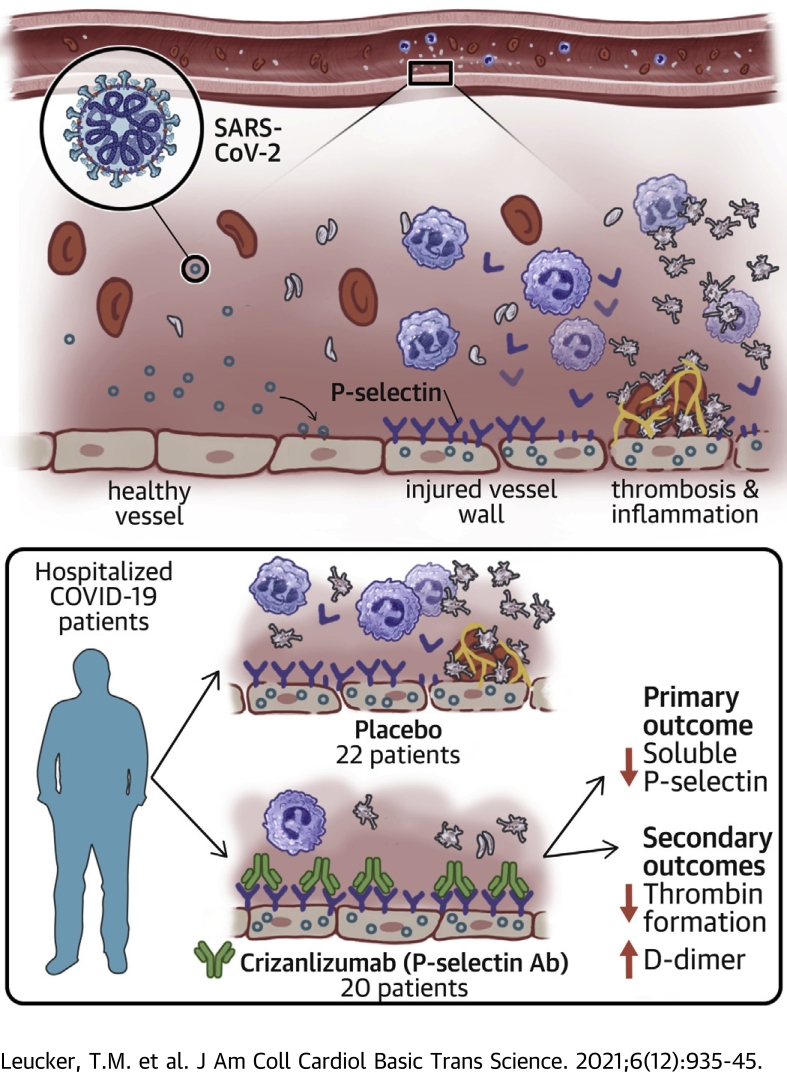
Visual Abstract
Key Words: coronavirus, crizanlizumab, endothelial, exocytosis, inflammation, thrombosis
Abbreviations and Acronyms: CRP, C-reactive protein; IL, interleukin; TAT, thrombin antithrombin; TNF, tumor necrosis factor; VTE, venous thromboembolism; VWF, von Willebrand factor
Highlights
-
•
Severe COVID-19 is characterized by vascular inflammation and thrombosis, including elevations of P-selectin, a marker released by activated endothelial cells that mediates vascular inflammation.
-
•
We tested the effect of crizanlizumab, an antibody to P-selectin, on biomarkers of inflammation and thrombosis in patients with COVID-19 in a randomized, placebo-controlled, double-blind clinical trial.
-
•
Crizanlizumab decreased soluble P-selectin levels in patients with COVID-19.
-
•
Crizanlizumab increased D-dimer and decreased prothrombin fragment 1.2 in patients with COVID-19.
-
•
Crizanlizumab may induce endogenous thrombolysis in the setting of COVID-19.
Summary
COVID-19 is characterized by vascular inflammation and thrombosis, including elevations in P-selectin, a mediator of inflammation released by endothelial cells. We tested the effect of P-selectin inhibition on biomarkers of thrombosis and inflammation in patients with COVID-19. Hospitalized patients with moderate COVID-19 were randomly assigned to receive either placebo or crizanlizumab, a P-selectin inhibitor, in a double-blind fashion. Crizanlizumab reduced P-selectin levels by 89%. Crizanlizumab increased D-dimer levels by 77% and decreased prothrombin fragment. There were no significant differences between crizanlizumab and placebo for clinical endpoints. Crizanlizumab was well tolerated. Crizanlizumab may induce thrombolysis in the setting of COVID-19. (Crizanlizumab for Treating COVID-19 Vasculopathy [CRITICAL]; NCT04435184)
SARS-CoV-2 is a highly infectious and pathogenic respiratory virus with a wide variety of respiratory, cardiovascular, and systemic manifestations. Clinical studies suggest that endothelial cell injury and activation are part of the pathogenesis of COVID-19 (1, 2, 3, 4, 5). Autopsies of patients who died of COVID-19 show systemic microvascular inflammation and microthrombi characteristic of the sequelae following activation of endothelial cells (6, 7, 8, 9). Severe cases of COVID-19 are characterized by elevated levels of P-selectin and von Willebrand factor (VWF), biomarkers of endothelial degranulation (7,10, 11, 12, 13, 14). However, the role of these vascular mediators in the pathogenesis of COVID-19 is unknown (15). We and others have hypothesized that endothelial cell release of vasoactive compounds may play a central role in the pathogenesis of severe COVID-19 (1,2,16).
Crizanlizumab is a humanized monoclonal antibody to P-selectin that decreases the occurrence of vaso-occlusive episodes in subjects with sickle cell disease (17). We tested whether crizanlizumab decreases P-selectin levels and suppresses levels of inflammatory and thrombotic biomarkers in hospitalized patients with moderate COVID-19.
Methods
Trial design and oversight
The CRITICAL (Crizanlizumab for Treating COVID-19 Vasculopathy) trial was a randomized, double-blind, placebo-controlled pilot trial. A steering committee designed and oversaw the conduct of the trial and data analysis. Data were collected, managed, and analyzed by the investigators. SOCAR Research was responsible for the generation and implementation of the randomization process and for data management using the eSOCDAT web-based system (SOCAR Research), and the data coordinating center at Brigham and Women’s Hospital was responsible for analyzing the data. The funder (Novartis AG) provided an institutional grant to the Johns Hopkins University School of Medicine as well as the study drug but had no role in the design, conduct, or analysis of the trial data. The first draft of the paper was prepared by the last author, who had complete access to the data. All authors made the decision to submit the paper for publication and vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol. This clinical trial was conducted at 3 hospitals within the Johns Hopkins Health System in Maryland. The Johns Hopkins University School of Medicine Institutional Review Board approved the protocol for the trial. An independent Data and Safety Monitoring Board monitored trial conduct and patient safety and reviewed unblinded data during the course of the trial (Supplemental Table 1).
Trial patients
Eligibility requirements at screening included age of 18 years or older, symptoms of acute respiratory infection, SARS-CoV-2 infection within the past 10 days of randomization as documented by a positive SARS-CoV-2 laboratory test result (reverse transcriptase polymerase chain reaction), hospitalization, need for supplemental oxygen or a peripheral capillary oxygenation saturation (Spo2) of <94% on room air, radiographic evidence of pulmonary infiltrates, and elevated D-dimer of >0.49 mg/L fibrinogen equivalent units. Exclusion criteria included mechanical ventilation, international normalized ratio of >3, or activated partial thromboplastin time of >60 seconds. Detailed inclusion and exclusion criteria are provided in Supplemental Table 2. All patients provided written informed consent.
Trial procedures
The trial consisted of a screening period followed by a double-blind treatment period. After the screening period, patients were randomly assigned in a 1:1 ratio to receive double-blind treatment with 1 intravenous dose of crizanlizumab 5.0 mg/kg or placebo. Randomization was performed using a secure, central, interactive, web-based response system (eSOCDAT). Crizanlizumab or saline placebo was prepared by the site pharmacist who was unblinded; after crizanlizumab or placebo was prepared, the study treatment was blinded by using darkened bags. After randomization, patients were evaluated on days 3, 7, and 14 if hospitalized, as well as on the day of discharge. The collection of blood samples stopped at hospital discharge. Patients were contacted 30 days after discharge by follow-up telephone call.
Trial outcomes
The primary outcome was the difference between treatment groups in levels of soluble P-selectin at day 3 after randomization or hospital discharge day, whichever occurred earlier. Secondary outcomes included changes between the treatment groups at days 7 and 14 for levels of soluble P-selectin and at days 3, 7, and 14 for levels of D-dimer, VWF, and C-reactive protein (CRP) (provided study participants remained in the hospital). No blood collections were obtained after discharge. Additional secondary outcomes also included changes between groups in the World Health Organization Ordinal Scale for COVID-19 Trials (18), the time to hospital discharge, and the safety of crizanlizumab. Exploratory endpoints included changes between the group receiving crizanlizumab and the group receiving placebo at days 3, 7, and 14 in levels of fibrinogen, interleukin (IL)-6, tumor necrosis factor (TNF) alpha, factor VIII, soluble ICAM-1, soluble VCAM-1, CCL2, troponin isoform T, and N-terminal pro–B-type natriuretic peptide (Supplemental Table 3). Exploratory endpoints included time to the following outcomes: resolution of fever, liberation from supplemental oxygen, mechanical ventilation, hospital death, arterial or venous vascular event, ischemic stroke, myocardial infarction, and hospital discharge. Additional biomarker assays that were not prespecified endpoints were added post hoc, including prothrombin fragment 1.2, thrombin-antithrombin (TAT) complex, plasmin-antiplasmin complex, IL-8, and IL-10.
Laboratory assay of primary endpoint
P-selectin levels were measured in plasma samples using an enzyme-linked immunosorbent assay (Human P-Selectin Quantikine ELISA Kit, DPSE00, R&D Systems) according to the manufacturer’s instructions. This assay has a detection range between 1.2 and 50 ng/mL. Undetectable samples were assigned a value of the lower limit of detection/2 (0.605 ng/mL). All subjects in the placebo group and the crizanlizumab group had detectable P-selectin levels on day 0. All 20 subjects in the placebo group had detectable P-selectin levels on day 3, but 3 of the 22 subjects in the crizanlizumab group had P-selectin levels below the limit of detection on day 3.
Statistical analyses
The prespecified primary, secondary, and exploratory analyses were based on a modified intention-to-treat population that consisted of all randomized patients who had a valid baseline P-selectin value before study drug administration and for whom at least 1 postbaseline P-selectin value was available on or before day 3. Adverse events were assessed in all randomized patients for whom study treatment was started.
The primary endpoint, absolute change from baseline of P-selectin levels, was summarized for each treatment group with descriptive statistics, including mean, SD, median, and interquartile range, as appropriate. Evaluation of the between-group difference was assessed in an analysis of covariance, with baseline soluble P-selectin and treatment group as covariates. A soluble P-selectin value greater than the assay maximum measurement was imputed as 150% of the upper limit of assessment. For all other biomarkers of interest, log-transformation was applied to both the baseline and follow-up values due to skewness of the distributions, and the treatment effect was quantified as the relative change, estimated by exponentiating the beta coefficient pertaining to the absolute treatment effect on the log-biomarker levels.
Characteristics between treatment groups were compared using Student’s t-tests for normally distributed continuous variables or Wilcoxon rank sum tests for nonnormally distributed continuous variables. Categorical variables were compared using the chi-square or Fisher exact tests, as appropriate. A P value of <0.05 was considered significant. Secondary and exploratory endpoints were not adjusted for multiplicity.
Based on a trial design with 2-sided α = 0.05 and assuming an SD of 30 ng/mL and a 10% dropout rate, a sample size of 40 patients (20 per treatment arm) was determined to provide 83% power to detect a between-group difference of 30 ng/mL and 93% power to detect a between- group difference of 35 ng/mL. All analyses were conducted using Stata, version 16 (StataCorp).
Results
Enrollment, randomization, and follow-up
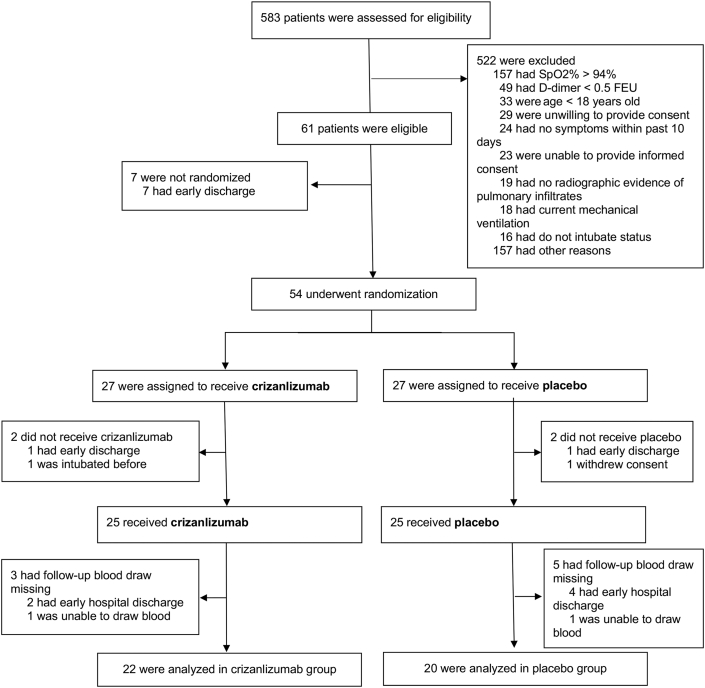
From July 15, 2020, through November 27, 2020, 583 patients were screened at 3 hospitals within the Johns Hopkins Health System (Figure 1). A total of 54 patients who fulfilled study entry criteria were randomized to receive crizanlizumab (n = 27) or placebo (n = 27). Of the 27 assigned to receive crizanlizumab, 2 did not receive crizanlizumab because of early discharge or early intubation; of the 25 who received crizanlizumab, 3 had follow-up blood draws missing because of early hospital discharge, and 22 patients were analyzed in the crizanlizumab group. Of the 27 assigned to receive placebo, 2 did not receive placebo because of early discharge or withdrawn consent; of the 25 who received placebo, 5 had follow-up blood draws missing because of early hospital discharge, and 20 patients were analyzed in the placebo group. Therefore, a total of 22 patients assigned to crizanlizumab and 20 patients assigned to placebo had blood available for biomarker analyses and were included in the primary analysis. At the end of the trial on December 27, 2020, outcomes were known for all patients.
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) Diagram
Patients were screened at 3 separate hospitals within 1 academic medical center system. Of the 583 screened patients, 522 were excluded. Under reasons for exclusion from the trial, “other reasons” includes needs interpreter and assigned to another clinical trial. Of the 61 eligible patients, 54 were randomized, 25 received placebo and 25 received crizanlizumab, and 22 were analyzed in the crizanlizumab group and 20 in the placebo group. FEU = fibrinogen equivalent units.
The baseline clinical characteristics of the patients between the treatment groups were moderately well balanced for a small sample size, except for a history of coronary artery disease and race (Table 1). Baseline levels of blood biomarkers were similar between groups (Table 2).
Table 1.
Characteristics of the Analyzed Patients at Baseline
| Placebo (n = 20) | Crizanlizumab (n = 22) | |
|---|---|---|
| Age, y | 58.0 ± 17.7 | 54.6 ± 13.4 |
| Male | 11 (55.0) | 13 (59.1) |
| Race | ||
| Asian | 1 (5.0) | 0 (0.0) |
| Black | 6 (30.0) | 14 (63.6) |
| White | 13 (65.0) | 8 (36.4) |
| Vital signs | ||
| BMI, kg/m2 | 32.7 ± 9.8 | 36.2 ± 8.0 |
| SBP, mm Hg | 128.3 ± 20.8 | 135.3 ± 23.4 |
| DBP, mm Hg | 70.4 ± 12.6 | 74.5 ± 14.5 |
| Heart rate, beats/min | 78.0 ± 18.2 | 84.3 ± 16.7 |
| Temperature, °C | 36.6 ± 0.5 | 36.9 ± 0.8 |
| O2, % saturation | 93.8 ± 2.9 | 93.3 ± 4.2 |
| COVID-19 symptoms | ||
| Fever | 12 (60.0) | 14 (63.6) |
| Cough | 17 (85.0) | 20 (90.9) |
| Dyspnea | 17 (85.0) | 21 (95.5) |
| Sore throat | 5 (25.0) | 2 (9.1) |
| Anosmia | 3 (15.0) | 2 (9.1) |
| Fatigue | 18 (90.0) | 17 (77.3) |
| Muscle ache | 9 (45.0) | 14 (63.6) |
| WHO status | ||
| 4 | 6 (30.0) | 2 (9.1) |
| 5 | 13 (65.0) | 19 (86.4) |
| 6 | 1 (5.0) | 1 (4.5) |
| Medical history | ||
| Hypertension | 14 (70.0) | 17 (77.3) |
| HF | 4 (20.0) | 1 (4.5) |
| CAD | 4 (20.0) | 0 (0.0) |
| PAD | 1 (5.0) | 0 (0.0) |
| Stroke/TIA | 0 (0.0) | 1 (4.5) |
| Arrhythmia | 0 (0.0) | 1 (4.5) |
| DVT/PE | 0 (0.0) | 1 (4.5) |
| Smoking | 3 (15.0) | 2 (9.1) |
| Diabetes | 8 (40.0) | 11 (50.0) |
| CKD (no dialysis) | 2 (10.0) | 1 (4.5) |
| CKD (dialysis) | 0 (0.0) | 2 (9.1) |
| Liver disease | 1 (5.0) | 0 (0.0) |
| Asthma | 1 (5.0) | 2 (9.1) |
| COPD | 3 (15.0) | 1 (4.5) |
Values are mean ± SD or n (%).
BMI = body mass index; CAD = coronary artery disease; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; DBP = diastolic blood pressure; DVT = deep vein thrombosis; HF = heart failure; PAD = peripheral artery disease; PE = pulmonary embolism; SBP = systolic blood pressure; TIA = transient ischemic attack; WHO = World Health Organization.
Table 2.
Biomarkers of the Analyzed Patients by Time
| Biomarker | Time | Placebo (n = 20) | Crizanlizumab (n = 22) |
|---|---|---|---|
| P-selectin, ng/mL | Baseline | 34 ± 15 | 30 ± 20 |
| Day 3 | 39 ± 18 | 7 ± 7 | |
| Day 7 | 48 ± 17 | 10 ± 8 | |
| Day 14 | 48 ± 24 | 12 ± 10 | |
| IL-6, pg/mL | Baseline | 71 (18-371) | 62 (36-87) |
| Day 3 | 115 (23-298) | 58 (29-134) | |
| Day 7 | 30 (28-123) | 26 (10-43) | |
| Day 14 | 78 (24-151) | 14 (6-27) | |
| TNF-α, pg/mL | Baseline | 15 (11-22) | 13 (7-26) |
| Day 3 | 14 (8-22) | 15 (10-21) | |
| Day 7 | 11 (5-25) | 12 (8-31) | |
| Day 14 | 12 (5-15) | 12 (10-20) | |
| VWF, IU/mL | Baseline | 2.5 (1.5-5.6) | 2.4 (1.8-4.1) |
| Day 3 | 2.8 (1.7-4.1) | 2.9 (1.9-4.9) | |
| Day 7 | 3.6 (2.1-6.8) | 3.9 (2.5-5.9) | |
| Day 14 | 4.6 (2.6-7.4) | 2.7 (1.9-4.2) | |
| CRP, mg/dL | Baseline | 5.8 (2.7-10.8) | 7.6 (3.2-11.7) |
| Day 3 | 4.5 (1.2-6.9) | 4.4 (2.1-5.6) | |
| Day 7 | 2.1 (0.6-4.2) | 2.4 (1.1-4.9) | |
| Day 14 | 1.3 (0.6-4.9) | 2.5 (1.1-5.1) | |
| D-dimer, mg/L | Baseline | 0.7 (0.6-1.1) | 0.9 (0.8-2.5) |
| Day 3 | 0.7 (0.5-1.4) | 1.6 (0.7-2.8) | |
| Day 7 | 0.7 (0.5-0.8) | 1.6 (0.6-2.0) | |
| Day 14 | 0.7 (0.5-0.8) | 1.5 (0.5-1.9) |
Values are mean ± SD or median (interquartile range).
CRP = C-reactive protein; IL = interleukin; TNF = tumor necrosis factor; VWF = von Willebrand factor.
Outcomes
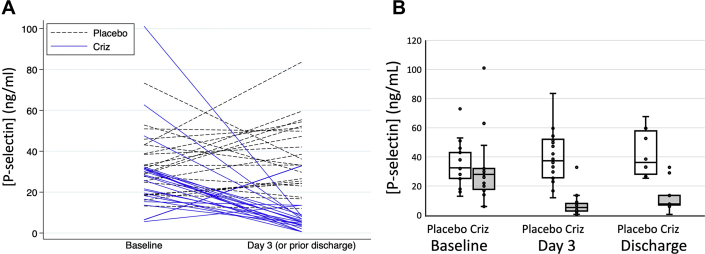
Baseline levels of P-selectin were similar between patient groups before receiving crizanlizumab (30 ± 20 ng/mL) and placebo (34 ± 15 ng/mL). The mean ± SD changes from baseline to day 3 or before discharge were -23 ± 23 ng/mL and +5 ± 18 ng/mL. In baseline-adjusted models, crizanlizumab reduced P-selectin levels by 32 ng/mL (95% CI: 24-41; P < 0.001) by day 3 or before discharge (89% reduction from baseline), 38 ng/mL: (95% CI: 28-48; P < 0.001) by day 7 (84% reduction from baseline), and 35 ng/mL: (95% CI: 19-51; P < 0.001) by day 14 (80% reduction from baseline) (Figure 2, Table 3). Crizanlizumab increased D-dimer levels by 77% (95% CI: 6%-194%; P = 0.029) on day 3 after treatment; D-dimer levels were not significantly different between groups on day 7 and on the day of discharge after day 3 (Table 4). Crizanlizumab had no significant effect on other secondary biomarker endpoints, including levels of IL-6, TNF-α, VWF, and CRP (Table 4).
Figure 2.
Crizanlizumab Decreases Soluble P-Selectin Levels
(A) Spaghetti plot. (B) Comparison of soluble P-selectin levels in groups treated with crizanlizumab (Criz) versus placebo at baseline, day 3 or before discharge, and day of discharge. n = 22 in the placebo group, and n = 20 in the crizanlizumab group.
Table 3.
Change From Baseline in Biomarkers by Time
| Biomarker | Time | Placebo (n = 20) | Crizanlizumab (n = 22) |
|---|---|---|---|
| P-selectin, ng/mL | Day 3 | +5 ± 18 | -23 ± 23 |
| Day 7 | +18 ± 13 | -17 ± 16 | |
| Day 14 | +19 ± 19 | -14 ± 19 | |
| IL-6, pg/mL | Day 3 | 0 (-21 to +13) | 0 (-18 to +35) |
| Day 7 | -22 (-89 to +10) | -12 (-50 to +3) | |
| Day 14 | -15 (-64 to +54) | -11 (-48 to +7) | |
| TNF-α, pg/mL | Day 3 | -1.7 (-5.9 to +0.4) | 4.2 (-2.6 to +9.8) |
| Day 7 | -1.7 (-5.5 to +0.7) | -0.3 (-4.4 to +12.3) | |
| Day 14 | -0.9 (-4.0 to +0.4) | 2.3 (-4.4 to +8.5) | |
| VWF, IU/mL | Day 3 | 0.0 (-2.8 to +1.7) | +0.1 (-1.2 to +2.2) |
| Day 7 | +0.4 (-1.4 to +1.7) | +1.1 (-0.3 to +2.6) | |
| Day 14 | +1.3 (0.1 to +3.7) | +0.8 (-0.1 to +2.2) | |
| CRP, mg/dL | Day 3 | -1.2 (-7.1 to +0.5) | -2.1 (-5.8 to -0.5) |
| Day 7 | -1.8 (-3.9 to -0.1) | -7.0 (-9.6 to -1.6) | |
| Day 14 | -1.5 (-8.8 to -0.1) | -3.6 (-8.9 to -1.6) | |
| D-dimer, mg/L | Day 3 | -0.1 (-0.5 to +0.2) | -0.1 (-0.3 to +1.1) |
| Day 7 | -0.1 (-0.3 to +0.1) | -0.1 (-0.6 to +0.8) | |
| Day 14 | -0.2 (-0.3 to +0.1) | -0.2 (-0.6 to +0.8) |
Values are mean ± SD or median (interquartile range). Values represent the absolute change in biomarker levels in patients treated with placebo and patients treated with crizanlizumab.
Abbreviations as in Table 2.
Table 4.
Effect of Treatment on Primary and Secondary Endpoint Biomarkers
| Biomarker | Day 3 or Before Discharge | Day 7 or Before Discharge | Day 14 or Before Discharge |
|---|---|---|---|
| P-selectin, ng/mL, absolute change | -32 (-41 to -24) P < 0.001, n = 42 |
-38 (-48 to -28) P < 0.001, n = 27 |
-35 (-51 to -19) P < 0.001, n = 21 |
| IL-6, relative change, % | +11 (-43 to +117) P = 0.76, n = 42 |
-41 (-74 to +34) P = 0.20, n = 27 |
-64 (-89 to +14) P = 0.08, n = 18 |
| TNF-α, relative change, % | +46 (-7 to +128) P = 0.10, n = 42 |
+56 (-24 to +218) P = 0.21, n = 26 |
+50 (-39 to +273) P = 0.36, n = 18 |
| VWF, relative change, % | +11 (-26 to +68) P = 0.60, n = 42 |
+10 (-38 to +98) P = 0.73, n = 27 |
0 (-57 to +128) P = 0.99, n = 18 |
| CRP, relative change | +1 (-40 to +68) P = 0.98, n = 40 |
-26 (-68 to +73) P = 0.47, n = 29 |
-18 (-68 to +110) P = 0.66, n = 25 |
| D-dimer, relative change | +77 (+6 to +194) P = 0.029, n = 40 |
+45 (-13 to +143) P = 0.15, n = 31 |
+16 (-41 to +127) P = 0.65, n = 28 |
Values are median (interquartile range) unless noted otherwise. The effect of crizanlizumab compared to placebo on biomarkers at day 3 or before discharge, day 7 or before discharge, and day 14 or before discharge is shown.
Abbreviations as in Table 2.
We observed no difference in clinical outcomes between treatment groups, including time to hospital discharge, or clinical status measured by the World Health Organization ordinal scale for COVID-19 trials (Table 5, Supplemental Table 4). There were no events for the endpoints of duration of mechanical ventilation, time to vascular event, stroke, or myocardial infarction.
Table 5.
Secondary Outcomes
| Clinical Status | Placebo (n = 20) | Crizanlizumab (n = 22) |
|---|---|---|
| WHO status: day 3 | ||
| ≤3 (discharged) | 2 (10) | 3 (14) |
| 4 | 7 (35) | 3 (14) |
| 5 | 7 (35) | 13 (59) |
| 6 | 4 (20) | 3 (14) |
| WHO status: day 7 | ||
| ≤3 (discharged) | 13 (65) | 9 (41) |
| 4 | 3 (15) | 5 (23) |
| 5 | 3 (15) | 6 (27) |
| 6 | 1 (5) | 1 (5) |
| 7 | 0 (0) | 1 (5) |
| WHO status: day 14 | ||
| ≤3 (discharged) | 19 (95) | 21 (95) |
| 6 | 0 (0) | 1 (5) |
| 8 | 1 (5) | 0 (0) |
| Days in hospital | ||
| Admission to randomization | 1.5 ± 0.9 | 1.7 ± 1.4 |
| Randomization to discharge | 4.8 ± 3.5 | 6.5 ± 4.1 |
| Admission to discharge | 6.2 ± 3.2 | 8.1 ± 4.4 |
Values are n (%) or mean ± SD. Clinical outcomes were measured by the WHO Clinical Scale and length of hospital stay.
WHO = World Health Organization.
Several post hoc analyses were performed to better understand the effect of crizanlizumab on markers of thrombosis and fibrinolysis. Crizanlizumab had no effect on a wide variety of cytokine and chemokine biomarkers, except for IL-8 and IL-10, which were both significantly increased on day 3 post-randomization (Table 6). To assess the effect of crizanlizumab on thrombin activation, we measured prothrombin fragment 1.2 and TAT complex. Crizanlizumab decreased prothrombin fragment 1.2 to 2.3 ng/mL (95% CI: 1.2-3.8) compared to placebo 3.6 ng/mL (95% CI: 0.6-6.1) with P = 0.007 on day 3 and nonsignificantly decreased TAT complex to 4.3 ng/mL (95% CI: 3.0-7.8) compared to placebo 8.4 ng/mL (95% CI: 4.8-9.98 ) with P = 0.08 on day 3 (Table 6). Crizanlizumab had no effect on plasmin-antiplasmin complex at 312 ng/mL (95% CI: 205-737) compared to placebo 345 ng/mL (95% CI: 180-788) with P = 0.93 on day 3 (Table 6).
Table 6.
Exploratory Biomarkers and Additional Biomarkers
| Analyte | Crizanlizumab Day 1 | Placebo Day 1 | Crizanlizumab Day 3 | Placebo Day 3 | Ratio (95% CI) | P Value |
|---|---|---|---|---|---|---|
| Exploratory biomarkers (prespecified) | ||||||
| CCL2, pg/mL (n = 38) | 257 (114-404) | 242 (147-293) | 243 (161-341) | 209 (142-460) | 0.98 (0.65-1.49) | 0.93 |
| Factor VIII, IU/mL (n = 42) | 35 (24-55) | 42 (26-83) | 56 (29-88) | 59 (32-83) | 1.25 (0.91-1.71) | 0.16 |
| IL-6, pg/mL (n = 38) | 6.3 (2.1-10.9) | 2.1 (1.0-10.3) | 4.8 (1.0-12.7) | 2.1 (0.6-5.1) | 1.14 (0.51-2.55) | 0.75 |
| TNF-α, pg/mL (n = 38) | 4.3 (3.3-5.9) | 4.7 (2.8-6.5) | 4.2 (3.4-6.1) | 4.0 (2.7-6.2) | 1.28 (0.95-1.73) | 0.10 |
| VCAM-1, μg/mL (n = 38) | 24.1 (21.1-26.6) | 24.5 (21.8-31.0) | 23.9 (21.9-30.2) | 23.0 (19.1-30.0) | 1.05 (0.92-1.21) | 0.45 |
| ICAM-1, μg/mL (n = 38) | 23.4 (18.5-26.9) | 22.6 (21.0-25.4) | 21.0 (19.2-27.6) | 20.7 (18.9-23.3) | 1.08 (0.99-1.18) | 0.10 |
| High sensitivity troponin T, ng/mL (n = 40) | 8 (3-12) | 10 (3-17) | 7 (3-11) | 9 (3-21) | 0.99 (0.72-1.38) | 0.97 |
| NT-proBNP, ng/mL (n = 36) | 0.19 (0.19-0.19) | 0.19 (0.19-0.19) | 0.19 (0.19-0.19) | 0.19 (0.19-0.19) | 0.99 (0.73-1.35) | 0.97 |
| Additional biomarkers (not prespecified) | ||||||
| CCL24, pg/mL (n = 38) | 195 (149-305) | 234 (197-301) | 182 (141-352) | 258 (201-351) | 0.81 (0.59-1.11) | 0.18 |
| CCL4, pg/mL (n = 38) | 92 (54-143) | 82 (56-116) | 128 (72-169) | 102 (68-136) | 1.03 (0.79-1.34) | 0.81 |
| CCL26, pg/mL (n = 38) | 20 (20-20) | 20 (20-20) | 20 (20-20) | 20 (20-20) | 1.31 (0.90-1.91) | 0.16 |
| CCL17, pg/mL (n = 38) | 103 (76-161) | 175 (82-257) | 103 (74-145) | 146 (94-240) | 0.74 (0.54-1.02) | 0.06 |
| CXCL10, pg/mL (n = 38) | 14,160 (3,335-14,160) | 3,935 (1,820-14,160) | 5,360 (2,762-11,125) | 1,675 (985-5,930) | 1.43 (0.86-2.39) | 0.16 |
| CCL3, pg/mL (n = 38) | 28 (28-28) | 28 (28-28) | 28 (28-28) | 28 (28-28) | 1.18 (0.93-1.49) | 0.16 |
| CCL22, pg/mL (n = 38) | 669 (509-963) | 832 (547-935) | 536 (444-654) | 630 (399-943) | 0.93 (0.70-1.23) | 0.60 |
| CCL13, pg/mL (n = 38) | 126 (76-180) | 106 (88-156) | 119 (86-186) | 129 (99-162) | 0.87 (0.68-1.13) | 0.29 |
| Interferon gamma, pg/mL (n = 38) | 22 (10-108) | 21 (8-64) | 17 (5-43) | 7 (2-12) | 2.10 (0.80-5.54) | 0.13 |
| IL-2, pg/mL (n = 38) | 0.9 (0.9-0.9) | 0.9 (0.9-0.9) | 0.9 (0.9-0.9) | 0.9 (0.9-0.9) | 0.97 (0.76-1.24) | 0.80 |
| IL-4, pg/mL (n = 38) | 0.2 (0.2-0.2) | 0.2 (0.2-0.2) | 0.2 (0.2-0.2) | 0.2 (0.2-0.2) | 1.12 (0.96-1.31) | 0.15 |
| IL-8, pg/mL (n = 38) | 15.7 (9.9-24.5) | 18.9 (15.2-21.9) | 21.7 (15.4-49.9) | 14.6 (11.2-21.5) | 1.88 (1.20-2.96) | 0.007 |
| IL-10, pg/mL (n = 38) | 2.9 (1.2-5.3) | 2.0 (1.1-3.2) | 2.8 (1.3-6.9) | 1.0 (0.3-1.8) | 2.64 (1.34-5.20) | 0.007 |
| IL-13, pg/mL (n = 38) | 4.2 (4.2-4.2) | 4.2 (4.2-4.2) | 4.2 (4.2-4.2) | 4.2 (4.2-4.2) | 1.00 (1.00-1.00) | 0.18 |
| SAA, μg/mL (n = 38) | 207 (207-207) | 207 (207-207) | 207 (160-207) | 207 (33-207) | 1.48 (0.98-2.22) | 0.06 |
| TAT complex, ng/mL(n = 41) | 6.0 (3.2-9.9) | 5.2 (3.4-8.8) | 4.3 (3.0-7.8) | 8.4 (4.8-9.8) | 0.73 (0.50-1.05) | 0.08 |
| Prothrombin fragment 1.2, ng/mL (n = 42) | 2.6 (1.6-4.0) | 2.5 (1.6-3.5) | 2.3 (1.2-3.8) | 3.6 (2.6-6.1) | 0.50 (0.31-0.82) | 0.007 |
| PAP complex, ng/mL (n = 42) | 409 (290-960) | 397 (260-755) | 312 (205-737) | 345 (180-788) | 0.98 (0.65-1.49) | 0.93 |
Values are median (interquartile range) unless noted otherwise. Absolute levels of biomarkers of patients treated with placebo and patients treated with crizanlizumab on baseline day 1 or on day 3 or before discharge (n = sample size).
IL = interleukin; NT-proBNP = N-terminal pro–B-type natriuretic peptide; PAP = plasmin-antiplasmin complex; SAA = serum amyloid A; TAT = thrombin-antithrombin.
Safety
After randomization, there were 6 serious and nonserious adverse events in the placebo-treated group of 25 patients and 7 serious and nonserious adverse events in the crizanlizumab-treated group of 25 patients, including 1 serious adverse event (multiorgan failure) in the placebo group and 0 serious adverse events in the crizanlizumab group (Table 7). Nonserious adverse events included headache, change in mental status, chest pain, urinary tract infection, and diarrhea.
Table 7.
Adverse Events
| Adverse Events | Placebo (n = 25) | Crizanlizumab (n = 25) | Total |
|---|---|---|---|
| Total adverse events | 6 | 7 | 13 |
| Patients with any adverse events | 6 | 4 | 10 |
| Total moderate or severe adverse events | 3 | 2 | 5 |
| Patients with any moderate or severe adverse events | 3 | 2 | 5 |
| Total serious adverse events | 1 | 0 | 1 |
| Patients with any serious adverse events | 1 | 0 | 1 |
Values are n. Adverse events in all subjects who received crizanlizumab or placebo are shown.
Discussion
We found that crizanlizumab treatment resulted in marked and sustained reduction in soluble P-selectin levels in patients with moderate COVID-19. Although not associated with changes in several cytokines, crizanlizumab increased D-dimer levels and decreased prothrombin fragment 1.2. Clinical outcomes and adverse events were similar between the crizanlizumab and placebo groups.
Crizanlizumab, a soluble P-selectin inhibitor, has previously been shown to reduce the rate of sickle cell–related vaso-occlusive crises in patients with sickle-cell disease (17). Elevation of P-selectin and VWF, both contained in endothelial granules, has been reported in COVID-19 and was associated with worse outcomes (7,10, 11, 12, 13, 14,19,20). Therefore, we hypothesized that blocking P-selectin with crizanlizumab would have beneficial downstream effects on inflammatory markers and markers of thrombosis, potentially by blocking leukocyte rolling, an early step in leukocyte trafficking that is mediated by P-selectin. We observed no compelling differences between treatment groups in markers of systemic inflammation such as CRP, IL-6, TNF-α, or interferon gamma, nor in markers of vascular inflammation released from endothelial cells, such as VCAM-1 or CCL2 (also called MCP1). Crizanlizumab did increase levels of IL-10, an anti-inflammatory cytokine, without suppressing targets of IL-10 such as TNF-α, IL-1β, and interferon gamma, so the significance of increased IL-10 levels is unclear. Crizanlizumab also increased levels of IL-8, a proinflammatory cytokine that recruits neutrophils. It is unclear how inhibition of P-selectin leads to changes in IL-8 and IL-10. The finding that markers of inflammation, particularly IL-6 and CRP, were not reduced by crizanlizumab suggests that P-selectin probably does not mediate the inflammatory response in COVID-19. We cannot exclude the possibility that endothelial granule release may be secondary to inflammatory processes rather than mediating them. Larger clinical trials are needed to evaluate the impact of crizanlizumab on systemic inflammation.
We found that crizanlizumab was associated with an increase in D-dimer, a marker that has been associated with the severity of COVID-19 (21, 22, 23). D-dimer, a fibrin degradation product, is typically elevated in the setting of thrombosis and concurrent thrombolysis (24,25), but can also be seen in the setting of therapies that stimulate thrombolysis (26, 27, 28, 29, 30). Because we observed no evidence of increased macrothrombosis or worsening of clinical status with crizanlizumab, we further explored the potential effects of the therapy on the thrombosis and fibrinolytic system. We found that crizanlizumab reduced both prothrombin fragment 1.2 and TAT complex, biomarkers of thrombin activation. In prior studies of patients with COVID-19, elevation of prothrombin fragment 1.2 was highly correlated with D-dimer (31), raising the possibility that the rise in D-dimer observed in association with a decrease in prothrombin fragment 1.2 may be due to fibrinolysis and reduced thrombin activation, rather than increased thrombosis, because it may indicate in the native state. However, we do not have direct evidence of increased thrombolysis in subjects treated with crizanlizumab. Another possible mechanism by which P-selectin inhibition might be beneficial in COVID-19 is through attenuation of neutrophil extracellular trap formation, which has been shown to be induced by P-selectin in mice and has been implicated in the pathophysiology of both sickle cell crisis and COVID-19 (32, 33, 34).
Endothelial cells release VWF and P-selectin from granules in response to vascular injury. Crizanlizumab did not affect levels of VWF in our subjects. We were unable to measure relative amounts of VWF multimers in our trial, and it is possible that crizanlizumab directly or indirectly changes the extent of VWF multimerization.
Although we observed no effect on clinical outcomes in patients treated with crizanlizumab, the patients enrolled in this trial was small in number and relatively healthy. There were no deaths during the study period. Average oxygen saturation levels in enrolled patients were between 87% and 93% for patients on room air and between 94% and 95% for patients on supplemental oxygen, CRP levels were between 5.8 and 7.6 mg/dL at baseline, and the clinical course in the majority of patients was relatively benign. Determining whether crizanlizumab would be of clinical benefit in COVID-19 would require testing in a higher-risk population with more severe vascular injury.
The role of P-selectin in COVID-19 is not completely understood. We have proposed that markers of endothelial injury such as P-selectin mediate microvascular inflammation and thrombosis in COVID-19 (1). We hypothesized that P-selectin inhibition would decrease microvascular inflammation and thrombosis and ultimately improve clinical outcomes in COVID-19. We designed the CRITICAL trial to enroll patients with moderate COVID-19, excluding subjects with mild COVID-19 who have minimal inflammation and are likely to recover rapidly and excluding subjects with severe COVID-19 who have such extensive systemic inflammation that any intervention is unlikely to change the course of the disease.
P-selectin levels have been shown to be higher in subjects with severe COVID-19 than in those with moderate COVID-19. However, reports of P-selectin levels in subjects with moderate COVID-19 are variable, with some reports showing P-selectin levels higher in moderate COVID-19 than in control subjects and other reports showing similar levels in moderate COVID-19 and control subjects (35). The cause of such differences between studies include the following: different assays for P-selectin, potential differences in serum versus plasma levels of P-selectin, different definitions of “moderate” COVID-19, and some reports measuring soluble P-selectin and others measuring platelet-expressed P-selectin. In our trial of patients with moderate COVID-19 levels, the baseline levels of plasma P-selectin were 34 ± 14 ng/mL for the placebo group and 30 ± 20 ng/mL for the crizanlizumab group. These levels are higher than levels reported for healthy control subjects in other studies (35). Nevertheless, it is possible that crizanlizumab could be more effective at decreasing inflammatory and thrombotic biomarkers in a study group with even higher levels of P-selectin.
Study limitations
Several additional limitations of this analysis should be noted. We administered only 1 dose of crizanlizumab, and it is possible that a higher dose at more frequent intervals may be necessary to produce significant effects. Nevertheless, the P-selectin reduction observed was substantial and sustained, suggesting that a higher dose is likely not needed. We administered crizanlizumab relatively early in the hospital stay, and it is possible that administration even earlier in the course of COVID-19, such as the time of admission, may have more impact on vascular inflammation. Finally, the patients enrolled in this pilot trial were at relatively low risk and had few clinical events. The role of crizanlizumab in reducing morbidity and mortality in COVID-19 will be tested as part of the NIH ACTIV-4a platform (NCT04505774).
Conclusions
In summary, a single infusion of crizanlizumab, a selective P-selectin inhibitor, resulted in a rapid, profound, and sustained reduction in P-selectin levels in hospitalized patients with mild to moderate COVID-19. These findings were associated with an increase in D-dimer levels and reduction in prothrombin fragment 1.2, raising the possibility that crizanlizumab might increase endogenous thrombolysis in this setting. The clinical significance of these findings needs to be assessed in a larger trial.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: COVID-19 is characterized by microvascular inflammation and thrombosis. P-selectin plays an important role in vascular inflammation by mediating platelet and neutrophil adherence to the endothelial lining of injured vessels.
TRANSLATIONAL OUTLOOK: Our randomized clinical trial suggests that targeting P-selectin might be a promising strategy to prevent or treat vascular and inflammatory complications of COVID-19.
Funding Support and Author Disclosures
This work was funded by Novartis. Dr Leucker has received grants from the American Heart Association Career Development Award (19CDA34760040), the Johns Hopkins Center for AIDS Research (P30AI094189 and 1704611701), the Johns Hopkins Older Americans Independence Center (P30AG021334), and Amgen; and is a coinvestigator of the CRITICAL trial, which tests the safety and efficacy of a P-selectin inhibitor in patients with COVID-19. Dr Gerstenblith has received grant support from Amgen, Novartis, and the National Institutes of Health; is also a member of the Data and Safety Monitoring Board for clinical trials sponsored by Salubris, Blade Therapeutics, and Capricor. Dr Hager has received a grant from Incyte Corporation for conduct of the RUXCOVID-DEVENT trial; has previously received salary support from the Marcus Foundation for conduct of the VICTAS trial; and is currently a coinvestigator in the Centers for Disease Control and Prevention–funded Influenza and Other Viruses in the Acutely Ill Network (75D30121F00002). Dr Streiff is supported by contracts from Patient Centered Outcomes Research Institute entitled “Preventing Venous Thromboembolism: Empowering Patients and Enabling Patient-Centered Care via Health Information Technology” (CE-12-11-4489), “Preventing Venous Thromboembolism (VTE): Engaging Patients to Reduce Preventable Harm From Missed/Refused Doses of VTE Prophylaxis” (DI-1603-34596), “Implementing Best-Practice, Patient-Centered Venous Thromboembolism (VTE) Prevention in Trauma Centers” (DI-2019C3-17859); is supported by a grant from the Agency for Healthcare Research and Quality (1R01HS024547) entitled “Individualized Performance Feedback on Venous Thromboembolism Prevention Practice,” a contract from Patient Centered Outcomes Research Institute entitled “Preventing Venous Thromboembolism (VTE): Engaging Patients to Reduce Preventable Harm From Missed/Refused Doses of VTE Prophylaxis,” a grant from the National Institutes of Health/National Heart, Lung, and Blood Institute (R21HL129028) entitled “Analysis of the Impact of Missed Doses of Venous Thromboembolism Prophylaxis,” and a grant from the Agency for Healthcare Research and Quality (R18HS025341) titled “Disseminating an Evidence-Based Venous Thromboembolism Prevention Bundle” (all of these grants/contract support studies outside the submitted work); has received research funding from Boehringer-Ingelheim, Janssen, Novo Nordisk, Roche, Sanofi, and Tremeau; has consulted for Bayer, Bristol Myers Squibb, Coagulo Medical Technologies, Janssen, Pfizer, and Portola; has received honoraria for CME presentations from Bayer, Pfizer, and Portola (outside the submitted work); and has given expert witness testimony in various medical malpractice cases. Dr Solomon has received grants and personal fees from Alnylam, Amgen, AstraZeneca, Bayer, Bristol Myers Squibb, Cytokinetics, Gilead, GlaxoSmithKline, MyoKardia, Novartis, and Theracos; grants from Bellerophon, Celladon, Eidos, Ionis, Lone Star Heart, Mesoblast, the National Institutes of Health/National Heart, Lung, and Blood Institute, and Sanofi Pasteur; personal fees from Akros, AoBiome, Arena, Cardiac Dimensions, Cardurion, Corvia, Daichi-Sankyo, Dinaqor, Ironwood, Janssen, Merck, Quantum Genomics, Roche, Takeda, Tenaya, Tremeau; and personal fees from Moderna (outside the submitted work) and is a coinvestigator of the CRITICAL trial, which tests the safety and efficacy of a P-selectin inhibitor in patients with COVID-19. Dr Lowenstein has received research grants from Novartis; the National Institutes of Health/National Heart, Lung, and Blood Institute (R01 HL134894, R61 HL14179); and the Michel Mirowski M.D. Professorship in Cardiology. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors gratefully acknowledge the work of members of the Johns Hopkins Investigational Drug Service, Laura Wachter, PharmD, and Lisa Ruppel, PharmD. We gratefully acknowledge the assistance of Shannon Kelley, BS, Johns Hopkins Hospital.
Footnotes
Drs Solomon and Lowenstein contributed equally to this work.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables, please see the online version of this paper.
Appendix
References
- 1.Lowenstein C.J., Solomon S.D. Severe COVID-19 is a microvascular disease. Circulation. 2020;142:1609–1611. doi: 10.1161/CIRCULATIONAHA.120.050354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libby P., Luscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. 2020;41:3038–3044. doi: 10.1093/eurheartj/ehaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dupont A., Rauch A., Staessens S., et al. Vascular endothelial damage in the pathogenesis of organ injury in severe COVID-19. Arterioscler Thromb Vasc Biol. 2021;41(5):1760–1773. doi: 10.1161/ATVBAHA.120.315595. [DOI] [PubMed] [Google Scholar]
- 4.Evans P.C., Rainger G.E., Mason J.C., et al. Endothelial dysfunction in COVID-19: a position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc Res. 2020;116:2177–2184. doi: 10.1093/cvr/cvaa230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varga Z., Flammer A.J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schurink B., Roos E., Radonic T., et al. Viral presence and immunopathology in patients with lethal COVID-19: a prospective autopsy cohort study. Lancet Microbe. 2020;1(7):e290–e299. doi: 10.1016/S2666-5247(20)30144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goshua G., Pine A.B., Meizlish M.L., et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7:e575–e582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bryce C., Grimes Z., Pujadas E., et al. Pathophysiology of SARS-CoV-2: the Mount Sinai COVID-19 autopsy experience. Mod Pathol. 2021;34(8):1456–1467. doi: 10.1038/s41379-021-00793-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrett T.J., Lee A.H., Xia Y., et al. Platelet and vascular biomarkers associate with thrombosis and death in coronavirus disease. Circ Res. 2020;127:945–947. doi: 10.1161/CIRCRESAHA.120.317803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fraser D.D., Patterson E.K., Slessarev M., et al. Endothelial injury and glycocalyx degradation in critically ill coronavirus disease 2019 patients: implications for microvascular platelet aggregation. Crit Care Explor. 2020;2 doi: 10.1097/CCE.0000000000000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venter C., Bezuidenhout J.A., Laubscher G.J., et al. Erythrocyte, platelet, serum ferritin, and P-selectin pathophysiology implicated in severe hypercoagulation and vascular complications in COVID-19. Int J Mol Sci. 2020;21(21):1–14. doi: 10.3390/ijms21218234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez-Castaneda S., Garcia-Larragoiti N., Cano-Mendez A., et al. Inflammatory and prothrombotic biomarkers associated with the severity of COVID-19 infection. Clin Appl Thromb Hemost. 2021;27:1–9. doi: 10.1177/1076029621999099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spadaro S., Fogagnolo A., Campo G., et al. Markers of endothelial and epithelial pulmonary injury in mechanically ventilated COVID-19 ICU patients. Crit Care. 2021;25:74. doi: 10.1186/s13054-021-03499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iba T., Connors J.M., Levy J.H. The coagulopathy, endotheliopathy, and vasculitis of COVID-19. Inflamm Res. 2020;69:1181–1189. doi: 10.1007/s00011-020-01401-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ataga K.I., Kutlar A., Kanter J., et al. Crizanlizumab for the prevention of pain crises in sickle cell disease. N Engl J Med. 2017;376:429–439. doi: 10.1056/NEJMoa1611770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO Working Group on the Clinical Characterisation and Management of COVID-19 Infection A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20:e192–e197. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Connors J.M., Iba T., Gandhi R.T. Thrombosis and COVID-19: controversies and (tentative) conclusions. Clin Infect Dis. Published online Feb 4, 2021 doi: 10.1093/cid/ciab096. https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciab096/6128795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karsli E., Sabirli R., Altintas E., et al. Soluble P-selectin as a potential diagnostic and prognostic biomarker for COVID-19 disease: a case-control study. Life Sci. 2021;277:119634. doi: 10.1016/j.lfs.2021.119634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berger J.S., Kunichoff D., Adhikari S., et al. Prevalence and outcomes of D-dimer elevation in hospitalized patients with COVID-19. Arterioscler Thromb Vasc Biol. 2020;40:2539–2547. doi: 10.1161/ATVBAHA.120.314872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smilowitz N.R., Nguy V., Aphinyanaphongs Y., et al. Multiple biomarker approach to risk stratification in COVID-19. Circulation. 2021;143:1338–1340. doi: 10.1161/CIRCULATIONAHA.120.053311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakka M., Connors J.M., Hekimian G., et al. Association between D-dimer levels and mortality in patients with coronavirus disease 2019 (COVID-19): a systematic review and pooled analysis. J Med Vasc. 2020;45:268–274. doi: 10.1016/j.jdmv.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adam S.S., Key N.S., Greenberg C.S. D-dimer antigen: current concepts and future prospects. Blood. 2009;113:2878–2887. doi: 10.1182/blood-2008-06-165845. [DOI] [PubMed] [Google Scholar]
- 25.Weitz J.I., Fredenburgh J.C., Eikelboom J.W. A test in context: D-dimer. J Am Coll Cardiol. 2017;70:2411–2420. doi: 10.1016/j.jacc.2017.09.024. [DOI] [PubMed] [Google Scholar]
- 26.Vaughan D.E., Goldhaber S.Z., Kim J., Loscalzo J. Recombinant tissue plasminogen activator in patients with pulmonary embolism: correlation of fibrinolytic specificity and efficacy. Circulation. 1987;75:1200–1203. doi: 10.1161/01.cir.75.6.1200. [DOI] [PubMed] [Google Scholar]
- 27.Ho C.H., Wang S.P. Serial thrombolysis-related changes after thrombolytic therapy with TPA in patients with acute myocardial infarction. Thromb Res. 1990;58:331–341. doi: 10.1016/0049-3848(90)90102-i. [DOI] [PubMed] [Google Scholar]
- 28.Cakar M.A., Gunduz H., Varim C., Ozdemir F., Vatan M.B., Akdemir R. Correlation between D-dimer levels and coronary artery reperfusion in acute myocardial infarction patients after thrombolytic treatment. Blood Coagul Fibrinolysis. 2013;24:608–612. doi: 10.1097/MBC.0b013e328360a53f. [DOI] [PubMed] [Google Scholar]
- 29.Koppensteiner R., Speiser W., Minar E., Ahmadi R., Ehringer H. D-dimer in local thrombolytic therapy with low doses of recombinant human tissue-type plasminogen activator (rt-PA) in patients with peripheral arterial occlusive disease. Thromb Haemost. 1990;64:192–195. [PubMed] [Google Scholar]
- 30.Francis C.W., Connaghan D.G., Marder V.J. Assessment of fibrin degradation products during fibrinolytic therapy for acute myocardial infarction. Circulation. 1986;74:1027–1036. doi: 10.1161/01.cir.74.5.1027. [DOI] [PubMed] [Google Scholar]
- 31.Al-Samkari H., Song F., Van Cott E.M., Kuter D.J., Rosovsky R. Evaluation of the prothrombin fragment 1.2 in patients with coronavirus disease 2019 (COVID-19) Am J Hematol. 2020;95:1479–1485. doi: 10.1002/ajh.25962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Etulain J., Martinod K., Wong S.L., Cifuni S.M., Schattner M., Wagner D.D. P-selectin promotes neutrophil extracellular trap formation in mice. Blood. 2015;126:242–246. doi: 10.1182/blood-2015-01-624023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barbu E.A., Mendelsohn L., Samsel L., Thein S.L. Pro-inflammatory cytokines associate with NETosis during sickle cell vaso-occlusive crises. Cytokine. 2020;127:1–9. doi: 10.1016/j.cyto.2019.154933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zuo Y., Yalavarthi S., Shi H., et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2021;9(6) doi: 10.1172/jci.insight.150111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agrati C., Sacchi A., Tartaglia E., et al. The role of P-selectin in COVID-19 coagulopathy: an updated review. Int J Mol Sci. 2021;22(15):7942. doi: 10.3390/ijms22157942. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.