Supplemental Digital Content is available in the text.
Keywords: blood pressure, echocardiography, health outcomes, heart failure
Abstract
It remains debated whether pulse pressure is associated with left ventricular traits and adverse outcomes over and beyond mean arterial pressure (MAP) in patients with heart failure (HF) with preserved ejection fraction. We investigated these associations in 3428 patients with HF with preserved ejection fraction (51.5% women; mean age, 68.6 years) enrolled in the TOPCAT trial (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist). We computed association sizes and hazards ratios with 1-SD increase in MAP and pulse pressure. In multivariable-adjusted analyses, association sizes (P≤0.039) for MAP were 0.016 cm and 0.014 cm for septal and posterior wall thickness, −0.15 for E/A ratio, −0.66 for E/e′, and −0.64% for ejection fraction, independent of pulse pressure. With adjustment additionally applied for MAP, E/A ratio and longitudinal strain increased with higher pulse pressure with association sizes amounting to 0.067 (P=0.026) and 0.40% (P=0.023). In multivariable-adjusted analyses of both placebo and spironolactone groups, lower MAP and higher pulse pressure predicted the primary composite end point (P≤0.028) and hospitalized HF (P≤0.002), whereas MAP was also significantly associated with total mortality (P≤0.007). Sensitivity analyses stratified by sex, median age, and region generated confirmatory results with exception for the association of adverse outcomes with pulse pressure in patients with age ≥69 years. In conclusion, the clinical application of MAP and pulse pressure may refine risk estimates in patients with HF with preserved ejection fraction. This finding may help further investigation for the development of HF with preserved ejection fraction preventive strategies targeting pulsatility and blood pressure control.
The blood pressure consists of a steady and pulsatile component, mean arterial pressure (MAP), and pulse pressure, respectively.1 The MAP is determined by cardiac output and peripheral arterial resistance.1 Pulse pressure defined as the difference between systolic and diastolic blood pressure depends on stroke volume, the elastic properties of the central arteries, and the timing and intensity of the backward wave in the peripheral circulation.1 Previous studies demonstrated that with aging, the stiffer large arteries lead to a gradual shift from diastolic to systolic blood pressure and then to pulse pressure as predictors of cardiovascular disease, indicating the transmission of pulsatile flow into the distal circulation.2–4
Although several previous studies showed that both MAP5–7 and pulse pressure6,8–11 predict adverse health outcomes in populations, in patients with cardiovascular or renal disease, and in critically ill patients, several other studies indicated that pulse pressure was associated with the risk of cardiovascular disease, beyond MAP.6,12–14 However, no previous studies systematically compared the effects of MAP and pulse pressure on adverse health outcomes in patients with heart failure with preserved ejection fraction (HFpEF). To address the above issue, we conducted a retrospective study to assess to what extent MAP and pulse pressure at baseline predicted adverse health outcomes by analyzing data from the TOPCAT trial (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist; URL: https://www.clinicaltrials.gov; Unique identifier: NCT00094302).15,16
Methods
Study Population
The TOPCAT study was an international, multicenter, randomized, double blind, placebo-controlled trial.15,16 The study was designed to investigate whether spironolactone improved clinical outcomes in patients with HFpEF compared with placebo. TOPCAT complied with the Declaration of Helsinki17 and received ethical clearance. All patients signed informed consent before randomization. This article was prepared using research materials obtained from the National Institutes of Heart, Lung, and Blood Institute’s Biologic Specimen and Data Repository Information Coordinating Center via an approved proposal and does not necessarily reflect the opinions and views of the TOPCAT investigators or the National Institutes of Heart, Lung, and Blood Institute’s. The requests to access the data set should be sent to the National Heart, Lung, and Blood Institute.
At 233 sites in 6 countries, 3445 patients with HFpEF were randomly assigned to spironolactone or placebo. Eligible patients were 50 years or older, had one or more signs and at least one symptom of HF with an EF not lower than 45%, controlled systolic blood pressure (defined as a systolic blood pressure of <140 mm Hg or <160 mm Hg if the patient was on 3 or more antihypertensive drugs), and a serum potassium concentration level of <5.0 mmol/L. We excluded 16 patients because covariables were missing. Thus, the number of patients included in the present analysis totaled 3428.
Clinical Measurements
Patients who participated in the TOPCAT trial underwent a detailed baseline evaluation. Blood pressure was measured manually in 75.6% of participants and by automated techniques in 24.4%. Body mass index was weight in kilograms divided by the height squared in meters. Study nurses also administered a standardized questionnaire inquiring into each participant’s medical history, smoking habits, and intake of medications.
Echocardiography
Echocardiography was performed according to the recommendations of the American Society of Echocardiography, as previously described.16,18 Dedicated analysts read all study echocardiograms at the core laboratory at the Brigham and Women’s Hospital, Boston, MA. The readers were blinded to clinical information and randomized assignment. Of 3445 randomized patients with HFpEF, 935 (27.1%) underwent echocardiography before the initiation of randomized treatment.16 Of the 935 analyzable imaging studies, complete 2-dimensional and Doppler data were available in 553 (59%), with all Doppler measures missing in 181 (19%), and tissue Doppler only missing in an additional 147 (16%) patients. Previous publications describe the procedures applied for acquisition and the off-line analysis of the echocardiographic measurements in detail.16,18 In this study, we statistically analyzed left ventricular (LV) structure, including LV dimensions, wall thickness, and mass index; diastolic function, including left atrial volume index, transmitral blood flow, and mitral annular tissue velocities; and systolic function, including EF and longitudinal strain.
Clinical Outcomes
The outcomes examined in the study included the primary end point, all-cause and cardiovascular death, hospitalization for HF, and stroke. The primary outcome was a composite of all-cause death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for HF. All-cause mortality included the composite of cardiovascular and noncardiovascular death. All events were adjudicated by a clinical end point committee. More detailed information on the evaluation of outcomes has been previously been described.19
Statistical Analysis
For database management and statistical analysis, we used SAS software, version 9.4 (SAS Institute Inc, Cary, NC), maintenance level 5. We applied the Kolmogorov-Smirnov test for assessing the normality of distributions. For between-group comparison of means and proportions, we applied the large-sample z test and Fisher exact test, respectively. For the pairwise comparison of correlation coefficients, we applied a published SAS program.20 For ease of interpretation, we used the absolute value of the longitudinal strain measurements, which were all negative. Significance was a 2-sided α level of ≤0.05.
We constructed heat maps to visualize the contribution of MAP and pulse pressure to the association with primary end point and HF hospitalization with adjustment applied for age. We expressed the association sizes of the echocardiographic indexes with blood pressure for a 1-SD increment in MAP or pulse pressure. In multivariable-adjusted analyses, in line with previous TOPCAT trial publications,16,18 we accounted for sex, age, ethnicity, body mass index, heart rate, estimated glomerular filtration rate, current smoking, New York Heart Association class, dyslipidemia, diabetes, use of antihypertensive medications by drug class, that is, diuretics, β-blockers, inhibitors of the renin-angiotensin, calcium-channel blockers, and intake of aspirin, lipid-lowering drugs, other cardiovascular medications, and antidiabetic agents. The single blood pressure component models include MAP or pulse pressure and twin models both. In the models, including both MAP and pulse pressure, we computed the variance inflation factor to examine to what extent parameter estimates for MAP and pulse pressure were affected by collinearity. Furthermore, we performed the analyses of primary end points associated with MAP and pulse pressure as primary analyses and the other analyses designated as confirmatory. Given the randomized design of TOPCAT, we computed the hazard ratios (HRs) with 95% CIs of clinical outcomes related to 1-SD increment in MAP and pulse pressure using Cox regression stratified by trial arm. In multivariable-adjusted Cox regression analyses, we assessed whether the association of clinical outcomes with MAP and pulse pressure by sex and median of age. Regarding that there were substantial regional differences in outcomes, we performed sensitivity analyses of adverse health outcomes associated with MAP and pulse pressure in participants enrolled in the Americas (United States, Canada, Argentina, and Brazil).
Results
Characteristics of Participants
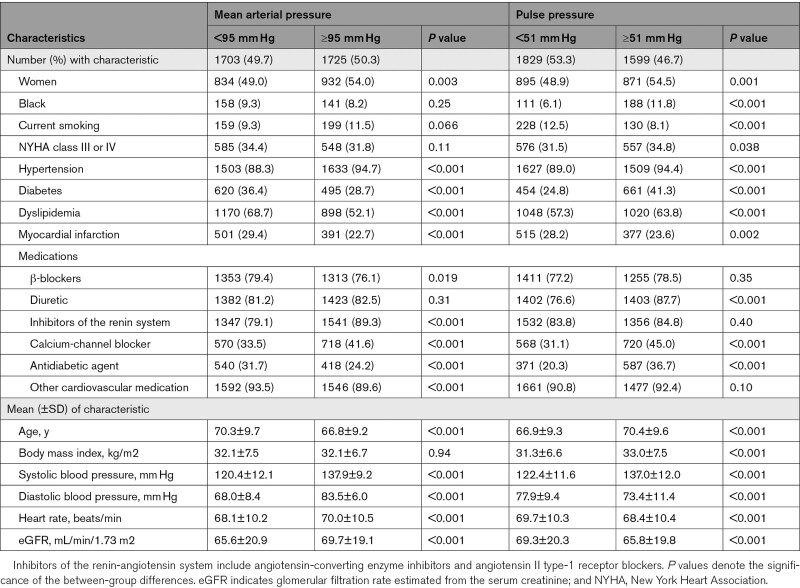
The 3428 patients with HFpEF included 1767 (51.5%) women and were predominantly White (89.0%). Mean±SD values in all patients were 68.6±9.6 years for age, 32.1±7.1 kg/m2 for body mass index, and 129.2±14.0/75.8±10.6 mm Hg for systolic/diastolic blood pressure. Table 1 shows the characteristics of the participants by the median of the distributions of MAP and pulse pressure, respectively. Table S1 in the Supplemental Material lists the echocardiographic traits by sex. Women compared with men had smaller LV end-diastolic and end-systolic volumes, septal and posterior wall thickness, and LV mass index, but higher EF and longitudinal strain (Table S1). Table S2 shows baseline cardiac structure and function by median of age, median of EF, and hypertension status. As shown in Table S3, MAP was related to systolic blood pressure (r=0.81; P<0.001), diastolic blood pressure (r=0.92; P<0.001), and pulse pressure (r=0.12; P<0.001) in placebo group and systolic blood pressure (r=0.81; P<0.001), diastolic blood pressure (r=0.92; P<0.001), and pulse pressure (r=0.11; P<0.001) in spironolactone group.
Table 1.
Characteristics of Participants by Median of Mean Arterial Pressure and Pulse Pressure
Echocardiographic Traits Associated With Blood Pressure
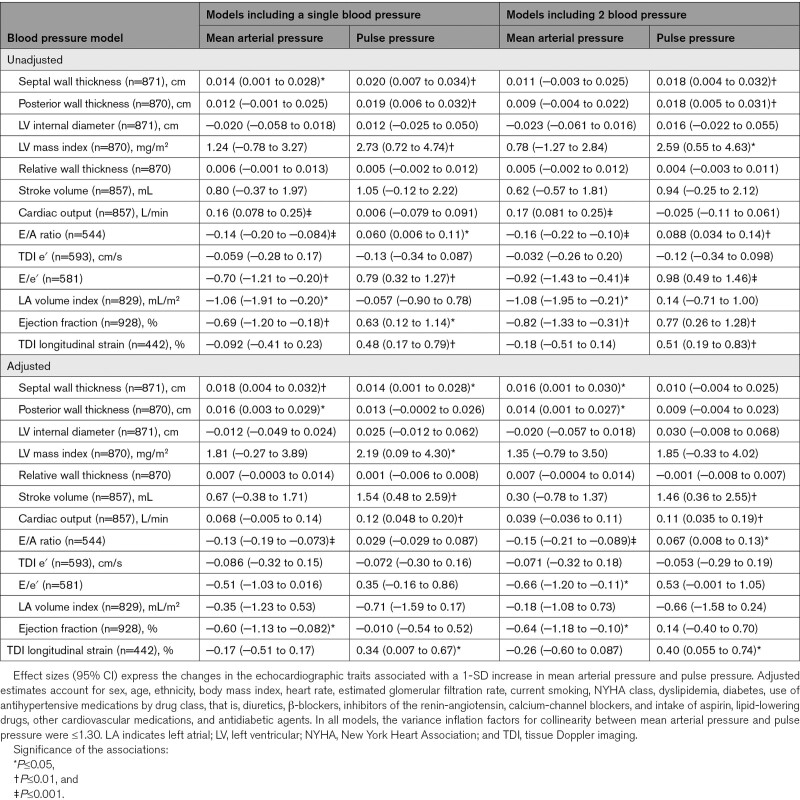
In multivariable-adjusted models (Table 2), septal and posterior wall thickness increased with higher MAP, whereas E/A ratio and EF decreased with higher MAP. The corresponding association sizes per 1-SD increment in MAP were 0.018 cm (P=0.009), 0.016 cm (P=0.013), −0.13 (P<0.001), and −0.60% (P=0.024), respectively. In multivariable-adjusted analyses additionally accounting for pulse pressure, those association sizes remained significant. Furthermore, E/e′ decreased with higher MAP (−0.66; P=0.020).
Table 2.
Cardiac Structure and Function in Relation to Mean Arterial Pressure and Pulse Pressure at Baseline
In multivariable-adjusted models (Table 2), septal wall thickness, LV mass index, and longitudinal strain increased with higher pulse pressure with association sizes amounting to 0.014 cm (P=0.041), 2.19 mg/m2 (P=0.041), and 0.34% (P=0.045), respectively. The association sizes with pulse pressure were 1.54 mL (P=0.004; Table 2) and 0.12 L/min (P=0.001) for Doppler stroke volume and cardiac output, respectively. With adjustment additionally applied for MAP, E/A ratio, stroke volume, cardiac output, and longitudinal strain increased with higher pulse pressure with association sizes amounting to 0.067 (P=0.026), 1.46 mL (P=0.009), 0.11 L/min (P=0.004), and 0.40% (P=0.023), respectively. There were no statistically significant associations of LV internal diameter with MAP and pulse pressure (P≥0.13; Table 2). In all models, the variance inflation factors for collinearity between MAP and pulse pressure were ≤1.30.
Adverse Outcomes Associated With Blood Pressure
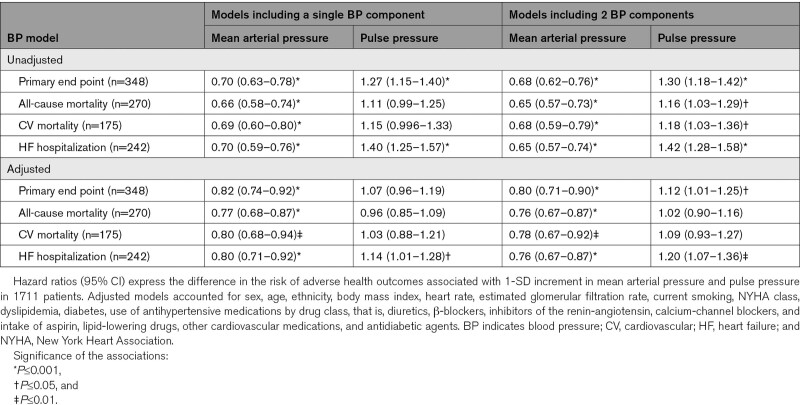
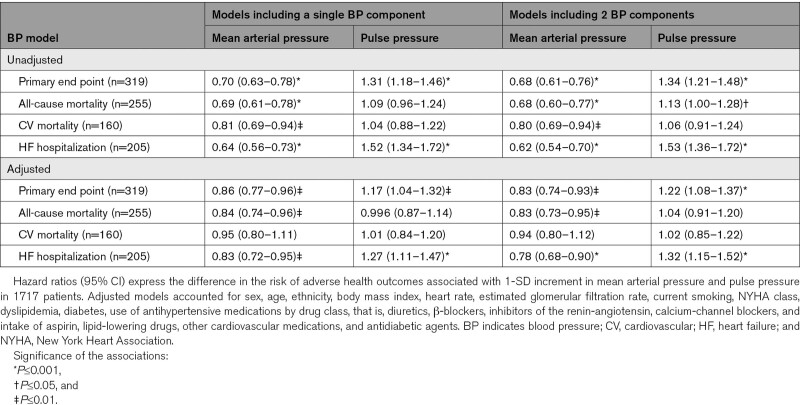
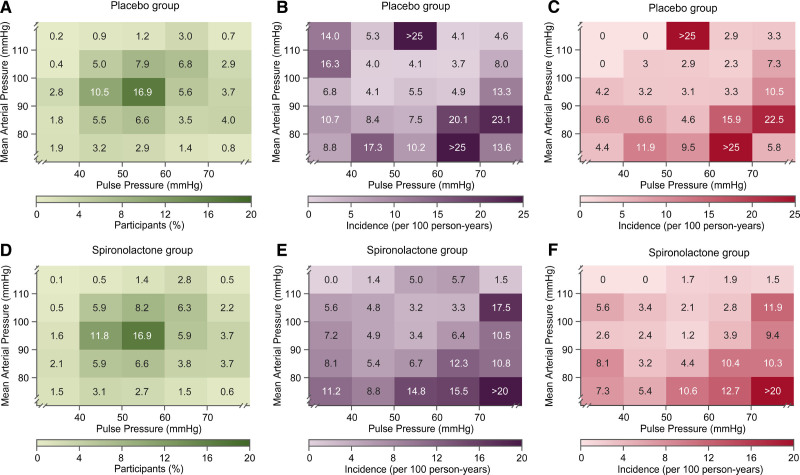
During a median follow-up of 3.0 years, 667 primary outcome events occurred. Heat maps (Figure) combining MAP and pulse pressure showed that the risk of the primary end point and hospitalization for HF increased with lower MAP along the vertical axis and increased with higher pulse pressure along the horizontal axis. In patients (n=29) with very low values of MAP (≤80 mm Hg) together with high values of pulse pressure (≥70 mm Hg), systolic and diastolic blood pressure averaged (±SD) 125.1±6.9 mm Hg and 48.9±4.7 mm Hg (Figure S1). In unadjusted analyses stratified by placebo (Table 3) or spironolactone (Table 4) groups, MAP was significantly (P<0.001) associated with primary end point, total and cardiovascular mortality, and hospitalization for HF with HRs for 1-SD increment in indexes of blood pressure components ranging from 0.64 to 0.81. With adjustments applied for confounders, those HRs remained significant in placebo group (Table 3) with exception for cardiovascular death in spironolactone group (Table 4). The multivariable-adjusted models additionally accounted for pulse pressure produced confirmatory results.
Table 3.
Associations of Adverse Health Outcomes With Mean Arterial Pressure and Pulse Pressure in Placebo Group
Table 4.
Associations of Adverse Health Outcomes With Mean Arterial Pressure and Pulse Pressure in Spironolactone Group
Figure.
Heat maps depicting the risk of primary end point and hospitalization for heart failure in relation to mean arterial pressure (MAP) and pulse pressure in the placebo and spironolactone groups.
Numbers in the (A) and (D) grids represent the percentage of participants within each blood pressure cross classification category; numbers in (B) and (E) represent the risk for primary end point; numbers in (C) and (F) indicates the risk for hospitalization for heart failure. Heat maps were derived by Cox proportional hazards regression with mean arterial pressure plotted along vertical axis and pulse pressure along the horizontal axis with adjustment applied for age. In patients (n=29) with very low values of MAP (≤80 mm Hg) together with high values of pulse pressure (≥70 mm Hg), systolic and diastolic blood pressure averaged (±SD) 125.1±6.9 mm Hg and 48.9±4.7 mm Hg.
In unadjusted models, pulse pressure was associated with primary end point and hospitalization for HF in placebo group (HRs, 1.27 and 1.40; Table 3) and in spironolactone group (HRs, 1.31 and 1.52; Table 4). The HRs remained significant in the models adjusted for potential confounders and pulse pressure. There was no difference in the prevalence of myocardial infarction at baseline between placebo group and spironolactone group (26.2% versus 25.9%; P=0.83). In the placebo group, in fully adjusted model additionally adjusted for a history of myocardial infarction, the HRs for association of hospitalization for HF with pulse pressure showed no material differences (1.21 [95% CI, 1.07–1.36]; P=0.002).
Sensitivity analyses stratified by sex (Table S4) confirmed the association of adverse health outcomes with MAP and pulse pressure. In participants younger than 69 (Table S5), irrespective of adjustments, pulse pressure was significantly associated with primary end point and hospitalization for HF. However, in participants with age of 69 and older (Table S5), these HRs related adverse outcomes to pulse pressure lost significance in multivariable-adjusted analyses. The analyses of adverse outcomes associated with MAP and pulse pressure remained confirmatory in Americas (Table S6). The interactions of randomization group with MAP (Tables S4 and S5) were not significant for all adverse health outcome (P≥0.12) with exception for association for cardiovascular death in Table S4 (P≤0.032). In patients younger than 69, the interaction of randomization group with pulse pressure was significant for primary end point in multivariable-adjusted model (P≤0.047; Table S5). In patients with age ≥69 years old, the interaction of randomization group with pulse pressure were significant for primary end point and hospitalization for HF in both unadjusted (P≤0.040; Table S5) and multivariable-adjusted models (P≤0.036; Table S5).
Discussion
The key findings of our study can be summarized as follows: (1) in patients with HFpEF, with multiple adjustments applied, septal and posterior wall thickness increased with higher MAP, while functional measures reflecting LV diastolic and systolic function were inversely associated with higher MAP, independent of pulse pressure; (2) tissue Doppler imaging longitudinal stain indicating systolic function increased with higher pulse pressure, independent of MAP; (3) in both the placebo and spironolactone groups, lower MAP and higher pulse pressure predicted the primary composite end point and hospitalized HF, whereas MAP was also significantly associated with total mortality. However, in participants aged 69 and older, pulse pressure did not substantially enhance risk stratification over and beyond the steady component, MAP.
Echocardiographic Traits in Relation to Blood Pressure
An elevated end-diastolic filling pressure, an increased left atrial volume and E/e′ ratio and lower stroke volume are among the hallmarks of HFpEF (Borlaug). With adjustments applied for potential confounders, LV mass index was positively associated with systolic blood pressure with an association size amounting to 2.42 mg/m2 (P=0.019). Thus, in line with previous studies,21,22 higher pulse pressure and systolic blood pressure increase LV afterload and, therefore, LV mass. By definition, the correlation between pulse pressure and systolic blood pressure is substantially higher than that between pulse pressure and MAP (P<0.001; Table S3). However, the positive associations in fully adjusted models of E/A, stroke volume, cardiac output, and longitudinal strain with the pulsatile blood pressure components are counterintuitive. Several interpretations come to mind. First, these associations were computed in HFpEF patients with low ejection fraction (median, 56%; interquartile range, 51%–61%) and, therefore, do not cover the whole range from low to high ejection fraction, which might have led to the unexpected associations. Other explanations might involve the selection criteria of patients and the heterogeneity of the various strata of patients enrolled in TOPCAT (Table S2).
Adverse Outcomes in Relation to Blood Pressure
Previous observational studies demonstrated that peripheral pulse pressure, as measured by conventional sphygmomanometry or ambulatory monitoring was an independent risk factor in populations,8,21,23 or in patients with cardiovascular9,10 or renal disease,11,24 whereas others failed to do so. Jackson et al25 investigated the prognostic value of pulse pressure in patients with HF including 22 038 patients with reduced ejection fraction (HFrEF) and 5008 with HFpEF, using data from 22 HF studies included in the Meta-Analysis Global Group in Chronic Heart Failure. In patients with HFrEF, compared with all other pulse pressure groups, patients in the lowest pulse pressure quintile had highest adjusted HRs for mortality (HR, 1.68 [95% CI, 1.53–1.84]). However, higher pulse pressure was associated with crude mortality in HFpEF, but this association was attenuated by multivariable adjustment.25 Laskey et al26 conducted a retrospective cohort study of patients with HF enrolled in the Get With The Guidelines-HF Program. There was a U-shaped relationship between pulse pressure at hospital discharge and 1-year mortality in both HFrEF and HFpEF, with a risk nadir at a pulse pressure of 50 mm Hg. In HFrEF, higher pulse pressure was associated with higher risk when pulse pressure was ≥50 mm Hg, but the association reversed when pulse pressure was <50 mm Hg. In HFpEF, higher pulse pressure was independently associated with increased risk when pulse pressure was ≥50 mm Hg but not with pulse pressure at levels of <50 mm Hg.26 However, those studies did not examine whether the association of adverse outcomes with pulse pressure was independent of MAP. Diastolic and MAP drive the perfusion of vital organs. Excessively lowering diastolic blood pressure exposes the myocardium to ischemia and further functional deterioration,27 which might explain the adverse health outcomes.28
Study Strengths and Limitations
To the best of our knowledge, our study is the first to report on the association of adverse health outcomes with pulse pressure in patients with HFpEF independent of the steady component, MAP. The multivariable-adjusted models and various analyses consistently yielded confirmatory results. We checked whether our multivariable-adjusted models, including both MAP and pulse pressure, were vulnerable to collinearity. In the absence of formal criteria, statisticians agree that variance inflation values >10 indicate collinearity among the explanatory variables warranting corrective action.29 However, in our analyses, the variance inflation factor between MAP and pulse pressure did not exceed 1.30. However, our study must also be interpreted within the context of its limitations. First, regarding a post hoc analysis of the TOPCAT trial, missing variables and residual confounding factors may still influence the results. Second, not all participants underwent echocardiography at baseline. Although compared with participants not included in the echocardiographic study, those included had relatively minor differences in some baseline characteristics,30 which may limit the generalizability. Third, we acknowledge that the main aim of the TOPCAT trial was not to assess the role of blood pressure in patients with HFpEF and that future studies targeting blood pressure in HFpEF might be warranted. Fourth, a thorough search of the TOPCAT publications did not identify a detailed quality control program of the blood pressure readings in this trial. However, the assumption can reasonably be made that the blood pressure readings in TOPCAT, a trial focused on HFpEF, were of clinical grade. Even if the blood pressure readings in TOPCAT were suboptimally standardized across centers, this would have weakened rather than strengthened the associations between adverse outcomes and the blood pressure components. Fifth, because information of the use of blood pressure lowering drugs during follow-up was unavailable, we could not adjust for their use as a time-dependent covariable. Sixth, as shown in the Figure, a minority of patients with MAP ≤80 mm Hg and pulse pressure ≥70 mm Hg showed higher risk, which in the absence of consensus guidelines might give some perception of the MAP and pulse pressure risk thresholds in patients with HFpEF. Finally, patient selection in clinical trials limits the external validity of our observations to community-based cohorts.
Perspectives
Our current observations highlight potentially important clinical implications. In view of differential efficacy of diverse antihypertensive drug classes for decreasing MAP and pulse pressure,31,32 it is of importance to evaluate these associations to optimize blood pressure lowering approaches for HF prevention. In patients with HFpEF, pulse pressure did not substantially add to risk stratification at age of 69 or older. However, lower MAP was associated with higher risk of adverse health outcomes across age strata. Paraphrasing a seminal review article,33 the inconvenient truth is that until recently there was no specific treatment for HFpEF, but to manage risk factors, such as hypertension and obesity. Ongoing placebo-controlled randomized clinical trials in patients with HFrEF or HFpEF, with or without type-2 diabetes, are further exploring the benefits of sodium-glucose cotransporter 2 is in terms of hard outcomes, such as in EMPEROR-Preserved (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Preserved Ejection Fraction; URL: https://www.clinicaltrials.gov; Unique identifier: NCT03057951) and DELIVER (Dapagliflozin Evaluation to Improve the Lives of Patients With Preserved Ejection Fraction Heart Failure; URL: https://www.clinicaltrials.gov; Unique identifier: NCT03619213) or in terms of LV remodeling or function. Given that there is no specific therapy for HFpEF, the results of EMPEROR-Preserved and DELIVER are eagerly awaited and expected to be published soon.
Article Information
Acknowledgments
We thank TOPCAT trial (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist) investigators for conducting this trial and making these data available.
Sources of Funding
The article was supported by the National Natural Science Foundation of China (81770392, 81770394, 81700344, 81800344, 81800345, and 82000372), Guangdong Natural Science Foundation (2016A030310180, 2017A030310311, and 2017A030313795), Science and Technology Program Foundation of Guangzhou (201610010125 and 201707010124), Science and Technology Program Foundation of Guangdong (2017A020215156), Medical Research Foundation of Guangdong Province (A2018107 and A2018082), China Postdoctoral Science Foundation (2019M663312). The NPA Alliance for the Promotion of Preventive Medicine (APPREMED), Mechelen, Belgium, received a nonbinding grant from OMRON Healthcare, Co, Ltd, Kyoto, Japan. TOPCAT trial (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist) was funded by the National Institutes of Health, National Heart, Lung, and Blood Institute (NHLBI), Bethesda, MD (N01 HC45207). This article was prepared using TOPCAT Research Materials obtained from the NHLBI Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of the TOPCAT or the NHLBI.
Disclosures
None.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- HFpEF
- heart failure with preserved ejection fraction
- HFrEF
- heart failure with reduced ejection fraction
- HR
- hazard ratio
- LV
- left ventricular
- MAP
- mean arterial pressure
- NYHA
- New York Heart Association
- TOPCAT
- Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist
This paper was sent to Marc L. De Buyzere, Guest Editor, for review by expert referees, editorial decision, and final disposition.
F.-F. Wei and Y. Wu are joint first authors.
J.A. Staessen and C. Liu are joint last authors.
The Supplemental Material is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.121.17782.
For Sources of Funding and Disclosures, see page 249.
Hypertension is available at www.ahajournals.org/journal/hyp
Contributor Information
Fang-Fei Wei, Email: fangfeimuxiang@163.com.
Yuzhong Wu, Email: yuzungng@qq.com.
Ruicong Xue, Email: xrcgrayson@163.com.
Xiao Liu, Email: liuch75@mail.sysu.edu.cn.
Xin He, Email: hejiangui@163.com.
Bin Dong, Email: dongxg@mail.sysu.edu.cn.
Zhe Zhen, Email: zhenzhe717@hotmail.com.
Xuwei Chen, Email: chenxuwei94@163.com.
Weihao Liang, Email: liangwh26@mail.sysu.edu.cn.
Jingjing Zhao, Email: zhaojingj@mail.sysu.edu.cn.
Jiangui He, Email: hejiangui@163.com.
Yugang Dong, Email: dongxg@mail.sysu.edu.cn.
Novelty and Significance
What Is New?
No longitudinal population study compared associations of echocardiographic traits and risks of adverse health outcomes with mean arterial pressure (MAP) versus pulse pressure. In 3428 patients with heart failure (HF) with preserved ejection fraction (51.5% women; mean age, 68.6 years), we examined these associations.
What Is Relevant?
In patients with HF with preserved ejection fraction, with multiple adjustments applied, septal and posterior wall thickness increased with higher MAP, while functional measures reflecting left ventricular diastolic and systolic function were inversely associated with higher MAP, independent of pulse pressure.
Tissue Doppler imaging longitudinal stain indicating systolic function increased with higher pulse pressure, independent of MAP.
In both placebo and spironolactone groups, lower MAP and higher pulse pressure predicted the primary composite end point and hospitalized HF, whereas MAP was also significantly associated with total mortality. However, in participants with age of 69 and older, pulse pressure does not substantially enhance risk stratification over and beyond the steady component, MAP.
Summary
In conclusion, the clinical application of MAP and pulse pressure may refine risk estimates in patients with HF with preserved ejection fraction. This finding may help further investigation for the development of HF with preserved ejection fraction preventive strategies targeting pulsatility and blood pressure control.
References
- 1.Safar ME, Levy BI, Struijker-Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation. 2003;107:2864–2869. doi: 10.1161/01.CIR.0000069826.36125.B4 [DOI] [PubMed] [Google Scholar]
- 2.Franklin SS, Larson MG, Khan SA, Wong ND, Leip EP, Kannel WB, Levy D. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation. 2001;103:1245–1249. doi: 10.1161/01.cir.103.9.1245 [DOI] [PubMed] [Google Scholar]
- 3.Mitchell GF, Wang N, Palmisano JN, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS. Hemodynamic correlates of blood pressure across the adult age spectrum: noninvasive evaluation in the Framingham Heart Study. Circulation. 2010;122:1379–1386. doi: 10.1161/CIRCULATIONAHA.109.914507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Safar ME, Asmar R, Benetos A, Blacher J, Boutouyrie P, Lacolley P, Laurent S, London G, Pannier B, Protogerou A, et al. ; French Study Group on Arterial Stiffness. Interaction between hypertension and arterial stiffness. Hypertension. 2018;72:796–805. doi: 10.1161/HYPERTENSIONAHA.118.11212 [DOI] [PubMed] [Google Scholar]
- 5.Melgarejo JD, Yang WY, Thijs L, Li Y, Asayama K, Hansen TW, Wei FF, Kikuya M, Ohkubo T, Dolan E, et al. ; International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcome Investigators*. Association of fatal and nonfatal cardiovascular outcomes with 24-hour mean arterial pressure. Hypertension. 2021;77:39–48. doi: 10.1161/HYPERTENSIONAHA.120.14929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kodama S, Horikawa C, Fujihara K, Yoshizawa S, Yachi Y, Tanaka S, Ohara N, Matsunaga S, Yamada T, Hanyu O, et al. Meta-analysis of the quantitative relation between pulse pressure and mean arterial pressure and cardiovascular risk in patients with diabetes mellitus. Am J Cardiol. 2014;113:1058–1065. doi: 10.1016/j.amjcard.2013.12.005 [DOI] [PubMed] [Google Scholar]
- 7.Houwink AP, Rijkenberg S, Bosman RJ, van der Voort PH. The association between lactate, mean arterial pressure, central venous oxygen saturation and peripheral temperature and mortality in severe sepsis: a retrospective cohort analysis. Crit Care. 2016;20:56. doi: 10.1186/s13054-016-1243-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glynn RJ, Chae CU, Guralnik JM, Taylor JO, Hennekens CH. Pulse pressure and mortality in older people. Arch Intern Med. 2000;160:2765–2772. doi: 10.1001/archinte.160.18.2765 [DOI] [PubMed] [Google Scholar]
- 9.Selvaraj S, Steg PG, Elbez Y, Sorbets E, Feldman LJ, Eagle KA, Ohman EM, Blacher J, Bhatt DL; REACH Registry Investigators. Pulse pressure and risk for cardiovascular events in patients with atherothrombosis: from the REACH registry. J Am Coll Cardiol. 2016;67:392–403. doi: 10.1016/j.jacc.2015.10.084 [DOI] [PubMed] [Google Scholar]
- 10.Bangalore S, Messerli FH, Franklin SS, Mancia G, Champion A, Pepine CJ. Pulse pressure and risk of cardiovascular outcomes in patients with hypertension and coronary artery disease: an INternational VErapamil SR-trandolapril STudy (INVEST) analysis. Eur Heart J. 2009;30:1395–1401. doi: 10.1093/eurheartj/ehp109 [DOI] [PubMed] [Google Scholar]
- 11.Liu JH, Chen CC, Wang SM, Chou CY, Liu YL, Kuo HL, Lin HH, Wang IK, Yang YF, Huang CC. Association between pulse pressure and 30-month all-cause mortality in peritoneal dialysis patients. Am J Hypertens. 2008;21:1318–1323. doi: 10.1038/ajh.2008.286 [DOI] [PubMed] [Google Scholar]
- 12.Verdecchia P, Schillaci G, Reboldi G, Franklin SS, Porcellati C. Different prognostic impact of 24-hour mean blood pressure and pulse pressure on stroke and coronary artery disease in essential hypertension. Circulation. 2001;103:2579–2584. doi: 10.1161/01.cir.103.21.2579 [DOI] [PubMed] [Google Scholar]
- 13.Zheng L, Sun Z, Li J, Zhang R, Zhang X, Liu S, Li J, Xu C, Hu D, Sun Y. Pulse pressure and mean arterial pressure in relation to ischemic stroke among patients with uncontrolled hypertension in rural areas of China. Stroke. 2008;39:1932–1937. doi: 10.1161/STROKEAHA.107.510677 [DOI] [PubMed] [Google Scholar]
- 14.Protogerou AD, Vlachopoulos C, Thomas F, Zhang Y, Pannier B, Blacher J, Safar ME. Longitudinal changes in mean and pulse pressure, and all-cause mortality: data from 71,629 untreated normotensive individuals. Am J Hypertens. 2017;30:1093–1099. doi: 10.1093/ajh/hpx110 [DOI] [PubMed] [Google Scholar]
- 15.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, et al. ; TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. doi: 10.1056/NEJMoa1313731 [DOI] [PubMed] [Google Scholar]
- 16.Shah AM, Shah SJ, Anand IS, Sweitzer NK, O’Meara E, Heitner JF, Sopko G, Li G, Assmann SF, McKinlay SM, et al. ; TOPCAT Investigators. Cardiac structure and function in heart failure with preserved ejection fraction: baseline findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist trial. Circ Heart Fail. 2014;7:104–115. doi: 10.1161/CIRCHEARTFAILURE.113.000887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 18.Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Liu L, Pitt B, Pfeffer MA, Solomon SD. Prognostic importance of impaired systolic function in heart failure with preserved ejection fraction and the impact of spironolactone. Circulation. 2015;132:402–414. doi: 10.1161/CIRCULATIONAHA.115.015884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solomon SD, Claggett B, Lewis EF, Desai A, Anand I, Sweitzer NK, O’Meara E, Shah SJ, McKinlay S, Fleg JL, et al. ; TOPCAT Investigators. Influence of ejection fraction on outcomes and efficacy of spironolactone in patients with heart failure with preserved ejection fraction. Eur Heart J. 2016;37:455–462. doi: 10.1093/eurheartj/ehv464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weaver B, Wuensch KL. SPSS and SAS programs for comparing Pearson correlations and OLS regression coefficients. Behav Res Methods. 2013;45:880–895. doi: 10.3758/s13428-012-0289-7 [DOI] [PubMed] [Google Scholar]
- 21.Palmieri V, Devereux RB, Hollywood J, Bella JN, Liu JE, Lee ET, Best LG, Howard BV, Roman MJ. Association of pulse pressure with cardiovascular outcome is independent of left ventricular hypertrophy and systolic dysfunction: the Strong Heart Study. Am J Hypertens. 2006;19:601–607. doi: 10.1016/j.amjhyper.2005.12.009 [DOI] [PubMed] [Google Scholar]
- 22.Celentano A, Palmieri V, Di Palma Esposito N, Pietropaolo I, Arezzi E, Mureddu GF, de Simone G. Relations of pulse pressure and other components of blood pressure to preclinical echocardiographic abnormalities. J Hypertens. 2002;20:531–537. doi: 10.1097/00004872-200203000-00030 [DOI] [PubMed] [Google Scholar]
- 23.Gu YM, Thijs L, Li Y, Asayama K, Boggia J, Hansen TW, Liu YP, Ohkubo T, Björklund-Bodegård K, Jeppesen J, et al. ; International Database on Ambulatory blood pressure in relation to Cardiovascular Outcomes (IDACO) Investigators. Outcome-driven thresholds for ambulatory pulse pressure in 9938 participants recruited from 11 populations. Hypertension. 2014;63:229–237. doi: 10.1161/HYPERTENSIONAHA.113.02179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amar J, Vernier I, Rossignol E, Bongard V, Arnaud C, Conte JJ, Salvador M, Chamontin B. Nocturnal blood pressure and 24-hour pulse pressure are potent indicators of mortality in hemodialysis patients. Kidney Int. 2000;57:2485–2491. doi: 10.1046/j.1523-1755.2000.00107.x [DOI] [PubMed] [Google Scholar]
- 25.Jackson CE, Castagno D, Maggioni AP, Køber L, Squire IB, Swedberg K, Andersson B, Richards AM, Bayes-Genis A, Tribouilloy C, et al. ; Meta-Analysis Global Group in Chronic Heart Failure MAGGIC. Differing prognostic value of pulse pressure in patients with heart failure with reduced or preserved ejection fraction: results from the MAGGIC individual patient meta-analysis. Eur Heart J. 2015;36:1106–1114. doi: 10.1093/eurheartj/ehu490 [DOI] [PubMed] [Google Scholar]
- 26.Laskey WK, Wu J, Schulte PJ, Hernandez AF, Yancy CW, Heidenreich PA, Bhatt DL, Fonarow GC. Association of arterial pulse pressure with long-term clinical outcomes in patients with heart failure. JACC Heart Fail. 2016;4:42–49. doi: 10.1016/j.jchf.2015.09.012 [DOI] [PubMed] [Google Scholar]
- 27.McEvoy JW, Chen Y, Rawlings A, Hoogeveen RC, Ballantyne CM, Blumenthal RS, Coresh J, Selvin E. Diastolic blood pressure, subclinical myocardial damage, and cardiac events: implications for blood pressure control. J Am Coll Cardiol. 2016;68:1713–1722. doi: 10.1016/j.jacc.2016.07.754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahman F, Al Rifai M, Blaha MJ, Nasir K, Budoff MJ, Psaty BM, Post WS, Blumenthal RS, McEvoy JW. Relation of diastolic blood pressure and coronary artery calcium to coronary events and outcomes (from the Multi-Ethnic Study of Atherosclerosis). Am J Cardiol. 2017;120:1797–1803. doi: 10.1016/j.amjcard.2017.07.094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kleinbaum DG, Kupper LL, Muller KE. Regression diagnostics. In: Applied Regression Analysis and Other Multivariate Methods. 1988. PWS-Kent Publishing Company; 181–227. [Google Scholar]
- 30.Wei FF, Xue R, Thijs L, Liang W, Owusu-Agyeman M, He X, Staessen JA, Dong Y, Liu C. Associations of left ventricular structure and function with blood pressure in heart failure with preserved ejection fraction: analysis of the TOPCAT trial. J Am Heart Assoc. 2020;9:e016009. doi: 10.1161/JAHA.119.016009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957–967. doi: 10.1016/S0140-6736(15)01225-8 [DOI] [PubMed] [Google Scholar]
- 32.Georgakis MK, Gill D, Webb AJS, Evangelou E, Elliott P, Sudlow CLM, Dehghan A, Malik R, Tzoulaki I, Dichgans M. Genetically determined blood pressure, antihypertensive drug classes, and risk of stroke subtypes. Neurology. 2020;95:e353–e361. doi: 10.1212/WNL.0000000000009814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Staessen JA, Janssens S, Van de Werf F. Critical questions in cardiovascular risk: do clinician trial data suggest a role for SGLT2-inhibitors in primary prevention of heart failure and chronic kidney disease? Int J Cardiol. 2021;10:200100. doi: 10.1016/j.ijcrp.2021.200100 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.