and MEP (%), as shown in table 4.
Table 4. Correlation of the variables of respiratory mechanics, spirometry and static respiratory pressures with the waist-to-hip ratio.
| Variables |
Morbid obese
(n=50) |
Non-obese
(n=30) |
|||
|---|---|---|---|---|---|
| r | P | r | P | ||
| R0 (kPa/l/s) | 0.2162 | 0.1315 | 0.3472 | 0.0601 | |
| Rm (kPa/l/s) | 0.1843 | 0.2002 | 0.3495 | 0.0584 | |
| TR (kPa/l/s) | 0.2531 | 0.0761 | 0.06626 | 0.7279 | |
| R5 (kPa/l/s) | 0.1109 | 0.4431 | 0.3367 | 0.0689 | |
| R20 (kPa/l/s) | 0.1719 | 0.2325 | 0.3887 | 0.0338 | |
| X5 (kPa/l/s) | 0.1613 | 0.2632 | 0.1907 | 0.3127 | |
| f0 (Hz) | -0.4358 | 0.0016 | -0.06562 | 0.7304 | |
| AX (kPa/l*Hz) | -0.3176 | 0.0246 | -0.1066 | 0.5749 | |
| X5ins (kPa/l/s) | 0.2266 | 0.1135 | 0.4051 | 0.0264 | |
| X5 exp (kPa/l/s) | 0.2353 | 0.0999 | 0.1052 | 0.5801 | |
| ∆X5 (kPa/l/s) | -0.1408 | 0.3294 | -0.4040 | 0.0268 | |
| dR/dF | 0.2677 | 0.0602 | 0.2670 | 0.1538 | |
| FVC (L) | -0.1046 | 0.4695 | -0.4276 | 0.0184 | |
| FVC (% pred) | -0.2612 | 0.0669 | -0.1596 | 0.3995 | |
| FEV1 (L) | -0.1525 | 0.2904 | -0.4045 | 0.0266 | |
| FEV1 (% pred) | -0.1902 | 0.1859 | -0.08580 | 0.6521 | |
| FEV1/FVC (L) | -0.1829 | 0.2036 | -0.2069 | 0.2727 | |
| P (L/s) | -0.1386 | 0.3370 | -0.3995 | 0.0287 | |
| PF (% pred) | -0.3715 | 0.0079 | -0.01331 | 0.9443 | |
| MVV (L) | -0.3663 | 0.0089 | -0.3824 | 0.0370 | |
| MVV (% pred) | -0.1856 | 0.1968 | -0.1457 | 0.4424 | |
| MIP (cmH2O) | -0.1979 | 0.1682 | -0.1133 | 0.5511 | |
| MIP (% pred) | -0.3036 | 0.0321 | -0.2380 | 0.2054 | |
| MEP (cmH2O) | -0.1061 | 0.4633 | -0.06478 | 0.7338 | |
| MEP (% pred) | -0.3764 | 0.0071 | -0.3791 | 0.0388 | |
FVC: forced vital capacity; FEV1: forced expiratory volume in the first second; PF: expiratory peak flow; MVV: maximal voluntary ventilation; MIP: maximal inspiratory pressure; MEP: maximal expiratory pressure. R0: resistance extrapolated for 0Hz; R5: resistance in 5Hz; R20: resistance in 20Hz; Rm: mean resistance; TR: tissue resistance; f0: resonance frequency; AX: reactance integral between 5Hz and resonance frequency; dR/dF: dependence of respiratory system resistance on frequency; X5ins: inspiratory reactance in 5Hz; X5exp: expiratory reactance in 5Hz; ∆X5: difference between inspiratory and expiratory reactance.
The BMI was correlated with spirometric variables FVC (%), FEV1 (%), PF (L/s) in the group of obese, and FEV1/FVC, in addition to MIP (cmH2O) and MEP (%) in the non-obese group (table 5).
Table 5. Correlation of the variables of respiratory mechanics, spirometry and static respiratory pressures with the body mass index.
| Variables |
Morbid obese
(n=50) |
Non-obese
(n=30) |
|||
|---|---|---|---|---|---|
| r | P | r | P | ||
| R0 (kPa/l/s) | 0.00009 | 0.9995 | 0.07414 | 0.6970 | |
| Rm (kPa/l/s) | 0.03030 | 0.8346 | 0.00081 | 0.9966 | |
| TR (kPa/l/s) | 0.1091 | 0.4506 | 0.4199 | 0.1209 | |
| R5 (kPa/l/s) | 0.03077 | 0.8320 | 0.05901 | 0.7567 | |
| R20 (kPa/l/s) | 0.1275 | 0.3777 | -0.1790 | 0.3438 | |
| X5 (kPa/l/s) | 0.06057 | 0.6760 | 0.1759 | 0.3526 | |
| f0 (Hz) | -0.02165 | 0.8813 | 0.4168 | 0.2219 | |
| AX (kPa/l*Hz) | -0.01857 | 0.8982 | 0.2543 | 0.1751 | |
| X5ins (kPa/l/s) | 0.01404 | 0.9229 | -0.03390 | 0.8589 | |
| X5 exp (kPa/l/s) | 0.00568 | 0.9687 | -0.1060 | 0.5773 | |
| ∆X5 (kPa/l/s) | -0.07194 | 0.6195 | -0.1080 | 0.5702 | |
| dR/dF | 0.04433 | 0.7599 | 0.2786 | 0.1360 | |
| FVC (L) | -0.04447 | 0.7591 | -0.3361 | 0.0694 | |
| FVC (% pred) | -0.3847 | 0.0058 | -0.2972 | 0.1107 | |
| FEV1 (L) | -0.02432 | 0.8669 | -0.2320 | 0.2174 | |
| FEV1 (% pred) | -0.3517 | 0.0122 | -0.06050 | 0.7508 | |
| FEV1/FVC (L) | -0.05293 | 0.7151 | -0.4029 | 0.0273 | |
| PF (L/s) | -0.2939 | 0.0383 | -0.3230 | 0.0817 | |
| PF (% pred) | -0.1123 | 0.4374 | -0.1225 | 0.5191 | |
| MVV (L) | -0.1098 | 0.4478 | -0.3298 | 0.0752 | |
| MVV (% pred) | -0.06098 | 0.6740 | -0.2338 | 0.2137 | |
| MIP (cmH2O) | -0.1951 | 0.1746 | -0.5408 | 0.0020 | |
| MIP (% pred) | -0.2941 | 0.0381 | -0.5191 | 0.1067 | |
| MEP (cmH2O) | -0.1746 | 0.6931 | -0.2949 | 0.1136 | |
| MEP (% pred) | -0.2715 | 0.0565 | -0.3627 | 0.0489 | |
FVC: forced vital capacity; FEV1: forced expiratory volume in the first second; PF: expiratory peak flow; MVV: maximal voluntary ventilation; MIP: maximal inspiratory pressure; MEP: maximal expiratory pressure. R0: resistance extrapolated for 0Hz; R5: resistance in 5Hz; R20: resistance in 20Hz; Rm: mean resistance; TR: tissue resistance; f0: resonance frequency; AX: reactance integral between 5Hz and resonance frequency; dR/dF: dependence of respiratory system resistance on frequency; X5ins: inspiratory reactance in 5Hz; X5exp: expiratory reactance in 5Hz; ∆X5: difference between inspiratory and expiratory reactance.
DISCUSSION
Even though in this study the patients with morbid obesity did not show obstructive spirometric patterns, the values of total (R0 and R5), airways (Rm), peripheral (dR/dF and PR) and tissue resistance (TR) were higher than the values presented by the control group. In addition, the results related to respiratory system reactance (AX, X5, X5insp, and X5exp) were different from those of the control group, what can be interpreted as increased peripheral resistance or reduced respiratory system compliance. These results corroborated with those of other authors that observed increased respiratory system, airways(18) and peripheral resistances(7) in obese individuals using the forced oscillations technique. Yap et al.(19) also observed increased peripheral resistance in obese; however, in our sample, the values are 18.6% higher than the group of morbid obese in their study. Such fact can be explained by higher BMI values in our study (50.7±8.9 kg/m2 vs. 43.6±2.5 kg/m2). Several authors suggested that the increased airways resistance in obese individuals is related to the reduction of pulmonary volume; however, its pathophysiology remains unclear. One hypothesis is that the airway structure can be remodeled due to the exposure do proinflammatory adipokines or to the lipid deposition.(6) Mahadev(20) observed that, in addition to the FRC reduction, the airways resistance in morbid obese individuals can also be increased due to the remodeling, which is characterized by the fat deposit in its interior, bronchial mucosa injury related to the stress of opening and closure of small airways and by the chronic exposure to adipocytokines.
This hypothesis agrees with the increased peripheral resistance observed in our study. Zerah et al.(18) also observed that the difference between the respiratory system and airways resistance did not increase significantly with the level of obesity. Based on these results, the authors raised the hypothesis that the thoracic resistance does not increase proportionally to the level of obesity. Even though we did not compare different levels of obesity, the patients with morbid obesity presented higher tissue resistance than the control group, suggesting that the amount of fat tissue in the thoracoabdominal region is associated with a higher dissipation of energy with the respiratory system movement. This result somehow disagrees with Zerah et al.(18) hypothesis. One of the hypothesis for this discordance is the higher BMI of the subjects included in our study, since the sample also had individuals considered super obese. Santana et al.(21) demonstrated how the pulmonary function of super obese can be more affected when compared to morbid obese; however with smaller BMI.
One of the aims of this study was to evaluate the occurrence of expiratory flow limitation in the group of morbid obese patients. According to Lin & Lin(22) the reduced functional residual capacity and expiratory reserve volume of patients with morbid obesity increase the risk of dynamic compression and collapse of the airways, even during rest. Thus, it can occur the expiratory flow limitation (EFL) and air trapping, resulting in increased respiratory effort and dyspnea. The occurrence of EFL has been assessed in obese individuals using the expiratory negative pressure method.(23) In our study, the occurrence of EFL was measured via respiratory system reactance, as described by Dellaca and cols. in 2004 who validated the method using the technique of negative pressure as the golden standard.(24) Using the difference between inspiratory and expiratory reactance, Mahadev et al.(20) evaluated 18 patients with BMI = 41.3±6.8 kg/m and only 1 showed EFL. Similarly, no patient from our sample (mean BMI of 50.7±8.9 kg/m2) presented EFL. These results showed that, despite the reduced FRC and increased peripheral resistance observed in the patients with morbid obesity, the EFL is a common finding only when these individuals are in supine position, corroborating with the study by Pankow et al.(25) In supine position, the abdomen compressive effect reduce even more the FRC and, consequently, the diameter of the airway, resulting in dynamic compression and/or collapse.
The consensus among authors(18,22,23,25) is that even when isolated from other comorbidities, obesity is a preponderant factor for respiratory mechanics impairments, either by analysis of resistance variables or respiratory system compliance. Based on this assumption, the only possible solution for such problems is the weight reduction.
As expected, differently from the hip circumference, the abdominal circumference was associated with several respiratory variables (FVC, FEV1, PF, MVV and respiratory pressures), probably due to the effect of restriction and increased intra-abdominal pressure that happens in morbid obese individuals, altering the elastic balance of the respiratory system and reducing the pulmonary volume.(9,26) The WHR was also associated with respiratory mechanics variables and maximal respiratory pressures, suggesting that not only BMI but also the pattern of body fat distribution affects the respiratory mechanics. This hypothesis corroborates with the findings of Chen et al.(27) that observed a negative correlation between the abdominal circumference ratio and the spirometric variables, regardless of the BMI. Canoy et al.(28) when analyzing 9,674 men and 11,876 women, observed that both FVC and FEV1 were linear and inversely correlated with WHR.
As limitations of this study, there is the lack of static pulmonary volume measures, which would contribute to the understanding of the mechanisms involved in the respiratory mechanics alterations. However, our results showed that, even with spirometric values within normal, the patients with morbid obesity may present respiratory mechanics alterations that can be detected by impulse oscillometry. In addition, not only the BMI but also the pattern of body fat distribution can influence the behavior of the respiratory variables. Thus, the respiratory mechanics assessment using the forced oscillations technique and measurement of anthropometric variables (circumference and WHR) significantly contribute to the follow-up of patients with morbid obesity, especially those with respiratory symptoms. Both methods are non-invasive and do not require special maneuvers. It is likely that the improvement of respiratory mechanics of these patients, especially the peripheral and tissue resistances (thoracic wall), may improve the exercise tolerance(29) with a positive impact on the functional independence and quality of life.
From the results of the present study, we conclude that patients with morbid obesity and no obstructive spirometric pattern have increased total, airways, peripheral, and tissue resistances of the respiratory system when compared to non-obese. These individuals, however, do not show expiratory flow limitation and reduced respiratory muscle strength.
Footnotes
Study carried out in the Hospital Universitário Clementino Fraga Filho, Universidade Federal do Rio de Janeiro, Rio de Janeiro (RJ) Brasil.
Financial support: Programa de Apoio a Núcleos de Excelência (PRONEX-FAPERJ), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Financiadora de Estudos e Projetos (FINEP).
REFERENCES
- 1.Lobato JC, Kale PL, Velarde LG, Szklo M, Costa AJ. Correlation between mean body mass index in the population and prevalence of obesity in Brazilian capitals: empirical evidence for a population-based approach of obesity. BMC Public Health. 2015;15(322):1–6. doi: 10.1186/s12889-015-1637-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang H, Yan Z, Chen Y, Liu F. A social contagious model of the obesity epidemic. Sci Rep. 2016;28(6):1–9. doi: 10.1038/srep37961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO: World Health Organization . Obesity: preventing and managing the global epidemic. Geneva: World Health Organization; 2000. (WHO Obesity Technical Report Series). [PubMed] [Google Scholar]
- 4.Teucher B, Rohrmann S, Kaaks R. Obesity: focus on all-cause mortality and cancer. Maturitas. 2010;65(2):112–116. doi: 10.1016/j.maturitas.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 5.Renquist K. Obesity classification. Obes Surg. 1997;7(6):523. doi: 10.1381/096089297765555331. [DOI] [PubMed] [Google Scholar]
- 6.Mafort TT, Rufino R, Costa CH, Lopes AJ. Obesity: systemic and pulmonary complications, biochemical abnormalities, and impairment of lung function. Multidiscip Respir Med. 2016;11(28):1–11. doi: 10.1186/s40248-016-0066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albuquerque CG, Andrade FMD, Rocha MAA, Oliveira AFF, Ladosky W, Victor EG, et al. Determining respiratory system resistence and reactance by impulse oscillometry in obese individuals. J Bras Pneumol. 2015;41(5):422–426. doi: 10.1590/S1806-37132015000004517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kopelman PG. Clinical complications of obesity. Clin Endocrinol Metab. 1984;13(3):613–634. doi: 10.1016/S0300-595X(84)80041-9. [DOI] [PubMed] [Google Scholar]
- 9.Jones R, Nzekwu M. The effects of body mass index on lung volumes. Chest. 2006;130(3):827–833. doi: 10.1378/chest.130.3.827. [DOI] [PubMed] [Google Scholar]
- 10.Oostveen E, MacLeod D, Lorino H, Farré R, Hantos Z, Desager K, et al. The forced oscillation technique in clinical practice: methodology, recommendations and future developments. Eur Respir J. 2003;22(6):1026–1041. doi: 10.1183/09031936.03.00089403. [DOI] [PubMed] [Google Scholar]
- 11.Bickel S, Popler J, Lesnick B, Eid N. Impulse oscillometry: interpretation and practical applications. Chest. 2014;146(3):841–847. doi: 10.1378/chest.13-1875. [DOI] [PubMed] [Google Scholar]
- 12.de Mesquita JA, Júnior, Lopes AJ, Jansen JM, de Melo PL. Using the forced oscillation technique to evaluate respiratory resistance in individuals with silicosis. J Bras Pneumol. 2006;32(3):213–220. [PubMed] [Google Scholar]
- 13.WHO: World Health Organization . Physical Status: the use and interpretation of anthropometry. Geneva: World Health Organization; 1995. (Technical Report Series). [PubMed] [Google Scholar]
- 14.Standardization of Spirometry 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152(3):1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 15.Sociedade Brasileira de Pneumologia e Tisiologia Diretrizes para testes de função pulmonar. J Bras Pneumol. 2002;28(3):1–238. [Google Scholar]
- 16.American Thoracic Society/European Respiratory Society ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166(4):518–624. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- 17.Neder JA, Andreoni S, Lerario MC, Nery LE. Reference values for lung function tests. II. Maximal respiratory pressures and voluntary ventilation. Braz J Med Biol Res. 1999;32(6):719–727. doi: 10.1590/S0100-879X1999000600007. [DOI] [PubMed] [Google Scholar]
- 18.Zerah F, Harf A, Perlemuter L, Lorino H, Lorino AM, Atlan G. Effects of obesity on respiratory resistance. Chest. 1993;103(5):1470–1476. doi: 10.1378/chest.103.5.1470. [DOI] [PubMed] [Google Scholar]
- 19.Yap JC, Watson RA, Gilbey S, Pride NB. Effects of posture on respiratory mechanics in obesity. J Appl Physiol. 1995;79(4):1199–1205. doi: 10.1152/jappl.1995.79.4.1199. [DOI] [PubMed] [Google Scholar]
- 20.Mahadev S, Salome CM, Berend N, King GG. The effect of low lung volume on airway function in obesity. Respir Physiol Neurobiol. 2013;188(2):192–199. doi: 10.1016/j.resp.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 21.Santana AN, Souza R, Martins AP, Macedo F, Rascovski A, Salge JM. The effect of massive weight loss on pulmonary function of morbid obese patients. Respir Med. 2006;100(6):1100–1104. doi: 10.1016/j.rmed.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 22.Lin CK, Lin CC. Work of breathing and respiratory drive in obesity. Respirology. 2012;17(3):402–411. doi: 10.1111/j.1440-1843.2011.02124.x. [DOI] [PubMed] [Google Scholar]
- 23.Ferretti A, Giampiccolo P, Cavalli A, Milic-Emili J, Tantucci C. Expiratory flow limitation and orthopnea in massively obese subjects. Chest. 2001;119(5):1401–1408. doi: 10.1378/chest.119.5.1401. [DOI] [PubMed] [Google Scholar]
- 24.Dellacà RL, Santus P, Aliverti A, Stevenson N, Centanni S, Macklem PT, et al. Detection of expiratory flow limitation in COPD using the forced oscillation technique. Eur Respir J. 2004;23(2):232–240. doi: 10.1183/09031936.04.00046804. [DOI] [PubMed] [Google Scholar]
- 25.Pankow W, Podszus T, Gutheil T, Penzel T, Peter J, Von Wichert P. Expiratory flow limitation and intrinsic positive end-expiratory pressure in obesity. J Appl Physiol (1985) 1998;85(4):1236–1243. doi: 10.1152/jappl.1998.85.4.1236. [DOI] [PubMed] [Google Scholar]
- 26.Steier J, Lunt A, Hart N, Polkey MI, Moxham J. Observational study of the effect of obesity on lung volumes. Thorax. 2014;69(8):752–759. doi: 10.1136/thoraxjnl-2014-205148. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Rennie D, Cormier YF, Dosman J. Waist circumference is associated with pulmonary function in normal-weight, overweight, and obese subjects. Am J Clin Nutr. 2007;85(1):35–39. doi: 10.1093/ajcn/85.1.35. [DOI] [PubMed] [Google Scholar]
- 28.Canoy D, Luben R, Welch A, Bingham S, Wareham N, Day N, et al. Abdominal obesity and respiratory function in men and women in the EPIC-Norfolk Study, United Kingdom. Am J Epidemiol. 2004;159(12):1140–1149. doi: 10.1093/aje/kwh155. [DOI] [PubMed] [Google Scholar]
- 29.Marinho CL, Maioli MCP, do Amaral JLM, Lopes AJ, Melo PL. Respiratory resistance and reactance in adults with sickle cell anemia: correlation with functional exercise capacity and diagnostic use. PLoS One. 2017;12(12):1–26. doi: 10.1371/journal.pone.0187833. [DOI] [PMC free article] [PubMed] [Google Scholar]


