Abstract
Plastics impact our daily lives. Unfortunately, it is the disuse and disposal of these items that may affect us the greatest. Plastic micro- and nanosized particles, likely from bulk degradation, have been identified in air pollution and water sources. Recently, plastic particles have also been identified in consumable products. The purpose of this review is to identify the likely routes of human exposure, the toxicological outcomes and concerns currently reported, and to provide some considerations for future assessments.
Keywords: microplastic, nanoplastic, toxicity, exposure
Graphical Abstract
Introduction
The production of synthetic polymers over the last half century has been astronomical. This production, subsequent use and disposal, has directly led to an increase in plastic waste. Reports have indicated that 50% of plastic products are single-use items [1]. It is commonly acknowledged that plastic pollution is a growing environmental concern. Once discarded, bulk plastics will break down to smaller pieces identified as micro- and nanosized plastic (MNP) particles [2]. These tiny microsized particles (< 5 mm) will continue to degrade to billions of smaller nanoplastic particles (<1 μm or <100 nm, depending on the source). At these size ranges, plastic particulate can become airborne where it may be inhaled or deposited miles down wind, entering waterways and the food chain. Microplastic particles have been identified in soil samples, fresh water, aquatic animals, atmospheric fallout, and arctic snow [3]. Furthermore, the expansive industrial use of plastic mingled with the increased processing of many consumable products, has led to the identification of microplastic particles in bottled water and food stuffs [4-6]. A natural step in the understanding the of plastic particle pollution is how these particles may affect human health.
Micro- and Nanoplastic Exposure Routes
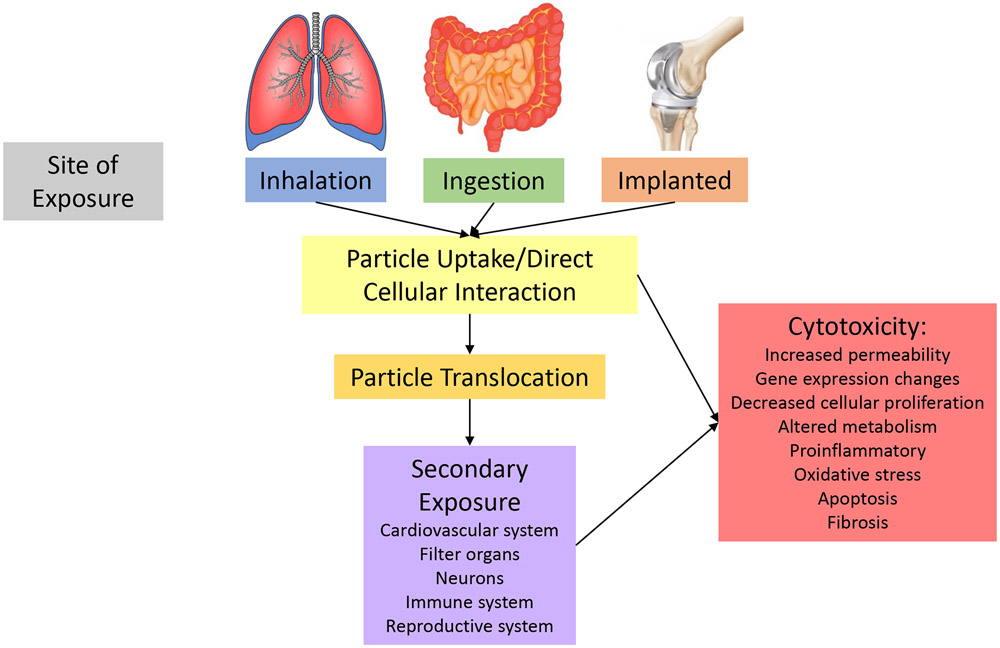
Human exposures occur through environmental interaction with the epithelial layers of the lungs, gastrointestinal tract, and skin. On rare occasions, particle-cellular interactions may occur through intentional plastic exposures. Therefore, evidence of MNP in the air, food or beverages, personal care products, or implanted devices would identify plausible human exposure routes including inhalation, ingestion, dermal, and implantation (Figure 1).
Figure 1:
Potential Fates of micro- and nanoplastic particles.
Inhalation
Microplastic particles have been identified and quantified in outdoor both and indoor air. The sources of aerosolized plastic particles include synthetic textiles (e.g., carpeting, furnishings, and clothing), roadway tire erosion and debris, and particle resuspension from waste, landfills, and emissions [7]. Wind transfer has been identified as a potential source for alpine and Antarctic plastic particles within snow samples and is estimated to contribute 7% of ocean contamination [8-10]. Plastic microfibers have been identified in atmospheric fallout, quantified as an average concentration of 0.9 m3 110 particles/m2 per day in Paris, ranging from 2.1-355.4 microplastic fibers/m2 per day, and making up approximately 29% of all outdoor aerosolized fibers [11]. The variability of these values is attributed to rainfall during sampling.
While outdoor environmental exposures are of concern, greater reports of plastic particle concentrations have been identified in indoor air [12]. Indoor air measures have reported deposition rates of 1,600-11,000 microplastic fibers/m2 per day, depending on the indoor environment (e.g., home or office) and lifestyle (e.g., tumble dryer vs. air dry clothing, air filtration of heating/cooling units) [12]. This is especially concerning given that humans spend an estimated 70-90% of their time indoors [13].
Inhalation of plastic particles allows for nasal and pulmonary deposition based on human anatomy and particle characteristics. As with other micro- and nanosized particles, smaller and lower density particles will be more likely to reach the lower airways and alveolar regions of the lung; whereas larger particles may be cleared through the mucociliary escalator [7,14]. Plastic fibers may be especially difficult to remove from the pulmonary system, due to their high surface area and high potential of penetration [15]. Pauly et al. [16] identified microplastic fibers, defined as a diameter ≥ 3 μm and a length of ≥ 5 μm, in 83% of the 81 non-neoplastic human lung samples analyzed. Interestingly, microplastic fibers were identified in 97% of the malignant samples evaluated. These microplastic fibers were not limited to specific pulmonary regions, but found to distribute throughout the lungs [16].
Occupational studies of synthetic textile workers can illustrate the possible human pulmonary consequences associated with MNP inhalation. One well-documented outcome is identified as “Flock’s disease” or flock worker’s lung, an interstitial lung disease caused by exposure to small plastic fibers (i.e., nylon, polyester, polyethylene, or polypropylene) as they are applied to an adhesive coating to produce velvet or fleece fabrics [17-19]. Overall, workers in this field carry an increased cancer incidence correlated with concentration and years of exposure [19]. This increased cancer risk is likely associated with chronic pulmonary inflammation and oxidative stress due to local particle deposition [7].
Laboratory recapitulation of flock exposure in rats has yielded conflicting reports wherein a single intratracheal instillation exposure revealed significant pulmonary inflammation [20]; yet a four-week nose-only inhalation studies demonstrated rapid clearance with no pulmonary effects [21]. Recent laboratory studies identified more pronounced effects at the local molecular level in the expression of inflammatory proteins in lung tissues after 14 days of MNP inhalation [22]. While these studies provide an initial foray into the inhalation toxicology of MNP particle inhalation there is much work that remains.
Ingestion
Environmental contamination and particle precipitation have been identified as likely sources of MNP in consumable products [23]. This connection was originally theorized as a risk through the consumption of marine and aquatic organisms which had ingested plastic particles and subsequent migration through the food chain [24,25]. It is now evident that processing and plastic packaging play a significant role in MNP particle migration into food products [5,6]. These products include less processed sources including salt, honey, rice, and granulated sugar, as well as highly processed canned goods [26-29]. As it pertains to beverages, MNP have been identified in tap and bottled water, beer, wine, and bagged tea [4,27,30-32]. Interestingly, bagged tea held the highest yields, estimating one would consume 14.7 billion MNP particles in a single cup of tea [31]. Cox et. al [5] reported through literature review that tap water contains 4 microparticles/L and bottled water contains 94 microparticles/L. This indicates that the source and pre-processing of an individual’s drinking water (i.e., bottled vs tap water) will impact MNP particle consumption dramatically. The range of microplastics reported in bottled water is very wide, fluctuating from 0.33 particles/L [33] to 325.33 particles/L [4]; these variations may be attributed to the size detection limits of the methodologies. While it is important to recognize that MNP particle are entering human mouths, it is equally as important to note that microplastic particles are also being excreted in human stool [34]. Therefore, analyses of consumable food and liquid sources of MNP exposures are ongoing.
Most studies have been completed using polystyrene particles to assess intestinal impairment and toxicity. In vitro models of gastrointestinal particle exposure demonstrate nanoplastic particle (40-90 nm) uptake by Caco-2 epithelial cells and capability to cross the intestinal barrier [35]. The use of pristine and fluorescent nanopolystyrene particles in these cellular studies indicate no digestive barrier impairments or epithelial cellular toxicity [35,36]. However, functionalization of the particle through carboxylation (−COOH) or amino groups (−NH2) permitted disruption of intestinal barrier function, easier epithelial uptake through endocytosis, and toxicity as identified by cellular autophagic death [37]. Furthermore, mixtures of polystyrene nanoparticles with other environmental pollutants, such as metals, markedly increases cellular uptake and toxicity [36].
In vivo models demonstrate a shift in intestinal oxidative and inflammatory balance due to direct epithelial-particle interactions; thus disrupting the gut microbiota, immune cell toxicity, nutritional uptake and impairing intestinal functions [38]. These models also echo the capability of nanopolystyrene absorption within and migration from the digestive system after oral exposure [37,39].
Dermal and Implanted Devices
While skin exposure to MNP particles is likely through personal care products and exposure to aerosolized particles. It is notable that plastic microbeads products were recently banned after disposal led to environmental contamination and accumulation. While it is unlikely that MNP will pass the healthy dermal barrier [40,41]; if the skin is damaged by small tears or sunburn, plastic particle penetration may occur. Furthermore, the mechanical wear of medical devices implanted into the human body (e.g. polyethylene articulating spacer in shoulder, knee, or hip replacement, dental implants and caps, and cosmetic implants) has been shown to allow for MNP particle production and translocation within the system [42-45].
Particle Uptake and Translocation
Micro- and nanoparticle translocation from the primary site of exposure has been a theory of how particle exposure can modulate toxicity and impact distant systems (Figure 1). Particle migration has been identified after gastric or pulmonary exposure to metallic and carbonaceous nano-sized materials [46]. Early work focused on MNP also indicate the propensity for MNP translocation and deposition.
Particle Translocation, Deposition, and Accumulation
Particle translocation from the primary site of exposure (i.e., gastric or pulmonary system) have been reported. Interestingly, intestinal disruption and increased cellular permeability was reported to be more severe after heterogeneous polystyrene oral exposure, where mice were exposed to both micro- (500 nm) and nanosized (50 nm) particles, allowed for greater intestinal accumulation and biodistribution [39]. Particle accumulation was reported in distant organs including the spleen, lung, kidney, brain, and reproductive system [37,39]. In vitro assays demonstrate nanopolystyrene particles can cross the alveolar epithelial barrier, an outcome influenced by particle physicochemical properties (i.e., size, density, and charge) [47]. We have identified nanopolystyrene accumulation in the maternal heart and spleen and fetal placenta, liver, lungs, heart, kidney, and brain after maternal pulmonary exposure, suggesting systemic translocation after pulmonary exposure in late-stage pregnancy [48]. These studies provide evidence of MNP migration and deposition, indicating that MNP toxicities may not be limited to the site of initial exposure. These outcomes coincide with concerns regarding cellular toxicity as evidenced by increased inflammatory and apoptotic markers [39]. Furthermore, how or if these plastics particles are removed from the system is unclear.
Secondary Exposure
Micro- and nanoplastic translocation and systemic deposition can lead to direct cell-cell interactions in the local environment. Studies pertaining to metallic and carbonaceous nanoparticles have identified secondary outcomes in the neurological, reproductive, immune, cardiovascular systems and their local cytotoxic outcomes (Figure 1). Few targeted MNP studies have been completed to date.
Neurological
Due to the capability of MNP particle translocation from the original site of exposure, there is the potential for neurotoxicity [49]. Initial studies evaluating this connection identify reduced neurotransmitter activity and neurotoxic effects with micro- and nanopolystyrene exposure [50]. Behavioral alterations in locomotion are reported in lower-level animal models but have not yet been replicated in mammalian studies [49,51]. However, more recent evidence has identified cognitive impairments in murine new-object recognition tests after nanopolystyrene IP injection exposure [51]. Interestingly, when the nanopolystyrene particles were mixed with zinc oxide nanoparticles, the cognitive impairment absolved [51].
Reproductive
The reproductive system is also impacted by MNP particle translocation and deposition. Micro- and nanopolystyrene particles have migrated to the testes, ovaries, and placenta from the original site of exposure [37,48,52]. An et al. identified that micropolystyrene uptake to the ovary reduced follicular growth and induced oxidative stress, thus promoting ovarian fibrosis [52]. Long term exposure was found to promote chromosomal abnormalities in oocytes and germ cell apoptosis, leading to transgenerational reproductive decline in C. elegans [53,54]. Lastly, nanopolystyrene translocation to the fetal compartment and fetal tissues may permit nanoplastic particle deposition in progeny, local cytotoxicity, and promote the development of disease within the offspring [48].
The placenta is thought to act as a barrier between maternal and fetal system, essentially to protect the fetus from the maternal environment while simultaneously providing nutrition and removing waste. Using an isolated ex vivo perfusion technique, Grafmueller et al. [55] demonstrated the bidirectional transfer of MNP particles within the human placenta. Recently, microplastic particles were identified using Raman microspectoscopy within samples of discarded human placenta after real-world exposure during pregnancy [56]. While it was unclear if these particles entered the maternal system through ingestion or inhalation routes; however, this study clearly demonstrates the capability of human exposure and systemic bioaccumulation.
Immune, Cytotoxicity, and Other Systems
Cellular-particle interactions occur at the local site of exposure and after systemic translocation and deposition. Many studies have identified modified gene expression, decreased cell proliferation rates, altered metabolism, increased proinflammatory cytokines, and oxidative stress production in hematological cells, human gastric epithelium, and lymphocytes after polystyrene exposure [57-61]. In vitro studies of particle interactions with immune cells resulted in increased oxidative stress and impaired lysosomes in RAW 264.7 macrophages [62]. Furthermore, in vivo studies have demonstrated cardiotoxicity induced by oxidative stress, resulting in cardiomyocyte apoptosis and structural damage to the myocardium [63,64].
Understanding the cellular internalization of MNP particles is paramount to discern their cytotoxicity [57,65]. Liu et al. [65] identified that micro- and nanosized polystyrene particles are endocytosed through differing pathways; where microplastics were often taken up through micropinocytosis and nanoparticles were endocytosed via clathrin and caveolae-mediated pathways [65]. We should keep in mind that there is significant selection variability associated with “representative” cell lines and plastic particles utilized within these initial studies [59]. Functionalized groups, metals, organics or other proteins on the surface of the plastic producing an eco-corona can also dramatically impact particle uptake, internalization, and release [65] [62,66-68].
Considerations
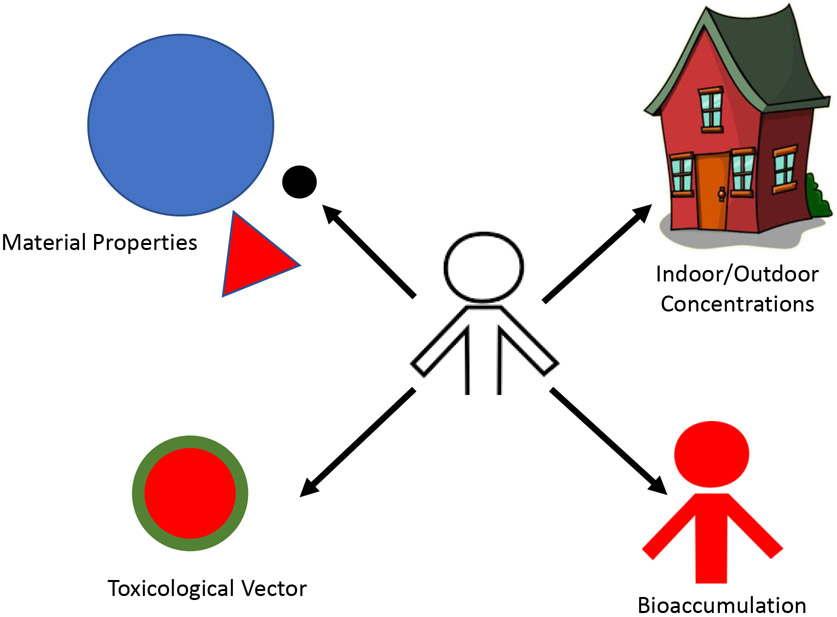
While it is clear that human exposures to MNP are inevitable and early toxicity studies are underway, there are clear elements of MNP to consider in future studies (Figure 2). The material properties and particle standards, real-world concentrations in indoor/outdoor environments, bioaccumulation, and the transportation and release of adsorbed surface coating or embedded chemicals to a biological environment must all be considered.
Figure 2:
Considerations for future assessments.
Material Properties
One area that remains under debate when assessing human risk is the size of the particles examined. Microplastic particles are described as those less than 5 mm in one dimension. Nanoplastics, however, have been described in environmental literature as less than 1 μm (1000 nm) and in laboratory studies as less than 0.1 μm (100 nm) [69]. Currently, most assessments of environmental or consumable products cannot report nanoplastic concentrations at either definition due filter pore limitations. These have been reported as low as of 125 μm for environmental studies [70] and 11 μm for consumable products [27]. Openings of this size would allow the passage on nanosized plastics, thus eliminating these particles from assessment and quantification. While nanoplastics would fall under the greater “microplastic” umbrella, and it is tempting to ignore these delineations, classifying these terms and size ranges is crucial for biological assessments. Particles within these micro- and nano-size ranges have significantly different biological interactions and physiochemical behaviors, thus greatly impacting cellular uptake, biodistribution, accumulation, and cytotoxicity [69,71].
Chemical construct, particle shape, and surface charge may also play a role in cellular interaction, uptake, translocation, and toxicity. There are numerous polymeric compounds that may be described as “plastics”. Each chemical transition between these composites begets a new toxicological profile. For example, polystyrene, nylon, polyethylene, and polypropylene particles may all behave differently in a biological environment. Furthermore, particle shape may also impact cellular interaction. Fibrous particles may lead to frustrated phagocytosis, thus increasing localized oxidative stress and inflammation, whereas spherical particles are easily internalized. Surface charge has also been shown to affect particle uptake and the binding of surface proteins in environmental or biological conditions. Given these properties, it is crucial that studies continue to characterize the MNP identified in environmental contamination and laboratory exposures.
Concentrations
Currently, there is a gap in the literature identifying the MNP particle concentrations in indoor and outdoor air, water, and consumable products. Recently a number of well-organized reviews have been published regarding MNP particle exposure, biological outcomes, and predicted human health consequences [3,7,57,68,72,73]; however, most laboratory exposures are not analogous to human toxicity studies. These studies utilize short duration, high concentration exposures while most humans are chronically exposed to low levels. Unfortunately, the real-world exposure paradigm of plastic particulate concentration and size ranges for humans in indoor or outdoor settings and urban or rural environments has not yet been established [74]. While literature reviews provide the basis for human exposure estimates [5], there is a lack of validated methods for collection, reference materials, and standardized analytical sampling and assessment techniques [57,72]. Furthermore, identifying the dosing concentrations of nanoplastic particles remains outside of our collective capabilities. Therefore, these experimental models provide a baseline start for which to build future studies. Until the environmental concentrations and doses are confirmed, the separation between laboratory exposures and real-world conditions will remain.
Bioaccumulation
Understanding MNP particle uptake, translocation, and bioaccumulation remains a barrier to human toxicology assessments. Few groups are applying imaging and microscopy approaches to visualize particle migration from the original site of exposure. Increasing the sensitivity of positron emission tomography (PET) to assess in vivo pharmacokinetic behavior of MNP particles will be crucial to aid in our understanding of human toxicology [75]. The expansion of Raman and darkfield microscopy techniques will also aid in the identification of nanoplastic intracellular uptake and tissue deposition [48,76].
Toxicological Vector
It is important to keep in mind that suspended MNP particles are within the heterogenous mixture [69]. Real-world exposures to these particles are not taking place in a pristine vacuum, but instead in a conglomerate mixture of carbonaceous, metallic, biological materials, and other plastics. It has been hypothesized that MNP particles may act as a toxicological vector for the transfer of heavy metals, volatile chemicals, or biological contaminants [3,77]. Furthermore, plastics are developed with a variety of chemical additives or extrudation processing to produce specific commercial characteristics (i.e., the difference between polystyrene foam and hard solids). These “plasticizers” may not be covalently bound to the polymer, thus capable of leaching from the plastic material [69]. Much work has been done on the health effects of these chemicals leaching from the plastic product into consumable products (i.e., bisphenol A, phthalates, nonylphenols, and perfluorooctanoic acid) or being released as volatile organic compounds (VOCs) [3]. Outcomes of these toxicological assessments identify carcinogenic compounds and endocrine disruption leading to metabolic, reproductive, and developmental effects [57,73]. It remains unclear if bioaccumulation of MNP particles would permit continued local release of these chemical additives at the site of deposition.
Conclusions
Human exposure to MNP is occurring; however, the toxicological consequences of these exposures in unclear. Laboratory studies are underway to assess the routes of exposure, particle uptake mechanisms, and secondary cytotoxicities. Gleaning an understanding of real-world exposure concentrations and primary particle chemistry is paramount. Until these exposure characteristics are revealed, selection variability, as it pertains to laboratory model and particle properties (i.e., size, material, shape, functionalization groups, particle corona) will continue to persist, clouding valuable experimental outcomes and delaying regulatory action [72]. This variability extends to the differentiation between pristine particles, functionalized groups, environmental contaminants, chemical additives, and biological corona, each having a distinct toxicological outcome. Given the capabilities for particle uptake from the primary exposure site, additional studies will need to focus on the toxicities at the initial site, secondary systemic signaling, and local secondary perturbations associated with deposition and bioaccumulation. Studies in these areas may permit for the identification of exposure biomarkers [74].
Overall, domestic and commercial plastic utilization continues to rise. Therefore, plastic disposal and subsequent particle pollution remains a worldwide concern. Human exposures via inhalation and ingestion have been documented, yet the toxicological considerations of these exposures remain elusive. Focused studies to determine environmental concentrations, human exposure dosage, particle physiochemical properties (i.e., material, size, shape, charge, and surface chemistry), mechanisms of cellular uptake, and in vivo outcomes are vital to determine human risk.
CONFLICT OF INTEREST AND ACKNOWLEDGEMENTS
We declare no conflicts. This work was supported by the National Institute of Environmental Health Sciences (R01-ES031285) and Rutgers Center for Environmental Exposures and Disease (P30-ES005022).
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
* indicate manuscripts of special interest
- 1.Hopewell J, Dvorak R, Kosior E: Plastics recycling: Challenges and opportunities. Philosophical transactions of the Royal Society of London Series B, Biological sciences (2009) 364(1526):2115–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang K, Hamidian AH, Tubić A, Zhang Y, Fang JKH, Wu C, Lam PKS: Understanding plastic degradation and microplastic formation in the environment: A review. Environ Pollut (2021) 274(116554. [DOI] [PubMed] [Google Scholar]
- 3. *Campanale C, Massarelli C, Savino I, Locaputo V, Uricchio VF: A detailed review study on potential effects of microplastics and additives of concern on human health. Int J Environ Res Public Health (2020) 17(4). *While providing a review of current literature, the authors focus on the plastics acting as a vector for human exposure to chemically added (i.e. plasticizers) or environmentally adhered (i.e., metals) toxicants.
- 4.Mason SA, Welch VG, Neratko J: Synthetic polymer contamination in bottled water. Frontiers in chemistry (2018) 6(407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. *Cox KD, Covernton GA, Davies HL, Dower JF, Juanes F, Dudas SE: Human consumption of microplastics. Environmental science & technology (2019) 53(12):7068–7074. **This is the first paper to unify microplastic measurements in environmental and consumable products to estimate human exposure. Authors further separate exposures to men, women, and children.
- 6.Toussaint B, Raffael B, Angers-Loustau A, Gilliland D, Kestens V, Petrillo M, Rio-Echevarria IM, Van den Eede G: Review of micro- and nanoplastic contamination in the food chain. Food additives & contaminants Part A, Chemistry, analysis, control, exposure & risk assessment (2019) 36(5):639–673. [DOI] [PubMed] [Google Scholar]
- 7. *Prata JC: Airborne microplastics: Consequences to human health? Environ Pollut (2018) 234(115–126. *This is a well-written review focusing on microplastic inhalation exposure.
- 8.Materić D, Kasper-Giebl A, Kau D, Anten M, Greilinger M, Ludewig E, van Sebille E, Röckmann T, Holzinger R: Micro- and nanoplastics in alpine snow: A new method for chemical identification and (semi)quantification in the nanogram range. Environmental science & technology (2020) 54(4):2353–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boucher J, Friot D: Primary microplastics in the oceans: A global evaluation of sources. 10. Iucn Gland, Switzerland, (2017). [Google Scholar]
- 10.Kelly A, Lannuzel D, Rodemann T, Meiners KM, Auman HJ: Microplastic contamination in east antarctic sea ice. Marine pollution bulletin (2020) 154(111130. [DOI] [PubMed] [Google Scholar]
- 11.Dris R, Gasperi J, Saad M, Mirande C, Tassin B: Synthetic fibers in atmospheric fallout: A source of microplastics in the environment? Marine pollution bulletin (2016) 104(1-2):290–293. [DOI] [PubMed] [Google Scholar]
- 12.Dris R, Gasperi J, Mirande C, Mandin C, Guerrouache M, Langlois V, Tassin B: A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environ Pollut (2017) 221(453–458. [DOI] [PubMed] [Google Scholar]
- 13.Alzona J, Cohen BL, Rudolph H, Jow HN, Frohliger JO: Indoor-outdoor relationships for airborne particulate matter of outdoor origin. Atmospheric Environment (1979) 13(55–60. [Google Scholar]
- 14.Facciolà A, Visalli G, Pruiti Ciarello M, Di Pietro A: Newly emerging airborne pollutants: Current knowledge of health impact of micro and nanoplastics. Int J Environ Res Public Health (2021) 18(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donaldson K, Tran CL: Inflammation caused by particles and fibers Inhalation Toxicology (2002) 14(1):5–27. [DOI] [PubMed] [Google Scholar]
- 16.Pauly JL, Stegmeier SJ, Allaart HA, Cheney RT, Zhang PJ, Mayer AG, Streck RJ: Inhaled cellulosic and plastic fibers found in human lung tissue. Cancer Epidemiol Biomarkers Prev (1998) 7(5):419–428. [PubMed] [Google Scholar]
- 17.Kern DG, Kuhn C 3rd, Ely EW, Pransky GS, Mello CJ, Fraire AE, Muller J: Flock worker's lung: Broadening the spectrum of clinicopathology, narrowing the spectrum of suspected etiologies. Chest (2000) 117(1):251–259. [DOI] [PubMed] [Google Scholar]
- 18.Kern DG, Crausman RS, Durand KT, Nayer A, Kuhn C 3rd: Flock worker's lung: Chronic interstitial lung disease in the nylon flocking industry. Annals of internal medicine (1998) 129(4):261–272. [DOI] [PubMed] [Google Scholar]
- 19.Turcotte SE, Chee A, Walsh R, Grant FC, Liss GM, Boag A, Forkert L, Munt PW, Lougheed MD: Flock worker's lung disease: Natural history of cases and exposed workers in kingston, ontario. Chest (2013) 143(6):1642–1648. [DOI] [PubMed] [Google Scholar]
- 20.Porter DW, Castranova V, Robinson VA, Hubbs AF, Mercer RR, Scabilloni J, Goldsmith T, Schwegler-Berry D, Battelli L, Washko R, Burkhart J et al. : Acute inflammatory reaction in rats after intratracheal instillation of material collected from a nylon flocking plant. J Toxicol Environ Health A (1999) 57(1):25–45. [DOI] [PubMed] [Google Scholar]
- 21.Warheit DB, Webb TR, Reed KL, Hansen JF, Kennedy GL Jr.: Four-week inhalation toxicity study in rats with nylon respirable fibers: Rapid lung clearance. Toxicology (2003) 192(2-3):189–210. [DOI] [PubMed] [Google Scholar]
- 22.Lim D, Jeong J, Song KS, Sung JH, Oh SM, Choi J: Inhalation toxicity of polystyrene micro(nano)plastics using modified oecd tg 412. Chemosphere (2021) 262(128330. [DOI] [PubMed] [Google Scholar]
- 23.Rist S, Carney Almroth B, Hartmann NB, Karlsson TM: A critical perspective on early communications concerning human health aspects of microplastics. Sci Total Environ (2018) 626(720–726. [DOI] [PubMed] [Google Scholar]
- 24.Elizalde-Velázquez GA, Gómez-Oliván LM: Microplastics in aquatic environments: A review on occurrence, distribution, toxic effects, and implications for human health. Sci Total Environ (2021) 780(146551. [DOI] [PubMed] [Google Scholar]
- 25.Waring RH, Harris RM, Mitchell SC: Plastic contamination of the food chain: A threat to human health? Maturitas (2018) 115(64–68. [DOI] [PubMed] [Google Scholar]
- 26.Dessì C, Okoffo ED, O'Brien JW, Gallen M, Samanipour S, Kaserzon S, Rauert C, Wang X, Thomas KV: Plastics contamination of store-bought rice. J Hazard Mater (2021) 416(125778. [DOI] [PubMed] [Google Scholar]
- 27.Kosuth M, Mason SA, Wattenberg EV: Anthropogenic contamination of tap water, beer, and sea salt. PLoS One (2018) 13(4):e0194970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karami A, Golieskardi A, Choo CK, Larat V, Karbalaei S, Salamatinia B: Microplastic and mesoplastic contamination in canned sardines and sprats. Sci Total Environ (2018) 612(1380–1386. [DOI] [PubMed] [Google Scholar]
- 29.Liebezeit G, Liebezeit E: Non-pollen particulates in honey and sugar. Food additives & contaminants Part A, Chemistry, analysis, control, exposure & risk assessment (2013) 30(12):2136–2140. [DOI] [PubMed] [Google Scholar]
- 30.Schymanski D, Goldbeck C, Humpf HU, Furst P: Analysis of microplastics in water by micro-raman spectroscopy: Release of plastic particles from different packaging into mineral water. Water Res (2018) 129(154–162. [DOI] [PubMed] [Google Scholar]
- 31.Hernandez LM, Xu EG, Larsson HCE, Tahara R, Maisuria VB, Tufenkji N: Plastic teabags release billions of microparticles and nanoparticles into tea. Environmental science & technology (2019) 53(21):12300–12310. [DOI] [PubMed] [Google Scholar]
- 32.Prata JC, Paco A, Reis V, da Costa JP, Fernandes AJS, da Costa FM, Duarte AC, Rocha-Santos T: Identification of microplastics in white wines capped with polyethylene stoppers using micro-raman spectroscopy. Food Chem (2020) 331(127323. [DOI] [PubMed] [Google Scholar]
- 33.Wiesheu AC, Anger PM, Baumann T, Niessner R, Ivleva NP: Raman microspectroscopic analysis of fibers in beverages. Analytical Methods (2016) 8(28):5722–5725. [Google Scholar]
- 34.Schwabl P, Köppel S, Königshofer P, Bucsics T, Trauner M, Reiberger T, Liebmann B: Detection of various microplastics in human stool: A prospective case series. Annals of internal medicine (2019) 171(7):453–457. [DOI] [PubMed] [Google Scholar]
- 35.Domenech J, Hernández A, Rubio L, Marcos R, Cortés C: Interactions of polystyrene nanoplastics with in vitro models of the human intestinal barrier. Arch Toxicol (2020) 94(9):2997–3012. [DOI] [PubMed] [Google Scholar]
- 36.Domenech J, Cortés C, Vela L, Marcos R, Hernández A: Polystyrene nanoplastics as carriers of metals. Interactions of polystyrene nanoparticles with silver nanoparticles and silver nitrate, and their effects on human intestinal caco-2 cells. Biomolecules (2021) 11(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu D, Ma Y, Han X, Chen Y: Systematic toxicity evaluation of polystyrene nanoplastics on mice and molecular mechanism investigation about their internalization into caco-2 cells. J Hazard Mater (2021) 417(126092. [DOI] [PubMed] [Google Scholar]
- 38.Hirt N, Body-Malapel M: Immunotoxicity and intestinal effects of nano- and microplastics: A review of the literature. Part Fibre Toxicol (2020) 17(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liang B, Zhong Y, Huang Y, Lin X, Liu J, Lin L, Hu M, Jiang J, Dai M, Wang B, Zhang B et al. : Underestimated health risks: Polystyrene micro- and nanoplastics jointly induce intestinal barrier dysfunction by ros-mediated epithelial cell apoptosis. Part Fibre Toxicol (2021) 18(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campbell CS, Contreras-Rojas LR, Delgado-Charro MB, Guy RH: Objective assessment of nanoparticle disposition in mammalian skin after topical exposure. Journal of Controlled Release (2012) 162(1):201–207. [DOI] [PubMed] [Google Scholar]
- 41.Revel M, Châtel A, Mouneyrac C: Micro (nano) plastics: A threat to human health? Current Opinion in Environmental Science & Health (2018) 1(17–23. [Google Scholar]
- 42.Urban RM, Jacobs JJ, Tomlinson MJ, Gavrilovic J, Black J, Peoc'h M: Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacement. J Bone Joint Surg Am (2000) 82(4):457–476. [DOI] [PubMed] [Google Scholar]
- 43.Suñer S, Gowland N, Craven R, Joffe R, Emami N, Tipper JL: Ultrahigh molecular weight polyethylene/graphene oxide nanocomposites: Wear characterization and biological response to wear particles. J Biomed Mater Res B Appl Biomater (2018) 106(1):183–190. [DOI] [PubMed] [Google Scholar]
- 44.Massin P: How does total knee replacement technique influence polyethylene wear? Orthop Traumatol Surg Res (2017) 103(1s):S21–s27. [DOI] [PubMed] [Google Scholar]
- 45.Massin P, Achour S: Wear products of total hip arthroplasty: The case of polyethylene. Morphologie (2017) 101(332):1–8. [DOI] [PubMed] [Google Scholar]
- 46.Kermanizadeh A, Balharry D, Wallin H, Loft S, Moller P: Nanomaterial translocation--the biokinetics, tissue accumulation, toxicity and fate of materials in secondary organs--a review. Crit Rev Toxicol (2015) 45(10):837–872. [DOI] [PubMed] [Google Scholar]
- 47.Yacobi NR, Demaio L, Xie J, Hamm-Alvarez SF, Borok Z, Kim KJ, Crandall ED: Polystyrene nanoparticle trafficking across alveolar epithelium. Nanomedicine (2008) 4(2):139–145. [DOI] [PubMed] [Google Scholar]
- 48.Fournier SB, D'Errico JN, Adler DS, Kollontzi S, Goedken MJ, Fabris L, Yurkow EJ, Stapleton PA: Nanopolystyrene translocation and fetal deposition after acute lung exposure during late-stage pregnancy. Part Fibre Toxicol (2020) 17(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prüst M, Meijer J, Westerink RHS: The plastic brain: Neurotoxicity of micro- and nanoplastics. Part Fibre Toxicol (2020) 17(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lei L, Wu S, Lu S, Liu M, Song Y, Fu Z, Shi H, Raley-Susman KM, He D: Microplastic particles cause intestinal damage and other adverse effects in zebrafish danio rerio and nematode caenorhabditis elegans. Sci Total Environ (2018) 619-620(1–8. [DOI] [PubMed] [Google Scholar]
- 51.Estrela FN, Guimarães ATB, Araújo A, Silva FG, Luz TMD, Silva AM, Pereira PS, Malafaia G: Toxicity of polystyrene nanoplastics and zinc oxide to mice. Chemosphere (2021) 271(129476. [DOI] [PubMed] [Google Scholar]
- 52.An R, Wang X, Yang L, Zhang J, Wang N, Xu F, Hou Y, Zhang H, Zhang L: Polystyrene microplastics cause granulosa cells apoptosis and fibrosis in ovary through oxidative stress in rats. Toxicology (2021) 449(152665. [DOI] [PubMed] [Google Scholar]
- 53.Yu CW, Luk TC, Liao VH: Long-term nanoplastics exposure results in multi and transgenerational reproduction decline associated with germline toxicity and epigenetic regulation in caenorhabditis elegans. J Hazard Mater (2021) 412(125173. [DOI] [PubMed] [Google Scholar]
- 54.Sun L, Liao K, Wang D: Comparison of transgenerational reproductive toxicity induced by pristine and amino modified nanoplastics in caenorhabditis elegans. Sci Total Environ (2021) 768(144362. [DOI] [PubMed] [Google Scholar]
- 55.Grafmueller S, Manser P, Diener L, Diener PA, Maeder-Althaus X, Maurizi L, Jochum W, Krug HF, Buerki-Thurnherr T, von Mandach U, Wick P: Bidirectional transfer study of polystyrene nanoparticles across the placental barrier in an ex vivo human placental perfusion model. Environ Health Perspect (2015) 123(12):1280–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. *Ragusa A, Svelato A, Santacroce C, Catalano P, Notarstefano V, Carnevali O, Papa F, Rongioletti MCA, Baiocco F, Draghi S, D'Amore E et al. : Plasticenta: First evidence of microplastics in human placenta. Environ Int (2021) 146(106274. *This is the first manuscript to identify real-world microplastic translocation in humans. Authors demonstrate particle accumulmation in the human placenta after natural maternal exposures during pregnancy.
- 57.Yee MS, Hii LW, Looi CK, Lim WM, Wong SF, Kok YY, Tan BK, Wong CY, Leong CO: Impact of microplastics and nanoplastics on human health. Nanomaterials (Basel) (2021) 11(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ding Y, Zhang R, Li B, Du Y, Li J, Tong X, Wu Y, Ji X, Zhang Y: Tissue distribution of polystyrene nanoplastics in mice and their entry, transport, and cytotoxicity to ges-1 cells. Environ Pollut (2021)280(116974. [DOI] [PubMed] [Google Scholar]
- 59.Rubio L, Barguilla I, Domenech J, Marcos R, Hernández A: Biological effects, including oxidative stress and genotoxic damage, of polystyrene nanoparticles in different human hematopoietic cell lines. J Hazard Mater (2020) 398(122900. [DOI] [PubMed] [Google Scholar]
- 60.Sun M, Ding R, Ma Y, Sun Q, Ren X, Sun Z, Duan J: Cardiovascular toxicity assessment of polyethylene nanoplastics on developing zebrafish embryos. Chemosphere (2021) 282(131124. [DOI] [PubMed] [Google Scholar]
- 61.Hwang J, Choi D, Han S, Choi J, Hong J: An assessment of the toxicity of polypropylene microplastics in human derived cells. Sci Total Environ (2019) 684(657–669. [DOI] [PubMed] [Google Scholar]
- 62.Florance I, Ramasubbu S, Mukherjee A, Chandrasekaran N: Polystyrene nanoplastics dysregulate lipid metabolism in murine macrophages in vitro. Toxicology (2021) 458(152850. [DOI] [PubMed] [Google Scholar]
- 63.Li Z, Zhu S, Liu Q, Wei J, Jin Y, Wang X, Zhang L: Polystyrene microplastics cause cardiac fibrosis by activating wnt/β-catenin signaling pathway and promoting cardiomyocyte apoptosis in rats. Environ Pollut (2020) 265(Pt A):115025. [DOI] [PubMed] [Google Scholar]
- 64.Wei J, Wang X, Liu Q, Zhou N, Zhu S, Li Z, Li X, Yao J, Zhang L: The impact of polystyrene microplastics on cardiomyocytes pyroptosis through nlrp3/caspase-1 signaling pathway and oxidative stress in wistar rats. Environ Toxicol (2021) 36(5):935–944. [DOI] [PubMed] [Google Scholar]
- 65.Liu L, Xu K, Zhang B, Ye Y, Zhang Q, Jiang W: Cellular internalization and release of polystyrene microplastics and nanoplastics. Sci Total Environ (2021) 779(146523. [DOI] [PubMed] [Google Scholar]
- 66.Barreto A, Santos J, Amorim MJB, Maria VL: Polystyrene nanoplastics can alter the toxicological effects of simvastatin on danio rerio. Toxics (2021) 9(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. *Schultz CL, Bart S, Lahive E, Spurgeon DJ: What is on the outside matters-surface charge and dissolve organic matter association affect the toxicity and physiological mode of action of polystyrene nanoplastics to c. Elegans. Environmental science & technology (2021) 55(9):6065–6075. *Using C. Elegans as a biological model and toxicological modeling, the studies in this manuscript demonstrate the toxicological variability associated with microplastic surface charge.
- 68.Yong CQY, Valiyaveetill S, Tang BL: Toxicity of microplastics and nanoplastics in mammalian systems. Int J Environ Res Public Health (2020) 17(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. *Gigault J, El Hadri H, Nguyen B, Grassl B, Rowenczyk L, Tufenkji N, Feng S, Wiesner M: Nanoplastics are neither microplastics nor engineered nanoparticles. Nature nanotechnology (2021) 16(5):501–507. *This is a perspective piece detailing the differences between engineered and incidental micro- and nanoplastic particles and the subsequent impact to toxicological investigations.
- 70.Mason SA, Garneau D, Sutton R, Chu Y, Ehmann K, Barnes J, Fink P, Papazissimos D, Rogers DL: Microplastic pollution is widely detected in us municipal wastewater treatment plant effluent. Environ Pollut (2016) 218(1045–1054. [DOI] [PubMed] [Google Scholar]
- 71.Mitrano DM, Wick P, Nowack B: Placing nanoplastics in the context of global plastic pollution. Nature nanotechnology (2021) 16(5):491–500. [DOI] [PubMed] [Google Scholar]
- 72. *Brachner A, Fragouli D, Duarte IF, Farias PMA, Dembski S, Ghosh M, Barisic I, Zdzieblo D, Vanoirbeek J, Schwabl P, Neuhaus W: Assessment of human health risks posed by nano-and microplastics is currently not feasible. Int J Environ Res Public Health (2020) 17(23). *This is well-written review focused on the limitations associated with human health risk assessment of micro- and nanoplastic exposures.
- 73.Lehner R, Weder C, Petri-Fink A, Rothen-Rutishauser B: Emergence of nanoplastic in the environment and possible impact on human health. Environmental science & technology (2019) 53(4):1748–1765. [DOI] [PubMed] [Google Scholar]
- 74. *Zarus GM, Muianga C, Hunter CM, Pappas RS: A review of data for quantifying human exposures to micro and nanoplastics and potential health risks. Sci Total Environ (2021) 756(144010. *This is a detailed systematic review highlighting micro- and nanoplastic exposure, translocation, and health effects.
- 75.Keinänen O, Dayts EJ, Rodriguez C, Sarrett SM, Brennan JM, Sarparanta M, Zeglis BM: Harnessing pet to track micro- and nanoplastics in vivo. Sci Rep (2021) 11(1):11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schmidt R, Nachtnebel M, Dienstleder M, Mertschnigg S, Schroettner H, Zankel A, Poteser M, Hutter HP, Eppel W, Fitzek H: Correlative sem-raman microscopy to reveal nanoplastics in complex environments. Micron (Oxford, England : 1993) (2021) 144(103034. [DOI] [PubMed] [Google Scholar]
- 77.Blackburn K, Green D: The potential effects of microplastics on human health: What is known and what is unknown. Ambio (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]