Abstract
Background
After recovery from acute infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), many patients experience long-term symptoms in different body systems. The aim of the present study was to identify these symptoms, their severity, and their duration as a first step in building a system to classify post-recovery long-term symptoms of coronavirus disease 2019 (COVID-19).
Methods
An online-based cross-sectional survey was administered between September and October 2020. Data regarding the severity of post-recovery symptoms and their duration were collected using an Arabic questionnaire divided into six categories encompassing the 20 most prevalent symptoms.
Results
A total of 979 patients recovered from COVID-19 in Saudi Arabia in the study period, of whom 53% were male and 47% were female. The most common symptoms included general fatigue and weakness (73% each), with moderate severity of neurological symptoms including mood changes (41%) and insomnia (39%). Among the special senses, loss of smell and taste of marked severity were reported by 64% and 55% among respiratory symptoms, cough of mild severity (47%), and dyspnea of moderate severity (43%). Loss of appetite of moderate severity was reported in 42%, and diarrhea, abdominal pain, and nausea of mild severity were reported by 53%, 50%, and 44% of respondents, respectively.
Conclusions
Long-term symptoms after recovery from COVID-19 warrant patient follow-up. The authors propose a classification system as a starting point to guide the identification and follow-up of long-term symptoms post-recovery, and recommend larger-scale studies to broaden the definition of recovery from COVID-19, which appears to have two phases, acute and chronic.
Introduction
An outbreak of a novel coronavirus, putatively termed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19), occurred in the city of Wuhan, China in December 2019 [1]. In March 2020, the World Health Organization declared the outbreak a pandemic. The identified virus, SARS-CoV-2, is similar to other fatal coronaviruses, namely SARS-CoV and Middle East Respiratory Syndrome coronavirus [2,3].
Coronavirus infection commonly causes respiratory and gastrointestinal symptoms; similarly, SARS-CoV-2 infection also leads to such symptoms, mainly fever, generalized weakness, lower respiratory tract symptoms, and anosmia caused by severe lung injury and acute respiratory distress syndrome, and affects multiple organs possibly causing organ failure, especially in old patients (>60 years) patients and/or those with specific comorbidities, which suggests that COVID-19 should be considered a systemic disease [4–6].
Many studies and reviews have focused on systems affected other than the respiratory system, including neurological, cardiac, vascular, gastrointestinal, kidney, and cutaneous manifestations [7–17]. Aside from the acute clinical challenges in those with severe illness, the long-term sequelae of pneumonia have been reported in many studies focusing on the decline in quality of life after pneumonia diagnosis that did not return to baseline—even after 1 year—and persistent late psychological effects, alongside the implications for rehabilitation and health care utilization [18–21]. The long-term effects of viral pneumonia among survivors, including those infected with SARS and MERS, have been documented. The same has been observed in those who recovered from COVID-19, in which survivors of acute illness experience long-term symptoms that persist for varying periods and varying degrees of severity. However, there are only a limited number of studies that followed patient symptoms after recovery from acute illness with COVID-19, and most focused on only one or a few symptoms, such as anosmia and cardiac conditions [22–27]. These symptoms impose a burden on patient health [28], and also affect physical and social status [29,30], which in turn have adverse effects on virtually all aspects of human activity and contribute to high burden on the economy and society. The pathogenesis of these post-recovery, long-term symptoms of COVID-19 remains unclear. As such, there is an urgent need to categorize these long-term symptoms according to the severity of their impact. This will raise awareness of the urgent need for studies to determine the underlying pathogenesis of the disease to better understand the process and, secondarily, for proper planning for patient management.
To date, published studies have focused on only one or a few symptoms, and have been smaller-scale investigations, which may increase the risk for missing other symptoms that lead to more serious longer-term effects on patients. Accordingly, the aim of the present study was to survey a larger number of patients who recovered from acute infection and illness with SARS-CoV-2/COVID-19 using different variables and an adequate number of post-recovery symptoms as a first step in building a classification system for COVID-19 post-recovery symptoms according to significance of importance, which should provide an opportunity for deeper understanding and future research to fill important knowledge gaps.
Materials and methods
Study design
Ethical approval for this study was obtained from the Subcommittee of Health Research Ethics, Deanship of Scientific Research, Qassim University (ref. # 20-03-04) and informed consent was obtained from all participants during the online-based cross-sectional survey. A survey was administered between September and October 2020 using Google Forms and Twitter as a forum, an anonymous Arabic survey was distributed. Saudi nationals and residents who had been recorded to be recovered from acute COVID-19 symptoms have been included in the study. There were no monetary benefits and participation was purely voluntary. Participants were told about the purpose of the questionnaire, the sample, and the significance of the score for each answer on the survey’s landing page, and were required to provide informed consent before proceeding to respond to the items. Participants were thanked for their participation on the final page. A new Twitter account was used to recruit the participants. Individuals and organizations received “tweets” requesting that they “retweet” the survey connection. According to Internet protocol restrictions, multiple enrolments by the same individual were prohibited.
Instrument development
The questionnaire used in this study was created using a combination of published literatures [31–35] and internal discussion among the research team to assess question format, comprehensiveness, clarity, and flow. Participants were assured that their responses would be collected anonymously, reducing the potential for bias introduced by self-reported results. The questionnaire was created and required approximately 3–5 minutes to complete with the aim of reducing survey fatigue. It was tested for validity using face validation by the survey research experts. The questionnaire was written in English by a native English speaker and translated into Arabic by two native Arabic speakers. The Arabic version of the questionnaire was piloted and distributed.
Instrument measures
The model was conducted on a group of 40 recovered COVID-19 cases in order to optimize the wording and clarification of the survey questions. The survey was widely distributed after slight changes to the format and vocabulary. The results of the pilot study were not included in any subsequent research but used for construct validity by employing the Pearson’s correlation coefficient r of the scores of respondents’ responses to an item with their total scores. According to the classification of r values as previously reported [36], the results showed that none of the questions presented poor correlations. All questions showed positive correlation that ranged from fair to very strong with significant relationships (Pearson’s r = 0.34–1.00; p<0.05). The instrument’s reliability was verified through Cronbach’s alpha as described in previously [37] by calculating the existing correlations. The result of this test was 0.870, which reflected a strong internal consistency. Final questionnaire was structured into 6 sections addressing specific symptoms potentially correlated with SARS-CoV-2 infection/COVID-19 as follows: (I) General symptoms (2 items: fatigue and weakness); (II) Skin and musculoskeletal symptoms (3 items: muscle ache[s], joint pain, and skin rash); (III) Psychological and neurological symptoms (4 items: headache, mood changes, insomnia, esthesia, and anesthesia); (IV) Special sense symptoms (5 items: hearing problems, visual disturbances, dry eyes, loss of smell, and loss of taste); (V) Respiratory system symptoms (3 items: cough, shortness of breath, and chest tightness); and (VI) Gastrointestinal symptoms (4 items: lack of appetite, nausea, diarrhea, and abdominal pain). Each section consisted of a group of related symptoms. For each symptom, participants were asked to score the severity of each on a three-point scale (mild, moderate, and severe, scored as 1, 2, and 3, respectively), and to report the duration of persistence of this symptom(s) after recovery in days. More than one symptom could be reported. Information regarding participant demographics and the method of diagnosis of SARS-CoV-2 infection was collected.
Sample size calculations
The estimated sample size was 384, which was obtained by a statistical calculation from the official Saudi Ministry of Health records, announced the number of recovered cases with a 95% confidence level and a 5% margin of error. We applied the online survey from September to October 2020 and a total of 992 Saudi subjects were approached. Out of them, 979 were included and the rest 13 subjects were omitted as they were made invalid selection. As reported previously [38,39], that sample size of 500 is very good, and 1000 or more is excellent, larger samples are always better than smaller, therefore it is recommended to utilize as large a sample size as is possible. Applying the same principle, 979 Saudi nationals were recruited in the study.
Data management and statistical analysis
The datasets were processed and analyzed using the PivotTable, data analysis within Microsoft excel version 2019. Descriptive statistics such as frequencies, percentage, mean and standard deviation (SD) were employed for the presentation of categorical and continuous variables to summarize respondent characteristics. Moreover, the data were further verified by SPSS version 25.0 (IBM Corporation, Armonk, NY, USA) using ANOVA and LSD tests. Differences with p ≤ 0.05 were considered to be statistically significant. Bar graphs were plotted for different variable including the age groups, methods of diagnosis, gender proportion and average days for each of the COVID-19 post recovery long-term symptoms.
Results
Of the 979 patients recovered from COVID-19, as they were post recovery group so none were experiencing acute symptoms or fever at the time the survey was administered. The males and females respondents in the study were 53% and 47%, respectively. Characteristics of the study population are summarized in Table 1. The frequency, degree of severity, and persistence of symptoms (in days) after recovery are summarized in Tables 2 and 3, and Figs 1–3. The largest age group comprised individuals 20 to 39 years of age (57.1%), with a mean age of 37.69. The most common long-term (i.e., persistent) symptoms were of general symptoms group, fatigue and weakness (73% each), which persisted for a mean of 7 and 8.11 days respectively, while muscle aches were the dominant symptom (66%) among the skin and musculoskeletal symptom groups, with a mean persistence of 7.8 days. Among psychological and neurological symptoms, headache was the most common (64%), with a mean persistence of 6.5 days. The presenting symptom among the special sense group was loss of smell (62%), with a mean persistence of 15.9 days (Table 2). Cough was the dominant symptom (47%) in the respiratory system group, and lack of appetite (46%) was the dominant symptom in the gastrointestinal group, with a mean persistence of 11 days and 9.4 days, respectively (Table 2). The most severe degree of symptom was scored “3”, which was recorded for both loss of smell and loss of taste, which also demonstrated the highest mean duration of symptom persistence (15.9 and 13.9 days, respectively) (Table 3). To study these findings in depth, we further categorized the studied cases in four age groups (Table 1). We observed a significant correlation of post-recovery COVID-19 symptoms with age, persistence of symptoms and degree of severity. Furthermore, we also found a statistically significant relation of age with the presence of post-recovery COVID-19 long term symptoms degree of severity and/or persistence, such as weakness degree (p = 0.003), persistence (p = 0.001), lack of appetite degree (p = 0.02), persistence (p = 0.003), Insomnia degree (p = 0.01), loss of smell degree (p = 0.002), loss of taste degree and Headache degree (p = 0.04 each), cough degree (p = 0.01) significant correlation was found between age and persistence of symptoms as fatigue (p = 0.004), joint pains (p = 0.01), mood changes (p = 0.03), nausea (p = 0.002) and abdominal pain (p = 0.02; Tables 2 & 3). According to the findings we proposed a scoring system that can be can be delivered online for post discharge follow-up, and the score of each case can be automatically calculated. The case that will reach the proposed score should be invited for follow up in the clinic, keeping in mind high concerns groups (as in Table 4 level 1A&B) should be the first priority follow up group, while lower concerns groups (as in Table 5 level 2 A&B) should be the second priority follow up. The cases will not reach the proposed score, no need for clinic follow up and should be reassured as mostly their symptoms are self-limited. By this scoring system we can easily pick up patients of concern to be followed up and also decrease load on health services as much as possible.
Table 1. Demographic and clinical characteristics of the study individuals.
| Characteristics | Value |
|---|---|
| Age, years (y)—mean (±SD) | 37.69 (±10.77) |
| Minimum | 10 y |
| Maximum | 84 y |
| Gender | Number of subjects (N) (%) |
| Male | 519 (53%) |
| Female | 460 (47%) |
| Method of diagnosis | N (%) |
| Swab only | 117 (12%) |
| Symptoms only | 158 (16%) |
| Swab & Symptoms | 704 (72%) |
| Age groups by years | N (%) |
| 10–19 | 31 (3.2%) |
| 20–29 | 177(18.1) |
| 30–39 | 383(39.1%) |
| 40–49 | 241(24.6%) |
| 50–59 | 113(11.5%) |
| 60–69 | 30 (3.1%) |
| 70–79 | 3 (0.3%) |
| 80–89 | 1(0.1%) |
| Age group categories | N (%) |
| >20 y | 32 (3.3%) |
| 20–39 y | 559 (57.1%) |
| 40–59 y | 354 (36.2) |
| 60 and above | 34 (3.5%) |
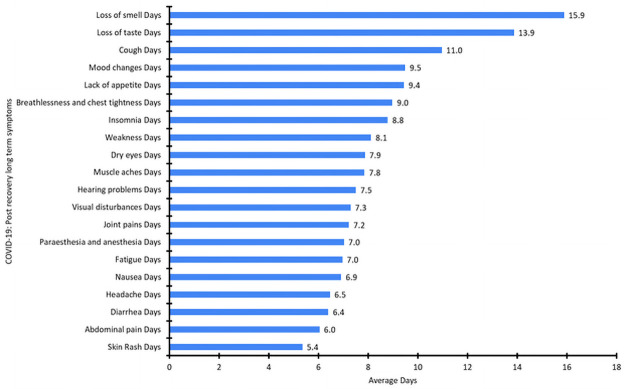
Table 2. Persistence days of post-recovery of COVD-19 recorded symptoms in 979 studied subjects.
| Descriptive Statistics | Mean (days) | Median (days) | Standard Deviation | Range (days) | No. | % | p-value | |
|---|---|---|---|---|---|---|---|---|
| I-General Symptoms | Fatigue Days | 7 | 5 | 8.6 | 120 | 719 | 73% | 0.004* |
| Weakness Days | 8.1 | 5 | 9.8 | 119 | 715 | 73% | 0.001* | |
| II- Skin and Musculoskeletal symptoms | Muscle aches Days | 7.8 | 5 | 12 | 119 | 646 | 66% | 0.14 |
| Joint pains Days | 7.2 | 5 | 9.4 | 119 | 595 | 61% | 0.01* | |
| Skin Rash Days | 5.4 | 2 | 11.7 | 119 | 164 | 17% | 0.62 | |
| III- Psychological and Neurological symptoms | Headache Days | 6.5 | 4 | 8.2 | 119 | 627 | 64% | 0.73 |
| Mood changes Days | 9.5 | 7 | 11.9 | 154 | 501 | 51% | 0.03* | |
| Insomnia Days | 8.8 | 7 | 10.6 | 89 | 432 | 44% | 0.21 | |
| Paraesthesia and anesthesia Days | 7 | 4 | 11.1 | 119 | 296 | 30% | 0.85 | |
| IV- Special sense symptoms | Hearing problems Days | 7.5 | 5 | 11.9 | 119 | 129 | 13% | 0.94 |
| Visual disturbances Days | 7.3 | 4 | 9.9 | 59 | 145 | 15% | 0.40 | |
| Dry eyes Days | 7.9 | 5 | 11 | 63 | 185 | 19% | 0.88 | |
| Loss of smell Days | 15.9 | 10 | 19.5 | 149 | 604 | 62% | 0.63 | |
| Loss of taste Days | 13.9 | 9 | 16.6 | 119 | 555 | 57% | 0.60 | |
| V- Respiratory system symptoms | Cough Days | 11 | 7 | 12.8 | 120 | 457 | 47% | 0.09 |
| Breathlessness and chest tightness Days | 9 | 6 | 10.7 | 119 | 388 | 40% | 0.45 | |
| VI- Gastrointestinal symptoms | Lack of appetite Days | 9.4 | 7 | 8 | 59 | 455 | 46% | 0.003* |
| Nausea Days | 6.9 | 5 | 7 | 59 | 313 | 32% | 0.002* | |
| Diarrhea Days | 6.4 | 4 | 8.8 | 119 | 402 | 41% | 0.42 | |
| Abdominal pain Days | 6 | 4 | 6.6 | 44 | 253 | 26% | 0.02* |
*p<0.05 considered statistically significant. P values were calculated by ANOVA and LSD tests using SPSS software.
The mean (±SD) age of the subjects was 37.69 (±10.767) years.
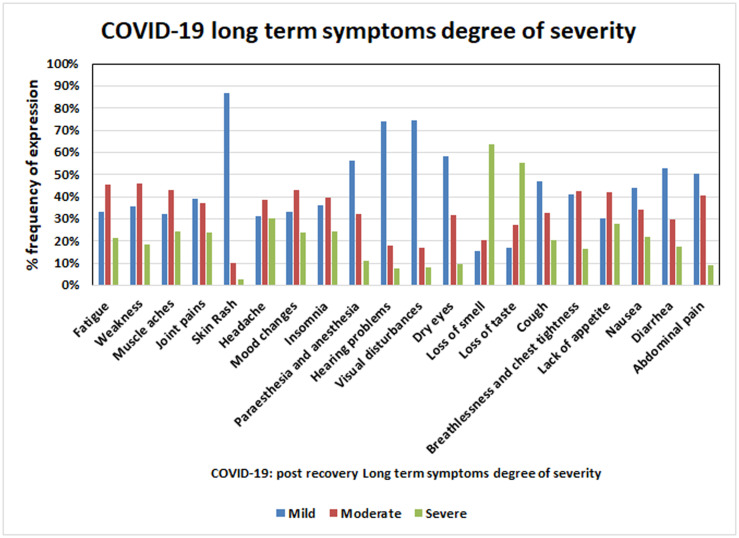
Table 3. Degree of severity of the recorded post recovery COVD-19 long-term symptoms with their frequency of expression.
| Degree of severity and its frequency of expression | p-value* | |||||
|---|---|---|---|---|---|---|
| Symptoms groups | Long term recorded symptom | Mild N (%) | Moderate N (%) | Severe N (%) | No. | |
| I-General Symptoms | Fatigue | 239 (33%) | 326(45%) | 154(21%) | 719 | 0.05 |
| Weakness | 254(36%) | 328(46%) | 133(19%) | 715 | 0.003* | |
| II- Skin and Musculoskeletal symptoms | Muscle aches | 208 (32%) | 279(43%) | 159(25%) | 646 | 0.36 |
| Joint pains | 232(39%) | 220(37%) | 143(24%) | 595 | 0.44 | |
| Skin Rash | 142(87%) | 17(10%) | 5(3%) | 164 | 0.86 | |
| III- Psychological & Neurological symptoms | Headache | 195(31%) | 242(39%) | 190(30%) | 627 | 0.04* |
| Mood changes | 166(33%) | 216(43%) | 119(24%) | 501 | 0.20 | |
| Insomnia | 157(36%) | 171(40%) | 105(24%) | 432 | 0.01* | |
| Paraesthesia and anesthesia | 167(56%) | 96(32%) | 33(11%) | 296 | 0.25 | |
| IV- Special sense symptoms | Hearing problems | 95 (74%) | 23(18%) | 10(8%) | 129 | 0.41 |
| Visual disturbances | 108(75%) | 25(17%) | 12(8%) | 145 | 0.34 | |
| Dry eyes | 108(58%) | 59(32%) | 18(10%) | 185 | 0.01* | |
| Loss of smell | 95(16%) | 124(21%) | 385(64%) | 604 | 0.002* | |
| Loss of taste | 95(17%) | 152(27%) | 308(55%) | 555 | 0.04* | |
| V- Respiratory system symptoms | Cough | 215(47%) | 149(33%) | 93(20%) | 457 | 0.01* |
| Breathlessness and chest tightness | 159(41%) | 166(43%) | 63(16%) | 388 | 0.42 | |
| VI- Gastrointestinal symptoms | Lack of appetite | 138(30%) | 191(42%) | 126(28%) | 455 | 0.02* |
| Nausea | 137(44%) | 108(34%) | 68(22%) | 313 | 0.22 | |
| Diarrhea | 212(53%) | 120(30%) | 70(17%) | 402 | 0.69 | |
| Abdominal pain | 127(50%) | 103(41%) | 23(9%) | 253 | 0.83 | |
*p<0.05 considered statistically significant. P values were calculated by ANOVA and LSD tests using SPSS software.
The mean (±SD) age of the subjects was 37.69 (±10.767) years.
Fig 1. Age groups and gender proportion for recovered COVID-19 patients.
Fig 3. Degree of severity and frequency expression for different COVID-19 post recovery long term symptoms.
Table 4.
(Level-1 A): Scoring Post-recovery COVID-19 long term symptoms persistence (High concerns groups). Scoring method: One point given for each item per symptom (one point for days and one point for age group). Interpretation: Score two for any symptom: follow up is recommended for the patient for each symptom got score 2. (Level 1 B): Scoring Post-recovery COVID-19 long term symptoms degree of severity (High concerns groups). Scoring method: One point given for each item per symptom (one point for severity and one point for age group). Interpretation: Score two for any symptom: follow up is recommended for the patient for each symptom got score 2.
| Long term symptom | Days (≥) * | Age groups (years, y) |
| Fatigue | 5 | ≥41 |
| Weakness | 5 | ≥40 |
| Joint pains | 5 | ≥20 |
| Mood changes | 7 | 20-59y |
| Lack of appetite | 7 | 20-59y |
| Nausea | 5 | 20-59y |
| Abdominal pain | 4 | 20-59y |
| Long term symptom | Degree of severity (≥) ** | Age group |
| Fatigue | 2 | Any age group* |
| Weakness | 2 | Any age group* |
| Headache | 2 | 20-59y |
| Insomnia | 2 | <20y or ≥ 40 y |
| Dry eyes | 1 | 20–39 or ≥60y |
| Loss of smell | 3 | 20–39 or ≥40y |
| Loss of taste | 3 | 10-39y |
| Cough | 1 | <20y or 40–59 y |
| Lack of appetite | 2 | <20y or ≥40y |
*Days here are the recorded median number for each symptom.
*Cases age between 10–84 years.
**Degree of severity Mild = 1, Moderate = 2, Severe = 3.
Table 5.
(Level 2 A): Scoring Post-recovery COVID-19 long term symptoms persistence (Lower concerns groups). Scoring method: One point given for each item per symptom (one point for days and one point for age group). Interpretation: Score two for any symptom: follow up is recommended for the patient for each symptom got score 2. (Level 2 B): Scoring Post-recovery COVID-19 long term symptoms degree of severity (Lower concerns groups). Scoring method: One point given for each item per symptom (one point for degree of severity and one point for age group) with exception for loss of smell and loss of taste. Interpretation: Score two for any symptom: follow up is recommended for the patient for each symptom got score 2.
| Long term symptom | No. of days (≥) ** | Age group (years) |
| Fatigue | 7 | 10-39y |
| Weakness | 8 | 10-39y |
| Muscle aches | 8 | Any age group* |
| Joint pains | 7 | <20y |
| Skin Rash | 5 | Any age group* |
| Headache | 7 | Any age group* |
| Mood changes | 10 | <20y or ≥60y |
| Insomnia | 9 | Any age group* |
| Paraesthesia and anesthesia | 7 | Any age group* |
| Hearing problems | 8 | Any age group* |
| Visual disturbances | 7 | Any age group* |
| Dry eyes | 8 | Any age group* |
| Loss of smell | 16 | Any age group* |
| Loss of taste | 14 | Any age group* |
| Cough | 11 | Any age group* |
| Breathlessness and chest tightness | 9 | Any age group* |
| Lack of appetite | 10 | <20y or ≥60y |
| Nausea | 7 | <20y or ≥60y |
| Diarrhea | 6 | Any age group* |
| Abdominal pain | 6 | <20y or ≥60y |
| Long term symptom | Degree of severity ** | Age group (years) |
| Muscle aches | >2 | Any age group* |
| Joint pains | >1 | Any age group* |
| Skin Rash | >1 | Any age group* |
| Headache | >2 | <20y or≥60y |
| Mood changes | >2 | Any age group* |
| Insomnia | >2 | 20-39y |
| Paraesthesia and anesthesia | >1 | Any age group* |
| Hearing problems | >1 | Any age group* |
| Visual disturbances | >1 | Any age group* |
| Dry eyes | >1 | <20y or 40-59y |
| ***Loss of smell | 3 in severity plus 9.7 days**** | <20y |
| ***Loss of taste | 3 in severity plus 12 days**** | ≥40y |
| Cough | >1 | 20-39y or ≥60y |
| Breathlessness and chest tightness | >2 | Any age group* |
| Lack of appetite | >2 | 20-39y |
| Nausea | >1 | Any age group* |
| Diarrhea | >1 | Any age group* |
| Abdominal pain | >1 | Any age group* |
*Cases age between 10–84 years.
**Days here are the average number of days recorded for each symptom.
*Cases age between 10–84 years.
**Degree of severity Mild = 1 Moderate = 2 Severe = 3.
****Degree plus persistence duration got one point.
****Days here are the average number of days for the mentioned age group.
Fig 2. Average days for different COVID-19 post recovery long term symptoms.
Discussion
To our knowledge, the present study is the first to describe the duration and severity of symptoms among individuals in Saudi Arabia following infection with SARS-CoV-2 and subsequent illness with COVID-19. This study was designed to address post-recovery symptoms in patients who experienced acute symptoms of COVID-19.
Twenty symptoms were included in the questionnaire and covered general symptoms, and skin, musculoskeletal, neurological and psychological symptoms. Fatigue, and weakness of moderate severity were the most commonly reported general symptoms (73% each), with a mean duration of 7 days for fatigue; however, symptoms of weakness persisted longer, with a mean duration of 8.1 days. These symptoms are usually observed in recovering patients with respiratory tract infections among old age (>60 years) patients, or after lengthy hospital stay or critical illness [40–45].
More than 80% of our participants comprised a relatively young age group (20 to 50 years) with good health status before contracting COVID-19, which indicates that weakness and fatigue are significant post-recovery symptoms of COVID-19, and is consistent with other studies that described fatigue as a long-term symptom and one of several common sequelae of COVID-19. Moreover, it is independent of the severity of the previous acute illness or levels of pro-inflammatory markers [46]. Muscle ache(s) of moderate severity and joint pain of mild severity were reported by 43% and 39% of respondents, respectively. Myalgia during the acute stages of COVID-19 has been well documented [47], ranging from 11% to 44% in other studies [48,49], and myalgia and arthralgia were also evident in various other coronavirus infections during acute illness [50].
The persistence of myalgia and arthralgia after acute illness may reflect the activation and triggering of excessive or uncontrolled cytokine responses, and a local inflammatory reaction in the respiratory tract, especially the alveoli, which progresses to involve other organs, especially the joints and muscles [51].
Neurological complications have also been studied in individuals infected with other human coronaviruses. Neuro-invasion causing neurological pathologies ranging from headache and anosmia to severe and fatal encephalopathy, encephalitis acute myelitis, Guillain-Barré syndrome, and other cerebrovascular pathologies have been documented [52].
In the present study the post-COVID-19 headache was reported by 64% of our participants, it was of moderate severity among 39% and persisted for a mean of 6.5 days, whereas anosmia was reported in severe degree by 64% and persisted for a mean of 15.9 days. Headache is considered one of the characteristic and cardinal symptoms of COVID-19, and is observed in 68.3% of patients in the emergency department [53]. Other symptoms addressed in our survey included mood changes of moderate degree in 43% and paresthesia in 56% of mild cases.
Anosmia is currently considered a cardinal and prominent symptom of COVID-19 [54,55], and may be the only presenting symptom [56] and is commonly associated with loss of taste (dysgeusia) [57]. Anosmia results from damage to the olfactory epithelium or, more commonly, the central olfactory pathway [58]. It has been observed in previous human coronavirus infections; however, the incidence is higher with SARS-CoV-2 infection [59]. Some cross-sectional studies have reported anosmia incidences ranging from 33.9% to 68% [60–63]. Among 114 swab-positive patients, Klopfenstein et al. [60] reported an anosmia incidence rate of 47%, with a mean duration of 8.9 days.
In this study, the incidence of anosmia was 62%, with a mean duration of 15.9 days and 64% of cases are of severe degree while the incidence of loss of taste was 57%, with a mean duration of 13.9 days and 55% of cases are of severe degree.
The higher percentages and longer durations found in our study may be attributed to mutations of SARS-CoV-2, which result in different genotypes and pathogenicity [61]. Other factors may be related to the currently increased awareness of anosmia in patients with COVID-19 compared to early in the outbreak. Another reason may be related to the varying pathogenicity of SARS-CoV-2 among humans; however, this remains speculative and needs more supportive evidence. In fact, the incidence of anosmia was as high as 98% in a study by Moein et al., who performed specific olfactory testing in patients positive for COVID-19, and identified a high percentage of patients (63%) who were unaware of their anosmia [62]. Other sensory effects include hearing and visual disturbances (17% and 19%, respectively), which are rarely investigated in those with acute COVID-19 illness. To the best of our knowledge, this is the first investigation to include patients who recovered from COVID-19.
Respiratory symptoms that persisted after acute illness included cough of mild severity (47%, with a mean duration of 11 days) and dyspnea of moderate severity (43%, duration 9 days). In 2020, a study by Garrigues et al. investigating discharged patients with COVID-19 found that the incidence of dyspnea was 42% and there was no difference between those admitted to the intensive care unit and general ward [63]. Gastrointestinal symptoms were significant in our study for lack of appetite (42%), with a mean duration of 9.4 days (moderate severity), while other symptoms, such as nausea (44% [6.9 days]), diarrhea (53%, [6.4 days]), and abdominal pain (50% [6 days]), were all mild.
To our knowledge, this is the first study to investigate post-recovery gastrointestinal symptoms in those with COVID-19. Most centers and hospitals considered COVID-19 case recovered by subsidence of acute symptoms. Follow up of hundreds of thousands of recovered cases is not practical and will add much extra load on health services which are barely coping with acute cases. Furthermore, we aren’t able to anticipate which patients will suffer long-term symptoms following their recovery, and if so, does all long-term symptoms cases need follow up. As our study highlights the importance of follow-up of patients who recover from acute illness to identify those who may be more likely to experience long-term symptoms that may require further care and investigation. To solve this issue, we proposed a scoring system for COVID-19 post recovery long-term persistent symptoms according to the degree of severity and the duration of persistence, which is best known to our knowledge, is the first time all over the world to classify and score COVID-19 post recovery long term symptoms (Tables 4 and 5).
Limitations
This study has few limitations including lack of knowledge of the severity of initial illness, details regarding hospitalization, obstacles to meet all survey participants, and the lack of a control group. In addition, the questionnaire used in this study has four limitations: (1) The first obvious one was the respondent’s previous experience filling out questionnaires may have an impact on the overall outcome, (2) who completed the questionnaires? The respondent or the surveyor, (3) to receive truthful responses from respondents who fill out the questionnaires, (4) the ways of online administration of the questionnaire such as Google and Twitter forums. In comparison to an interview room, an on-site survey may generate distraction owing to noise and task.
Conclusions
This study confirmed that the long-term persistent symptoms are evident among individuals who recover from COVID-19. This should raise awareness of the importance of post-recovery follow-up of cases to manage persistent symptoms and reduce the burden on patients and the community. Further studies, however, are needed to investigate the pathogenesis of these persistent symptoms. As we recorded long term persistent symptoms with different degrees of severity and variable persistence, we propose to classify COVID-19 illness into two phases: acute and chronic so we can consider long term persistent symptoms after recovery from acute illness and not to miss any case. Our proposed scoring system will encourage wider scale studies to confirm and refine the findings by considering geographical distribution and a larger number of COVID-19 cases. This will help to identify priorities in follow-up among patients according to our proposed scoring system and to avoid prolonged suffering in those who considered recovered from COVID-19 “acute illness”.
Supporting information
(XLSX)
(DOCX)
Acknowledgments
The authors thank all health services personnel and volunteers who are on the front lines of the pandemic and have expended great effort to fight the disease all over the world. Special thanks to those who sacrificed their lives to save thousands of others. The authors also thank those who devoted their time and exerted extraordinary effort to create vaccines to ease—if not eliminate—suffering around the world.
Data Availability
All relevant data are within the manuscript.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Na Zhu, Dingyu Zhang, Wenling Wang, Xingwang Li, Bo Yang, Jingdong Song, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8): 727–733. doi: 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3): 105924. doi: 10.1016/j.ijantimicag.2020.105924 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrosillo N, Viceconte G, Ergonul O, Ippolito G, Petersen E. COVID-19, SARS and MERS: are they closely related? Clin Microbiol Infect. 2020. Mar [Cited 2020 May 16]. Available: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7176926/;Volume 26: 729–734. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu D, Wu T, Liu Q, Yang Z. The SARS-CoV-2 outbreak: what we know. Int J Infect Dis. 2020;94: 44–48). doi: 10.1016/j.ijid.2020.03.004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lake MA. What we know so far: COVID-19 current clinical knowledge and research. Clin Med (Lond). 2020;20: 124–127. doi: 10.7861/clinmed.2019-coron . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020. Mar 28;395(10229): 1033–1034. doi: 10.1016/S0140-6736(20)30628-0 Epub 2020 Mar 16. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed MU, Hanif M, Ali MJ, Haider MA, Kherani D, Memon GM, et al. Neurological Manifestations of COVID-19 (SARS-CoV-2): A Review. Front Neurol. 2020. May 22;11: 518. doi: 10.3389/fneur.2020.00518 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A, et al. Neurological associations of COVID-19. Lancet Neurol. 2020. Sep;19(9): 767–783. doi: 10.1016/S1474-4422(20)30221-0 Epub 2020 Jul 2. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kochi AN, Tagliari AP, Forleo GB, Fassini GM, Tondo C. Cardiac and arrhythmic complications in patients with COVID-19. J Cardiovasc Electrophysiol. 2020. May;31(5): 1003–1008. doi: 10.1111/jce.14479 Epub 2020 Apr 13. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, et al. Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020. Jul 1;5(7): 819–824. doi: 10.1001/jamacardio.2020.1096 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pons S, Fodil S, Azoulay E, Zafrani L. The vascular endothelium: the cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit Care. 2020. Jun 16;24(1): 353. doi: 10.1186/s13054-020-03062-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oudkerk M, Kuijpers D, Oudkerk SF, van Beek EJ. The vascular nature of COVID-19. Br J Radiol. 2020. Sep 1;93(1113): 20200718. doi: 10.1259/bjr.20200718 Epub 2020 Jul 31. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel KP, Patel PA, Vunnam RR, Hewlett AT, Jain R, Jing R, et al. Gastrointestinal, hepatobiliary, and pancreatic manifestations of COVID-19. J Clin Virol. 2020. Jul;128: 104386. doi: 10.1016/j.jcv.2020.104386 Epub 2020 Apr 29. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gabarre P, Dumas G, Dupont T, Darmon M, Azoulay E, Zafrani L. Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med. 2020. Jul;46(7): 1339–1348. doi: 10.1007/s00134-020-06153-9 Epub 2020 Jun 12. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ronco C, Reis T. Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nat Rev Nephrol. 2020. Jun;16(6): 308–310. doi: 10.1038/s41581-020-0284-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao Q, Fang X, Pang Z, Zhang B, Liu H, Zhang F. COVID-19 and cutaneous manifestations: a systematic review. J Eur Acad Dermatol Venereol. 2020. Jun 28;Volume 34: 2505–2510. doi: 10.1111/jdv.16778 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020. May;34(5): e212–e213. doi: 10.1111/jdv.16387 . [DOI] [PubMed] [Google Scholar]
- 18.Glick HA, Miyazaki T, Hirano K, Gonzalez E, Jodar L, Gessner BD, et al. One-Year Quality of Life Post-Pneumonia Diagnosis in Japanese Adults. Clin Infect Dis. 2020. May 24: ciaa595. doi: 10.1093/cid/ciaa595 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kapfhammer HP, Rothenhäusler HB, Krauseneck T, Stoll C, Schelling G. Posttraumatic stress disorder and health-related quality of life in long-term survivors of acute respiratory distress syndrome. Am J Psychiatry. 2004. Jan;161(1): 45–52. doi: 10.1176/appi.ajp.161.1.45 . [DOI] [PubMed] [Google Scholar]
- 20.Denke C, Balzer F, Menk M, Szur S, Brosinsky G, Tafelski S, et al. Long-term sequelae of acute respiratory distress syndrome caused by severe community-acquired pneumonia: Delirium-associated cognitive impairment and post-traumatic stress disorder. J Int Med Res. 2018. Jun;46(6): 2265–2283. doi: 10.1177/0300060518762040 Epub 2018 Apr 2. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmed H, Patel K, Greenwood DC, Halpin S, Lewthwaite P, Salawu A, et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: A systematic review and meta-analysis. J Rehabil Med. 2020. May 31;52(5): jrm00063. doi: 10.2340/16501977-2694 . [DOI] [PubMed] [Google Scholar]
- 22.Chan KS, Zheng JP, Mok YW, Li YM, Liu YN, Chu CM, et al. SARS: prognosis, outcome and sequelae. Respirology. 2003;8 Supplement: S36–S40. doi: 10.1046/j.1440-1843.2003.00522.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park WB, Jun KI, Kim G, Choi JP, Rhee JY, Cheon S, et al. Correlation between pneumonia severity and pulmonary complications in Middle East respiratory syndrome. J Korean Med Sci. 2018;33: e169. doi: 10.3346/jkms.2018.33.e169 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chary E, Carsuzaa F, Trijolet JP, Capitaine AL, Roncato-Saberan M, Fouet K, et al. Prevalence and Recovery From Olfactory and Gustatory Dysfunctions in Covid-19 Infection: A Prospective Multicenter Study. Am J Rhinol Allergy. 2020. Sep;34(5): 686–693. doi: 10.1177/1945892420930954 Epub 2020 Jun 12. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hopkins C, Surda P, Whitehead E, Kumar BN. Early recovery following new onset anosmia during the COVID-19 pandemic—an observational cohort study. J Otolaryngol Head Neck Surg. 2020. May 4;49(1): 26. doi: 10.1186/s40463-020-00423-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klok FA, Boon GJAM, Barco S, Endres M, Geelhoed JJM, Knauss S, et al. The Post-COVID-19 Functional Status scale: a tool to measure functional status over time after COVID-19. Eur Respir J. 2020. Jul 2;56(1):2001494, doi: 10.1183/13993003.01494-2020 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitrani RD, Dabas N, Goldberger JJ. COVID-19 cardiac injury: Implications for long-term surveillance and outcomes in survivors. Heart Rhythm. 2020. Jun 26:S1547;5271(20): 30625–30621. doi: 10.1016/j.hrthm.2020.06.026 Epub ahead of print. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ong KC, Ng AW, Lee LS, Kaw G, Kwek SK, Leow MK, et al. Pulmonary function and exercise capacity in survivors of severe acute respiratory syndrome. Eur Respir J. 2004. Sep;24(3): 436–442. doi: 10.1183/09031936.04.00007104 . [DOI] [PubMed] [Google Scholar]
- 29.Mak IWC, Chu CM, Pan PC, Yiu MGC, Chan VL. Long-term psychiatric morbidities among SARS survivors. Gen Hosp Psychiatry. 2009;31: 318–326. doi: 10.1016/j.genhosppsych.2009.03.001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan E, Dowdy DW, Colantuoni E, Mendez-Tellez PA, Sevransky JE, Shanholtz C, et al. Physical complications in acute lung injury survivors: a two-year longitudinal prospective study. Crit Care Med. 2014;42: 849–859. doi: 10.1097/CCM.0000000000000040 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garrigues E, Janvier P, Kherabi Y, Le Bot A, Hamon A, Gouze H, Doucet L, Berkani S, Oliosi E, Mallart E, Corre F, Zarrouk V, Moyer JD, Galy A, Honsel V, Fantin B, Nguyen Y. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. 2020. Dec;81(6):e4–e6. doi: 10.1016/j.jinf.2020.08.029 Epub 2020 Aug 25. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halpin SJ, McIvor C, Whyatt G, Adams A, Harvey O, McLean L, Walshaw C, Kemp S, Corrado J, Singh R, Collins T, O’Connor RJ, Sivan M. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: A cross-sectional evaluation. J Med Virol. 2021. Feb;93(2):1013–1022. doi: 10.1002/jmv.26368 Epub 2020 Aug 17. . [DOI] [PubMed] [Google Scholar]
- 33.Turkmen D, Altunisik N, Sener S, Colak C. Evaluation of the effects of COVID-19 pandemic on hair diseases through a web-based questionnaire. Dermatol Ther. 2020. Nov;33(6):e13923. doi: 10.1111/dth.13923 Epub 2020 Jul 16. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horemans HL, Nollet F, Beelen A, Lankhorst GJ. A comparison of 4 questionnaires to measure fatigue in postpoliomyelitis syndrome. Arch Phys Med Rehabil. 2004. Mar;85(3):392–8. doi: 10.1016/j.apmr.2003.06.007 . [DOI] [PubMed] [Google Scholar]
- 35.Jesús G, Francisco S, Loreto C, Validation of questionnaires, Reumatología Clínica (English Edition). 2009. Dec; 5(4):171–177. 10.1016/S2173-5743(09)70115-7. [DOI] [PubMed] [Google Scholar]
- 36.Chan YH. Biostatistics 104: correlational analysis. Singapore Med J. 2003. Dec;44(12):614–9. . [PubMed] [Google Scholar]
- 37.Taber K.S. The Use of Cronbach’s Alpha When Developing and Reporting Research Instruments in Science Education. Res Sci Educ 48, 1273–1296 (2018). doi: 10.1007/s11165-016-9602-2 [DOI] [Google Scholar]
- 38.Comfrey A.L., & Lee H.B. (1992). A First Course in Factor Analysis (2nd ed.). Psychology Press. 10.4324/9781315827506 [DOI] [Google Scholar]
- 39.Tsang S, Royse CF, Terkawi AS. Guidelines for developing, translating, and validating a questionnaire in perioperative and pain medicine. Saudi J Anaesth. 2017;11(Suppl 1):S80–S89. doi: 10.4103/sja.SJA_203_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wyrwich KW, Yu H, Sato R, Powers JH. Observational longitudinal study of symptom burden and time for recovery from community-acquired pneumonia reported by older adults surveyed nationwide using the CAP Burden of Illness Questionnaire. Patient Relat Outcome Meas. 2015. Jul 30;6: 215–223. doi: 10.2147/PROM.S85779 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El Moussaoui R, Opmeer BC, de Borgie CA, Nieuwkerk P, Bossuyt PM, Speelman P, et al. Long-term symptom recovery and health-related quality of life in patients with mild-to-moderate-severe community-acquired pneumonia. Chest. 2006. Oct;130(4): 1165–1172. doi: 10.1378/chest.130.4.1165 . [DOI] [PubMed] [Google Scholar]
- 42.Townsend L, Dyer AH, Jones K, Dunne J, Mooney A, Gaffney F, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLOS ONE. 2020;15(11): e0240784. Published 2020 Nov 9. doi: 10.1371/journal.pone.0240784 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020. Feb 15;395(10223): 507–513. doi: 10.1016/S0140-6736(20)30211-7 Epub 2020 Jan 30. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020. Feb 15;395(10223): 497–506. doi: 10.1016/S0140-6736(20)30183-5 Epub 2020 Jan 24. . Erratum in: Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Jan 30;: 31986264; PMCID: PMC7159299;Volume 395: 497–506. 10.1016/S0140-6736(20)30183-5, 31986264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Memish ZA, Perlman S, Van Kerkhove MD, Zumla A. Middle East respiratory syndrome. Lancet. 2020;395: 1063–1077. doi: 10.1016/S0140-6736(19)33221-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li S, Wang R, Zhang Y, Zhang X, Layon AJ, Li Y, et al. Symptom combinations associated with outcome and therapeutic effects in a cohort of cases with SARS. Am J Chin Med. 2006;34(6): 937–947. doi: 10.1142/S0192415X06004417 . [DOI] [PubMed] [Google Scholar]
- 47.Schett G, Manger B, Simon D, Caporali R. COVID-19 revisiting inflammatory pathways of arthritis. Nat Rev Rheumatol. 2020;16(8): 465–470. doi: 10.1038/s41584-020-0451-z . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carod-Artal FJ. Neurological complications of coronavirus and COVID-19. Rev Neurol. 2020. May 1;70(9): 311–322. English. doi: 10.33588/rn.7009.2020179 , Carod-Artal FJ. Neurological complications of coronavirus and COVID-19. Rev Neurol. 2020;Volume 70: 311–322. 10.33588/rn.7009.2020179. 32329044. [DOI] [PubMed] [Google Scholar]
- 49.Membrilla JA, de Lorenzo Í, Sastre M, Díaz de Terán J. Headache as a Cardinal Symptom of Coronavirus Disease 2019: A Cross-Sectional Study. Headache. 2020. Nov;60(10): 2176–2191. doi: 10.1111/head.13967 Epub 2020 Sep 28. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heidari F, Karimi E, Firouzifar M, Khamushian P, Ansari R, Mohammadi Ardehali M, et al. Anosmia as a prominent symptom of COVID-19 infection. Rhinology. 2020;58: 302–303. doi: 10.4193/Rhin20.140 . [DOI] [PubMed] [Google Scholar]
- 51.Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020; Volume 277: 2251–2261. doi: 10.1007/s00405-020-05965-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gane SB, Kelly C, Hopkins C. Isolated sudden onset anosmia in COVID-19 infection. A novel syndrome? Rhinology. 2020;58: 299–301. doi: 10.4193/Rhin20.114 . [DOI] [PubMed] [Google Scholar]
- 53.Lee DJ, Lockwood J, Das P, Wang R, Grinspun E, Lee JM. Self-reported anosmia and dysgeusia as key symptoms of coronavirus disease 2019. CJEM. 2020;22(5): 595–602. doi: 10.1017/cem.2020.420 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hummel T, Whitcroft KL, Andrews P, Altundag A, Cinghi C, Costanzo RM. Position paper on olfactory dysfunction. Rhinol Suppl. 2017;54: 1–30. doi: 10.4193/Rhino16.248 [DOI] [PubMed] [Google Scholar]
- 55.Hwang CS. Olfactory neuropathy in severe acute respiratory syndrome: report of a case. Acta Neurol Taiwan. 2006;15: 26–28. . [PubMed] [Google Scholar]
- 56.Giacomelli A, Pezzati L, Conti F, Bernacchia D, Siano M, Oreni L, et al. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross-sectional study. Clin Infect Dis. 2020; Volume 71: 889–890. doi: 10.1093/cid/ciaa330 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bagheri SHR, Asghari AM, Farhadi M, Shamshiri AR, Kabir A, Kamrava SK. Coincidence of COVID-19 epidemic and olfactory dysfunction outbreak. medRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Menni C, Valdes A, Freydin MB, Ganesh S, El-Sayed Moustafa J, Visconti A. Loss of smell and taste in combination with other symptoms is a strong predictor of COVID-19 infection. medRxiv. 2020. [Google Scholar]
- 59.Yan CH, Faraji F, Prajapati DP, Boone CE, DeConde AS. Association of chemosensory dysfunction and Covid-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol. 2020;Volume 10: 806–813. doi: 10.1002/alr.22579 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klopfenstein T, Kadiane-Oussou NJ, Toko L, Royer PY, Lepiller Q, Gendrin V, et al. Features of anosmia in COVID-19. Med Mal Infect. 2020;Volume 50, Apr 17: 436–439. doi: 10.1016/j.medmal.2020.04.006 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Forster P, Forster L, Renfrew C, Forster M. Phylogenetic network analysis of SARS-CoV-2 genomes. Proc Natl Acad Sci U S A. 2020;117: 9241–9243. doi: 10.1073/pnas.2004999117 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moein ST, Hashemian SMR, Mansourafshar B, Khorram-Tousi A, Tabarsi P, Doty RL. Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol. 2020;Volume 10: 944–950. doi: 10.1002/alr.22587 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garrigues E, Kherabi Y, Le Bot A, Hamon A, Gouze H, Janvier P et al. post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infectol. 2020. Aug 25. doi: 10.1016/j.jinf.2020.08.029 Epub ahead of print. . [DOI] [PMC free article] [PubMed] [Google Scholar]