Abstract
Background:
New combination vaccines reduce the number of injections needed for immunization. However, possible drawbacks include higher prices, extra doses of vaccine antigens and increased minor adverse events. Our objective was to measure parental and societal values for attributes of childhood combination vaccines.
Methods:
We conducted a discrete choice experiment using an online survey of adults administered by Knowledge Networks. Values were measured for attributes of combination vaccines for a hypothetical child aged 6 months: (1) number of injections, (2) extra dose of hepatitis B vaccine, (3) 20% higher chance of fever, (4) community-level immunization coverage of 2-year-olds of 90% or 80%, and (5) cost per visit. Logistic regression with generalized estimating equations was used to analyze the value of different attributes and generate a marginal willingness-to-pay for a change in attribute level.
Results:
The response rate was 64% (N = 558). Most respondents were parents (63%) and most respondents agreed that combination vaccines were safe (77%). Respondents were willing to pay $7.68 to avoid an injection (compared to $9.94 when looking at parents only). However, respondents were willing to pay $41.57 to avoid higher risk of fever after one set of immunizations (10% versus 30%) and $65.42 for higher immunization coverage rates. These results were very similar for parents only. There was no significant preference to avoid an extra dose of hepatitis B vaccine.
Conclusions:
Respondents were willing to pay larger amounts to avoid increased risk of minor adverse events and to increase community-level immunization coverage than to avoid injections. These values should be taken into account when determining the risks and benefits of combination vaccines.
Keywords: Vaccines, Combination vaccines, Childhood vaccines, Economic analysis, Discrete choice experiment
1. Introduction
Combination vaccines are designed to reduce the number of injections required for routine childhood immunizations. Several new combination vaccines have been introduced over the past decade, including diphtheria-tetanus-acellular pertussis, hepatitis B, and inactivated polio vaccine, or DTaP-HepB-IPV (Pediarix®)*; hepatitis B and Hib, or HepB-Hib (COMVAX®); measles, mumps, rubella, and varicella, or MMR-V (ProQuad®); and most recently DTaP-IPV/Hib (Pentacel®) and DTaP-IPV (Kinrix®).
Under the current immunization schedule, an infant could receive as many as 6 injections at a single visit [1]. Some parents believe this to be an excessive number of injections [2] and some choose to defer some vaccines to a later visit or decline them altogether [3]. With combination vaccines, the number of injections could be reduced by 2 or more per visit, which may decrease vaccine refusal and increase immunization coverage [4,5]. Improved immunization coverage benefits the entire community, as vaccine-preventable diseases are less likely to circulate and cause infection.
Combination vaccines also have drawbacks. Depending on the combination vaccine and immunization schedule used, they may result in the administration of extra doses of antigen. This can occur when 3 doses of DTaP-HepB-IPV are given on schedule at 2, 4, and 6 months, in addition to the hepatitis B dose recommended at birth [6]. Although the Advisory Committee on Immunization Practices considers a fourth dose of hepatitis B safe for children [7], some parents may remain concerned about the risks of an unneeded vaccine antigen. Administration of unnecessary antigens can also occur during vaccine shortages. During the recent Hib shortage, DTaP-IPV/Hib was recommended for the last dose of Hib if it was the only Hib-containing product available, even if it resulted in an unnecessary dose of DTaP [8]. In addition, some combination vaccines are associated with higher rates of minor adverse events, such as fever [9,10]. Although these rates are considered trivial enough that the Advisory Committee on Immunization Practices generally recommends combination vaccines over separate injections of the equivalent vaccines [11], parents may still have strong preferences to avoid even minor adverse events [12].
Typically, combination vaccines cost more than the equivalent component vaccines. In order to make informed choices about combination vaccines, decision-makers must take into account the public’s willingness to pay for different attributes of combination vaccines, such as fewer injections, increased immunization coverage, avoidance of extra vaccine antigens, and avoidance of adverse events. These attributes are important to both parents and pediatricians, but are often not measured and explicitly factored into policy decisions about combination vaccines.
Discrete choice experiments have been used to measure values for many different healthcare interventions and outcomes, including specific immunizations such as HPV [13] and influenza illness [14]. This approach has the advantage of allowing several aspects of combination vaccines to be evaluated simultaneously, allowing for understanding of how respondents trade off between attributes. We conducted a discrete choice experiment (DCE) using an online survey to measure parental and societal values and willingness-to-pay for childhood combination vaccines.
2. Methods
2.1. Study population and data collection
Data for the discrete choice experiment were collected using a 20-min online survey during March to April 2010. The survey was administered by Knowledge Networks, a firm that maintains an online panel that is representative of the general adult population in the United States and includes people from all 50 states. People are recruited into the panel using random digit dialing and address-based sampling [15]. Knowledge Networks provides participants with web-enabled TV if they do not have Internet access in the home. We deliberately chose not to exclude non-parents from the survey, as we wanted to obtain a societal perspective on the value of combination vaccines. The survey protocol was approved by the Human Subjects Committee at Harvard Pilgrim Health Care.
2.2. Survey design
Discrete choice experiments use a survey-based approach in which respondents are asked to make a choice between two or more discrete alternatives that use attributes and levels to describe a good or service [16]. At least one attribute of the alternatives is then systematically varied in such a way that allows information related to trade-offs between the attributes describing the good or service to be inferred. The discrete choice experiment tasks contained in the survey are the mechanism by which possible profiles are presented to respondents for the purpose of preference elicitation. We adhered to the International Society for Pharmacoeconomics and Outcomes Research checklist for conjoint analysis applications in health [17]. We identified important attributes of combination vaccines based on a review of the literature, as well as qualitative interviews with pediatricians [18], policymakers [18], and community members. The key attributes included in the survey were: (1) number of shots per visit and over 6 months, (2) immunization coverage in respondent’s community (80% versus 90%), (3) extra hepatitis B dose, (4) risk of fever > 100.4 after receiving vaccines (10% versus 30%), and (5) total cost over 6 months (Table 1).
Table 1.
Attributes and levels.
| Attribute | Levels | Variable coding |
|---|---|---|
| Number of shots per visit and total number of shots over 6 months | 3 visits with 4 shots (total 12 shots) | Continuous |
| 3 visit with 3 shots (total 9 shots) | ||
| 3 visits with 2 shots (total 6 shots) | ||
| 3 visits with 1 shot (total 3 shots) | ||
| Immunization coverage at age 2 years in respondent’s community | 80% | Categorical |
| 90% | ||
| Extra hepatitis B dose | Yes | Categorical |
| No | ||
| Risk of fever > 100.4 after receiving each set of vaccines over 6 months | 30% | Categorical |
| 10% | ||
| Cost per visit and total cost over 6 months | $20 (total $60) | Continuous |
| $40 (total $120) | ||
| $80 (total $240) | ||
| $160 (total $480) |
The survey instrument was developed through an iterative process of extensive one-on-one pretesting followed by cognitive interviews during which we asked respondents to explain what they were thinking as they answered the questions. A formal pilot test (N = 57) was conducted to evaluate the range of attribute levels for the cost attribute and used simple regression models to evaluate main effects in the pilot dataset.
The survey’s introduction consisted of an explanation of the discrete choice tasks, which included a description of the attributes and a practice choice task. Respondents were instructed to think about a specific child when making their choices. Choices were presented as unlabeled forced-choice discrete choice questions with no explicit opt-out option (Fig. 1). An opt-out option was intentionally not included in the choice task, because vaccination is largely required for school entry for children. However, to acknowledge that some respondents would prefer to “opt out” of vaccines, we did ask whether respondents would vaccinate a child in real life so that we could conduct sensitivity analyses to explore the potential impact on preferences.
Fig. 1.
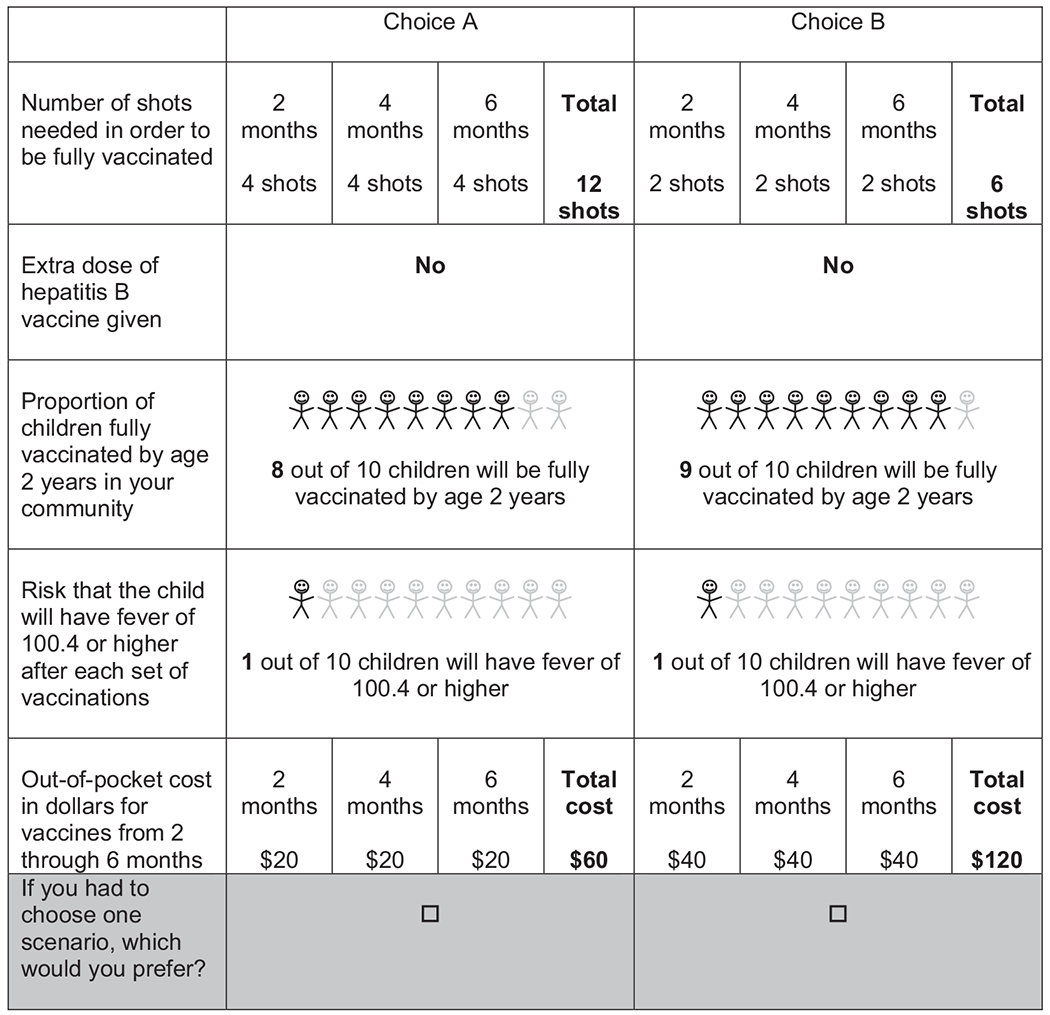
Sample discrete choice experiment question.
Respondents were asked to complete 17 choice pairs in total. A fractional factorial design was used to generate the choices taking into account the mathematical design properties needed to estimate the main effects of each attribute [19]. Dominated choices, in which a choice would offer respondents worse health for more money, were deleted from the set of choice tasks. One dominated choice was retained in each block as a test of internal validity.
In the next section of the survey, respondents were randomized to traditional time trade-off (TTO) or willingness-to-pay (WTP) questions to elicit willingness-to-pay amounts, described here as “direct valuation” questions [20]. Time trade-off questions were asked as the amount of the respondent’s life that the respondent would be willing to give up to avoid a child receiving injections [21]. Respondents were also asked for the maximum amount of money that they would be willing to pay to avoid a child receiving injections [22]. Depending on the answer to an initial opening bid, the respondent was asked either a higher or lower second bid (dichotomous-choice double-bounded question), which was then followed by a final open-ended question about their maximum willingness to pay. To minimize response burden, we limited our direct valuation questions to ask about number of injections. We asked respondents about their preferences for a child to receive 2 instead of 4 injections and 3 instead of 4 injections. Respondents were randomized as to which question they received first.
A final section included questions on sociodemographic characteristics; perceived safety of vaccines; whether the respondents would split up vaccines for a child; and whether the respondent would vaccinate a child in real life.
2.3. Statistical methods
We conducted multivariate logistic regression with generalized estimating equations (to account for multiple responses per respondent) using SAS statistical software (SAS 9.1, SAS Institute, Cary, NC). Unadjusted models were run for parents only and for all respondents, and included only variables that represented attributes of the discrete choice experiment. The adjusted models also included (1) sociodemographic characteristics (age, gender, race, education, income, and insurance status), (2) respondent’s parental status, (3) perceived safety of combination vaccines, and (4) whether respondents would split up vaccines between visits.
The primary analysis excluded respondents who did not answer more than half of the discrete choice questions. Sensitivity analyses explored the robustness of the results to changes in the inclusion criteria for respondents. One sensitivity analysis re-estimated unadjusted and adjusted models after excluding respondents who selected a dominated choice. Respondents who chose scenarios in which every attribute was associated with worse outcomes (i.e., more shots, higher risk of fever, lower immunization coverage, and higher costs) were identified as having selected a dominated choice. Additional sensitivity analyses re-estimated the unadjusted models after (1) excluding those respondents who would not vaccinate a child in real life; and (2) running the unadjusted model with number of shots and cost as categorical instead of continuous variables.
Marginal willingness-to-pay amounts for each attribute were calculated using the beta estimates for the attributes from the unadjusted model (in which the number of shots and cost were both used as continuous variables). We then divided the estimate for the attribute of interest by the estimate for the total cost attribute to derive the marginal willingness to pay for the attribute of interest [16]. Confidence intervals for marginal willingness-to-pay amounts were calculated using the multivariate delta method, which estimates the variance of a non-linear function of two or more random variables by assuming they have a joint normal distribution [23].
For the direct valuation questions, confidence intervals for the mean time trade-off and willingness-to-pay amounts were calculated using a bootstrap procedure. Time traded off at the end of life was discounted at 3% per year.
3. Results
Of the 877 potential respondents, 558 completed the survey for a response rate of 64%. Compared with non-respondents, respondents were significantly (p < 0.001) older (48 versus 44 years), more highly educated (30% with a bachelor’s degree or higher versus 20%), and more likely to have had Internet access prior to enrolling in Knowledge Networks (67% versus 55%). Responders were less likely to be black (8% versus 17%), or Hispanic (10% versus 26%), and to have a child less than 18 years of age residing in the house (29% versus 41%).
Most respondents (63%) were parents, and a third of all respondents were thinking about their own child when answering the questions (Table 2). The remaining respondents were thinking about a grandchild (24%), niece/nephew (24%) or other (16%).
Table 2.
Respondent characteristics.
| N (%) | |
|---|---|
| Male gender | 272 (48.8%) |
| Age | |
| 18–40 | 189 (33.9%) |
| 41–64 | 276 (49.5%) |
| 65–79 | 76 (13.6%) |
| 80+ | 17 (3.0%) |
| Education | |
| Less than high school | 69 (12.4%) |
| High school | 174 (31.2%) |
| Some college | 145 (26.0%) |
| Bachelor’s degree or higher | 170 (30.5%) |
| Race | |
| White, non-Hispanic | 427 (76.5%) |
| Hispanic | 54 (9.7%) |
| Black, non-Hispanic | 42 (7.5%) |
| Other | 35 (6.3%) |
| Marital status | |
| Married | 291 (52.2%) |
| Never married | 139 (24.9%) |
| Divorced | 61 (10.9%) |
| Widow | 30 (5.4%) |
| Living with partner | 28 (5.0%) |
| Separated | 9 (1.6%) |
| Household with low income (<3 times the poverty threshold) | 275 (49.6%) |
| Households with Internet | 372 (66.7%) |
| Type of insurance | |
| Private insurance | 276 (52.2%) |
| Medicare/SCHIP | 90 (17.0%) |
| Do not have health insurance | 63 (11.9%) |
| Other | 59 (11.2%) |
| Medicaid | 41 (7.8%) |
| Parent of a child | 333 (62.6%) |
| Relationship to child that the respondent thought about for scenarios | |
| Son/daughter | 187 (35.8%) |
| Grandchild | 125 (23.9%) |
| Niece/nephew | 127 (24.3%) |
| Other | 84 (16.1%) |
| Difficulty with questions | |
| Discrete choice experiment questions were hard to answer | 146 (27.0%) |
| Time trade-off questions were hard to answera | 131 (49.2%) |
| Willingness-to-pay questions were hard to answerb | 94 (34.2%) |
N = 266 because only half the sample were randomized to TTO.
N = 275 because only half the sample were randomized to WTP.
Most respondents agreed or strongly agreed that the benefits of vaccines outweighed the risks (Table 3). About 77% agreed or strongly agreed that combination vaccines were safe. Twenty-six percent stated that they had or would split up vaccines so that some were given at an extra visit. When specifically asked about the reasons for splitting vaccines, the most commonly cited concern was overloading the child’s immune system (66%). Only 4% of respondents stated that they would not vaccinate the child they were thinking of in real life.
Table 3.
Perceived risks and adverse effects associated with vaccines.
| N (%) | |
|---|---|
| Agree or strongly agree that the benefits of vaccines outweigh the risks | 470 (88.0%) |
| Agree or strongly agree that combination vaccines are safe | 409 (77.0%) |
| Would split up vaccines so that some shots are given at an extra visit | 140 (26.5%) |
| If yes: | |
| Because of concern for overloading child’s immune system | 93 (66.4%)a |
| Because of concern about safety of one or more components of vaccine | 63 (45.0%)a |
| Because of pain of multiple injections | 61 (43.6%)a |
| Other | 9 (6.4%)a |
| Would not vaccinate the child they were thinking about in real life | 19 (3.6%) |
Frequencies do not add up to 100% because respondents could choose more than one answer.
3.1. Attributes of the discrete choice experiment
Respondents were significantly more likely to select profiles that reduced the number of injections, minimized risk of fever, increased immunization coverage, and minimized cost. There was no significant preference for avoiding an extra dose of hepatitis B antigen (Table 4). When adjusted for demographic characteristics, parenthood, insurance status, perceived safety of combination vaccines, and splitting of vaccines, the results were similar except for an increased preference to avoid fever (estimate 1.464 instead of 0.773) (see Appendix).
Table 4.
Multivariate regression model for probability of choosing a scenario by attributes of the discrete choice experiment, adjusted only for attributes (N = 554).
| Attribute | Beta estimatea | 95% CI | p-Value |
|---|---|---|---|
| Increased risk of fever avoided after receiving each set of vaccines over 6 months (10% risk compared to 30% risk) | 0.773 | 0.667, 0.880 | < 0.001 |
| Immunization coverage at age 2 years increased in community (90% compared to 80%) | 0.405 | 0.328, 0.483 | < 0.001 |
| Number of injections per visit | −0.048 | −0.059, −0.036 | < 0.001 |
| Cost per visit | −0.006 | −0.007, −0.006 | <0.001 |
| Extra dose of hepatitis B avoided | 0.057 | −0.030, 0.143 | 0.20 |
The beta estimate is a measure of how much change there is in the dependent variable (choice of scenario A versus B in this case) given a 1-unit change in the independent variable (attribute in this case).
3.2. Willingness-to-pay from the discrete choice experiment
Based on the unadjusted model, respondents were willing to pay about $8 to avoid an injection (Table 5). However, they were willing to pay much higher amounts to increase immunization coverage in their community from 80% to 90% ($65) and to reduce risk of fever from 30% to 10% for the child after each of 3 sets of vaccines over the first 6 months of life ($125 over the first 6 months, or $42 after one set of vaccines).
Table 5.
Discrete choice experiment with marginal WTP for benefits of combination vaccines over the first 6 months of life, using multivariate regression model adjusted only for attributes.
| Valuing combination vaccine attributes for a child |
||||
|---|---|---|---|---|
| All respondents (N = 554) |
Parents only (N = 333) |
|||
| WTP | 95% CIa | WTP | 95% CIa | |
| Increased risk of fever avoided after receiving one set of vaccines (10% risk compared to 30% risk) | $41.57 | $35.30, $47.87 | $41.09 | $33.21, $45.92 |
| Immunization coverage at age 2 years increased in community (90% compared to 80%) | $65.42 | $52.30, $78.54 | $63.90 | $48.95, $77.82 |
| Each injection avoided | $7.68 | $5.75, $9.60 | $9.95 | $8.10, $12.36 |
| Extra dose of hepatitis B avoided | $9.13 | −$4.82, $23.08 | $14.56 | −$0.21, $27.01 |
95% confidence intervals calculated using the delta method.
When the model included parents only, there was almost no change in the results (Table 5). Parents were willing to pay minimally more to avoid an injection ($9.95 compared to $7.68). Willingness to pay to avoid an extra dose of hepatitis B vaccine was different, but this preference was not significant in either model.
3.3. Sensitivity analyses for the discrete choice experiment
Sensitivity analyses on the unadjusted model excluding those people who would not vaccinate a child in real life showed no change in the model results. When excluding those respondents who selected a dominated choice (meaning they chose a scenario in which every attribute was associated with worse outcomes), respondents were more likely to choose scenarios that did not include an extra dose of hepatitis B (p = 0.022). Results for the unadjusted model with number of shots and cost as categorical instead of continuous variables were similar (see Table A2).
3.4. Direct valuation questions
In the conventionally formatted direct valuation questions on WTP and TTO, respondents were willing to pay a median of $50 for a child to have 3 instead of 4 shots, and to trade a median of 0.17 days from the end of their life for the same benefit (Table 6). In addition, respondents were willing to pay a median of $80 for a child to have 2 instead of 4 shots, and to trade a median of 0.23 days from the end of their life. We found no significant difference in willingness-to-pay and time traded based on the order in which the respondents answered these questions.
Table 6.
Time traded (TTO) and willingness-to-pay (WTP) to avoid additional injections using direct valuation questions.
| Median | Mean (95% CI)a | |
|---|---|---|
| TTO (days) – 2 instead of 4 injections | 0.23 | 1.15 [0.93, 1.37] |
| TTO (days) – 3 instead of 4 injections | 0.17 | 1.08 [0.87, 1.29] |
| WTP – 2 instead of 4 injections | $80 | $136 [$116, $159] |
| WTP – 3 instead of 4 injections | $50 | $126 [$103, $151] |
95% confidence intervals calculated using the bootstrap method.
4. Discussion
Our study suggests that society is willing to pay a significant amount of money to avoid an increased risk of a minor vaccine adverse event such as fever, as well as to increase immunization coverage in their community. They are also willing to pay to avoid injections, but the absolute amount for this benefit is far smaller. The public appears to have no significant preference to avoid an extra dose of hepatitis B vaccine.
We found little difference in our results when comparing parents to the general population, except that parents were slightly more willing to pay to avoid one extra injection ($2.27). We chose to use the societal perspective as the primary perspective because the Vaccines for Children (VFC) Program spending – which represents the bulk of vaccine purchase – is funded from taxpayer dollars. The increased costs of combination vaccines are likely be funded from this pool, not from out of pocket co-pays from parents. Regardless, the willingness-to-pay for attributes of combination vaccines were remarkably similar for parents, indicating that parental status may not strongly influence such values.
Our findings overall are consistent with the available literature examining willingness-to-pay for comparable aspects of childhood vaccines. A study conducted by Prosser et al. [24] of a similar national sample found that they were willing to pay $50 to avoid an episode of fever to 101 °F in a 4-month-old baby that requires no additional medical attention and resolves by the next day. This is quite similar to our estimate of $42. Previous work conducted by Lieu et al. [25] identified a higher willingness-to-pay to avoid an injection using an approach where parents were asked directly what they would be willing to pay to avoid different risks of combination vaccines. Parents said they were willing to pay between $25 and $50 to avoid an additional injection, depending on the total number of injections to be administered. These responses were felt to overestimate the amounts people would actually pay, most likely because patients do not pay for medical services and their estimates were not well-calibrated.
The discrete choice experiment offers a unique and fitting approach for assessing the value of combination vaccines from the public’s perspective. It has the advantage of allowing several attributes of a choice to be evaluated at the same time and quantify the trade-offs that people make between different aspects of a vaccine. Our current study found a lower willingness-to-pay to avoid injections [24,25]. This may be because the DCE approach is well-suited to combination vaccines that confer a set of risks and benefits that are best evaluated simultaneously, whereas most approaches only enable respondents to value one attribute at a time.
Within the current study, we identified heterogeneity of the willingness-to-pay estimates depending on elicitation method. Values to avoid injections were substantially higher when elicited using the direct valuation methods compared to the discrete choice experiment method. In contrast, other studies have found that willingness-to-pay tends to be higher when using a discrete choice experiment compared to conventional direct valuation questions [14,26]. We include both estimates for willingness-to-pay to avoid injections as policymakers may require different types of estimates to inform policy.
4.1. Limitations
One limitation of our study is that respondents differed some-what from the general population. Respondents were more likely than non-respondents to be educated, less likely to be black or Hispanic, more likely to have Internet access prior to joining the online panel, and less likely to have a child less than 18 years of age residing in the household. These response differences are consistent with those observed in other studies, including surveys fielded by Knowledge Networks [27]. Income was not significantly different between the two groups, decreasing the likelihood of income effect leading to higher willingness-to-pay observed in other studies [28].
In our main analysis, the preference to avoid an extra dose of Hepatitis B was not significant. However, in a sensitivity analysis excluding those who picked a dominated choice, the preference to avoid this extra dose became significant. Of note, the extra dose of hepatitis B could have some benefit as some non-responders may respond to a fourth dose. In addition, a fourth dose results in higher antibody titers which may translate in longer-lasting immunity. However, because of the lack of data on these benefits, we did not include them.
Based on our extensive pretesting, we inflated the increased risk of fever to a difference of 20% (from 8%, based on the safety profile of DTaP-HepB-IPV [29]) to make this attribute more salient to respondents. Although we can generally assume linearity to generate estimates for smaller differences in fever, this assumption may not hold when extrapolating our results to much smaller differences in fever (e.g. 3% instead of 20%). We also relied on the linearity assumption to extrapolate our results from willingness-to-pay to avoid fever after 3 sets of vaccines over the first 6 months of life, to generate a result for just one set of vaccines. Regardless of these assumptions, our results highlight the degree of concern related to even minor safety aspects of vaccines. The value to avoid an increased risk of fever – whether specific to combination vaccines or not – can now be taken into consideration by decision-makers whose considerations include safety.
Finally, we did ask respondents to think about the risks and benefits of combination vaccines at the individual infant level as well as at the community level, which is a complex task. In any stated preference study, choices and scenarios are hypothetical and may not reflect actual preferences. Despite these challenges, stated preference methods have become an established approach for valuing goods that do not have a real market [16].
5. Conclusions
This study is the first to examine willingness to pay for the risks and benefits of combination vaccines. We used a discrete choice experiment that allowed respondents to assess the risks and benefits of combination vaccines simultaneously, evaluate both individual and community level attributes, and understand the trade-offs respondents made between the attributes. Respondents in this survey place more value on avoiding fever than avoiding injections, and results for parents only were very similar. This information can help guide decisions for groups such as Advisory Committee on Immunization Practices, and allows for a better understanding of the public’s valuation of side effects such as fever.
Acknowledgements
We would like to thank Ken Kleinman, PhD (Department of Population Medicine, Harvard Pilgrim Health Care Institute), for his statistical advice. We would also like to acknowledge Acham Gebremariam, MS (University of Michigan) and Ping Shi, MA (formerly of the Department of Population Medicine, Harvard Pilgrim Health Care Institute) for their assistance with the analysis of the data.
Disclosures
The authors have no financial disclosures or conflict of interest to report. This study was funded by cooperative agreement U01 IP000143-01 from the Centers for Disease Control and Prevention (CDC), National Center for Immunization and Respiratory Diseases. The views in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention, US Department of Health and Human Services. Dr. Gidengil was supported by Grant T32 HS000063-13 from the Agency for Healthcare Research and Quality (AHRQ) through the Harvard Pediatric Health Services Research Fellowship Program. The funders had no role in the design and conduct of the study; or collection, management, analysis, and interpretation of the data. The CDC reviewed the manuscript. All authors made substantial contributions to all of the following: (1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, (3) final approval of the version to be submitted. All authors have approved the final article.
Abbreviations:
- DTaP
diphtheria-tetanus-acellular pertussis
- HepB
hepatitis B
- Hib
Haemophilus influenzae type b
- IPV
inactivated polio vaccine
- DCE
discrete choice experiment
- TTO
time trade-off
- WTP
willingness-to-pay.
Appendix.
Table A1.
Adjusted multivariate regression model for main attributes of discrete choice experiment and covariates.a
| Estimate | 95% CI | p-Value | |
|---|---|---|---|
| Increased risk of fever avoided after receiving each set of vaccines over 6 months (10% risk compared to 30% risk) | 1.464 | 1.000, 1.928 | <0.001 |
| Immunization coverage at age 2 years increased in community (90% compared to 80%) | 0.505 | 0.262, 0.749 | <0.001 |
| Number of injections per visit | −0.063 | −0.109, −0.016 | 0.009 |
| Cost per visit | −0.006 | −0.008, −0.005 | <0.001 |
| Unnecessary dose of hepatitis B avoided | −0.292 | −0.605, 0.021 | 0.07 |
| Age | |||
| 18–40 years | – | – | – |
| 41–64 years | 0.023 | −0.308, 0.354 | 0.892 |
| 65–79 years | −0.168 | −0.779, 0.442 | 0.588 |
| 80+ years | 0.134 | −0.508, 0.776 | 0.682 |
| Male gender | −0.341 | −0.639, −0.043 | 0.025 |
| Education | |||
| Less than high school | 0.322 | −0.245, 0.890 | 0.27 |
| High school | 0.032 | −0.392, 0.455 | 0.88 |
| Some college | 0.120 | −0.268, 0.508 | 0.54 |
| Bachelor’s degree or higher | – | – | – |
| Race | |||
| Black, non-Hispanic | −0.388 | −1.038, 0.262 | 0.24 |
| Hispanic | −0.618 | −1.105, −0.130 | 0.013 |
| Other | −0.147 | −0.736, 0.443 | 0.62 |
| White, non-Hispanic | – | – | – |
| Parent | 0.258 | −0.078, 0.593 | 0.13 |
| Insurance status | |||
| Medicaid | −0.601 | −0.688, −0.568 | 0.85 |
| Medicare/SCHIP | 0.084 | −0.475, 0.642 | 0.77 |
| Other | −0.065 | −0.543, 0.413 | 0.79 |
| None | 0.165 | −0.333, 0.663 | 0.52 |
| Private insurance | – | – | – |
| Low income (<3× poverty threshold) | −0.097 | −0.443, 0.248 | 0.58 |
| Believe that combination vaccines are safe | 0.203 | −0.163 0.569 | 0.28 |
| Have or would split up vaccines into separate injections given at different times | −0.041 | −0.397, 0.316 | 0.82 |
Interaction effects were also included in the model, but not described here due to space constraints. Interaction effects were run for each level of each DCE attribute by each level of each covariate.
Table A2.
Unadjusted multivariate regression model for main attributes of discrete choice experiment, with number of injections and cost as categorical variables.
| Estimate | 95% CI | p-Value | |
|---|---|---|---|
| Increased risk of fever avoided after receiving each set of vaccines over 6 months (10% risk compared to 30% risk) | 0.823 | 0.715, 0.931 | <0.001 |
| Immunization coverage at age 2 years increased in community (90% compared to 80%) | 0.443 | 0.364, 0.522 | <0.001 |
| Number of injections per visit | |||
| 1 injection | – | – | – |
| 2 injections | −0.175 | −0.260, −0.091 | <0.001 |
| 3 injections | −0.211 | −0.284, −0.138 | <0.001 |
| 4 injections | −0.509 | −0.631, −0.388 | <0.001 |
| Cost per visit | |||
| $20 | – | – | – |
| $40 | −1.024 | −1.142, −0.907 | <0.001 |
| $80 | −1.281 | −1.397, −1.166 | <0.001 |
| $160 | −2.943 | −3.168, −2.719 | <0.001 |
| Unnecessary dose of hepatitis B avoided | 0.046 | −0.043, 0.134 | 0.31 |
References
- [1].Centers for Disease Control and Prevention. Recommended immunization schedules for persons aged 0–18 years – United States, 2010. MMWR Morb Mortal Wkly Rep 2010;58(51 & 52):1–4. [Google Scholar]
- [2].Woodin KA, Rodewald LE, Humiston SG, Carges MS, Schaffer SJ, Szilagyi PG. Physician and parent opinions. Are children becoming pincushions from immunizations? Arch Pediatr Adolesc Med 1995;149(August (8)):845–9. [DOI] [PubMed] [Google Scholar]
- [3].Melman ST, Chawla T, Kaplan JM, Anbar RD. Multiple immunizations. Ouch! Arch Fam Med 1994;3(July (7)):615–8. [DOI] [PubMed] [Google Scholar]
- [4].Happe LE, Lunacsek OE, Marshall GS, Lewis T, Spencer S. Combination vaccine use and vaccination quality in a managed care population. Am J Manag Care 2007;13(September(9)):506–12. [PubMed] [Google Scholar]
- [5].Marshall GS, Happe LE, Lunacsek OE, Szymanski MD, Woods CR, Zahn M, et al. Use of combination vaccines is associated with improved coverage rates. Pediatr Infect Dis J 2007;26(June (6)):496–500. [DOI] [PubMed] [Google Scholar]
- [6].Centers for Disease Control and Prevention. Pediarix vaccine: questions and answers, http://www.cdc.gov/vaccines/vpd-vac/combo-vaccines/pediarix/faqs-hcp-pediarix.htm; 2009. [accessed 18.01.12].
- [7].Centers for Disease Control and Prevention. Combination vaccines for childhood immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP), the American Academy of Pediatrics (AAP), and the American Academy of Family Physicians (AAFP). MMWR Morb Mortal Wkly Rep 1999;48(RR05):1–15. [PubMed] [Google Scholar]
- [8].Updated recommendations for use of Haemophilus influenzae type b (Hib) vaccine: reinstatement of the booster dose at ages 12–15 months. MMWR Morb Mortal Wkly Rep 2009;58(June (24)):673–4. [PubMed] [Google Scholar]
- [9].Marin M, Broder KR, Temte JL, Snider DE, Seward JF. Use of combination measles, mumps, rubella, and varicella vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2010;59(May (RR-3)):1–12. [PubMed] [Google Scholar]
- [10].Centers for Disease Control and Prevention. Notice to readers: FDA licensure of diphtheria and tetanus toxoids and acellular pertussis adsorbed, hepatitis B (recombinant), and poliovirus vaccine combined, (PEDIARIX™) for use in infants. MMWR Morb Mortal Wkly Rep 2003;52(10):203–4. [PubMed] [Google Scholar]
- [11].General recommendations on immunization – recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011;60:1–64. [PubMed] [Google Scholar]
- [12].Lee GM, Salomon JA, Gay C, Hammitt JK. Preferences for health outcomes associated with Group A Streptococcal disease and vaccination. Health Qual Life Outcomes 2010;8:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].de Bekker-Grob EW, Hofman R, Donkers B, van Ballegooijen M, Helmerhost TJ, Raat H, et al. Girls’ preferences for HPV vaccination: a discrete choice experiment. Vaccine 2010;28(September (41)):6692–7. [DOI] [PubMed] [Google Scholar]
- [14].Prosser LA, Rusinak D, Payne K, Uyeki T, Messonnier M. Eliciting time trade-off amounts for health states in hypothetical individuals of different ages using a discrete choice experiment. In: 14th annual international meeting, International Society for Pharmacoeconomics and Outcomes Research. 2009. [Google Scholar]
- [15].Knowledge Networks. Knowledge networks methodology, http://www.knowledgenetworks.com/ganp/docs/knowledge%20networks%20methodology.pdf; 2011. [accessed 18.01.12].
- [16].Lancsar E, Louviere J. Conducting discrete choice experiments to inform healthcare decision making: a user’s guide. Pharmacoeconomics 2008;26(8):661–77. [DOI] [PubMed] [Google Scholar]
- [17].Bridges JF, Hauber AB, Marshall D, Lloyd A, Prosser LA, Regier DA, et al. A Checklist for conjoint analysis applications in health: a report of the ISPOR good research practices for conjoint analysis task force. Value Health 2011;14(4):403–13. [DOI] [PubMed] [Google Scholar]
- [18].Gidengil CA, Rusinak D, Allred NJ, Luff D, Lee GM, Lieu TA. Financial barriers to implementing combination vaccines: perspectives from pediatricians and policy makers. Clin Pediatr (Phila) 2009;48(5):539–47. [DOI] [PubMed] [Google Scholar]
- [19].Street DJ, Burgess L. The construction of optimal stated choice experiments. Hoboken, NJ: Wiley-Interscience; 2007. [Google Scholar]
- [20].Neumann PJ, Goldie SJ, Weinstein MC. Preference-based measures in economic evaluation in health care. Annu Rev Public Health 2000;21:587–611. [DOI] [PubMed] [Google Scholar]
- [21].Torrance GW, Thomas WH, Sackett DL. A utility maximization model for evaluation of health care programs. Health Serv Res 1972;7(2):118–33. [PMC free article] [PubMed] [Google Scholar]
- [22].Olsen JA, Smith RD, Sackett DL. Theory versus practice: a review of ‘willingness-to-pay’ in health and health care. Health Econ 2001;10(1):39–52. [DOI] [PubMed] [Google Scholar]
- [23].Hole AR. A comparison of approaches to estimating confidence intervals for willingness to pay measures. Health Econ 2007;16(August (8)):827–40. [DOI] [PubMed] [Google Scholar]
- [24].Prosser LA, Ray GT, O’Brien M, Kleinman K, Santoli J, Lieu TA. Preferences and willingness to pay for health states prevented by pneumococcal conjugate vaccine. Pediatrics 2004;113(February (2)):283–90. [DOI] [PubMed] [Google Scholar]
- [25].Lieu TA, Black SB, Ray GT, Martin KE, Shinefield HR, Weniger BG. The hidden costs of infant vaccination. Vaccine 2000;19(August (1)):33–41. [DOI] [PubMed] [Google Scholar]
- [26].Ryan M A comparison of stated preference methods for estimating monetary values. Health Econ 2004;13(March (3)):291–6. [DOI] [PubMed] [Google Scholar]
- [27].Huggins VJ, Dennis MJ, Seryakova K. An evaluation of nonresponse bias in Internet surveys conducted using the knowledge networks panel. Paper presented at: Joint Statistical Meetings – Section on Survey Research Methods; 2002. [Google Scholar]
- [28].Guimaraes C, Marra CA, Colley L, Gill S, Simpson S, Meneilly G, et al. Socioeconomic differences in preferences and willingness-to-pay for insulin delivery systems in type 1 and type 2 diabetes. Diabetes Technol Ther 2009;11(September (9)):567–73. [DOI] [PubMed] [Google Scholar]
- [29].GlaxoSmithKline. Full Prescribing Information, Pediarix®, http://us.gsk.com/products/assets/us_pediarix.pdf; 2010. [accessed 18.01.12].


