Abstract
Orthopaedics pioneered the expansion of gene therapy beyond its traditional scope of diseases that are caused by rare single-gene defects. Orthopaedic applications of gene therapy are most developed in the areas of arthritis and regenerative medicine, but several additional possibilities exist.
Invossa, an ex vivo gene therapeutic for osteoarthritis, was approved in South Korea in 2017, but its approval was retracted in 2019 and remains under appeal; a Phase-III clinical trial of Invossa has restarted in the U.S.
There are several additional clinical trials for osteoarthritis and rheumatoid arthritis that could lead to approved gene therapeutics for arthritis.
Bone-healing and cartilage repair are additional areas that are attracting considerable research; intervertebral disc degeneration and the healing of ligaments, tendons, and menisci are other applications of interest. Orthopaedic tumors, genetic diseases, and aseptic loosening are additional potential targets.
If successful, these endeavors will expand the scope of gene therapy from providing expensive medicines for a few patients to providing affordable medicines for many.
Although the conceptual origins of gene therapy lie with the treatment of rare diseases, it has the potential for much wider application in treating common, complex, acquired disorders. In the orthopaedic context, this includes diseases such as arthritis as well as the regeneration of bone, cartilage, meniscus, and other musculoskeletal tissues. We described these possibilities in a forward-looking review that was published in The Journal of Bone & Joint Surgery in 19951. However, at that time, there had been modest progress toward orthopaedic application. Gene transfer to the joints of laboratory animals had been achieved2-4, and the first clinical trial of gene therapy for arthritis had just been approved by the United States Food and Drug Administration (FDA)5; there were some preliminary laboratory data concerning gene transfer to chondrocytes6-10 but little else. The present article reviews the progress in orthopaedic gene therapy during the 25 years that have elapsed since our initial review article. Emphasis is placed on translation and progress toward clinical application.
Gene Therapy Basics and Scope
Successful gene therapy requires the safe delivery of genes, usually as their complementary (c) DNAs, to specific cells in a manner that ensures expression of the transferred DNA at sufficient levels for the appropriate period of time in the correct location. Related molecular therapies, such as gene editing (e.g., CRISPR) and RNA therapeutics, have emerged recently, but since their orthopaedic development remains preclinical, discussion of these approaches lies outside the scope of this review. However, gene transfer can enable these technologies by delivering therapeutic species of non-coding RNA as well as components of the gene-editing apparatus.
Major advances in viral-vector design have greatly improved the efficiency and safety of viral gene transfer known as transduction. Although a dozen or more different types of viruses have been modified as potential gene delivery vectors11, those that are used most commonly in human clinical trials are derived from adeno-associated viruses (AAVs), adenoviruses, retroviruses, and lentiviruses12. Marketing approval for gene therapeutics using these vectors has occurred in various jurisdictions worldwide (Table I).
TABLE I.
Gene Therapy Approvals Worldwide
| Indication | Vector (Delivery Method) |
Gene Product | Name | Jurisdiction | Year Approved |
|---|---|---|---|---|---|
| Head and neck cancer | Adenovirus (in vivo) | p53 | Gendicine (recombinant human p53 adenovirus) | People’s Republic of China | 2003 |
| Solid tumors | Retrovirus (in vivo) | Mutant cyclin G1 | Rexin-G | Philippines | 2007 |
| Peripheral artery disease | Plasmid (in vivo) | Vascular endothelial growth factor | Neovasculgen Cambiogeneplasmid | Russia | 2011 |
| Lipoprotein lipase deficiency | AAV (in vivo) | Lipoprotein lipase | Glybera (alipogene tiparvovec) | EMA | 2012 |
| Melanoma | Herpes simplex virus (in vivo) | Granulocyte-macrophage colony stimulating factor | Imlygic (talimogene laherparepvec) | FDA, EMA | 2015 |
| Adenosine deaminase deficiency | Retrovirus (ex vivo) | Adenosine deaminase | Strimvelis | EMA | 2016 |
| Restoration of host immune system | Retrovirus (ex vivo) | Low affinity nerve growth factor receptor | Zalmoxis* | EMA | 2016 |
| Osteoarthritis | Retrovirus (ex vivo) | Transforming growth factor-β | Invossa† (tonogenchoncel-L) | South Korea | 2017 |
| Acute lymphoblastic leukemia | Lentivirus (ex vivo) | Chimeric antigen receptor | Kymriah (tisagenlecleucel) | FDA, EMA | 2017, 2018 |
| Large B-cell lymphoma | Retrovirus (ex vivo) | Chimeric antigen receptor | Yescarta (axicabtagene ciloleucel) | FDA, EMA | 2017, 2018 |
| Biallelic RPE65 mutation-associated retinal dystrophy | AAV (in vivo) | Retinal pigment epithelium-specific 65 kDa protein | Luxturna (voretigene neparvovec-rzyl) | FDA, EMA | 2017, 2018 |
| Spinal muscular atrophy | AAV (in vivo) | Survival motor neuron-1 | Zolgensma (onasemnogene abeparvovec) | FDA | 2019 |
| β-thalassemia | Lentivirus (ex vivo) | β-globin | Zynteglo‡ (betibeglogene autotemcel) | EMA | 2019 |
| Critical limb ischemia | Plasmid (in vivo) | Hepatocyte growth factor | Collategene (beperminogene perplasmid) | Japan | 2019 |
| Multiple myeloma | Lentivirus (ex vivo) | Chimeric antigen receptor | Abecma (idecabtagene vicleucel) | FDA | 2021 |
Zalmoxis, comprising genetically modified allogeneic T cells, was conditionally approved by the EMA for the restoration of the host immune system after hematopoietic stem cell treatment pending the outcome of a Phase-III trial. This trial was suspended because an interim analysis suggested that the primary end point had not been met. The EMA withdrew Zalmoxis authorization in 2019.
Invossa was withdrawn in 2019. Phase-II trials have started in the U.S.
Zynteglo was conditionally approved for β-thalassemia pending additional clinical data. Its deployment is presently on hold because of 2 malignancies occurring in a related clinical trial of sickle cell anemia. Reproduced, with modification, from: Evans CH. The vicissitudes of gene therapy. Bone Joint Res. 2019 Nov 2;8(10):469-471. © The Authors under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) license.
AAV is an increasingly popular vector because it is relatively safe and transduces nondividing cells, thus allowing in vivo delivery (Fig. 1). Although the recombinant viral genome remains episomal in transduced cells, extended periods of transgene expression are possible if the host cells do not divide. Various serotypes of AAV with different tropisms provide the opportunity to target specific cell populations and avoid the neutralizing humoral immune response that is present in many individuals as a result of prior asymptomatic infection with AAV. Because AAV is difficult to produce under conditions of good manufacturing practice (GMP), costs are high. For example, Zolgensma (onasemnogene abeparvovec), an AAV-based gene therapeutic that was approved in 2019 for treating spinal muscular atrophy, costs >$2 million U.S. dollars per dose13.
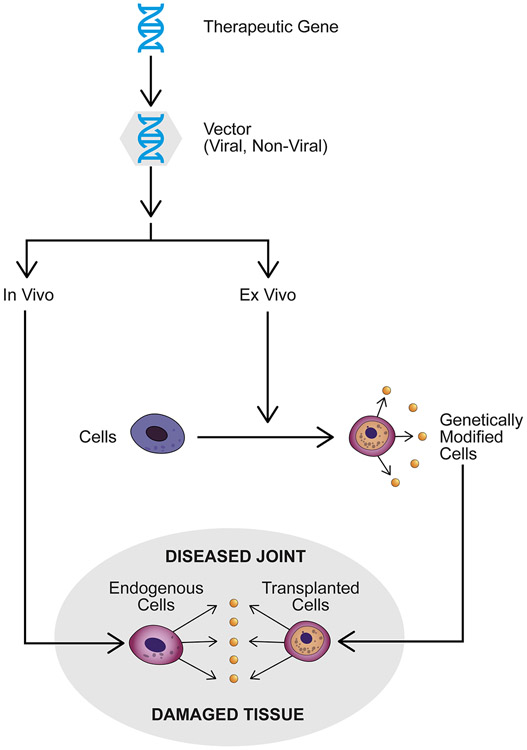
Fig. 1.
Principles of local gene therapy for the treatment of orthopaedic conditions. The therapeutic gene, usually in its cDNA form, is incorporated into a viral or non-viral vector and delivered to a site of disease or damage in an in vivo or ex vivo fashion. For in vivo delivery, the vector is administered directly to the relevant site. For ex vivo delivery, the vector transfers genes to cells outside the body, and the genetically modified cells are then administered to the relevant site.
Adenoviruses transduce a wide range of cell types, including nondividing cells, and can be readily produced in high titers. They have not proved to be a useful vector for treating monogenic disorders because they are inflammatory and immunogenic and do not sustain prolonged transgene expression14. Nevertheless, there is interest in using adenoviruses for regenerative medicine purposes because they can deliver morphogens to sites of injury and express them locally at high concentrations for 2 to 3 weeks, which might be ideal for triggering a lasting reparative response. However, immune and inflammatory responses to a virus may inhibit regeneration15. Immune responses to cells that are transduced with adenovirus can be minimized by eliminating all viral coding elements from the adenovirus genome, producing high-capacity or “gutted” adenovirus vectors that can support extended periods of transgene expression16.
Retroviruses were the first viruses that were developed as vectors for human gene therapy. While relatively straightforward to produce and manipulate, the type of retrovirus that was used in this early work (Moloney murine leukemia virus, a gammaretrovirus) requires target cell division for efficient transduction, which largely limits its use to ex vivo gene therapy (Fig. 1). Moreover, because the retroviral genome inserts itself into the host-cell genome at unpredictable sites, there is a stochastic possibility of insertional mutagenesis. Instances of this have occurred in clinical trials17, which largely restrict the clinical application of retroviruses to serious conditions (Table I), where the risk-benefit ratios justify their use. For one application in osteoarthritis (OA), which is discussed later, the retrovirally transduced cells are irradiated prior to injection to prevent the cells from dividing and thereby creating malignancy. Another option is to include a suicide gene that can be activated to kill cells that are undergoing malignant transformation.
Lentiviruses are also members of the retrovirus family, but unlike gammaretroviruses, they transduce nondividing cells. This has led to their use as vectors for transferring genes to hematopoietic stem cells for potentially treating diseases such as thalassemia, severe combined immunodeficiency disease, and Fanconi anemia18. They are very efficient vectors but, like other retroviruses, run the risk of insertional mutagenesis and are thus unlikely candidates for applications in orthopaedics.
Despite improvements in design and delivery, non-viral vectors remain much less efficient than viral vectors but continue to attract attention because of their relative simplicity, safety, lower cost, and ease of use19. Two plasmid-based gene therapies have received regulatory approval (Table I).
Regardless of the vector, transgene expression can be driven by promoters that are constitutively active in many cell types or those that confer tissue specificity of expression. Inducible promoters of various kinds allow the level of transgene expression to be regulated by exogenous or endogenous stimuli20.
Present Status of Gene Therapy
After nearly half a century of research and several major reversals, gene therapy is finally coming of age21. The FDA has given marketing approval for 6 gene therapy products (Table I), and additional gene therapeutics have been approved in the European Union (by the European Medicines Agency [EMA]) and other jurisdictions. Most of these products target cancer or Mendelian genetic disorders; among the exceptions is Invossa for treating OA, whose strange history is described below. The pipeline of additional gene therapy products is very large, and the FDA expects to be approving 10 to 20 new cell and gene therapeutics a year within 5 years.
Despite these successes, the complexities of manufacturing make for a high cost of goods and expensive drugs. As noted above, Zolgensma costs >$2 million a dose, and the chimeric antigen receptor (CAR) T cells that are used for cancer therapy cost around $300,000 to $450,000 per treatment. Envisaged orthopaedic applications of gene therapy have the advantage of local delivery to individual locations such as joints or sites of tissue injury, for example (Fig. 1). This massively reduces the required amount of vector, improves safety, and lowers costs.
Orthopaedic Applications of Gene Therapy
Arthritis
Other than cancer, arthritis was the first non-genetic disease that has been targeted by gene therapy4. The intra-articular delivery and expression of transgenes22 offer a technology for overcoming the pharmacokinetics of the joint, whereby intra-articularly injected drugs are typically cleared within a few hours23. Moreover, local delivery to individual joints is safer and far less expensive than systemic delivery. Seventeen clinical trials in the gene therapy of rheumatoid arthritis (RA) or OA, using ex vivo or in vivo gene delivery, have been completed or are in progress (Table II).
TABLE II.
Clinical Trials in Gene Therapy for Arthritis
| Indication | Transgene | Vector (Delivery Method) |
Phase | National Clinical Trial (NCT) Identifier |
Status |
|---|---|---|---|---|---|
| RA24 | IL-1Ra | Retrovirus Ex vivo | I | Predates the establishment of the NCT | Completed |
| RA25 | IL-1Ra | Retrovirus Ex vivo | I | Predates the establishment of the NCT | Completed |
| RA | Etanercept | AAV In vivo | I | 00617032 | Completed |
| RA, psoriatic arthritis, ankylosing spondylitis | Etanercept | AAV In vivo | I/II | 00126724 | Completed |
| OA | TGF-β | Retrovirus Ex vivo | I, II, and III | 02341391 02341378 02072070 01671072 00599248 03291470 03203330 |
Completed Completed Completed Completed Completed Not yet recruiting Not yet recruiting |
| RA, OA | IFN-β | AAV In vivo | I | 02727764 | Not yet recruiting |
| RA | IFN-β | AAV In vivo | I | 03445715 | Unknown |
| OA | IL-1Ra | AAV In vivo | I | 02790723 | Completed Recruiting |
| OA | IL-1Ra | Adenovirus In vivo | I | 04119687 | Recruiting |
| OA | IL-10 | Plasmid In vivo | I and II | 03477487 04124042 |
Completed Active, not recruiting |
The first clinical trial targeted the metacarpophalangeal joints of 9 patients with RA. This followed an ex vivo protocol using retrovirus to express the interleukin-1 receptor antagonist (IL-1Ra) in culture-expanded autologous synovial fibroblasts, which were then returned to the patient by intra-articular injection. One week later, the joints were removed during the surgical implantation of silicone protheses5. Analysis of the recovered joint tissues confirmed successful gene transfer and expression of a biologically active gene product24. There were no safety issues. A related trial in Germany reported equivalent results with clinical improvement in 2 patients25 before it was terminated because of severe adverse events in an unrelated clinical trial elsewhere that used a similar retrovirus17.
The ex vivo intra-articular strategy was revived in a modified form for treating OA by Kim et al.26. To avoid the cost and complexity of ex vivo delivery using autologous cell cultures, these investigators established a line of chondrocytes from an infant with polydactyly and used the cells as allografts. One population of cells was transduced with retrovirus-carrying transforming growth factor-β1 (TGF-β1) and irradiated, prior to intra-articular injection, at a radiation dose that permitted transgene expression but inhibited cell division. Clinical trials (Table II) met their primary end point of symptomatic relief, and the treatment was approved by the South Korean authorities in 2017 as the drug Invossa. After a Phase-III clinical trial was initiated in the U.S., a monumental mistake was discovered. The genetically modified cells were not chondrocytes, but were from an epithelial cell line that is derived from human embryonic kidney known as HEK 293. Invossa was withdrawn from the market, and its future remains uncertain. The U.S. clinical trial was suspended but has now been allowed to continue on the basis that, despite using the wrong cells, the clinical data showed no severe adverse events and the cells that were used in the U.S. studies, unlike those in South Korea, were HEK 293 cells from the beginning. Amazingly, they met their clinical end points.
The first arthritis trials using in vivo gene transfer employed AAV2 to deliver a cDNA encoding etanercept, a tumor necrosis factor-α (TNF-α) antagonist, into the joints of patients with RA27,28. The Phase-I trial proceeded unproblematically28, but a fatality from histoplasmosis29 occurred in the subsequent Phase-II study30. The trial was suspended while the death was investigated; it was determined to be unrelated to the gene therapy. The FDA allowed the trial to continue to completion with certain modifications. The results showed some promising trends, but failed to provide significant clinical improvement27, possibly because patients who were enrolled in the trial were already taking TNF-α inhibitors. Moreover, it is not known whether the vector succeeded in transducing cells within the injected joints. To our knowledge, there has been no further development of this product.
Two subsequent trials were initiated in which AAV5 was used to deliver interferon (IFN)-β cDNA under the transcriptional control of a nuclear factor-kappa B (NF-κB)-inducible promoter into the joints of patients with RA of the wrist (NCT03445715) or with OA or RA of the hand (NCT02727764). No data from these trials have yet been published.
AAV is being used in a Phase-I clinical trial that was initiated in 2019 to deliver IL-1Ra to the knee joints of 9 patients with mid-stage OA (NCT02790723). All 9 of the patients have received doses without serious adverse events. A similar trial (NCT04119687) in which IL-1Ra is delivered to the knee joints of patients with OA using high-capacity adenovirus with expression of IL-1Ra that is driven by an NF-κB-inducible promoter was subsequently started (NCT04119687).
A Phase-I trial has been completed in which plasmid DNA encoding IL-10 was injected into the knee joints of patients with OA (NCT03477487); a Phase-II trial of this material is underway (NCT04124042).
As well as treating OA and RA, intra-articular gene transfer has therapeutic potential in other conditions that affect joints, including gout, pseudogout, hemarthrosis, arthrofibrosis, pigmented villonodular synovitis, and the articular sequelae of certain lysosomal storage diseases31. Cartilage repair is discussed later in this article.
Regenerative Orthopaedics
Injuries to bone, cartilage, ligament, tendon, meniscus, and other tissues of orthopaedic interest are common and do not always heal well. There is much optimism that application of the appropriate growth factors to sites of injury will prompt robust regenerative responses32. However, their recombinant proteins are difficult to localize and have short biological half-lives. Gene delivery has the potential to overcome these hurdles. As described below, gene therapy has shown promise in animal models; 3 human studies have been initiated (NCT02293031, NCT01825811, and NCT03076138).
Bone-Healing
Deficiencies in bone-healing are remarkably recalcitrant33; the clinical treatment of choice, autograft bone, was introduced >100 years ago34 and has yet to be supplanted.
Much of the initial research into gene therapy for bone-healing used bone morphogenetic protein (BMP)-2 or BMP-7. They were among the first osteogenic genes that were cloned, and the FDA has approved their recombinant proteins for clinical use in certain indications where it is necessary to grow bone. Gene transfer of BMP-2 or BMP-7 offers the possibility to deliver these morphogens in a fashion that enhances bone-healing without the side effects of the recombinant proteins35,36.
The pioneering research by Lieberman et al. demonstrated convincing healing of femoral segmental defects in rodent models by ex vivo gene transfer37,38. The laboratory used adenovirus to transfer BMP-2 cDNA to autologous mesenchymal stromal cells (MSCs)39 that were grown on a collagen scaffold and implanted into the defect. Healing was efficient, and the regenerate lacked the “eggshell” appearance that occurred with recombinant (r) BMP-2. Subsequent research using a lentivirus vector to deliver higher amounts of BMP-2 for a longer period of time was also successful40. Because of the theoretical possibility of insertional mutagenesis, the lentivirus was subsequently modified to include a suicide gene, with good results41. The use of lentivirus allows abbreviated, “same day”42 or “next day”43 ex vivo approaches in which autologous cells are harvested, transduced, and returned to the patient without expansion.
To avoid ex vivo culture of autologous cells44, we first concentrated on in vivo delivery of BMP-2 using adenovirus45,46. This approach showed efficacy in rabbit and rat femoral defects47,48 but not those of sheep49. Because of the high intrinsic osteogenic properties of muscle, we evaluated an abbreviated ex vivo method in which muscle grafts were transduced with adenovirus before implantation into rat femoral defects50. This showed high promise and was also successful using grafts of modified fat, a rich source of endogenous MSCs. Tracking experiments confirmed that implanted muscle cells became chondrocytes, osteoblasts, and vascular endothelial cells in the healing bone51. Healing by genetically modified muscle was enhanced under immunosuppression that had little effect on healing by rBMP-215, suggesting that immune reactions to the adenovirus vector compromise bone-healing.
We have consistently noted that bone-healing by gene therapy requires much less BMP-2 than healing by rBMP-215,52. This is the exact opposite of the expectation at the outset, which assumed a need for high BMP-2 expression for an extended period of time. A striking in vivo example of this is shown in data from the laboratory of Dr. D. Gazit that used a tibial segmental defect model in the pig53. Ultrasound-enabled transfection of plasmid DNA encoding BMP-6 achieved healing under conditions where sub-nanogram amounts of BMP-6 were expressed for only 5 to 10 days. Healing may have been helped by delayed gene transfer54 and by the fact that BMP-6, unlike BMP-2, is not inhibited by noggin. Efficacy in a large animal model is very important because rodents heal bones readily using rBMPs whereas bigger animals do not33.
Allograft revitalization, a concept introduced by the laboratory of Dr. E.M. Schwarz, offers the possibility of an “off-the-shelf” gene-based product for forming bone. Taking advantage of the relative stability of AAV, vectors that encode osteogenic products are freeze-dried onto the surface of the allograft. Success has been demonstrated after implantation of such constructs in mice using vectors that encode the receptor activator of NF-κB ligand (RANKL) and vascular endothelial growth factor (VEGF)55, as well as BMP-256.
Gene-activated matrices (GAMs) are combinations of scaffolds and vectors that also provide off-the-shelf products57. One such GAM called “Nucleostim” has advanced to clinical trials in Russia. It delivers the plasmid from the product Neovasculgen (Table I) that encodes VEGF on a collagen-hydroxyapatite scaffold to treat maxillofacial bone defects. A promising case report from 1 study (NCT02293031) was published in 201658, but no additional details have been forthcoming. However, promising data from a similar study (NCT03076138) using the same GAM have recently been published59.
A variety of additional transgenes have been explored in animal models of long bone, cranial defects and mandibular healing, and spinal fusion. A number of recent review articles cover gene therapy for bone-healing more comprehensively than is possible here52,60-64.
Cartilage Repair
Unlike bone, cartilage has little or no ability to regenerate spontaneously and, thus, there is no natural biology to follow when developing reparative strategies. A number of procedures are used clinically to repair cartilage, including microfracture, autologous chondrocyte implantation (ACI), allografting, and autografting. Most gene therapy approaches to healing cartilage are based on augmenting the effectiveness of one of these existing techniques.
Initial attention was focused on augmenting ACI by genetically modifying chondrocytes before their implantation into defects. This was shown to be feasible in animal models using a retrovirus6, an adenovirus7, an AAV65, and liposome-associated plasmids66. Success was reported in repairing cartilage defects using BMP-767, insulin-like growth factor-1 (IGF-1)66,68, or fibroblast growth factor-2 (FGF-2)69 in this fashion. A combination of IGF-1 and FGF-2 was shown to be superior to either growth factor used alone70. There has been 1 Phase-I/II clinical trial in which Invossa cells were encapsulated in fibrin and inserted into cartilage lesions in the knee joints of patients with OA (NCT01825811). It is not known whether the implanted cells were chondrocytes or HEK 293 cells (see above), and the data do not seem to have been published.
Marrow-stimulation technologies are popular because they are straightforward, inexpensive, 1-step procedures that produce good short- to medium-term benefit in many patients. They are based on facilitating the ingress of MSCs from the underlying marrow into the lesion with the expectation that the MSCs will differentiate into chondrocytes, produce new matrix, and heal the defect. However, MSCs fail to differentiate into authentic articular chondrocytes under these conditions, instead producing a fibrocartilaginous scar with inferior mechanical properties.
The chondrogenic differentiation of MSCs can be enhanced by gene transfer71,72, supporting the idea that gene delivery can augment microfracture by expressing chondrogenic genes in these cells as they enter the defect. There have been 2 approaches to this. Cucchiarini et al. applied recombinant AAV directly to the emerging marrow and have reported promising results using transgenes that express IGF-173 or FGF-274 in rabbit osteochondral defects and TGF-β in minipigs75. An alternative approach removes marrow from the animal, adds vector as the marrow clots, and then press-fits the resulting “gene plug” into the lesion76. Improved chondrogenesis has been noted in a rabbit model using transgenes that express Indian hedgehog (Ihh) and BMP-277. In the “gene plug” method, the clotted marrow provides an autologous fibrin scaffold that acts as a type of GAM. The use of scaffolds to guide the delivery of vectors for cartilage repair recently has been reviewed by Cucchiarini and Madry78.
Cartilage repair in the arthritic joint is much more challenging than in the acutely injured but otherwise normal joint. To achieve success in the former, it may be helpful to combine genetic enhancement of cartilage repair with the arthritis gene therapy strategies that were discussed earlier. IL-1, for example, is a powerful inhibitor of chondrogenesis79, suggesting that co-delivery of IL-1Ra with a chondrogenic growth factor would provide powerful synergy80.
Intervertebral Disc Degeneration
Intervertebral disc degeneration (IDD) is an attractive target because, like OA, it is common, debilitating, expensive, and very difficult to treat. Loss of extracellular matrix in the nucleus pulposus is a major pathological feature of the degenerating disc, and stimulating its resynthesis by applying the appropriate growth factors via gene transfer is an attractive strategy81.
Efficient gene transfer to cells within the nucleus pulposus of rabbits has been reported with adenovirus82, AAV83, and lentivirus84 vectors. Moreover, transgene expression persists for over a year85, which is remarkable, especially in the case of adenovirus given its high antigenicity and that of the β-galactosidase marker that is used to demonstrate expression. This suggests that the interior of the intervertebral disc is protected from immune surveillance, possibly on account of its avascularity and dense extracellular matrix. Adenoviral delivery of TGF-β markedly enhanced proteoglycan synthesis by the disc86.
Leckie et al. were able to protect discs from undergoing IDD after a puncture wound in a rabbit model by the transfer of tissue inhibitor of metalloproteinase-1 (TIMP-1) or BMP-2 using AAV83. Intradiscal injection of lentivirus encoding the transcription factor SOX9 or a short-hairpin RNA suppressing the expression of matrix metalloproteinase-3 also dramatically delayed IDD in this model84, as did lentivirus delivery of a combination of TGF-β3, TIMP-1, and connective tissue growth factor87. Chen et al. have recently published a review of gene delivery to the disc88.
While providing grounds for optimism, there are several points to consider for clinical development. One is the poor cellularity of the degenerating disc, which may necessitate the introduction of cells as well as genes to stimulate matrix production. This raises the issue of which cells to use, a matter that may be informed by the increasing clinical use of intradiscal cell therapy for treating IDD89. Because extracellular matrix becomes depleted in the degenerating disc, its immunoprivileged status may be compromised.
Ligaments, Tendons, and Menisci
Injured ligaments and tendons provide a range of regenerative challenges90. Three strategies are being explored for harnessing gene transfer to improve clinical outcomes. The first delivers cDNAs that encode regenerative growth factors to the site of a lesion91,92. The second delivers them to reconstructed tissues to enhance performance93. The third uses gene transfer to aid ligament or tendon-to-bone healing94-96.
Preliminary gene transfer experiments also have been performed in the context of meniscal repair97-100 but, as with ligaments and tendons, no human clinical trials seem imminent.
Other Applications
There has been exploratory research into the use of gene therapy for treating orthopaedic malignancies101 and certain genetic diseases102, without clinical translation. Aseptic loosening also has been studied103-105, and a Phase-I trial demonstrated that the pseudosynovium around loosened hip prostheses was ablated by genetic means106,107. No subsequent development of the latter strategy seems to have occurred.
Perspective
Like the field of gene therapy as a whole, progress in orthopaedic gene therapy has been fitful, and the process of bringing applications into clinical trials has been tortuous21,108,109. Nevertheless, 17 clinical trials of arthritis gene therapies have been completed or are underway, and several human trials have been initiated for other indications (Table III).
TABLE III.
Progress in the Clinical Development of Orthopaedic Gene Therapy Applications*
| Application | Preclinical | Clinical Trial | Approval | ||
|---|---|---|---|---|---|
| Phase I | Phase II | Phase III | |||
| Osteoarthritis | Yes | Yes | Yes | Yes | * |
| Rheumatoid arthritis | Yes | Yes | Yes | ||
| Cartilage repair | Yes | † | † | ||
| Bone-healing | Yes | Yes | |||
| Aseptic loosening | Yes | Yes | |||
| Intervertebral disc degeneration | Yes | ||||
| Ligament, tendon | Yes | ||||
| Mendelian disorders, cancer | Yes | ||||
Invossa, an ex vivo gene therapeutic for osteoarthritis, was approved in South Korea in 2017, but its approval was retracted in 2019. It is currently in Phase-III trials in the U.S.
Invossa has been studied in 1 Phase-I/II clinical trial for cartilage repair. The results have not been published.
The application of gene therapy to regenerative orthopaedics has generated much interest and a large literature, but clinical translation remains slow. However, there has been sufficient progress in the areas of bone-healing and cartilage repair to provide optimism about future clinical development. Other applications remain at an early experimental stage.
The degree to which gene therapy will be used by clinicians depends not only on its safety and efficacy but also on its cost. The gene therapies that have been approved so far are extremely expensive because they are delivered systemically in large amounts, they require the ex vivo expansion of autologous cells, or both. Most clinical applications in orthopaedics, in contrast, will involve the local application of relatively small, inexpensive amounts of GMP material. Therefore, orthopaedic gene therapy promises to expand the scope of gene therapy from providing expensive medicines for a few patients to providing affordable medicines for many110.
Source of Funding
The authors’ work in this area has been supported by the Orthopaedic Trauma Association, the National Institute of Arthritis, Musculoskeletal and Skin Diseases, the Department of Defense, and the AO Foundation. The research by Dr. Evans is supported in part by the John and Posy Krehbiel Professorship in Orthopedics.
Footnotes
Disclosure: The Disclosure of Potential Conflicts of Interest forms are provided with the online version of the article (http://links.lww.com/JBJSREV/A741).
References
- 1.Evans CH, Robbins PD. Possible orthopaedic applications of gene therapy. J Bone Joint Surg Am. 1995. July;77(7):1103–14. [DOI] [PubMed] [Google Scholar]
- 2.Bandara G, Mueller GM, Galea-Lauri J, Tindal MH, Georgescu HI, Suchanek MK, Hung GL, Glorioso JC, Robbins PD, Evans CH. Intraarticular expression of biologically active interleukin 1-receptor-antagonist protein by ex vivo gene transfer. Proc Natl Acad Sci U S A. 1993. November 15;90(22):10764–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roessler BJ, Allen ED, Wilson JM, Hartman JW, Davidson BL. Adenoviral-mediated gene transfer to rabbit synovium in vivo. J Clin Invest. 1993. August;92(2):1085–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bandara G, Robbins PD, Georgescu HI, Mueller GM, Glorioso JC, Evans CH. Gene transfer to synoviocytes: prospects for gene treatment of arthritis. DNA Cell Biol. 1992. April;11(3):227–31. [DOI] [PubMed] [Google Scholar]
- 5.Evans CH, Robbins PD, Ghivizzani SC, Herndon JH, Kang R, Bahnson AB, Barranger JA, Elders EM, Gay S, Tomaino MM, Wasko MC, Watkins SC, Whiteside TL, Glorioso JC, Lotze MT, Wright TM. Clinical trial to assess the safety, feasibility, and efficacy of transferring a potentially anti-arthritic cytokine gene to human joints with rheumatoid arthritis. Hum Gene Ther. 1996. June 20;7(10):1261–80. [DOI] [PubMed] [Google Scholar]
- 6.Kang R, Marui T, Ghivizzani SC, Nita IM, Georgescu HI, Suh JK, Robbins PD, Evans CH. Ex vivo gene transfer to chondrocytes in full-thickness articular cartilage defects: a feasibility study. Osteoarthritis Cartilage. 1997. March;5(2):139–43. [DOI] [PubMed] [Google Scholar]
- 7.Baragi VM, Renkiewicz RR, Qiu L, Brammer D, Riley JM, Sigler RE, Frenkel SR, Amin A, Abramson SB, Roessler BJ. Transplantation of adenovirally transduced allogeneic chondrocytes into articular cartilage defects in vivo. Osteoarthritis Cartilage. 1997. July;5(4):275–82. [DOI] [PubMed] [Google Scholar]
- 8.Baragi VM, Renkiewicz RR, Jordan H, Bonadio J, Hartman JW, Roessler BJ. Transplantation of transduced chondrocytes protects articular cartilage from interleukin 1-induced extracellular matrix degradation. J Clin Invest. 1995. November;96(5):2454–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arai Y, Kubo T, Kobayashi K, Takeshita K, Takahashi K, Ikeda T, Imanishi J, Takigawa M, Hirasawa Y. Adenovirus vector-mediated gene transduction to chondrocytes: in vitro evaluation of therapeutic efficacy of transforming growth factor-beta 1 and heat shock protein 70 gene transduction. J Rheumatol. 1997. September;24(9):1787–95. [PubMed] [Google Scholar]
- 10.Tomita T, Hashimoto H, Tomita N, Morishita R, Lee SB, Hayashida K, Nakamura N, Yonenobu K, Kaneda Y, Ochi T. In vivo direct gene transfer into articular cartilage by intraarticular injection mediated by HVJ (Sendai virus) and liposomes. Arthritis Rheum. 1997. May;40(5):901–6. [DOI] [PubMed] [Google Scholar]
- 11.Lundstrom K Viral Vectors in Gene Therapy. Diseases. 2018. May 21;6(2):E42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ginn SL, Amaya AK, Alexander IE, Edelstein M, Abedi MR. Gene therapy clinical trials worldwide to 2017: An update. J Gene Med. 2018. May;20(5):e3015. Epub 2018 Apr 19. [DOI] [PubMed] [Google Scholar]
- 13.Dyer O Health ministers condemn Novartis lottery for Zolgensma, the world’s most expensive drug. BMJ. 2020. February 12;368:m580. [DOI] [PubMed] [Google Scholar]
- 14.Appaiahgari MB, Vrati S. Adenoviruses as gene/vaccine delivery vectors: promises and pitfalls. Expert Opin Biol Ther. 2015. March;15(3):337–51. Epub 2014 Dec 22. [DOI] [PubMed] [Google Scholar]
- 15.De la Vega RE, Coenen MJ, Müller SA, Nagelli CV, Quirk NP, Lopez de Padilla C, Evans CH. Effects of FK506 on the healing of diaphyseal, critical size defects in the rat femur. Eur Cell Mater. 2020. October 6;40:160–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ricobaraza A, Gonzalez-Aparicio M, Mora-Jimenez L, Lumbreras S, Hernandez-Alcoceba R. High-Capacity Adenoviral Vectors: Expanding the Scope of Gene Therapy. Int J Mol Sci. 2020. May 21;21(10):E3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E, Clappier E, Caccavelli L, Delabesse E, Beldjord K, Asnafi V, MacIntyre E, Dal Cortivo L, Radford I, Brousse N, Sigaux F, Moshous D, Hauer J, Borkhardt A, Belohradsky BH, Wintergerst U, Velez MC, Leiva L, Sorensen R, Wulffraat N, Blanche S, Bushman FD, Fischer A, Cavazzana-Calvo M. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest. 2008. September;118(9):3132–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milone MC, O’Doherty U. Clinical use of lentiviral vectors. Leukemia. 2018. July;32(7):1529–41. Epub 2018 Mar 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slivac I, Guay D, Mangion M, Champeil J, Gaillet B. Non-viral nucleic acid delivery methods. Expert Opin Biol Ther. 2017. January;17(1):105–18. Epub 2016 Nov 9. [DOI] [PubMed] [Google Scholar]
- 20.Guo ZS, Li Q, Bartlett DL, Yang JY, Fang B. Gene transfer: the challenge of regulated gene expression. Trends Mol Med. 2008. September;14(9):410–8. Epub 2008 Aug 7. [DOI] [PubMed] [Google Scholar]
- 21.Evans CH. The vicissitudes of gene therapy. Bone Joint Res. 2019. November 2;8(10):469–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans CH, Ghivizzani SC, Robbins PD. Gene Delivery to Joints by Intra-Articular Injection. Hum Gene Ther. 2018. January;29(1):2–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans CH, Kraus VB, Setton LA. Progress in intra-articular therapy. Nat Rev Rheumatol. 2014. January;10(1):11–22. Epub 2013 Nov 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evans CH, Robbins PD, Ghivizzani SC, Wasko MC, Tomaino MM, Kang R, Muzzonigro TA, Vogt M, Elder EM, Whiteside TL, Watkins SC, Herndon JH. Gene transfer to human joints: progress toward a gene therapy of arthritis. Proc Natl Acad Sci U S A. 2005. June 14;102(24):8698–703. Epub 2005 Jun 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wehling P, Reinecke J, Baltzer AW, Granrath M, Schulitz KP, Schultz C, Krauspe R, Whiteside TW, Elder E, Ghivizzani SC, Robbins PD, Evans CH. Clinical responses to gene therapy in joints of two subjects with rheumatoid arthritis. Hum Gene Ther. 2009. February;20(2):97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim MK, Ha CW, In Y, Cho SD, Choi ES, Ha JK, Lee JH, Yoo JD, Bin SI, Choi CH, Kyung HS, Lee MC. A Multicenter, Double-Blind, Phase III Clinical Trial to Evaluate the Efficacy and Safety of a Cell and Gene Therapy in Knee Osteoarthritis Patients. Hum Gene Ther Clin Dev. 2018. March;29(1):48–59. [DOI] [PubMed] [Google Scholar]
- 27.Mease PJ, Wei N, Fudman EJ, Kivitz AJ, Schechtman J, Trapp RG, Hobbs KF, Greenwald M, Hou A, Bookbinder SA, Graham GE, Wiesenhutter CW, Willis L, Ruderman EM, Forstot JZ, Maricic MJ, Dao KH, Pritchard CH, Fiske DN, Burch FX, Prupas HM, Anklesaria P, Heald AE. Safety, tolerability, and clinical outcomes after intraarticular injection of a recombinant adeno-associated vector containing a tumor necrosis factor antagonist gene: results of a phase 1/2 Study. J Rheumatol. 2010. April;37(4):692–703. Epub 2009 Dec 23. [DOI] [PubMed] [Google Scholar]
- 28.Mease PJ, Hobbs K, Chalmers A, El-Gabalawy H, Bookman A, Keystone E, Furst DE, Anklesaria P, Heald AE. Local delivery of a recombinant adenoassociated vector containing a tumour necrosis factor alpha antagonist gene in inflammatory arthritis: a phase 1 dose-escalation safety and tolerability study. Ann Rheum Dis. 2009. August;68(8):1247–54. Epub 2008 Aug 4. [DOI] [PubMed] [Google Scholar]
- 29.Frank KM, Hogarth DK, Miller JL, Mandal S, Mease PJ, Samulski RJ, Weisgerber GA, Hart J. Investigation of the cause of death in a gene-therapy trial. N Engl J Med. 2009. July 9;361(2):161–9. [DOI] [PubMed] [Google Scholar]
- 30.Evans CH, Ghivizzani SC, Robbins PD. Arthritis gene therapy’s first death. Arthritis Res Ther. 2008;10(3):110. Epub 2008 May 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evans CH, Ghivizzani SC, Robbins PD. Getting arthritis gene therapy into the clinic. Nat Rev Rheumatol. 2011. April;7(4):244–9. Epub 2010 Dec 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evans CH, Huard J. Gene therapy approaches to regenerating the musculoskeletal system. Nat Rev Rheumatol. 2015. April;11(4):234–42. Epub 2015 Mar 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evans CH, Stoddart MJ. Why does bone have TERM limits? Injury. 2016. June;47(6):1159–61. [DOI] [PubMed] [Google Scholar]
- 34.de Boer HH. The history of bone grafts. Clin Orthop Relat Res. 1988. January;(226):292–8. [PubMed] [Google Scholar]
- 35.James AW, LaChaud G, Shen J, Asatrian G, Nguyen V, Zhang X, Ting K, Soo C. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng Part B Rev. 2016. August;22(4):284–97. Epub 2016 Apr 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garrison KR, Shemilt I, Donell S, Ryder JJ, Mugford M, Harvey I, Song F, Alt V. Bone morphogenetic protein (BMP) for fracture healing in adults. Cochrane Database Syst Rev. 2010. June 16;(6):CD006950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lieberman JR, Daluiski A, Stevenson S, Wu L, McAllister P, Lee YP, Kabo JM, Finerman GA, Berk AJ, Witte ON. The effect of regional gene therapy with bone morphogenetic protein-2-producing bone-marrow cells on the repair of segmental femoral defects in rats. J Bone Joint Surg Am. 1999. July;81(7):905–17. [DOI] [PubMed] [Google Scholar]
- 38.Lieberman JR, Le LQ, Wu L, Finerman GA, Berk A, Witte ON, Stevenson S. Regional gene therapy with a BMP-2-producing murine stromal cell line induces heterotopic and orthotopic bone formation in rodents. J Orthop Res. 1998. May;16(3):330–9. [DOI] [PubMed] [Google Scholar]
- 39.Peterson B, Zhang J, Iglesias R, Kabo M, Hedrick M, Benhaim P, Lieberman JR. Healing of critically sized femoral defects, using genetically modified mesenchymal stem cells from human adipose tissue. Tissue Eng. 2005. Jan-Feb;11(1-2):120–9. [DOI] [PubMed] [Google Scholar]
- 40.Sugiyama O, An DS, Kung SP, Feeley BT, Gamradt S, Liu NQ, Chen IS, Lieberman JR. Lentivirus-mediated gene transfer induces long-term transgene expression of BMP-2 in vitro and new bone formation in vivo. Mol Ther. 2005. March;11(3):390–8. [DOI] [PubMed] [Google Scholar]
- 41.Alaee F, Sugiyama O, Virk MS, Tang H, Drissi H, Lichtler AC, Lieberman JR. Suicide gene approach using a dual-expression lentiviral vector to enhance the safety of ex vivo gene therapy for bone repair. Gene Ther. 2014. February;21(2):139–47. Epub 2013 Nov 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Virk MS, Sugiyama O, Park SH, Gambhir SS, Adams DJ, Drissi H, Lieberman JR. “Same day” ex-vivo regional gene therapy: a novel strategy to enhance bone repair. Mol Ther. 2011. May;19(5):960–8. Epub 2011 Feb 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bougioukli S, Alluri R, Pannell W, Sugiyama O, Vega A, Tang A, Skorka T, Park SH, Oakes D, Lieberman JR. Ex vivo gene therapy using human bone marrow cells overexpressing BMP-2: “Next-day” gene therapy versus standard “two-step” approach. Bone. 2019. November;128:115032. Epub 2019 Aug 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Evans CH, Palmer GD, Pascher A, Porter R, Kwong FN, Gouze E, Gouze JN, Liu F, Steinert A, Betz O, Betz V, Vrahas M, Ghivizzani SC. Facilitated endogenous repair: making tissue engineering simple, practical, and economical. Tissue Eng. 2007. August;13(8):1987–93. [DOI] [PubMed] [Google Scholar]
- 45.Baltzer AW, Lattermann C, Whalen JD, Wooley P, Weiss K, Grimm M, Ghivizzani SC, Robbins PD, Evans CH. Genetic enhancement of fracture repair: healing of an experimental segmental defect by adenoviral transfer of the BMP-2 gene. Gene Ther. 2000. May;7(9):734–9. [DOI] [PubMed] [Google Scholar]
- 46.Baltzer AW, Lattermann C, Whalen JD, Braunstein S, Robbins PD, Evans CH. A gene therapy approach to accelerating bone healing. Evaluation of gene expression in a New Zealand white rabbit model. Knee Surg Sports Traumatol Arthrosc. 1999;7(3):197–202. [DOI] [PubMed] [Google Scholar]
- 47.Betz OB, Betz VM, Nazarian A, Pilapil CG, Vrahas MS, Bouxsein ML, Gerstenfeld LC, Einhorn TA, Evans CH. Direct percutaneous gene delivery to enhance healing of segmental bone defects. J Bone Joint Surg Am. 2006. February;88(2):355–65. [DOI] [PubMed] [Google Scholar]
- 48.Baltzer AW, Lattermann C, Whalen JD, Ghivizzani S, Wooley P, Krauspe R, Robbins PD, Evans CH. Potential role of direct adenoviral gene transfer in enhancing fracture repair. Clin Orthop Relat Res. 2000. October;(379)(Suppl):S120–5. [DOI] [PubMed] [Google Scholar]
- 49.Egermann M, Lill CA, Griesbeck K, Evans CH, Robbins PD, Schneider E, Baltzer AW. Effect of BMP-2 gene transfer on bone healing in sheep. Gene Ther. 2006. September;13(17):1290–9. Epub 2006 Apr 27. [DOI] [PubMed] [Google Scholar]
- 50.Evans CH, Liu FJ, Glatt V, Hoyland JA, Kirker-Head C, Walsh A, Betz O, Wells JW, Betz V, Porter RM, Saad FA, Gerstenfeld LC, Einhorn TA, Harris MB, Vrahas MS. Use of genetically modified muscle and fat grafts to repair defects in bone and cartilage. Eur Cell Mater. 2009. December 31;18:96–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De La Vega RE, De Padilla CL, Trujillo M, Quirk N, Porter RM, Evans CH, Ferreira E. Contribution of Implanted, Genetically Modified Muscle Progenitor Cells Expressing BMP-2 to New Bone Formation in a Rat Osseous Defect. Mol Ther. 2018. January 3;26(1):208–18. Epub 2017 Oct 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De la Vega RE, Atasoy-Zeybek A, Panos JA, Griensven MV, Evans CH, Balmayor ER. Gene therapy for bone healing: lessons learned and new approaches. Transl Res. 2021. May 5:S1931–5244(21)00105-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bez M, Sheyn D, Tawackoli W, Avalos P, Shapiro G, Giaconi JC, Da X, David SB, Gavrity J, Awad HA, Bae HW, Ley EJ, Kremen TJ, Gazit Z, Ferrara KW, Pelled G, Gazit D. In situ bone tissue engineering via ultrasound-mediated gene delivery to endogenous progenitor cells in mini-pigs. Sci Transl Med. 2017. May 17;9(390):eaal3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Betz OB, Betz VM, Nazarian A, Egermann M, Gerstenfeld LC, Einhorn TA, Vrahas MS, Bouxsein ML, Evans CH. Delayed administration of adenoviral BMP-2 vector improves the formation of bone in osseous defects. Gene Ther. 2007. July;14(13):1039–44. Epub 2007 Apr 26. [DOI] [PubMed] [Google Scholar]
- 55.Ito H, Koefoed M, Tiyapatanaputi P, Gromov K, Goater JJ, Carmouche J, Zhang X, Rubery PT, Rabinowitz J, Samulski RJ, Nakamura T, Soballe K, O’Keefe RJ, Boyce BF, Schwarz EM. Remodeling of cortical bone allografts mediated by adherent rAAV-RANKL and VEGF gene therapy. Nat Med. 2005. March;11(3):291–7. Epub 2005 Feb 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yazici C, Takahata M, Reynolds DG, Xie C, Samulski RJ, Samulski J, Beecham EJ, Gertzman AA, Spilker M, Zhang X, O’Keefe RJ, Awad HA, Schwarz EM. Self-complementary AAV2.5-BMP2-coated femoral allografts mediated superior bone healing versus live autografts in mice with equivalent biomechanics to unfractured femur. Mol Ther. 2011. August;19(8):1416–25. Epub 2011 Jan 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.D’Mello S, Atluri K, Geary SM, Hong L, Elangovan S, Salem AK. Bone Regeneration Using Gene-Activated Matrices. AAPS J. 2017. January;19(1):43–53. Epub 2016 Sep 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bozo IY, Deev RV, Drobyshev AY, Isaev AA, Eremin II. World’s First Clinical Case of Gene-Activated Bone Substitute Application. Case Rep Dent. 2016;2016:8648949. Epub 2016 Nov 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bozo IY, Drobyshev AY, Redko NA, Komlev VS, Isaev AA, Deev RV. Bringing a Gene-Activated Bone Substitute Into Clinical Practice: From Bench to Bedside. Front Bioeng Biotechnol. 2021. February 4;9:599300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Atasoy-Zeybek A, Kose GT. Gene Therapy Strategies in Bone Tissue Engineering and Current Clinical Applications. Adv Exp Med Biol. 2018;1119:85–101. [DOI] [PubMed] [Google Scholar]
- 61.Shapiro G, Lieber R, Gazit D, Pelled G. Recent Advances and Future of Gene Therapy for Bone Regeneration. Curr Osteoporos Rep. 2018. August;16(4):504–11. [DOI] [PubMed] [Google Scholar]
- 62.Bougioukli S, Evans CH, Alluri RK, Ghivizzani SC, Lieberman JR. Gene Therapy to Enhance Bone and Cartilage Repair in Orthopaedic Surgery. Curr Gene Ther. 2018;18(3):154–70. [DOI] [PubMed] [Google Scholar]
- 63.Balmayor ER, van Griensven M. Gene therapy for bone engineering. Front Bioeng Biotechnol. 2015. February 2;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Evans CH. Gene delivery to bone. Adv Drug Deliv Rev. 2012. September;64(12):1331–40. Epub 2012 Mar 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yokoo N, Saito T, Uesugi M, Kobayashi N, Xin KQ, Okuda K, Mizukami H, Ozawa K, Koshino T. Repair of articular cartilage defect by autologous transplantation of basic fibroblast growth factor gene-transduced chondrocytes with adeno-associated virus vector. Arthritis Rheum. 2005. January;52(1):164–70. [DOI] [PubMed] [Google Scholar]
- 66.Madry H, Zurakowski D, Trippel SB. Overexpression of human insulin-like growth factor-I promotes new tissue formation in an ex vivo model of articular chondrocyte transplantation. Gene Ther. 2001. October;8(19):1443–9. [DOI] [PubMed] [Google Scholar]
- 67.Hidaka C, Goodrich LR, Chen CT, Warren RF, Crystal RG, Nixon AJ. Acceleration of cartilage repair by genetically modified chondrocytes over expressing bone morphogenetic protein-7. J Orthop Res. 2003. July;21(4):573–83. [DOI] [PubMed] [Google Scholar]
- 68.Goodrich LR, Hidaka C, Robbins PD, Evans CH, Nixon AJ. Genetic modification of chondrocytes with insulin-like growth factor-1 enhances cartilage healing in an equine model. J Bone Joint Surg Br. 2007. May;89(5):672–85. [DOI] [PubMed] [Google Scholar]
- 69.Kaul G, Cucchiarini M, Arntzen D, Zurakowski D, Menger MD, Kohn D, Trippel SB, Madry H. Local stimulation of articular cartilage repair by transplantation of encapsulated chondrocytes overexpressing human fibroblast growth factor 2 (FGF-2) in vivo. J Gene Med. 2006. January;8(1):100–11. [DOI] [PubMed] [Google Scholar]
- 70.Orth P, Kaul G, Cucchiarini M, Zurakowski D, Menger MD, Kohn D, Madry H. Transplanted articular chondrocytes co-overexpressing IGF-I and FGF-2 stimulate cartilage repair in vivo. Knee Surg Sports Traumatol Arthrosc. 2011. December;19(12):2119–30. Epub 2011 Feb 25. [DOI] [PubMed] [Google Scholar]
- 71.Steinert AF, Palmer GD, Pilapil C, Nöth U, Evans CH, Ghivizzani SC. Enhanced in vitro chondrogenesis of primary mesenchymal stem cells by combined gene transfer. Tissue Eng Part A. 2009. May;15(5):1127–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Palmer GD, Steinert A, Pascher A, Gouze E, Gouze JN, Betz O, Johnstone B, Evans CH, Ghivizzani SC. Gene-induced chondrogenesis of primary mesenchymal stem cells in vitro. Mol Ther. 2005. August;12(2):219–28. [DOI] [PubMed] [Google Scholar]
- 73.Cucchiarini M, Madry H. Overexpression of human IGF-I via direct rAAV-mediated gene transfer improves the early repair of articular cartilage defects in vivo. Gene Ther. 2014. September;21(9):811–9. Epub 2014 Jul 3. [DOI] [PubMed] [Google Scholar]
- 74.Cucchiarini M, Madry H, Ma C, Thurn T, Zurakowski D, Menger MD, Kohn D, Trippel SB, Terwilliger EF. Improved tissue repair in articular cartilage defects in vivo by rAAV-mediated overexpression of human fibroblast growth factor 2. Mol Ther. 2005. August;12(2):229–38. [DOI] [PubMed] [Google Scholar]
- 75.Cucchiarini M, Asen AK, Goebel L, Venkatesan JK, Schmitt G, Zurakowski D, Menger MD, Laschke MW, Madry H. Effects of TGF-β Overexpression via rAAV Gene Transfer on the Early Repair Processes in an Osteochondral Defect Model in Minipigs. Am J Sports Med. 2018. July;46(8):1987–96. Epub 2018 May 24. [DOI] [PubMed] [Google Scholar]
- 76.Pascher A, Palmer GD, Steinert A, Oligino T, Gouze E, Gouze JN, Betz O, Spector M, Robbins PD, Evans CH, Ghivizzani SC. Gene delivery to cartilage defects using coagulated bone marrow aspirate. Gene Ther. 2004. January;11(2):133–41. [DOI] [PubMed] [Google Scholar]
- 77.Sieker JT, Kunz M, Weißenberger M, Gilbert F, Frey S, Rudert M, Steinert AF. Direct bone morphogenetic protein 2 and Indian hedgehog gene transfer for articular cartilage repair using bone marrow coagulates. Osteoarthritis Cartilage. 2015. March;23(3):433–42. Epub 2014 Nov 13. [DOI] [PubMed] [Google Scholar]
- 78.Cucchiarini M, Madry H. Biomaterial-guided delivery of gene vectors for targeted articular cartilage repair. Nat Rev Rheumatol. 2019. January;15(1):18–29. [DOI] [PubMed] [Google Scholar]
- 79.Wehling N, Palmer GD, Pilapil C, Liu F, Wells JW, Müller PE, Evans CH, Porter RM. Interleukin-1beta and tumor necrosis factor alpha inhibit chondrogenesis by human mesenchymal stem cells through NF-kappaB-dependent pathways. Arthritis Rheum. 2009. March;60(3):801–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haupt JL, Frisbie DD, McIlwraith CW, Robbins PD, Ghivizzani S, Evans CH, Nixon AJ. Dual transduction of insulin-like growth factor-I and interleukin-1 receptor antagonist protein controls cartilage degradation in an osteoarthritic culture model. J Orthop Res. 2005. January;23(1):118–26. [DOI] [PubMed] [Google Scholar]
- 81.Evans C Potential biologic therapies for the intervertebral disc. J Bone Joint Surg Am. 2006. April;88(Suppl 2):95–8. [DOI] [PubMed] [Google Scholar]
- 82.Nishida K, Kang JD, Suh JK, Robbins PD, Evans CH, Gilbertson LG. Adenovirus-mediated gene transfer to nucleus pulposus cells. Implications for the treatment of intervertebral disc degeneration. Spine (Phila Pa 1976). 1998. November 15;23(22):2437–42, discussion 2443. [DOI] [PubMed] [Google Scholar]
- 83.Leckie SK, Bechara BP, Hartman RA, Sowa GA, Woods BI, Coelho JP, Witt WT, Dong QD, Bowman BW, Bell KM, Vo NV, Wang B, Kang JD. Injection of AAV2-BMP2 and AAV2-TIMP1 into the nucleus pulposus slows the course of intervertebral disc degeneration in an in vivo rabbit model. Spine J. 2012. January;12(1):7–20. Epub 2011 Oct 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhao Z, Li S, Huang H, Fang J, Wei H, Xi Y. In vivo delivery of MMP3-shRNA and Sox9 lentivirus cocktail enhances matrix synthesis to prevent lumbar disc degeneration. Adv Clin Exp Med. 2020. June;29(6):639–47. [DOI] [PubMed] [Google Scholar]
- 85.Nishida K, Gilbertson LG, Moon S, Robbins PD, Evans CH, Kand JD. Transgene expression one year after in vivo adenovirus-mediated gene transfer to the intervertebral discs of an immunocompetent animal. Presented at a poster session at the 46th Annual Meeting of the Orthopaedic Research Society; 2000. Mar 12-15; Orlando, Florida. Abstract number 341. [Google Scholar]
- 86.Nishida K, Kang JD, Gilbertson LG, Moon SH, Suh JK, Vogt MT, Robbins PD, Evans CH. Modulation of the biologic activity of the rabbit intervertebral disc by gene therapy: an in vivo study of adenovirus-mediated transfer of the human transforming growth factor beta 1 encoding gene. Spine (Phila Pa 1976). 1999. December 1;24(23):2419–25. [DOI] [PubMed] [Google Scholar]
- 87.Liu Y, Yu T, Ma XX, Xiang HF, Hu YG, Chen BH. Lentivirus-mediated TGF-β3, CTGF and TIMP1 gene transduction as a gene therapy for intervertebral disc degeneration in an in vivo rabbit model. Exp Ther Med. 2016. April;11(4):1399–404. Epub 2016 Feb 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen S, Luo M, Kou H, Shang G, Ji Y, Liu H. A Review of Gene Therapy Delivery Systems for Intervertebral Disc Degeneration. Curr Pharm Biotechnol. 2020;21(3):194–205. [DOI] [PubMed] [Google Scholar]
- 89.Ju DG, Kanim LE, Bae HW. Intervertebral Disc Repair: Current Concepts. Global Spine J. 2020. April;10(2)(Suppl):130S–6S. Epub 2020 May 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Docheva D, Müller SA, Majewski M, Evans CH. Biologics for tendon repair. Adv Drug Deliv Rev. 2015. April;84:222–39. Epub 2014 Nov 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tang JB, Zhou YL, Wu YF, Liu PY, Wang XT. Gene therapy strategies to improve strength and quality of flexor tendon healing. Expert Opin Biol Ther. 2016;16(3):291–301. Epub 2016 Feb 6. [DOI] [PubMed] [Google Scholar]
- 92.Majewski M, Porter RM, Betz OB, Betz VM, Clahsen H, Flückiger R, Evans CH. Improvement of tendon repair using muscle grafts transduced with TGF-β1 cDNA. Eur Cell Mater. 2012. February 16;23:94–101, discussion 101-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wei X, Mao Z, Hou Y, Lin L, Xue T, Chen L, Wang H, Yu C. Local administration of TGFβ-1/VEGF165 gene-transduced bone mesenchymal stem cells for Achilles allograft replacement of the anterior cruciate ligament in rabbits. Biochem Biophys Res Commun. 2011. March 11;406(2):204–10. Epub 2011 Feb 15. [DOI] [PubMed] [Google Scholar]
- 94.Martinek V, Latterman C, Usas A, Abramowitch S, Woo SL, Fu FH, Huard J. Enhancement of tendon-bone integration of anterior cruciate ligament grafts with bone morphogenetic protein-2 gene transfer: a histological and biomechanical study. J Bone Joint Surg Am. 2002. July;84(7):1123–31. [DOI] [PubMed] [Google Scholar]
- 95.Zhang X, Ma Y, Fu X, Liu Q, Shao Z, Dai L, Pi Y, Hu X, Zhang J, Duan X, Chen W, Chen P, Zhou C, Ao Y. Runx2-Modified Adipose-Derived Stem Cells Promote Tendon Graft Integration in Anterior Cruciate Ligament Reconstruction. Sci Rep. 2016. January 8;6:19073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bez M, Kremen TJ, Tawackoli W, Avalos P, Sheyn D, Shapiro G, Giaconi JC, Ben David S, Snedeker JG, Gazit Z, Ferrara KW, Gazit D, Pelled G. Ultrasound-Mediated Gene Delivery Enhances Tendon Allograft Integration in Mini-Pig Ligament Reconstruction. Mol Ther. 2018. July 5;26(7):1746–55. Epub 2018 Apr 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cucchiarini M, McNulty AL, Mauck RL, Setton LA, Guilak F, Madry H. Advances in combining gene therapy with cell and tissue engineering-based approaches to enhance healing of the meniscus. Osteoarthritis Cartilage. 2016. August;24(8):1330–9. Epub 2016 Apr 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang H, Leng P, Zhang J. Enhanced meniscal repair by overexpression of hIGF-1 in a full-thickness model. Clin Orthop Relat Res. 2009. December;467(12):3165–74. Epub 2009 Jun 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Goto H, Shuler FD, Lamsam C, Moller HD, Niyibizi C, Fu FH, Robbins PD, Evans CH. Transfer of lacZ marker gene to the meniscus. J Bone Joint Surg Am. 1999. July;81(7):918–25. [DOI] [PubMed] [Google Scholar]
- 100.Steinert AF, Palmer GD, Capito R, Hofstaetter JG, Pilapil C, Ghivizzani SC, Spector M, Evans CH. Genetically enhanced engineering of meniscus tissue using ex vivo delivery of transforming growth factor-beta 1 complementary deoxyribonucleic acid. Tissue Eng. 2007. September;13(9):2227–37. [DOI] [PubMed] [Google Scholar]
- 101.Witlox MA, Lamfers ML, Wuisman PI, Curiel DT, Siegal GP. Evolving gene therapy approaches for osteosarcoma using viral vectors: review. Bone. 2007. April;40(4):797–812. Epub 2006 Dec 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Niyibizi C, Wang S, Mi Z, Robbins PD. Gene therapy approaches for osteogenesis imperfecta. Gene Ther. 2004. February;11(4):408–16. [DOI] [PubMed] [Google Scholar]
- 103.Yang SY, Mayton L, Wu B, Goater JJ, Schwarz EM, Wooley PH. Adeno-associated virus-mediated osteoprotegerin gene transfer protects against particulate polyethylene-induced osteolysis in a murine model. Arthritis Rheum. 2002. September;46(9):2514–23. [DOI] [PubMed] [Google Scholar]
- 104.Ulrich-Vinther M, Carmody EE, Goater JJ, S balle K, O’Keefe RJ, Schwarz EM. Recombinant adeno-associated virus-mediated osteoprotegerin gene therapy inhibits wear debris-induced osteolysis. J Bone Joint Surg Am. 2002. August;84(8):1405–12. [DOI] [PubMed] [Google Scholar]
- 105.Yang S, Wu B, Mayton L, Evans CH, Robbins PD, Wooley PH. IL-1Ra and vIL-10 gene transfer using retroviral vectors ameliorates particle-associated inflammation in the murine air pouch model. Inflamm Res. 2002. July;51(7):342–50. [DOI] [PubMed] [Google Scholar]
- 106.de Poorter JJ, Hoeben RC, Obermann WR, Huizinga TW, Nelissen RG. Gene therapy for the treatment of hip prosthesis loosening: adverse events in a phase 1 clinical study. Hum Gene Ther. 2008. October;19(10):1029–38. [DOI] [PubMed] [Google Scholar]
- 107.de Poorter JJ, Hoeben RC, Hogendoorn S, Mautner V, Ellis J, Obermann WR, Huizinga TW, Nelissen RG. Gene therapy and cement injection for restabilization of loosened hip prostheses. Hum Gene Ther. 2008. January;19(1):83–95. [DOI] [PubMed] [Google Scholar]
- 108.Evans CH, Ghivizzani SC, Robbins PD. Orthopedic gene therapy—lost in translation? J Cell Physiol. 2012. February;227(2):416–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Evans CH, Ghivizzani SC, Robbins PD. The 2003 Nicolas Andry Award. Orthopaedic gene therapy. Clin Orthop Relat Res. 2004. December;(429):316–29. [DOI] [PubMed] [Google Scholar]
- 110.Evans CH. Orthopaedics: gene therapy’s dark horse. Gene Ther. 2004. February;11(4):343. [DOI] [PubMed] [Google Scholar]