Abstract
Objective
The prognostic value of interleukin-6 (IL-6) in patients with atrial fibrillation (AF) has not been fully elucidated. Therefore, we conducted a cohort study and a meta-analysis to assess the predictive value of IL-6 for stroke and mortality in patients with AF.
Methods
A cohort study was performed in newly diagnosed non-valvular patients with AF. A total of 217 patients with AF were followed up for a mean of 27 months. A multivariate Cox regression analysis was used to evaluate the association between IL-6 and stroke/all-cause mortality. The incremental value was also assessed by adding IL-6 to the CHA2DS2-VASc score. Besides, a meta-analysis of all reported cohort studies and our cohort study was conducted to validate the association of circulating IL-6 and stroke/mortality in patients with AF.
Results
Our cohort study showed that elevated plasma level of IL-6 was an independent risk factor for predicting stroke [hazard ratio (HR)=3.81; 95% confidence interval (CI), 1.11–13.05; p=0.033] and all-cause mortality (HR=3.11; 95% CI, 1.25–7.72; p=0.015) in patients with AF. Adding IL-6 levels to CHA2DS2-VASc score showed limited improvement of the predictive power for stroke [area under curve (AUC) from 0.81 to 0.88, p=0.006]. Meta-analysis confirmed that increased circulating level of IL-6 was significantly associated with increased risk of stroke (pooled HR=1.97; 95% CI, 1.22–3.17; p=0.006) and all-cause mortality (pooled HR=2.73; 95% CI, 2.29–3.25; p<0.001).
Conclusion
Increased circulating level of IL-6 was significantly associated with greater risk of stroke and all-cause mortality in patients with AF. Adding IL-6 biomarker to the CHA2DS2-VASc score may help to determine the management of AF treatment.
Keywords: atrial fibrillation, prognosis, interleukin-6, meta-analysis, stroke, mortality
Introduction
Atrial fibrillation (AF) is the most common arrhythmia in clinical practice (1). The prevalence of AF is estimated to be 0.4% to 1.0% in the general population (2). AF increases the risk of stroke, heart failure, and overall mortality (3). In patients with AF, the annual incidence of ischemic stroke is about 5%, 2–7 times of that in patients without AF (4), and a doubling in mortality rate (5). Therefore, it is crucial to prevent the occurrence of adverse cardiovascular events and improve the prognosis of patients with AF.
Thromboembolism is the main cause of death and disability in patients with AF, and stroke is the most common type of manifestation (6). The mechanism of occurrence and development of AF is considered to be multifactorial. Several risk stratifications scoring schemes have been developed to determine which patients with AF have higher stroke risk. The most widely used scoring scheme is the CHA2DS2-VASC score (7). However, there remains a need for improving the predictive ability of CHA2DS2-VASc score. Studies have found that adding biomarkers such as von Willebrand factor, D-dimer, N-terminal proB-type natriuretic peptide, and high-sensitivity troponin T to the CHADS2 score improved the predictive accuracy (8, 9).
Although there is some evidence indicating that inflammation may be associated with AF (10), it may underlie pathogenesis of arrhythmia and vascular events (11). IL-6 is a pleiotropic cytokine, which can induce a pro-thrombotic state by increasing the expression of fibrinogen, tissue factor, factor VIII, and von Willebrand factor, as well as by activating endothelial cells and increasing platelet production, which may contribute to the adverse outcome of AF (12, 13). Several studies have evaluated the associations between circulating level of IL-6 and stroke/mortality in patients with AF (14, 15), but the results lack consistency. Therefore, we conducted a prospective cohort study to determine the association of plasma level of IL-6 with stroke and all-cause mortality in patients with AF. In addition, we performed a meta-analysis to further validate the prognostic value of IL-6 in AF.
Methods
Cohort study
Patients
We consecutively recruited newly diagnosed patients with AF aged at least 18 years and hospitalized in Southwest Hospital of the Army Medical University in Chongqing, China, from December 2013 to August 2015. The diagnosis of AF was made according to the 2012 European Guidelines for Atrial Fibrillation (16). Exclusion criteria included mitral rheumatic valve disease or a prosthetic valve, infections, malignant tumors, connective tissue diseases (such as rheumatoid arthritis), other acute or chronic inflammatory diseases (such as giant cell arteritis). We collected demographic, epidemiological, and medical information from medical records and structured interviews. The study was approved by the Ethics Committee of Southwest Hospital of the Army Medical University. Written informed consent was obtained from all the patients.
Outcomes and follow up
Primary outcomes included all-cause mortality and stroke. All the patients were actively followed up annually by phone interviews. Stroke was defined as the first neurologic deficit that lasted for at least 24 hours and sub-classified as ischemic (with or without hemorrhagic conversion), hemorrhagic, or uncertain after discharge. The diagnosis of stroke was confirmed by screening the medical records. Next-of-kin reports, electronic medical notes, and the national register of death were used to verify the annual vital status and the cause of death of the patients. Patients known to be alive were censored at the time of last contact.
Measurement of IL-6
A fasting blood sample (5 mL) was drawn from each patient before any treatment. The plasma was separated into aliquots of 500-μL straws and frozen at −80°C immediately after processing until use. The plasma level of IL-6 was measured using MILLIPLEX MAP Human Th17 Magnetic Bead Panel kits (Millipore, Billerica, MA., USA) based on the Luminex xMAP technology (Luminex Corporation, Austin, TX., USA). Plates were run on the Luminex MagPix machine (Luminex Corporation). Raw data were collected using the Luminex xPONENT 4.2 software and analyzed using MILLIPLEX Analyst 5.1 software (Millipore). Concentrations of IL-6 were calculated using a standard curve. Two duplicate samples were run for quality control (replicate QC1 samples, low level; replicate QC2 samples, high level). The coefficients of variation of all repeated quality control samples were less than 10%.
Statistical analysis
Frequency counts and proportions were used to report categorical data. For the description of continuous variables, means and standard deviation were used for normally distributed data, and median and interquartile ranges (25th–75th percentile) were used for non-normally distributed data. The best cutoff value of IL-6 was determined by X-tile software, which is a free software available from Yale University School of Medicine that can determine a cutoff point for continuous data with a time-dependent outcome (17). Univariate and multivariate Cox proportional hazards regression models were used to evaluate the association of IL-6 with stroke and all-cause mortality in patients with AF. Baseline variables with p<0.05 in univariate Cox regression model were included as covariates in the multivariate Cox regression model. Hazard ratios (HR) and 95% confidence interval (CI) were estimated. Kaplan-Meier curves were used to depict the cumulative risk of stroke and all-cause mortality in patients with AF by different IL-6 levels, and the significance of their differences was assessed using log-rank tests. A CHA2DS2-VASc stroke risk score (including congestive heart failure; hypertension; age ≥75 years; diabetes; stroke, transient ischemic attack, or thromboembolism; vascular disease; age 65–74 years, and sex) was assigned to each patient. However, these schemes have only a modest predictive value for predicting “high-risk” patients. C-statistics were estimated to compare the predictive power between modified models, using methods recommended by Pencina et al. (18). Besides, we used the time-dependent area under the receiver operating characteristic curve (AUC) to compare the predictive ability between modified models (adding IL6 to the CHA2DS2-VASc score) (19). A 2-sided p value <0.05 was considered to be statistically significant. All these statistical analyses were conducted using the Statistical Package for the Social Sciences statistical software (version 20.0; IBM Corp., Armonk, NY, USA) and R 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria).
Search strategy and inclusion criteria of meta-analysis
We searched PubMed, EMBASE, Springer Link, Web of Science, CNKI, and supplemented with Google scholar search engine for relevant studies published from database establishment to January 2020. We used the following keywords, “interleukin 6,” “inflammatory markers,” “biomarker,” “prognosis,” and “atrial fibrillation.” In addition, relevant review articles were also cross-referenced. The included studies had to satisfy the following predefined criteria: (i) the study was a cohort study; (ii) the study enrolled patients with AF; (iii) stroke or mortality events were assessed as outcomes; (iv) the study investigated the association between IL-6 and outcome events. If studies were duplicated, the one with the most complete data was chosen.
Data extraction and quality assessment of meta-analysis
Two investigators (Xiaoyue Jia and Na Wu) independently extracted the data using a standard data extraction form. We extracted general data (first author’s name, year of publication), study characteristics (country and study design), types of AF, mean age of participants, mean follow-up time, adverse outcomes, and information related to effect size like HR, 95% CI, and cutoff values. If both univariate analysis and multivariate analysis were available for the studies, the adjusted HR (95% CI) from the multivariate analysis with a maximum number of adjusted variables were extracted priority.
The quality of studies was independently assessed by two reviewers (Xiaoyue Jia and Xi Cheng). The assessment was done using the primary criteria for non-randomized studies described in the Newcastle-Ottawa scale. The total scores ranged from 0 to 9 for these studies (Supplementary Table 1). Any disagreement between the reviewers was discussed and resolved by consensus.
Statistical analysis of meta-analysis
Statistical heterogeneity across studies was assessed using the I2 statistic, and heterogeneity was considered to be significant if I2 >50%. A fixed-effects model was used to calculate pooled effect sizes when I2 ≤50%. Otherwise, a random-effects model was applied. Funnel plot with or without contour enhancement was applied to detect publication bias owing to small study effects. RevMan 5.3 software (version 5.3, Cochrane Collaboration, Oxford, United Kingdom) was used to perform the meta-analysis.
Results
Cohort study
A total of 232 patients with newly diagnosed non-valvular AF were enrolled in the cohort, and 217 (54.38% men) patients with a mean age of 63.41 years were followed up. The median follow-up time was 27 months (IQR 23–30 months). During follow-up, all-cause mortality rate was 11.06% and the incidence rate of stroke was 6.91% (Table 1). The optimal IL-6 cut-off values was 55.20 pg/mL.
Table 1.
Baseline characteristics of patients with AF and factors associated with death or stroke
| Patient characteristics | Number of patients (%) |
|---|---|
| Age, mean ± SD, years | 63.41±12.20 |
| Sex | |
| Male | 118 (54.38) |
| Female | 99 (45.62) |
| BMI, mean ± SD, kg/m2 | 24.32±3.59 |
| Education | |
| Junior school and below | 173 (79.72) |
| High school and above | 44 (20.27) |
| Income (10,000 RMB/year) | |
| <2.5 | 110 (50.69) |
| ≥2.5 | 107 (49.31) |
| AF type | |
| Paroxysmal | 68 (31.34) |
| Persistent | 149 (68.66) |
| Smoking status | |
| Former/current | 73 (33.64) |
| Never | 144 (66.36) |
| Drinking status | |
| Former/current | 76 (35.02) |
| Never | 141 (64.98) |
| Warfarin treatment | |
| Yes | 65 (29.96) |
| No | 152 (70.04) |
| Statin treatment | |
| Yes | 97 (44.70) |
| No | 120 (55.30) |
| History of combined diseases | |
| Hypertension | 109 (50.23) |
| Diabetes | 33 (15.21) |
| Coronary heart disease | 83 (38.25) |
| Cardiomyopathy | 22 (10.14) |
| Heart failure | 78 (35.94) |
| TIA | 9 (4.15) |
| Vascular diseases | 14 (6.45) |
| Previous stroke | 18 (8.29) |
| IL-6, median (range), pg/mL | 28.3 (0.3–216.2) |
| ≤55.2 | 184 (84.79) |
| >55.2 | 33 (15.21) |
| CHA2DS2-VASc score | |
| <2 | 68 (32.72) |
| ≥2 | 146 (67.28) |
| Follow-up time, mean (IQR), month | 27 (23–30) |
| stroke | 15 (6.91) |
| All-cause mortality | 24 (11.06) |
AF - atrial fibrillation; BMI - body mass index; IL - interleukin; SD - standard deviation; TIA - transient ischemic attack
Age and left atrial diameter (LAD) in patients with AF and stroke or death was significantly larger than that without stroke or survivor (both p<0.05) (Supplementary Table 2). According to the result of univariate Cox regression analysis, age, history of coronary heart disease, and heart failure were significantly associated with all-cause mortality. Age, history of stroke, and diabetes were significant risk factors of stroke (Supplementary Tables 3, 4). These significant variables were further adjusted in the multivariate Cox regression model. The results of multivariate cox regression analysis indicated that the increased plasma level of IL-6 was significantly associated with increased risk of all-cause mortality and stroke with adjusted HR 3.81 (95% CI 1.11–13.05; p=0.033) and 3.11 (95% CI 1.25–7.72; p=0.015), respectively (Table 2). The Kaplan-Meier curve showed that patients with elevated IL-6 had a higher risk of all-cause mortality (p<0.05) and for stroke (p>0.05) (Fig. 1 and 2).
Supplementary Table 2.
Baseline information of patients with AF with different outcomes
| Variable | Death outcome | Stroke outcome | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Death (24) | Survivor (193) | P-value | Stroke (15) | Without stroke (202) | P-value | |
| Age (years) | 73.71±8.73 | 62.13±11.97 | <0.001 | 69.93±9.77 | 62.93±12.24 | 0.032 |
| Sex | 0.982 | 0.993 | ||||
| Male | 13 (54.17%) | 105 (44.40%) | 8 (53.33%) | 110 (54.46%) | ||
| Female | 11 (45.83%) | 88 (45.60%) | 7(46.67%) | 92 (45.54%) | ||
| BMI (kg/m 2 ) | 25.06±4.29 | 24.23±3.50 | 0.289 | 23.18±3.24 | 24.40±3.61 | 0.205 |
| Income (10,000 RMB/year) | 0.170 | 0.391 | ||||
| <2.5 | 9 (37.50%) | 101 (52.33%) | 6 (40.00%) | 104 (51.49%) | ||
| ≥2.5 | 15 (62.50%) | 92 (47.27%) | 9 (60.00%) | 98 (48.51%) | ||
| Smoking | 7 (29.17%) | 66 (34.20%) | 0.623 | 3 (20.00%) | 70 (34.65%) | 0.246 |
| Drinking | 8 (33.33%) | 68 (35.23%) | 0.854 | 3 (20.00%) | 73 (36.14%) | 0.206 |
| Treatment | ||||||
| Warfarin | 8 (33.33%) | 57 (29.53%) | 0.702 | 7 (46.67%) | 58 (28.71%) | 0.153 |
| Statins | 14 (58.33%) | 83 (43.01%) | 0.154 | 8 (53.33%) | 89 (44.06%) | 0.486 |
| Comorbidity | ||||||
| Hypertension | 16(66.67%) | 93 (48.19%) | 0.088 | 10 (66.67%) | 99 (49.01%) | 0.187 |
| Diabetes | 5 (20.83%) | 28 (14.51%) | 0.379 | 6 (40.00%) | 27 (13.37%) | 0.014 |
| Coronary heart disease | 14(58.33%) | 69 (35.75%) | 0.032 | 7 (46.67%) | 76 (37.62%) | 0.487 |
| Cardiomyopathy | 4 (16.67%) | 18 (9.33%) | 0.278 | 0 (0.00%) | 22 (10.89%) | 0.374 |
| Heart failure | 19(79.17%) | 59 (30.57%) | <0.001 | 7 (46.67%) | 71 (35.15%) | 0.370 |
| TIA | 0 (0.00%) | 9 (4.66%) | 0.602 | 2 (13.33%) | 7 (3.47%) | 0.121 |
| Vascular diseases | 2 (8.33%) | 12 (6.22%) | 0.657 | 2 (13.33%) | 12 (5.94%) | 0.250 |
| Previous stroke | 2 (8.33%) | 16 (8.29%) | 1.000 | 5 (33.33%) | 13 (6.44%) | 0.004 |
| Echocardiograhic parameters | ||||||
| LAD, mm | 49.74±8.37 | 44.28±9.76 | 0.001 | 50.2±11.14 | 44.47±9.54 | 0.028 |
| LVEDD, mm | 57.47±10.69 | 50.63±8.23 | 0.007 | 51.07±8.09 | 51.43±8.85 | 0.879 |
| Left ventricular EF, % | 45.52±12.94 | 56.65±12.81 | <0.001 | 55.87±13.26 | 55.37±13.30 | 0.888 |
AF - atrial fibrillation; BMI - body mass index; EF - ejection fraction; LAD - left atrial diameter; LVEDD - left ventricular end diastolic diameter; TIA - transient ischemic attack
Table 2.
Associations between plasma IL-6 level and stroke and all-cause mortality in patients with AF
| Variable | Univariate Cox regression | Multivariate Cox regression | ||
|---|---|---|---|---|
|
| ||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Stroke | ||||
| IL-6 (pg/mL) | ||||
| ≤55.2 | Reference | Reference | ||
| >55.2 | 1.69 (0.61, 4.66) | 0.312 | 3.81 (1.11, 13.05) | 0.033 |
| All-cause mortality | ||||
| IL-6 (pg/mL) | ||||
| ≤55.2 | Reference | Reference | ||
| >55.2 | 2.46 (1.02, 5.93) | 0.045* | 3.11 (1.25, 7.72) | 0.015* |
P<0.05, the difference was statistically significant.
Cox proportional hazards model for stroke adjusted for age, types of AF, history of diabetes and history of stroke; for all-cause mortality, adjusted for age, types of AF, history of coronary disease and history of heart failure; using a backward selection strategy.
AF - atrial fibrillation; CI - confidence interval; HR - hazard ratio; IL - interleukin
Figure 1.
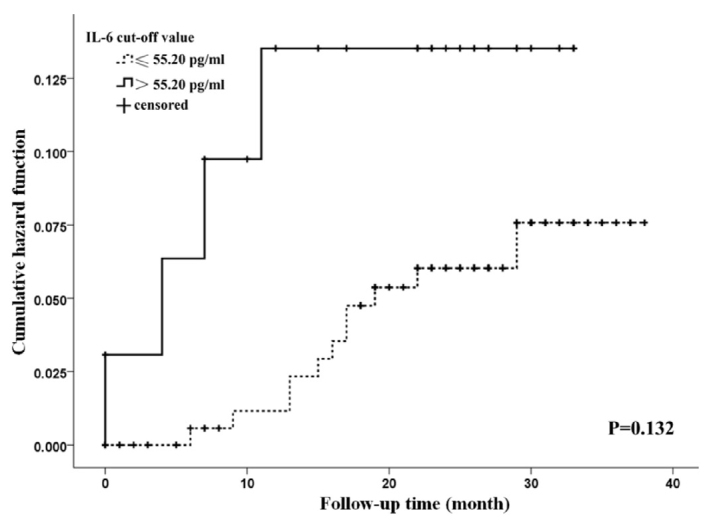
Cumulative risk of stroke in patients with atrial fibrillation at different plasma interleukin-6 levels
Figure 2.
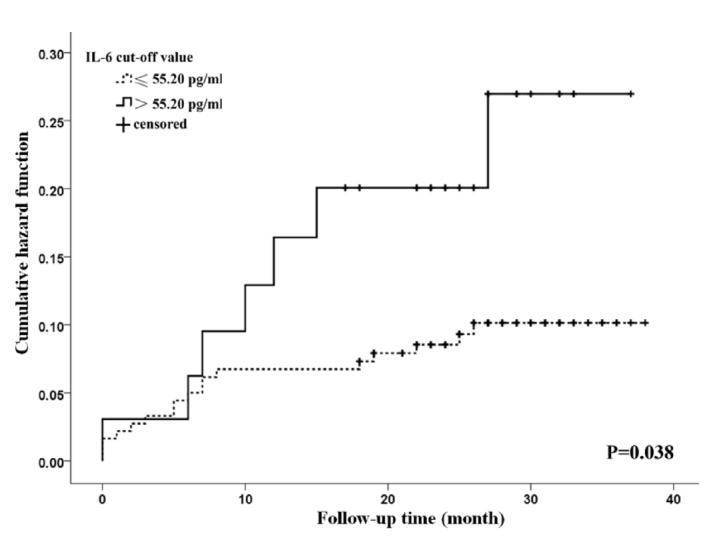
Cumulative risk of all-cause mortality in patients with atrial fibrillation at different plasma interleukin-6 levels
We further evaluated the predictive power of the model by adding IL-6 bio-markers to the CHA2DS2-VASc score. Adding high IL-6 to the CHA2DS2-VASC score had a better predictive power for stroke, AUC was improved from 0.81 to 0.88 significantly at 12-month follow-up time (p=0.006), whereas the improvement was non-statistically significant in all-cause mortality (AUC from 0.76 to 0.77, p=0.635) (Fig. 3). For stroke, the c-statistic increased from 0.79 (95% CI, 0.69–0.88) to 0.83 (95% CI, 0.74–0.93) when high IL-6 was added (p=0.213). As for the all-cause mortality, the discrimination index c-statistic was 0.75 (95% CI, 0.68–0.82) in the prediction model consisting only of the CHA2DS2-VASc score. Adding high IL-6 to the score increased the c-statistic to 0.77 (95% CI, 0.69–0.84), while it was not statistically significant (p=0.460).
Figure 3.
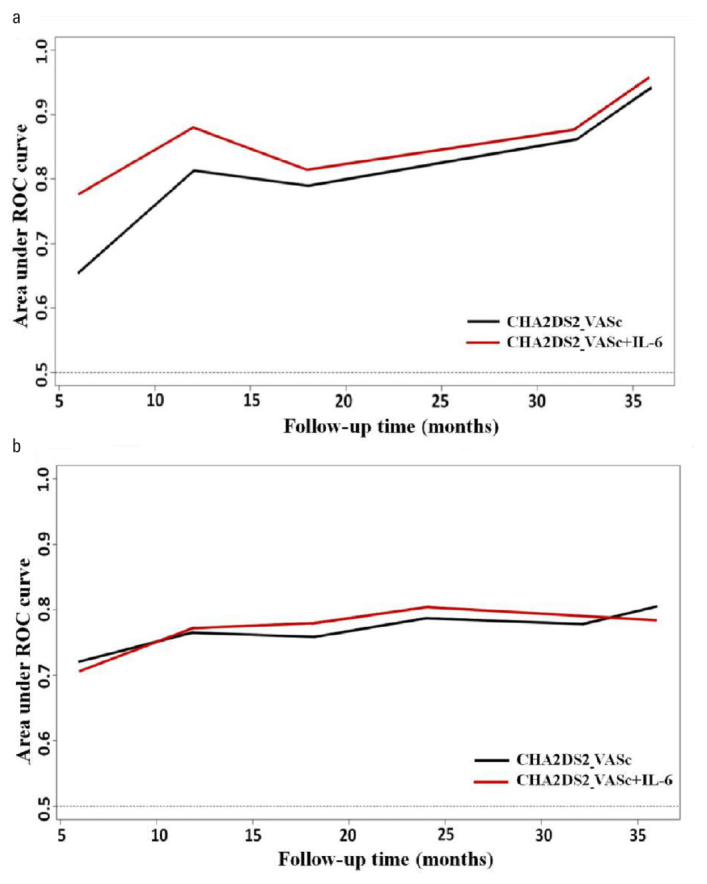
Area under the receiver operating curves (AUC) using a time-dependent receiver operating curve analysis. (a) Time-dependent AUC of the two scores for stroke. (b) Time-dependent AUC of the two scores for all-cause mortality
Meta-analysis
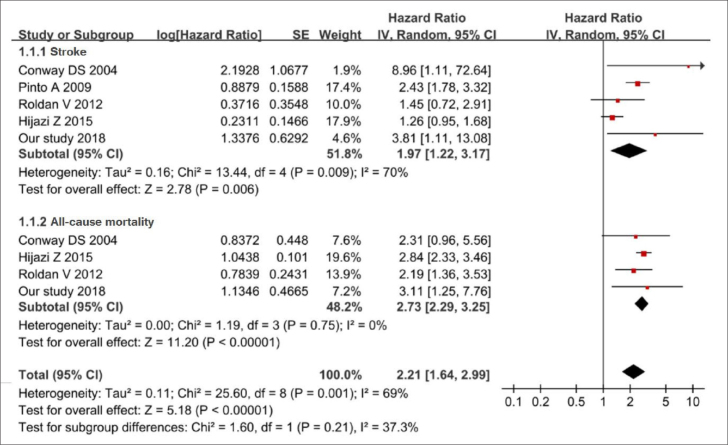
A total of 299 studies were retrieved after initial search. After screening the titles and abstracts, 270 studies were excluded. The remaining 29 studies were retrieved for the full text. Finally, 4 studies (20–23) met the inclusion criteria and a total of 16,334 participants were included in the final analysis (Supplementary Fig. 1). All the 4 included cohort studies investigated the predictive role of IL-6 for stroke, and 3 studies (20, 21, 23) with 15,961 patients investigated the predictive role of IL-6 for all-cause mortality.
The mean age ranged from 63.0 to 76.0 years. The duration of follow-up for cohort studies ranged from 1.8 to 6.4 years, and all studies had a quality score of at least 5. The detailed characteristics and NOS score are depicted in Supplementary Table 5. Meta-analysis of 4 published studies and our cohort study showed that higher level of IL-6 was significantly associated with an increased risk of stroke events. The pooled HR is 1.97 (95% CI, 1.22–3.17; p<0.05) with a medium heterogeneity (I2=70%, p=0.001) across the studies by random effects model (Fig. 4). A sensitivity analysis was performed, and the result showed that the study of Hijazi et al. (23) might be the source of heterogeneity. Compared with other studies, the study by Hijazi et al. (23) had shorter follow-up time (less than 2 two years), and there is one more inclusion criterion of patients with AF (at least one of the risk factors for stroke was required), which may lead to clinical heterogeneity. After excluding the study of Hijazi et al. (23), the pooled HR of stroke was 2.34 (95% CI, 1.78–3.09; p<0.05) with a low heterogeneity (I2=26%, p=0.001) (Supplementary Fig. 2).
Figure 4.
Forest plot of the relationship between the interleukin-6 level and outcomes. The squares and horizontal lines represent the hazards ratio (HR) and 95% confidence interval (CI), respectively. The area of the squares reflects the weight of each study. The diamond represents the pooled HR and 95% CI
Four studies (including our study) examined the association between IL-6 and all-cause mortality. The pooled HR of all-cause mortality was 2.73 (95% CI, 2.29–3.25; p<0.001) with no significant heterogeneity (I2=0%, p=0.75) across the studies, indicating that elevated plasma level of IL-6 was significantly associated with increased risk of all-cause mortality.
Discussion
We explored the association between IL-6 and the prognosis of patients with AF in a cohort study and meta-analysis. Our results showed that elevated IL-6 levels were significantly associated with higher risk of stroke and all-cause mortality in patients with AF. Adding plasma level of IL-6 could limit the improvement of the predictive power of CHA2DS2-VASc scores for stroke and all-cause mortality.
At present, the association of IL-6 with AF prognosis remains controversial. Most studies show that the increase of IL-6 is an independent risk factor for stroke or mortality in patients with AF (24, 25). However, a few studies (23) reported that IL-6 level was not related to the risk of stroke in patients with AF. Our cohort study and meta-analysis consistently revealed that elevated IL-6 level was related to higher risk of stroke and death, independent of established clinical risk factors.
The pathophysiological mechanism involved in inflammation and the prognosis of AF remains undetermined. IL-6 is an established inflammatory biomarker, which is a circulating cytokine produced by monocytes, T lymphocytes, and epithelial cells (26). IL-6 also increases platelet production and platelet sensitivity to thrombin, stimulates transcription of fibrinogen, and is linked to endothelial cell activation and damage (27, 28). As a pleiotropic cytokine, IL-6 can induce CRP production by hepatocytes and is involved in immuno-inflammatory reaction by promoting neutrophil adhesion, atherosclerotic plaque rupture, and thrombus formation (29). It is therefore plausible that IL-6 inducing inflammation might mediate the prothrombic or hypercoagulable state that exists in AF (30), with consequences for left atrial thrombosis, stroke, and vascular events.
On the basis of clinical risk factors, the CHA2DS2-VASc score is the most commonly used stratification scheme to assess the risk of stroke in patients with AF and guide the use of anticoagulants, although its assessment ability and predictive accuracy is modest (31, 32). To increase prediction ability, incorporation of inflammatory biomarkers to CHA2DS2-VASc score become a hot research field (33, 34). Our cohort study indicated that modifying the CHA2DS2-VASc score by adding IL-6 could limit the improvement of the prediction ability for stroke and all-cause mortality in patients with AF. Further epidemiological studies with large samples are needed to verify the clinical significance of our results.
Study limitations
Several limitations of this study should be considered in interpreting our results. Heterogeneity tests showed that there was significant heterogeneity for some studies investigating the predictive role of IL-6 for stroke, and no significant heterogeneity for studies examining the association between IL-6 and all-cause mortality. Because of the limited number of studies, our sensitivity analysis showed that the study of Hijazi et al. (23) might be the potential source of heterogeneity. Comparing the characteristics of the included studies, we found that mean age of participants, mean follow-up time, time points of the sample assay, and cut-off point varied among studies. In previous studies of meta-analysis, the cut-off values of IL-6 for predicting adverse outcomes were 3.35, 20, 0.89, and 2.3 pg/mL, respectively, (20–23) lower than our study. The cut-off value of IL6 in Hijazi et al. (23) and Roldan et al. (20) studies was the median of anticoagulant patients. The difference in assay methods might also partly contribute to the high heterogeneity among the studies. As a single-center study, our cohort study might have selection bias. Because of anticoagulant treatment rate in China is relatively low (35, 36), only 65 (30.0%) patients received warfarin anti-coagulation in our cohort study, which may also introduce bias. Another limitation is the small sample size and insufficient follow-up time, which may have led to insufficient statistical power. Finally, although funnel plots showed no obvious publication bias (Supplementary Fig. 3) in our meta-analysis, the efficiency of publishing bias test is low because of the limited number of studies. Lau et al. (37) doubted that the widely used method of the funnel plot accurately predicts publication bias. A true standard measure of publication bias would require prospective registries of studies with detailed knowledge of which studies had been published and which were unpublished. Therefore, publication bias cannot be completely excluded.
Conclusion
In conclusion, our study suggested that high levels of IL-6 were significantly associated with stroke and death and other adverse outcomes in patients with AF. Moreover, compared to the classical CHADS-VASC score, adding IL-6 levels to CHADS-VASC score showed limited improvement of the predictive power. IL-6 is a promising prognostic biomarker to help optimize the strategies for risk stratification, treatment, and prevention of adverse outcomes in patients with AF.
HIGHLIGHTS.
The elevated plasma level of interleukin (IL) 6 was an independent risk factor for predicting stroke and all-cause mortality in patients with atrial fibrillation (AF).
Adding IL-6 biomarker to the CHA2DS2-VASc score may help determine the management of AF treatment.
IL-6 is a promising prognostic biomarker to help optimize the strategies for risk stratification in patients with AF.
Supplementary Data
Flowcharts presenting the pipeline of the search and data extraction
Sensitivity analysis on association between plasma interleukin-6 level and stroke events
Funnel plot for publication bias analysis. a) Stroke events and b) all-cause mortality events
Supplementary Table 1.
Quality assessment score scale
| Item | Score |
|---|---|
| NOS score scale for cohort studies * | |
| Selection | |
| (1) Representativeness of the exposed cohort | |
| Truly representative of the average status in the community | 1 |
| Somewhat representative of the average status in the community | 1 |
| Selected group of users (e.g. nurses, volunteers) | 0 |
| No description of the derivation of the cohort | 0 |
| (2) Selection of the non-exposed cohort | |
| Drawn from the same community as the exposed cohort | 1 |
| Drawn from a different source | 0 |
| No description of the derivation of the non-exposed cohort | 0 |
| (3) Ascertainment of exposure | |
| Secure record (e.g. surgical records) | 1 |
| Structured interview | 1 |
| Written self-report | 0 |
| No description | 0 |
| (4) Demonstration that outcome of interest was not present at the start of study | |
| Yes | 1 |
| No | 0 |
| Comparability | |
| Comparability of cohorts on the basis of the design or analysis | |
| Study controls for the most important factor | 1 |
| Study controls for any additional factor | 1 |
| Outcome | |
| (1) Assessment of outcome | |
| Independent blind assessment | 1 |
| Record linkage | 1 |
| Self-report | 0 |
| No description | 0 |
| (2) Was follow-up long enough for outcomes to occur? | |
| Yes | 1 |
| No | 0 |
| (3) Adequacy of follow up of cohorts | |
| Complete follow-up - all subjects accounted for | 1 |
| Subjects lost to follow-up unlikely to introduce bias | 1 |
| Follow-up rate is low and no description of those lost | 0 |
| No statement | 0 |
A study can be awarded a maximum of one score for each numbered item within the Selection and Outcome categories. A maximum of two scores can be given for Comparability.
Supplementary Table 3.
Univariate Cox regression analysis on association between plasma IL-6 level and stroke and all-cause mortality in patients with AF
| Variable | Stroke | All-cause mortality | ||
|---|---|---|---|---|
|
| ||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age (years) | 2.02 (1.10, 3.71) | 0.024* | 3.29 (1.94, 5.60) | <0.001* |
| <65 | Reference | Reference | ||
| 65~74 | 1.72 (0.46, 6.41) | 0.170 | 3.16 (0.89, 11.21) | 0.074 |
| ≥75 | 4.03 (1.22, 13.32) | 0.022* | 10.71 (3.51, 32.63) | < 0.001* |
| Sex | ||||
| Male | Reference | Reference | ||
| Female | 1.09 (0.47, 2.51) | 0.848 | 1.02 (0.46, 2.28) | 0.955 |
| BMI (kg/m 2 ) | ||||
| <24 | Reference | Reference | ||
| ≥24 | 0.34 (0.11, 1.08) | 0.067 | 0.96 (0.43, 2.15) | 0.929 |
| AF type | ||||
| Paroxysmal | Reference | Reference | ||
| Persistent | 1.36 (0.43, 4.28) | 0.597 | 3.35 (0.99, 11.22) | 0.050 |
| Education | ||||
| Junior school and below | Reference | Reference | ||
| High school and above | 1.05 (0.29, 3.77) | 0.940 | 0.81 (0.28, 2.40) | 0.709 |
| Income (10,000 RMB/year) | ||||
| <2 | Reference | Reference | ||
| ≥2 | 1.61 (0.57, 4.53) | 0.365 | 1.79 (0.78, 4.09) | 0.169 |
| Smoker | ||||
| No | Reference | Reference | ||
| Yes | 0.29 (0.07, 1.31) | 0.107 | 0.81 (0.34, 1.96) | 0.640 |
| Drinking | ||||
| No | Reference | Reference | ||
| Yes | 0.29 (0.07,1.29) | 0.103 | 0.94 (0.40, 2.20) | 0.942 |
| Warfarin | ||||
| No | Reference | Reference | ||
| Yes | 2.09 (0.76, 5.75) | 0.156 | 1.15 (0.49, 2.69) | 0.748 |
| Statins | ||||
| No | Reference | Reference | ||
| Yes | 1.48 (0.54, 4.08) | 0.450 | 1.77 (0.79, 3.99) | 0.167 |
| Comorbidity | ||||
| Hypertension | 2.08 (0.71, 6.09) | 0.181 | 2.06 (0.88, 4.82) | 0.095 |
| Diabetes | 4.16 (1.48, 11.69) | 0.007* | 1.52 (0.56, 4.06) | 0.409 |
| Coronary disease | 1.54 (0.56, 4.25) | 0.404 | 2.40 (1.07, 5.41) | 0.034* |
| Cardiomyopathy | 0.04 (0.00, 66.41) | 0.399 | 1.82 (0.62, 5.33) | 0.274 |
| Heart failure | 1.79 (0.65, 4.94) | 0.260 | 7.45 (2.78, 9.97) | <0.001* |
| TIA | 1.86 (0.88, 3.92) | 0.102 | 0.22 (0.01, 15.60) | 0.482 |
| Vascular diseases | 2.57 (0.58, 11.41) | 0.214 | 1.40 (0.33, 5.95) | 0.650 |
| Stroke | 2.41 (1.41, 4.12) | 0.001* | 0.98 (0.47, 2.01) | 0.949 |
| IL-6 (pg/mL) | ||||
| ≤55.2 | Reference | Reference | ||
| >55.2 | 1.69 (0.61, 4.66) | 0.312 | 2.46 (1.02, 5.93) | 0.045* |
P<0.05, the difference was statistically significant
AF - atrial fibrillation; BMI - body mass index; CI - confidence interval; HR - hazards ratio; IL - interleukin; TIA - transient ischemic attack
Supplementary Table 4.
Multivariate Cox regression analysis on association between plasma IL-6 level and stroke and all-cause mortality in patients with AF
| Variable | Stroke | All-cause mortality | ||
|---|---|---|---|---|
|
| ||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age (years) | 2.06 (1.08, 3.90) | 0.027* | 3.10 (1.70, 5.67) | 0.001* |
| <65 | Reference | Reference | ||
| 65~74 | 1.55 (0.41, 5.84) | 0.519 | 2.41 (0.67, 8.68) | 0.180 |
| ≥75 | 4.22 (1.23, 14.54) | 0.022* | 8.91 (2.64, 30.03) | 0.001* |
| Comorbidity | ||||
| Diabetes | 3.34 (1.13, 9.82) | 0.029* | - | - |
| Coronary disease | - | - | 1.10 (0.45, 2.64) | 0.833 |
| Heart failure | - | - | 5.41 (1.99, 14.65) | 0.001* |
| Stroke | 2.11 (1.21, 3.70) | 0.009* | - | - |
| IL-6 (pg/mL) | ||||
| ≤55.2 | Reference | Reference | ||
| >55.2 | 3.81 (1.11, 13.05) | 0.033* | 3.11 (1.25, 7.72) | 0.015* |
P<0.05, the difference was statistically significant; - unavailable.
Cox proportional hazards model for stroke adjusted for age, types of AF, history of diabetes and history of stroke; for all-cause mortality, adjusted for age, types of AF, history of coronary disease and history of heart failure; using a backward selection strategy.
AF - atrial fibrillation; CI - confidence interval; HR - hazard ratio; IL - interleukin
Supplementary Table 5.
Characteristics of studies included for meta-analysis
| First author | Year | Country | Study design | AF patients (n) | Male (%) | Mean age | Mean follow-up time (days) | Adverse outcome | Cut-off value | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|
| Roldan et al. (20) | 2012 | UK | Cohort | 930 | 470 (50.5) | 76 | 975 | Stroke/TIA, Heart failure, Cardiovascular events | 3.35 pg/mL | 8 |
| Conway et al. (21) | 2004 | UK | Cohort | 77 | 44 (57.1) | 68 | 2305 | Stroke, death, heart failure | 20 pg/mL | 6 |
| Pinto et al. (22) | 2009 | Italy | Cohort | 373 | 237 (63.5) | 66.08 | 1095 | stroke | 0.89 pg/mL | 9 |
| Hijazi et al. (23) | 2015 | Sweden | Cohort | 14954 | 9630 (67.1) | 70 | 694 | Stroke/embolism, Myocardial infarctions bleeding, death | 2.3 pg/mL | 6 |
| Our study | 2018 | China | Cohort | 217 | 118 (54.4) | 63.41 | 760 | Stroke, death | 55.20 pg/mL | 7 |
NOS - Newcastle-Ottawa scale; AF - atrial fibrillation; IL-6 - interleukin-6; TIA - transient ischemic attack
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Institutional and financial support: This study was supported by the National Natural Science Foundation of China (No. 81502883 and 82073649 to N.W).
Author contributions: Concept – L.Z., Y.L.; Design – X.J., X.C., N.W., L.Z., Y.L.; Supervision – X.J., X.C., N.W., L.Z., Y.L.; Fundings – N.W.; Materials – X.C., B.X.; Data collection &/or processing – X.J., X.C., N.W., C.L., Z.Z., S.T.; Analysis &/or interpretation – X.J., X.C., N.W., Y. X.; Literature search – Y. X., L.W.; Writing – X.J., X.C., N.W.; Critical review – L.Z., Y.L.
References
- 1.Nattel S. New ideas about atrial fibrillation 50 years on. Nature. 2002;415:219–26. doi: 10.1038/415219a. [DOI] [PubMed] [Google Scholar]
- 2.Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol. 2011;57:e101–98. doi: 10.1161/CIR.0b013e318214876d. [DOI] [PubMed] [Google Scholar]
- 3.Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med. 1995;98:476–84. doi: 10.1016/S0002-9343(99)80348-9. [DOI] [PubMed] [Google Scholar]
- 4.Bai Y, Bai R, Wu JH, Zhang T, Liu N, Shi XB, et al. Differences in Quality of Life Between Atrial Fibrillation Patients with Low Stroke Risk Treated With and Without Catheter Ablation. J Am Heart Assoc. 2015;4:e002130. doi: 10.1161/JAHA.115.002130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–8. doi: 10.1161/01.STR.22.8.983. [DOI] [PubMed] [Google Scholar]
- 6.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–4. doi: 10.1001/jama.1994.03510350050036. [DOI] [PubMed] [Google Scholar]
- 7.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–70. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 8.Sadanaga T, Kohsaka S, Ogawa S. D-dimer levels in combination with clinical risk factors can effectively predict subsequent thromboembolic events in patients with atrial fibrillation during oral anticoagulant therapy. Cardiology. 2010;117:31–6. doi: 10.1159/000319626. [DOI] [PubMed] [Google Scholar]
- 9.Christersson C, Wallentin L, Andersson U, Alexander JH, Ansell J, De Caterina R, et al. D-dimer and risk of thromboembolic and bleeding events in patients with atrial fibrillation--observations from the ARISTOTLE trial. J Thromb Haemost. 2014;12:1401–12. doi: 10.1111/jth.12638. [DOI] [PubMed] [Google Scholar]
- 10.Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719–47. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- 11.Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol. 2012;60:2263–70. doi: 10.1016/j.jacc.2012.04.063. [DOI] [PubMed] [Google Scholar]
- 12.Harada M, Van Wagoner DR, Nattel S. Role of inflammation in atrial fibrillation pathophysiology and management. Circ J. 2015;79:495–502. doi: 10.1253/circj.CJ-15-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96:1180–4. doi: 10.1161/01.CIR.96.4.1180. [DOI] [PubMed] [Google Scholar]
- 14.Grönefeld GC, Lilienthal J, Kuck KH, Hohnloser SH Pharmacological Intervention in Atrial Fibrillation (PIAF) Study investigators. Impact of rate versus rhythm control on quality of life in patients with persistent atrial fibrillation. Results from a prospective randomized study. Eur Heart J. 2003;24:1430–6. doi: 10.1016/s0195-668x(03)00261-6. [DOI] [PubMed] [Google Scholar]
- 15.The Stroke Risk in Atrial Fibrillation Working Group. Independent predictors of stroke in patients with atrial fibrillation. A systematic review. Neurology. 2007;69:546–54. doi: 10.1212/01.wnl.0000267275.68538.8d. [DOI] [PubMed] [Google Scholar]
- 16.Overbeck P Europäische Gesellschaft für Kardiologie. Update 2012: Neue europäische Leitlinien bei Vorhofflimmern [Update 2012: new European guidelines for atrial fibrillation] MMW Fortschr Med. 2012;154:18–9. doi: 10.1007/s15006-012-1048-5. [Article in German] [DOI] [PubMed] [Google Scholar]
- 17.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252–9. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
- 18.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 19.Blanche P, Dartigues JF, Jacqmin-Gadda H. Estimating and comparing time-dependent areas under receiver operating characteristic curves for censored event times with competing risks. Stat Med. 2013;32:5381–97. doi: 10.1002/sim.5958. [DOI] [PubMed] [Google Scholar]
- 20.Providência R. High sensitivity cardiac troponin T and interleukin-6 predict adverse cardiovascular events and mortality in anticoagulated patients with atrial fibrillation: a rebuttal. J Thromb Haemost. 2012;10:2413. doi: 10.1111/j.1538-7836.2012.04904.x. ; author reply 2014–5. [DOI] [PubMed] [Google Scholar]
- 21.Conway DS, Buggins P, Hughes E, Lip GY. Prognostic significance of raised plasma levels of interleukin-6 and C-reactive protein in atrial fibrillation. Am Heart J. 2004;148:462–6. doi: 10.1016/j.ahj.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 22.Pinto A, Tuttolomondo A, Casuccio A, Di Raimondo D, Di Sciacca R, Arnao V, et al. Immuno-inflammatory predictors of stroke at follow-up in patients with chronic non-valvular atrial fibrillation (NVAF) Clin Sci (Lond) 2009;116:781–9. doi: 10.1042/CS20080372. [DOI] [PubMed] [Google Scholar]
- 23.Hijazi Z, Aulin J, Andersson U, Alexander JH, Gersh B, Granger CB, et al. ARISTOTLE Investigators. Biomarkers of inflammation and risk of cardiovascular events in anticoagulated patients with atrial fibrillation. Heart. 2016;102:508–17. doi: 10.1136/heartjnl-2015-308887. [DOI] [PubMed] [Google Scholar]
- 24.Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98:731–3. doi: 10.1161/01.CIR.98.8.731. [DOI] [PubMed] [Google Scholar]
- 25.Ridker PM. Role of inflammatory biomarkers in prediction of coronary heart disease. Lancet. 2001;358:946–8. doi: 10.1016/S0140-6736(01)06112-8. [DOI] [PubMed] [Google Scholar]
- 26.Turagam MK, Mirza M, Werner PH, Sra J, Kress DC, Tajik AJ, et al. Circulating Biomarkers Predictive of Postoperative Atrial Fibrillation. Cardiol Rev. 2016;24:76–87. doi: 10.1097/CRD.0000000000000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burstein SA, Peng J, Friese P, Wolf RF, Harrison P, Downs T, et al. Cytokine-induced alteration of platelet and hemostatic function. Stem Cells. 1996;14(Suppl 1):154–62. doi: 10.1002/stem.5530140720. [DOI] [PubMed] [Google Scholar]
- 28.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972–8. doi: 10.1161/01.ATV.19.4.972. [DOI] [PubMed] [Google Scholar]
- 29.Paquissi FC. The Predictive Role of Inflammatory Biomarkers in Atrial Fibrillation as Seen through Neutrophil-Lymphocyte Ratio Mirror. J Biomark. 2016;2016:8160393. doi: 10.1155/2016/8160393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung MK, Martin DO, Sprecher D, Wazni O, Kanderian A, Carnes CA, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104:2886–91. doi: 10.1161/hc4901.101760. [DOI] [PubMed] [Google Scholar]
- 31.European Heart Rhythm Association; European Association for Cardio-Thoracic Surgery. Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Eur Heart J. 2010;31:2369–429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 32.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–72. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 33.Senoo K, Lau YC, Lip GY. Updated NICE guideline: management of atrial fibrillation (2014) Expert Rev Cardiovasc Ther. 2014;12:1037–40. doi: 10.1586/14779072.2014.943189. [DOI] [PubMed] [Google Scholar]
- 34.Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SA, et al. Document Reviewers: Osmar A. Centurion (Paraguay), Karl-Heinz Kuck (Germany), Kristen K. Patton (USA), John L. Sapp (Canada), Martin Stiles (New Zealand), Jesper Hastrup Svendsen (Denmark), and Gaurav A. Upadhyay (USA); Review coordinator: Alena Shantsila (UK) EHRA/HRS/APHRS/SOLAECE expert consensus on Atrial cardiomyopathies: Definition, characterisation, and clinical implication. J Arrhythm. 2016;32:247–78. doi: 10.1016/j.joa.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang SS, Dong JZ, Ma CS, Du X, Wu JH, Tang RB, et al. Current Status and Time Trends of Oral Anticoagulation Use Among Chinese Patients With Nonvalvular Atrial Fibrillation: The Chinese Atrial Fibrillation Registry Study. Stroke. 2016;47:1803–10. doi: 10.1161/STROKEAHA.116.012988. [DOI] [PubMed] [Google Scholar]
- 36.Chao TF, Chiang CE, Lin YJ, Chang SL, Lo LW, Hu YF, et al. Evolving Changes of the Use of Oral Anticoagulants and Outcomes in Patients With Newly Diagnosed Atrial Fibrillation in Taiwan. Circulation. 2018;138:1485–7. doi: 10.1161/CIRCULATIONAHA.118.036046. [DOI] [PubMed] [Google Scholar]
- 37.Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ. 2006;333:597–600. doi: 10.1136/bmj.333.7568.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flowcharts presenting the pipeline of the search and data extraction
Sensitivity analysis on association between plasma interleukin-6 level and stroke events
Funnel plot for publication bias analysis. a) Stroke events and b) all-cause mortality events
Supplementary Table 1.
Quality assessment score scale
| Item | Score |
|---|---|
| NOS score scale for cohort studies * | |
| Selection | |
| (1) Representativeness of the exposed cohort | |
| Truly representative of the average status in the community | 1 |
| Somewhat representative of the average status in the community | 1 |
| Selected group of users (e.g. nurses, volunteers) | 0 |
| No description of the derivation of the cohort | 0 |
| (2) Selection of the non-exposed cohort | |
| Drawn from the same community as the exposed cohort | 1 |
| Drawn from a different source | 0 |
| No description of the derivation of the non-exposed cohort | 0 |
| (3) Ascertainment of exposure | |
| Secure record (e.g. surgical records) | 1 |
| Structured interview | 1 |
| Written self-report | 0 |
| No description | 0 |
| (4) Demonstration that outcome of interest was not present at the start of study | |
| Yes | 1 |
| No | 0 |
| Comparability | |
| Comparability of cohorts on the basis of the design or analysis | |
| Study controls for the most important factor | 1 |
| Study controls for any additional factor | 1 |
| Outcome | |
| (1) Assessment of outcome | |
| Independent blind assessment | 1 |
| Record linkage | 1 |
| Self-report | 0 |
| No description | 0 |
| (2) Was follow-up long enough for outcomes to occur? | |
| Yes | 1 |
| No | 0 |
| (3) Adequacy of follow up of cohorts | |
| Complete follow-up - all subjects accounted for | 1 |
| Subjects lost to follow-up unlikely to introduce bias | 1 |
| Follow-up rate is low and no description of those lost | 0 |
| No statement | 0 |
A study can be awarded a maximum of one score for each numbered item within the Selection and Outcome categories. A maximum of two scores can be given for Comparability.
Supplementary Table 3.
Univariate Cox regression analysis on association between plasma IL-6 level and stroke and all-cause mortality in patients with AF
| Variable | Stroke | All-cause mortality | ||
|---|---|---|---|---|
|
| ||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age (years) | 2.02 (1.10, 3.71) | 0.024* | 3.29 (1.94, 5.60) | <0.001* |
| <65 | Reference | Reference | ||
| 65~74 | 1.72 (0.46, 6.41) | 0.170 | 3.16 (0.89, 11.21) | 0.074 |
| ≥75 | 4.03 (1.22, 13.32) | 0.022* | 10.71 (3.51, 32.63) | < 0.001* |
| Sex | ||||
| Male | Reference | Reference | ||
| Female | 1.09 (0.47, 2.51) | 0.848 | 1.02 (0.46, 2.28) | 0.955 |
| BMI (kg/m 2 ) | ||||
| <24 | Reference | Reference | ||
| ≥24 | 0.34 (0.11, 1.08) | 0.067 | 0.96 (0.43, 2.15) | 0.929 |
| AF type | ||||
| Paroxysmal | Reference | Reference | ||
| Persistent | 1.36 (0.43, 4.28) | 0.597 | 3.35 (0.99, 11.22) | 0.050 |
| Education | ||||
| Junior school and below | Reference | Reference | ||
| High school and above | 1.05 (0.29, 3.77) | 0.940 | 0.81 (0.28, 2.40) | 0.709 |
| Income (10,000 RMB/year) | ||||
| <2 | Reference | Reference | ||
| ≥2 | 1.61 (0.57, 4.53) | 0.365 | 1.79 (0.78, 4.09) | 0.169 |
| Smoker | ||||
| No | Reference | Reference | ||
| Yes | 0.29 (0.07, 1.31) | 0.107 | 0.81 (0.34, 1.96) | 0.640 |
| Drinking | ||||
| No | Reference | Reference | ||
| Yes | 0.29 (0.07,1.29) | 0.103 | 0.94 (0.40, 2.20) | 0.942 |
| Warfarin | ||||
| No | Reference | Reference | ||
| Yes | 2.09 (0.76, 5.75) | 0.156 | 1.15 (0.49, 2.69) | 0.748 |
| Statins | ||||
| No | Reference | Reference | ||
| Yes | 1.48 (0.54, 4.08) | 0.450 | 1.77 (0.79, 3.99) | 0.167 |
| Comorbidity | ||||
| Hypertension | 2.08 (0.71, 6.09) | 0.181 | 2.06 (0.88, 4.82) | 0.095 |
| Diabetes | 4.16 (1.48, 11.69) | 0.007* | 1.52 (0.56, 4.06) | 0.409 |
| Coronary disease | 1.54 (0.56, 4.25) | 0.404 | 2.40 (1.07, 5.41) | 0.034* |
| Cardiomyopathy | 0.04 (0.00, 66.41) | 0.399 | 1.82 (0.62, 5.33) | 0.274 |
| Heart failure | 1.79 (0.65, 4.94) | 0.260 | 7.45 (2.78, 9.97) | <0.001* |
| TIA | 1.86 (0.88, 3.92) | 0.102 | 0.22 (0.01, 15.60) | 0.482 |
| Vascular diseases | 2.57 (0.58, 11.41) | 0.214 | 1.40 (0.33, 5.95) | 0.650 |
| Stroke | 2.41 (1.41, 4.12) | 0.001* | 0.98 (0.47, 2.01) | 0.949 |
| IL-6 (pg/mL) | ||||
| ≤55.2 | Reference | Reference | ||
| >55.2 | 1.69 (0.61, 4.66) | 0.312 | 2.46 (1.02, 5.93) | 0.045* |
P<0.05, the difference was statistically significant
AF - atrial fibrillation; BMI - body mass index; CI - confidence interval; HR - hazards ratio; IL - interleukin; TIA - transient ischemic attack
Supplementary Table 4.
Multivariate Cox regression analysis on association between plasma IL-6 level and stroke and all-cause mortality in patients with AF
| Variable | Stroke | All-cause mortality | ||
|---|---|---|---|---|
|
| ||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age (years) | 2.06 (1.08, 3.90) | 0.027* | 3.10 (1.70, 5.67) | 0.001* |
| <65 | Reference | Reference | ||
| 65~74 | 1.55 (0.41, 5.84) | 0.519 | 2.41 (0.67, 8.68) | 0.180 |
| ≥75 | 4.22 (1.23, 14.54) | 0.022* | 8.91 (2.64, 30.03) | 0.001* |
| Comorbidity | ||||
| Diabetes | 3.34 (1.13, 9.82) | 0.029* | - | - |
| Coronary disease | - | - | 1.10 (0.45, 2.64) | 0.833 |
| Heart failure | - | - | 5.41 (1.99, 14.65) | 0.001* |
| Stroke | 2.11 (1.21, 3.70) | 0.009* | - | - |
| IL-6 (pg/mL) | ||||
| ≤55.2 | Reference | Reference | ||
| >55.2 | 3.81 (1.11, 13.05) | 0.033* | 3.11 (1.25, 7.72) | 0.015* |
P<0.05, the difference was statistically significant; - unavailable.
Cox proportional hazards model for stroke adjusted for age, types of AF, history of diabetes and history of stroke; for all-cause mortality, adjusted for age, types of AF, history of coronary disease and history of heart failure; using a backward selection strategy.
AF - atrial fibrillation; CI - confidence interval; HR - hazard ratio; IL - interleukin
Supplementary Table 5.
Characteristics of studies included for meta-analysis
| First author | Year | Country | Study design | AF patients (n) | Male (%) | Mean age | Mean follow-up time (days) | Adverse outcome | Cut-off value | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|
| Roldan et al. (20) | 2012 | UK | Cohort | 930 | 470 (50.5) | 76 | 975 | Stroke/TIA, Heart failure, Cardiovascular events | 3.35 pg/mL | 8 |
| Conway et al. (21) | 2004 | UK | Cohort | 77 | 44 (57.1) | 68 | 2305 | Stroke, death, heart failure | 20 pg/mL | 6 |
| Pinto et al. (22) | 2009 | Italy | Cohort | 373 | 237 (63.5) | 66.08 | 1095 | stroke | 0.89 pg/mL | 9 |
| Hijazi et al. (23) | 2015 | Sweden | Cohort | 14954 | 9630 (67.1) | 70 | 694 | Stroke/embolism, Myocardial infarctions bleeding, death | 2.3 pg/mL | 6 |
| Our study | 2018 | China | Cohort | 217 | 118 (54.4) | 63.41 | 760 | Stroke, death | 55.20 pg/mL | 7 |
NOS - Newcastle-Ottawa scale; AF - atrial fibrillation; IL-6 - interleukin-6; TIA - transient ischemic attack