Purpose of review
The resistance of immune checkpoint inhibitors (ICIs) has become an obstacle to further improve the survival of patients with advanced cancer. This review provides an overview of recent advances in primary resistance mechanisms of ICIs.
Recent findings
With the improvement of study approach, new characteristics and trends have emerged in the classification of tumor immune subtypes. The effects of germline genetic on tumor microenvironment and the efficacy of immunotherapy have been further studied. Exosomal programmed death-ligand 1 (PD-L1) is an increasing focus of research in primary resistance mechanisms of ICIs. In addition to antibiotics and steroids, the influence of other concomitant medications on the efficacy of ICIs has recently gained more attention.
Summary
Exploring the resistance mechanisms of ICIs is one of the great challenges in the field of tumor immunotherapy. Continued work to understand the resistance mechanism of ICIs is ongoing.
Keywords: exosomal programmed death-ligand 1, germline genetic, immune checkpoint inhibitors, immune subtype, medications, resistance
INTRODUCTION
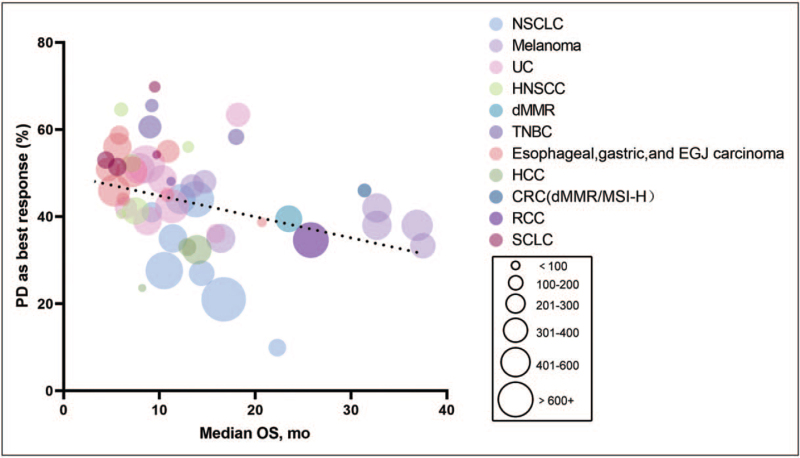
The emergence of immune checkpoint inhibitors (ICIs) has greatly improved the survival of patients with advanced cancer. However, resistance of ICIs has created a bottleneck in the application of ICIs. According to the criterions of the American Society for Immunotherapy of Cancer [1▪▪], primary resistance for advanced patients receiving ICIs needs to meet the following three requirements: (1) drug exposure ≥6 weeks, (2) progressive disease (PD) or stable disease (SD) for <6 months as best response, (3) confirmatory scan for PD is required at least 4 weeks after initial disease progression. An important feature of the definition of primary resistance is to be able to reflect the population that does not benefit from initial immunotherapy, which is essential to distinguish patients who do not benefit from initial and longer exposure to monotherapy of programmed death receptor 1 (PD-1)/programmed death-ligand 1 (PD-L1) inhibitors. We only summarized the rate of ‘PD as best response ‘, because it is difficult to distinguish the patients with the best response of SD < 6months based on the current literature. It can be seen that the rate of ‘PD as best response’ of Hodgkin's lymphoma is the lowest, less than 15%, whereas the rates of other tumors, including melanoma, nonsmall cell lung cancer (NSCLC), urothelial carcinoma (UC) and hepatocellular carcinoma (HCC) and more, are generally high (Table 1). It appears to be a negative relationship between the rate of ‘PD as best response’ and median overall survival (OS) (Fig. 1). It is important to note that the actual proportion of patients with primary resistance of ICIs is higher than our data. However, the response and prognosis of the patients with PD in our statistics are much worse.
Table 1.
The rate of ‘PD as best response’ and the median overall survival of cancer patients treated with ICIs in clinical trials
| Cancer type | Trial Name | Group number | Treatment | Line of Therapy | Median OS (95% CI), mo | ORR (%) | PD as best response (%) | Reference |
| NSCLC | Keynote 001 | 101 | Pembrolizumab(treatment-naïve) | 1 | 22.3 (17.1–32.3) | 41.6 | 9.9 | [2,3] |
| Keynote 001 | 449 | Pembrolizumab(previously treated) | 2+ | 10.5 (8.6–13.2) | 22.9 | 27.6 | [2,3] | |
| Keynote 042 | 637 | Pembrolizumab | 1 | 16.7 (13.9–19.7) | 27 | 21 | [4] | |
| OAK | 425 | Atezolizumab | 2+ | 13 8 (11 8–15 7) | 14 | 44 | [5,6] | |
| CheckMate 057 (nonsquamous) | 292 | Nivolumab | 2+ | 12.2 (9.7–15.0) | 19 | 44 | [7] | |
| CheckMate 017 (squamous) | 135 | Nivolumab | 2+ | 9.2 (7.3–13.3) | 20 | 41 | [8] | |
| CheckMate 026 | 211 | Nivolumab | 1 | 14.4 (11.7–17.4) | 26 | 27 | [9] | |
| Javelin 200 Lung | 264 | Avelumab | 2+ | 11 4 (9 4–13 9) | 19 | 35 | [10] | |
| Melanoma | Keynote 002 | 180 | Pembrolizumab(2mg/kg) | 2+ | 13.4 (11.0–16.4) | 21 | 47 | [11,12] |
| Keynote 002 | 181 | Pembrolizumab(10mg/kg) | 2+ | 14.7 (11.3–19.5) | 26 | 48 | [11,12] | |
| Keynote 006 | 277 | Pembrolizumab(10mg/kg Q3W) | 1+ | 32.7 (24 5–41.6) | 36 | 42 | [13,14] | |
| Keynote 006 | 279 | Pembrolizumab(10mg/kg Q2W) | 1+ | 32.7 (24 5–41.6) | 37 | 38 | [13,14] | |
| CheckMate 037 | 272 | Nivolumab | 2+ | 16.4 (12.9–20.3) | 31.7 | 35 | [15,16] | |
| CheckMate 066 | 210 | Nivolumab | 1 | 37.5 (25.5-NR) | 42.9 | 33.3 | [17,18] | |
| CheckMate 067 | 316 | Nivolumab | 1 | 36.9 (28.2–58.7) | 45 | 38 | [19–21] | |
| UC | Keynote 052 | 370 | Pembrolizumab | 1 | 11.3 (9.7–13.1) | 28.6 | 42.4 | [22,23] |
| Keynote 045 | 270 | Pembrolizumab | 2+ | 10.3 (8.0–11.8) | 21.1 | 48.5 | [24] | |
| IMvigor210 | 119 | Atezolizumab | 1 | 15.9 (10.4-NE) | 23 | 36.1 | [25] | |
| IMvigor210 Cohort2 | 310 | Atezolizumab | 2+ | 7.9 (6.6–9.3) | 15 | 51 | [26] | |
| IMvigor211 | 467 | Atezolizumab | 2+ | 8.6 (7.8–9.6) | 13 4 | 52 | [27] | |
| CheckMate 275 | 265 | Nivolumab | 2+ | 8.74 (6.05-NR) | 19.6 | 39 | [28] | |
| Study 1108 | 191 | Durvalumab | 2+ | 18.2 (8.1-NE) | 17.8 | 63.4 | [29] | |
| JAVELIN Solid Tumor | 161 | Avelumab | 2+ | 6.5 (4.8–9.5) | 17 | 42 | [30] | |
| HNSCC | Keynote 012 | 45 | Pembrolizumab | 2+ | 13 (5-NR) | 18 | 56 | [31] |
| CheckMate 141 | 240 | Nivolumab | 2+ | 7 0.5 (5.5–9.1) | 13.3 | 41.3 | [32,33] | |
| CONDOR | 65 | Durvalumab | 2+ | 6.0 (4.0–1.3) | 9.2 | 64.6 | [34] | |
| HAWK | 111 | Durvalumab | 2+ | 7.1 (4.9–9.9) | 16.2 | 52.3 | [35] | |
| NCT01375842 | 32 | Atezolizumab | 1+ | 6.0 (0.5–51.6) | 22 | 40.6 | [36] | |
| dMMR | Keynote 158 | 233 | Pembrolizumab | 2+ | 23.5 (13.5-NR) | 34.3 | 39.5 | [37] |
| TNBC | Keynote 012 | 27 | Pembrolizumab | 1+ | 11.2 (5.3-NR) | 18.5 | 48.1 | [38] |
| Keynote 086 cohort A | 170 | Pembrolizumab | 2+ | 9.0 (7.7–11.2) | 5.3 | 60.6 | [39] | |
| Keynote 086 cohort B | 84 | Pembrolizumab | 1 | 18 (12.9–23.0) | 21.4 | 58.3 | [40] | |
| JAVELIN Solid Tumor | 58 | Avelumab | 1+ | 9.2 (4.3-NE) | 5.2 | 65.5 | [41] | |
| ESCC | ATTRACTION-3 | 171 | Nivolumab | 2 | 10 9 (9 2–13 3) | 19 | 55 | [42] |
| ONO-4538-07 | 64 | Nivolumab | 3+ | 10.8 (7.4–13.3) | 17 | 45 | [43] | |
| ESCC/EAC/GEJC | Keynote 181 | 314 | Pembrolizumab | 2 | 7.1 (6.2–8.1) | 13.1 | 50.3 | [44] |
| Keynote 180 | 121 | Pembrolizumab | 3+ | 5.8 (4.5–7.2) | 9.9 | 58.7 | [45] | |
| GC/GEJC | JAVELIN Gastric 300 | 185 | Avelumab | 3 | 4.6 (3.6–5.7) | 2.2 | 50.8 | [46] |
| Keynote 059 cohort 3 | 31 | Pembrolizumab | 1 | 20.7 (9.2–20.7) | 25.8 | 38.7 | [47] | |
| ATTRACTION-2 | 330 | Nivolumab | 3+ | 5 26 (4 60–6 37) | 11.2 | 46 | [48] | |
| Keynote 059 cohort 1 | 259 | Pembrolizumab | 3 | 5.6 (4.3–6.9) | 11.6 | 56 | [49] | |
| GC/ESCA/GEJC | CheckMate 032 | 59 | Nivolumab | 2+ | 6.2 (3.4–12.4) | 7 | 44 | [50] |
| HCC | Keynote 224 | 104 | Pembrolizumab | 2 | 12 9 (9 7–15 5) | 17 | 33 | [51] |
| Keynote 240 | 278 | Pembrolizumab | 2 | 13.9 (11.6–16.0) | 18.3 | 32.4 | [52] | |
| CRC (dMMR/MSI-H) | Keynote 177 | 153 | Pembrolizumab | 1 | NR | 43.8 | 29.4 | [53] |
| Keynote 164 cohort A | 61 | Pembrolizumab | 3+ | 31.4 (21.4-NR) | 33 | 46 | [54] | |
| Keynote 164 cohort B | 63 | Pembrolizumab | 2+ | NR (19.2-NR) | 33 | 40 | [54] | |
| CheckMate 142 | 74 | Nivolumab | 2+ | NR | 32 | 28 | [55] | |
| RCC | CheckMate 025 | 410 | Nivolumab | 2+ | 25.8 (22.2–29.8) | 22.9 | 34.6 | [56,57] |
| SCLC | Keynote 028 | 24 | Pembrolizumab | 2+ | 9.7 (4.1-NR) | 33.3 | 54.2 | [58] |
| CheckMate 032 | 98 | Nivolumab | 2+ | 4.4 (3.0–9.3) | 10 | 53 | [59] | |
| CheckMate 032 | 109 | Nivolumab | 3+ | 5.6 (3.1–6.8) | 11.9 | 51.4 | [60] | |
| IFCT-1603 | 43 | Atezolizumab | 2+ | 9.5 (3.2–14.4) | 2.3 | 69.8 | [61] | |
| cHL | CheckMate 039 | 23 | Nivolumab | 3+ | NR | 87 | 0 | [62] |
| CheckMate 205 | 243 | Nivolumab | 2+ | NR | 69 | 9 | [63] | |
| Keynote 013 | 31 | Pembrolizumab | 3+ | NR | 65 | 13 | [64] | |
| Keynote 087 | 210 | Pembrolizumab | 4+ | NR | 69 | 14.3 | [65] |
ICIs, immune checkpoint inhibitors; OS, overall survival; ORR, objective response rate; PD, progressive disease; NSCLC, nonsmal cell lung cancer; UC, urothelial carcinoma; HNSCC, head and neck squamoucel carcinom; dMMR, deficient mismatch repair tumors; TNBC, triple-negative breast cancer; ESCC, esophageal squamous cell carcinoma; EAC, esophageal adenocarcinoma; GEJC, gastroesophageal junction cancer; GC, gastric cancer; ESCA, esophageal carcinoma; HCC, hepatocellular carcinoma; CRC, colorectal cancer; dMMR, deficient mismatch repair; MSI-H, microsatellite instability-high; RCC, renal cell carcinoma, SCLC, smal cell lung cancer; cHL, classical Hodgkin lymphoma; NR, not rearch; NE, not estimable.
FIGURE 1.
The rate of ‘PD as best response’ and the median overall survival of cancer patients treated with immune checkpoint inhibitors (ICIs). The colored circles represent different tumor types, and the size of the circles represents the number of cancer patients. Trials that did not reach the median overall survival in Table 1 are not included in the figure.
Box 1.
no caption available
Exploring the mechanisms of ICIs resistance has become one of the significant challenges in the field of tumor immunotherapy. The known and putative mechanisms of primary resistance to ICIs include: lack of antigen mutations or tumor antigen expression, loss of human leukocyte antigen expression, mitogen-activated protein kinase pathway activation, loss of phosphatase and tensin homolog (PTEN) expression leads to enhancement of phosphatidylinositide 3-kinases (PI3K) signaling pathway; WNT/β-catenin signaling pathway activation; lack of interferon-γ (INF-γ) signaling pathway, mutation or deletion of INF-γ signaling pathway-related receptor chains janus kinase 1 (JAK1), JAK2, signal transducer and activators of transcription (STAT) and INF regulatory factor 1, mutation of the epidermal growth factor receptor/anapastic lymphoma kinase, and constitutive PD-L1 expression. Tumor immune microenvironment components, such as myeloid-derived suppressor cells, regulatory T cells (Tregs), M2 type macrophages and immunosuppressive substances. In addition, many host factors have been identified to be associated with the efficacy of ICIs.
MECHANISMS OF PRIMARY RESISTANCE OF IMMUNE CHECKPOINT INHIBITORS
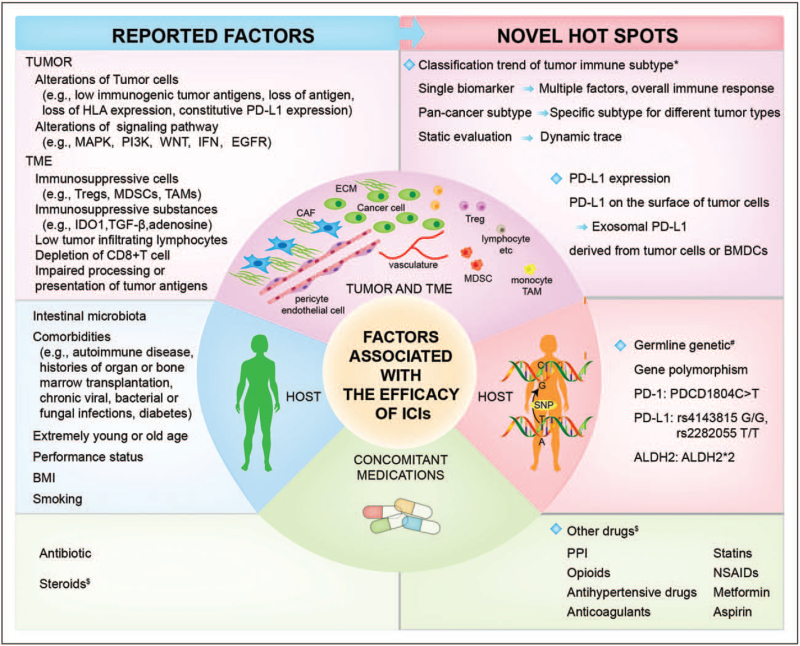
The underlying reason for primary resistance of ICIs is that immunotherapy cannot initiate an antitumor immune response, or tumor-induced immunosuppression cannot be relieved. In this review, we summarize the latest advances in mechanisms of primary resistance of ICIs and some other factors which are relatively easy to ignore (Fig. 2).
FIGURE 2.
Novel hot spots in tumor and TME, host, concomitant medications related to the efficacy and resistance of ICIs. ∗The current classification methods are no longer limited to traditional methods, such as tumor immunogenicity or PD-L1 expression and tumor infiltrating lymphocytes (TILs) or characteristics of tumor tissue sections. Multiple omics analysis has also become a very promising method. # Germline genetic has recently become a research hotspot that affects the efficacy of immunotherapy, we only list a few gene polymorphisms directly related to ICIs resistance. $ There is controversy about the effects of the above-mentioned drugs on the efficacy of ICIs. ICIs, immune checkpoint inhibitors; PD, progressive disease; TME, tumor microenvironment.
Tumor immune subtype
Since tumor immune response is a dynamic and complex process, it is difficult to rely on any single immune biomarker to accurately predict the prognosis of patients and chose suitable treatment plan. The nature of immune microenvironment is closely related to treatment response and prognosis, and immunosuppressive microenvironment is currently recognized as a major factor that mediates the primary resistance of tumor to ICIs. Researchers have divided tumor immune subtypes from different perspective, such as tumor immunogenicity or PD-L1 expression and tumor infiltrating lymphocytes (TILs) or characteristics of tumor tissue sections [66–68]. In 2018, based on immunogenomic analysis, researchers divided the tumor microenvironment (TME) into six immune subtypes [69]. Recently, by integrating transcriptomic and genomic data, researchers have described tumor structure, mutation burden, immune composition, antitumor immunity, immune suppression or escape mechanisms, and divided tumors into four different microenvironments [70▪▪]. The characteristic of immune-enriched, fibrotic (IE/F) melanomas subtype is that the high expression of functional gene expression signatures (FGES) related to angiogenesis and activation of cancer-associated fibroblasts (CAFs). The immune-enriched, nonfibrotic (IE) subtype is characterized by high degree of immune infiltration and significantly elevated cytolysis scores, the highest mutation burden, CD8+ T cell/Tregs ratio and M1/M2 macrophage ratio, JAK/STAT pathway activation increased. Fibrotic (F) and depletion (D) subtype have little or no leukocyte/lymphocyte infiltration, and D subtype contains the highest percentage of malignant cells. In contrast, melanoma classified as subtype F shows increased expression of FGES and increased CAF associated with angiogenesis. Fibroblasts become powerful immunosuppressive agents by secreting transforming growth factor-β (TGF-β). Patients with subtype IE melanoma have significantly longer OS and progress free survival (PFS) than subtype F and D, and patients with subtype F have the worst OS. Interestingly, the researchers dynamically observed the evolution of TME during treatment and found that people who responded to anti-PD-1 treatment mainly had IE/F and IE subtypes which remained unchanged during treatment or became immune enriched environment. In contrast, the TME of most patients who did not respond to PD-1 treatment seemed to maintain or tend to be immune-unfavorable TME, with weaker immune function and increasing fibrosis [70▪▪]. With the improvement of analysis methods and continuous increase of integrated factors, tumor immune subtypes have been further refined and the accuracy of prediction of therapeutic response and prognosis has been improved. What is more, the characteristics of tumor immune subtypes with poor prognosis can enable us to understand the resistance mechanism of ICIs more deeply, and it may be a breakthrough for researchers to find more efficient strategies to overcome resistance of ICIs.
Different tumors may have their own characteristics in the tumor immune microenvironment, which is of great importance for elucidating the distinction in the effects of different tumor types of ICIs. Many studies are trying to classify different immune subtypes for specific tumors to reveal the reasons for the differences in efficacy. Some researchers have classified lung adenocarcinoma into two distinct subtypes which were characterized by significant differences in survival outcomes. High-risk subtype is more likely to respond to ICIs treatment which is characterized by lower tumor immune dysfunction and exclusion score, up-regulated expression of PD-L1, higher tumor mutation burden, and significantly increased mutations in cell cycle regulatory factors CDK4/CDK6 and TP53 [71]. In gastroesophageal adenocarcinoma (GEA), the subtypes of severely inflammatory microsatellite instability (MSI) or Epstein–Barr virus positive respond well to treatment with ICIs, whereas chromosomal instable (CIN) and diffuse/genome-stable (GS) have a significantly lower response to ICIs. Further studies have found that CIN-GEAs not only have a lower density of CD8+ T cell, but they are mainly present at the invasive edge, whereas CD68+ macrophages were more evenly distributed within the tumor, indicating that T cell exclusion is the main mechanism of immunosuppression but not T cell suppression. In addition, the immunological ‘cold’ CIN GEAs was characterized by the enrichment of MYC and cell cycle pathways including CCNE1 amplification. The GS subtype showed enrichment of CD4+ T cells, macrophages and B cells, and tertiary lymphoid structure was seen in about 50% of cases [72▪▪]. These may provide new directions for overcoming resistance of ICIs. Gastric cancer is classified into immune-activation, immunosuppressive and nonimmune subtypes. Immunosuppressive subtype has high immune infiltration, stromal enrichment and activation of TGF-β signaling pathway, which is related to the nonresponse of checkpoint blocking therapy, and may be suitable for anti-PD-L1 and anti-TGF-β combined therapy [73]. The above results not only illustrate the heterogeneity of the immune environment of different tumors, but also provide opportunities for more personalized targeted or combined immunotherapy.
GERMLINE GENETIC
There is growing evidence that host immunity is affected by inherited factors. Genetic germline factors may affect cancer immune responsiveness (CIR) in many ways, such as mutations in gene involved in life style habits or DNA repair genes, polymorphisms of genes related to INF signaling, T and B cell differentiation, variants in genes controlling antigen presentation and related to the function of macrophages, natural killer (NK) cells and granulocyte [74]. Recently, the question of whether PD-(L)1 gene polymorphism affects the efficacy of ICIs has received much attention. It has been reported that the OS of patients with the germline variant PDCD1804C>T (rs2227981) deteriorated significantly, and the 3-year survival rate was 51.8%, whereas that of wild-type patients was 71.0% (OR 2.366; 95% CI 1.111–5.036; P = 0.026). Initial studies on mechanism have shown that this single nucleotide polymorphism may affect the clinical efficacy of ICIs by reducing the transcription initiation and expression of PD-1 in T cells [75]. Compared with A/G genotype, patients with PD1.3 (rs11568821) G/G genotype have a higher complete response (16.5% vs. 2.6%) [76]. PD-L1 rs4143815 G/G and rs2282055 T/T are associated with worse objective response rate (ORR) and PFS in NSCLC patients receiving nivolumab [77–79]. Aldehyde dehydrogenase 2 (ALDH2) serves a key role in the detoxification of endogenous acetaldehyde. ALDH2∗2 is a variant allele of ALDH2 polymorphism rs671, which provoked reduced enzyme activity. ALDH2∗2 can enhance the presentation of tumor antigens caused by acetaldehyde-induced DNA damage, whereas inhibiting peripheral blood T cell count and T cell activation. ALDH2∗2 may be a negative predictor of the short-term prognosis of ICIs in thoracic malignancies. The best response rate of rs671(−) patients to ICIs (PR/SD/PD) was 36%/50%/14%, whereas that of rs671(+) patients was relatively lower (27%/29%/45%) (P = 0.002), the hazard ratio of disease progression within 6 months of rs671(+) patients was much higher than rs671(−). Researchers speculated that ALDH2∗2 inhibited the PI3K-Akt pathway in T cells through the accumulation of endogenous aldehydes, which negatively affected the initial efficacy of ICIs [80]. Recent studies have shown that germline gene variations impact the richness of immune cells and infiltration in tumor, which significantly affect the composition and functional localization of tumor immune microenvironment. Some loci of immune traits with significant heritability are related to leukocytes subset enrichment and IFN signal, which may affect the effect of immunotherapy [81▪▪]. The above-mentioned initial research results aroused our keen interest to explore the key molecular mechanisms of germline genetic variation that may regulate antitumor immunity. In the future, combining germline data with somatic alterations, epigenetics and other information may improve the accuracy of CIR prediction and provide new targets for immunotherapy.
EXOSOMAL PROGRAMMED DEATH-LIGAND 1
Many studies have shown that exosomal PD-L1 derived from tumor cells can also inhibit the activation of CD8+ T cells. In addition, the exosomal PD-L1 acquired more characteristics than PD-L1 on the surface of tumor cells and may play a role in tumor lymphatic metastasis [82–85]. Some studies have suggested that the exosomal expression of PD-L1 is one of the mechanisms of primary resistance of ICIs. On one hand, PD-L1 inhibitors can bind to exosomal PD-L1, resulting in inability to inhibit PD-L1 on the surface of tumor cells or weakening of the inhibitory effect, and on the other hand, exosomal PD-L1 can directly bind to PD-1 on effector T cells. Both of the above conditions will affect the blocking effect of the antibody, leading to the persistence of PD-L1-mediated immunosuppression [86▪▪]. A recent study revealed that in addition to tumor cells, exosome of bone marrow-derived cells (BMDCs) can also carry PD-L1 in tumor-bearing mice, which has biological functions and can inhibit the proliferation and activation of CD8+ T cells both in vivo and in vitro, playing a major role in tumor immunosuppression. This may be useful to understand that some patients whose tumor cells do not express PD-L1 can also respond to anti-PD-1 treatment. Anti-PD-L1 therapy can abolish immunosuppression caused by exosomal PD-L1 of BMDCs, thereby activating antitumor immunity [87▪]. However, the PD-L1 expressed by exosomes derived from tumor cells has not always been the same as the PD-L1 expressed on tumor cells [83,88–92]. Whether the factors that regulate the expression of PD-L1 on the surface of tumor cells will regulate the level of exosomal PD-L1, and how to regulate it also need more research to clarify.
CONCOMITANT MEDICATIONS
Antibiotics and steroids are the most investigated concomitant medications during ICIs therapy. It is currently accepted that antibiotics use is an independent risk factor for primary resistance of ICIs [93], which leads to worse OS and PFS [94,95,96▪▪,97,98▪], lower ORR [99▪▪]and higher risk of progression and death [100▪]. The time window [101▪,102–111] and course [112] of antibiotics use may have varying degrees of impact on the efficacy of ICIs. Previous studies have shown that baseline or early use of steroids (equivalent to>10 mg of prednisone/d) was associated with worse ORR, OS and PFS [113–116,117▪]. However, recent studies suggest that only patients treated with steroids for tumor-related symptoms have deleterious effects on OS and PFS in NSCLC [118], intercurrent introduction of steroids for the treatment of cancer unrelated symptoms or immune-related adverse events (irAE) has no harmful effect on clinical outcomes [119–121,122▪].
Many other nononcological medications have been speculated to influnce the TME, and then affect the depth, duration of response, and survival of patients receiving ICIs (Table 2). Proton pump inhibitors (PPI) may cause immunosuppression by reducing the expression of adhesion molecules of inflammatory cell or changing the secretion of pro-inflammatory cytokines. On the other hand, PPI use can affect the intestinal microbiota composition, reduce the diversity of intestinal microbiota and induce positive and negative selection of specific bacterial species. For example, the use of PPI is related to the greater species abundance of bifidobacteria, which may increase the effectiveness of ICIs, but it also leads to the decrease of the alpha diversity of the gut microbiota, which seems to be related to the higher response rate of melanoma patients treated with ICIs [96▪▪,97,98▪,100▪,128,129]. The analgesic effect of opioids is achieved by targeting μ receptors in the central nervous system, but opioid receptors are also expressed on intestinal epithelial cells and immune cells, which means that opioids may cause changes in the intestinal microflora and alter immune response. Therefore, it is not surprising that the exposure of opioids during ICIs treatment will impact the effect of immunotherapy. However, it is also necessary to consider that patients taking opioids may have lower body mass index, higher prevalence of alcohol consumption and, and worse Eastern Cooperative Oncology Group performance [97]. The impact of antihypertensive drugs on the efficacy of ICIs is not consistent in the literature [123,124,125▪]. One of the papers reported that patients using angiotensin-converting enzyme inhibitors (ACEI) were in an immunosuppressive state with decrease of M1 macrophages, activated mast cells, NK cells and memory activated T cells. Captopril induced the expression of M2 marker CD206, when monocytes were involved in the differentiation of M1 macrophages in vitro. Animal experiments showed the same results that the therapeutic effect of anti-PD-1 monoclonal antibody was inhibited when used in combination with captopril [125▪]. Current research is mainly focused on observing the effect of concomitant medications on the efficacy of ICIs. However, there are few studies describing the biological mechanism of these drugs affecting the effect of ICIs. It is urgent to clarify the possible mechanisms of the interaction between ICIs and concomitant medications.
Table 2.
The impact of concomitant medications on the efficacy of ICIs
| Reference (year) | Cancer type | ICIs | Concomitant medications | Effect of concomitant medications on ICIs | |
| [96▪▪] (2020) | NSCLC | Atezolizumab | PPIs (234/757) | PPI use was associated with shorter OS (9.6 vs. 14.5 months, HR 1.45, 95% CI 1.20–1.75, P = 0.0001) and PFS (1.9 vs. 2.8 months, HR 1.30, 95% CI 1.10–1.53, P = 0.001). | |
| [97] (2020) | NSCLC Kidney Bladder Melanoma Head and neck Others | Nivolumab Pembrolizumab Atezolizumab Nivolumab + Ipilimumab | PPIs (78/102) Opioids (55/102) | PPIs use did not affect clinical outcome of ICIs. Opioids use was significantly associated with shorter PFS (4.5 vs. 8.1 months, P = 0.010) and OS (8.6 vs. 26.3 months, P<0.001). | |
| [98▪] (2021) | NSCLC Melanoma Head and neck Renal and urothelial Others | Nivolumab Pembrolizumab Ipilimumab Nivolumab+ipilimumab | PPIs(149/372) Opioids(173/372) Metformin(17/372) NSAIDs(23/372) Statins(83/372) | AVKs (16/372) Levothyrox (40/372) Cholecalciferol(59/372/) Phloroglucinol(19/372) Antiarrhythmics(20/372) | PPIs use did not affect OS, but tumor response is lower (18.8% vs. 30.1%, P = 0.036). opioids use was significantly associated with shorter OS (36.6 vs. 126.4 months, P < 0.001) and lower ORR (16.2% vs. 33.7%, P < 0.001). Metformin use did not affect OS, but tumor response is higher (47.1% vs. 24.5%, P = 0.020). The use of NSAIDs, statins, AVK anticoagulants, levothyroxine, cholecalciferol, phloroglucinol, or antiarrhythmics did not affect OS. |
| [100▪] (2020) | NSCLC Melanoma Renal cell carcinoma Others | Pembrolizumab Nivolumab Atezolizumab Others | H2 antagonists(56/1012) PPIs(491/1012) Statins(196/1012) Aspirin(189/1012) Other lipid lowerings(48/1012) Anticoagulants(145/1012) | NSAIDs (59/1012) ACEI/ARBs(313/1012) Calcium antagonist(140/1012) Beta blockers (114/1012) Metformin (114/943) Opioids(68/921) | Baseline statins (HR 1.60, 95% CI 1.14–2.25, P = 0.0064), aspirin (HR 1.47, 95% CI 1.04–2.08, P = 0.0267) and β-blockers (HR 1.76, 95% CI 1.16–2.69, P = 0.0080) were associated with an increased ORR. Prophylactic gastric acid suppressants (HR 1.29, 95% CI 1.09–1.53, P = 0.0021), PPIs (HR 1.26, 95% CI 1.07–1.48, P = 0.0050), anticoagulants (HR 1.43, 95% CI 1.16–1.77, P = 0.0007) and opioids (HR 1.71, 95% CI 1.28–2.28, P = 0.0002) were associated with a significantly higher risk of disease progression. Prophylactic gastric acid suppressants (HR 1.29, 95% CI 1.06–1.57, P = 0.0091), PPI (HR 1.26, 95% CI 1.04–1.52, P = 0.0172), anticoagulants (HR 1.45,95% CI 1.14–1.84, P = 0.0024) and opioids (HR 1.53, 95% CI 1.11–2.11, P = 0.0098) were confirmed to have a significantly higher risk of death. |
| [116] (2020) | NSCLC | Nivolumab | PPIs(64/224) NSAIDs(45/224) | Statin(31/224) Metformin(18/224) | The risk of progression in patients who are not taking NSAIDs is 1.596 times that of patients taking NSAIDs.A possible positive effect of the concomitant use of NSAIDs at the initiation of nivolumab treatment was revealed. |
| [123] (2021) | NSCLC Renal cell carcinoma Urothelial cancers | Atezolizumab | Renin--angiotensin system inhibitor(604/2539) Other classes of antihypertensives | No statistically significant difference in OS (HR 0.92, 95% CI 0.79–1.07, P = 0.29), PFS (HR 0.95, 95% CI 0.84–1.08, P = 0.42) between renin--angiotensin system inhibitor users and nonusers. Other classes of antihypertensives were also not associated with survival. | |
| [124] (2021) | NSCLC | Anti-PD-1/PD-L1 Antibodies monotherapy | Renin–angiotensin system inhibitors(37/256) | The median PFS of patients treated with renin–angiotensin system inhibitors was significantly longer than that of patients treated without (HR = 0.59, 95% CI = 0.40–0.88). The median OS of patients treated with Renin–angiotensin system inhibitors tended to be longer than that of patients treated without (HR = 0.71, 95% CI = 0.45–1.11). | |
| [125▪] (2020) | NSCLC | Pembrolizumab Nivolumab Durvalumab | ACEI (22/178) | ACEI use was associated with shorter median PFS (1.97 vs. 2.56 months, HR = 1.8, 95% CI 1.1–2.8, P = 0.01). | |
| [126] (2020) | Advanced melanoma | Anti-PD-1 therapy | NSAIDs (122/330) Metformin(34/330) Beta blocker(65/330) | The use of NSAIDs has a tendency to improve PFS (median PFS 8.5 vs. 5.2 months; P = 0.054). Multivariate analysis did not reveal an association with NSAID, metformin or beta blockers with ORR, PFS, or OS. | |
| [127▪] (2021) | MPM NSCLC | PD-1 inhibitors | Statin(67/261) | statin use was associated with increased ORR (32% vs. 18%, P = 0.02), PFS (median 6.7 vs. 2.9 months, HR 0.57, 95% CI 0.39–0.83, P < 0.01), and OS (median 13.1 vs. 8.7 months, HR 0.67, 95% CI 0.45–1.00, P = 0.05) in an intensity-dependent manner. | |
ICIs, immune checkpoint inhibitors; CI, confidence interval; HR, hazard ratio; NSAIDs, nonsteroidal anti-inflammatory drugs; AVKs, antivitamin K; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blockers; MPM, malignant pleural mesothelioma.
Additionally, inter- and intra-class differences between PD-1 inhibitors and PD-L1 inhibitors, including molecular, pharmacodynamics and pharmacokinetics characteristics, will affect their efficacy [130–138]. For example, pembrolizumab seems to have the best affinity and engagement among PD-1 inhibitors. Avelumab seems to have the best affinity, and atezolizumab has the longest half-life among the PD-L1 inhibitors [130]. In some cases, antidrug antibody will neutralize the activity of the antibody, which is also a reason for resistance of ICIs in some patients [137].
CONCLUSION
The huge advantages of immunotherapy over traditional treatment have made it an effective treatment for various malignant tumors. However, drug resistance has created a bottleneck in the application of immunotherapy. At present, there are endless combination treatment strategies for drug resistance, but the successful clinical application is quite limited. In the future, it will be necessary to deeply understand the mechanism of resistance and adopt appropriate methods to avoid resistance in order to achieve better treatment effects.
Acknowledgements
None.
Financial support and sponsorship
This work was supported by the project fund of Shaanxi Province Science and Technology Key Projects (No.2021JZ-35) and National Natural Science Foundation of China (No.81702554).
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1▪▪.Kluger HM, Tawbi HA, Ascierto ML, et al. Defining tumor resistance to PD-1 pathway blockade: recommendations from the first meeting of the SITC Immunotherapy Resistance Taskforce. J Immunother Cancer 2020; 8:e000398. [DOI] [PMC free article] [PubMed] [Google Scholar]; For the first time, this article provides a clear definition of different types of drug resistance at different stages of ICIs treatment.
- 2.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of nonsmall-cell lung cancer. N Engl J Med 2015; 372:2018–2028. [DOI] [PubMed] [Google Scholar]
- 3.Garon EB, Hellmann MD, Rizvi NA, et al. Five-year overall survival for patients with advanced nonsmall-cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 study. J Clin Oncol 2019; 37:2518–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic nonsmall-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019; 393:1819–1830. [DOI] [PubMed] [Google Scholar]
- 5.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated nonsmall-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017; 389:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazieres J, Rittmeyer A, Gadgeel S, et al. Atezolizumab versus docetaxel in pretreated patients with nsclc: final results from the randomized phase 2 POPLAR and phase 3 OAK clinical trials. J Thorac Oncol 2021; 16:140–150. [DOI] [PubMed] [Google Scholar]
- 7.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous nonsmall-cell lung cancer. N Engl J Med 2015; 373:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell nonsmall-cell lung cancer. N Engl J Med 2015; 373:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carbone DP, Reck M, Paz-Ares L, et al. First-line nivolumab in stage IV or recurrent nonsmall-cell lung cancer. N Engl J Med 2017; 376:2415–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barlesi F, Vansteenkiste J, Spigel D, et al. Avelumab versus docetaxel in patients with platinum-treated advanced nonsmall-cell lung cancer (JAVELIN Lung 200): an open-label, randomised, phase 3 study. Lancet Oncol 2018; 19:1468–1479. [DOI] [PubMed] [Google Scholar]
- 11.Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015; 16:908–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamid O, Puzanov I, Dummer R, et al. Final analysis of a randomised trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. Eur J Cancer 2017; 86:37–45. [DOI] [PubMed] [Google Scholar]
- 13.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015; 372:2521–2532. [DOI] [PubMed] [Google Scholar]
- 14.Schachter J, Ribas A, Long GV, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 2017; 390:1853–1862. [DOI] [PubMed] [Google Scholar]
- 15.Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015; 16:375–384. [DOI] [PubMed] [Google Scholar]
- 16.Larkin J, Minor D, D’Angelo S, et al. Overall survival in patients with advanced melanoma who received nivolumab versus investigator's choice chemotherapy in CheckMate 037: a randomized, controlled, open-label phase III trial. J Clin Oncol 2018; 36:383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015; 372:320–330. [DOI] [PubMed] [Google Scholar]
- 18.Ascierto PA, Long GV, Robert C, et al. Survival outcomes in patients with previously untreated braf wild-type advanced melanoma treated with nivolumab therapy: three-year follow-up of a randomized phase 3 trial. JAMA Oncol 2019; 5:187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017; 377:1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hodi FS, Chiarion-Sileni V, Gonzalez R, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol 2018; 19:1480–1492. [DOI] [PubMed] [Google Scholar]
- 21.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2019; 381:1535–1546. [DOI] [PubMed] [Google Scholar]
- 22.Balar AV, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol 2017; 18:1483–1492. [DOI] [PubMed] [Google Scholar]
- 23.Vuky J, Balar AV, Castellano D, et al. Long-term outcomes in KEYNOTE -052: phase II study investigating first-line pembrolizumab in cisplatin-ineligible patients with locally advanced or metastatic urothelial cancer. J Clin Oncol 2020; 38:2658–2666. [DOI] [PubMed] [Google Scholar]
- 24.Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 2017; 376:1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017; 389:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016; 387:1909–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018; 391:748–757. [DOI] [PubMed] [Google Scholar]
- 28.Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 2017; 18:312–322. [DOI] [PubMed] [Google Scholar]
- 29.Powles T, O’Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open-label study. JAMA Oncol 2017; 3:e172411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel MR, Ellerton J, Infante JR, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol 2018; 19:51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol 2016; 17:956–965. [DOI] [PubMed] [Google Scholar]
- 32.Ferris RL, Blumenschein G, Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 2016; 375:1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferris RL, Blumenschein G, Jr, Fayette J, et al. Nivolumab vs investigator's choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol 2018; 81:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siu LL, Even C, Mesía R, et al. Safety and efficacy of durvalumab with or without tremelimumab in patients with PD-L1-low/negative recurrent or metastatic HNSCC: the phase 2 CONDOR randomized clinical trial. JAMA Oncol 2019; 5:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zandberg DP, Algazi AP, Jimeno A, et al. Durvalumab for recurrent or metastatic head and neck squamous cell carcinoma: Results from a single-arm, phase II study in patients with ≥25% tumour cell PD-L1 expression who have progressed on platinum-based chemotherapy. Eur J Cancer 2019; 107:142–152. [DOI] [PubMed] [Google Scholar]
- 36.Colevas AD, Bahleda R, Braiteh F, et al. Safety and clinical activity of atezolizumab in head and neck cancer: results from a phase I trial. Ann Oncol 2018; 29:2247–2253. [DOI] [PubMed] [Google Scholar]
- 37.Marabelle A, Le DT, Ascierto PA, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol 2020; 38:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nanda R, Chow LQ, Dees EC, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol 2016; 34:2460–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adams S, Schmid P, Rugo HS, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol 2019; 30:397–404. [DOI] [PubMed] [Google Scholar]
- 40.Adams S, Loi S, Toppmeyer D, et al. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: cohort B of the phase II KEYNOTE-086 study. Ann Oncol 2019; 30:405–411. [DOI] [PubMed] [Google Scholar]
- 41.Dirix LY, Takacs I, Jerusalem G, et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Res Treat 2018; 167:671–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kato K, Cho BC, Takahashi M, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019; 20:1506–1517. [DOI] [PubMed] [Google Scholar]
- 43.Kudo T, Hamamoto Y, Kato K, et al. Nivolumab treatment for oesophageal squamous-cell carcinoma: an open-label, multicentre, phase 2 trial. Lancet Oncol 2017; 18:631–639. [DOI] [PubMed] [Google Scholar]
- 44.Kojima T, Shah MA, Muro K, et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol 2020; 38:4138–4148. [DOI] [PubMed] [Google Scholar]
- 45.Shah MA, Kojima T, Hochhauser D, et al. Efficacy and safety of pembrolizumab for heavily pretreated patients with advanced, metastatic adenocarcinoma or squamous cell carcinoma of the esophagus: the phase 2 KEYNOTE-180 study. JAMA Oncol 2019; 5:546–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bang YJ, Ruiz EY, Van Cutsem E, et al. Phase III, randomised trial of avelumab versus physician's choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol 2018; 29:2052–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bang YJ, Kang YK, Catenacci DV, et al. Pembrolizumab alone or in combination with chemotherapy as first-line therapy for patients with advanced gastric or gastroesophageal junction adenocarcinoma: results from the phase II nonrandomized KEYNOTE-059 study. Gastric Cancer 2019; 22:828–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017; 390:2461–2471. [DOI] [PubMed] [Google Scholar]
- 49.Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol 2018; 4:e180013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Janjigian YY, Bendell J, Calvo E, et al. CheckMate-032 study: efficacy and safety of nivolumab and nivolumab plus ipilimumab in patients with metastatic esophagogastric cancer. J Clin Oncol 2018; 36:2836–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a nonrandomised, open-label phase 2 trial. Lancet Oncol 2018; 19:940–952. [DOI] [PubMed] [Google Scholar]
- 52.Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol 2020; 38:193–202. [DOI] [PubMed] [Google Scholar]
- 53.André T, Shiu KK, Kim TW, et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med 2020; 383:2207–2218. [DOI] [PubMed] [Google Scholar]
- 54.Le DT, Kim TW, Van Cutsem E, et al. Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: KEYNOTE-164. J Clin Oncol 2020; 38:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 2017; 18:1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015; 373:1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Motzer RJ, Escudier B, George S, et al. Nivolumab versus everolimus in patients with advanced renal cell carcinoma: Updated results with long-term follow-up of the randomized, open-label, phase 3 CheckMate 025 trial. Cancer 2020; 126:4156–4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ott PA, Elez E, Hiret S, et al. Pembrolizumab in patients with extensive-stage small-cell lung cancer: results from the phase Ib KEYNOTE-028 study. J Clin Oncol 2017; 35:3823–3829. [DOI] [PubMed] [Google Scholar]
- 59.Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016; 17:883–895. [DOI] [PubMed] [Google Scholar]
- 60.Ready N, Farago AF, de Braud F, et al. Third-line nivolumab monotherapy in recurrent SCLC: CheckMate 032. J Thorac Oncol 2019; 14:237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pujol JL, Greillier L, Audigier-Valette C, et al. A Randomized noncomparative phase II study of antiprogrammed cell death-ligand 1 atezolizumab or chemotherapy as second-line therapy in patients with small cell lung cancer: results from the IFCT-1603 trial. J Thorac Oncol 2019; 14:903–913. [DOI] [PubMed] [Google Scholar]
- 62.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 2015; 372:311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Armand P, Engert A, Younes A, et al. Nivolumab for relapsed/refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow-up of the multicohort single-arm phase II CheckMate 205 trial. J Clin Oncol 2018; 36:1428–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Armand P, Shipp MA, Ribrag V, et al. Programmed death-1 blockade with pembrolizumab in patients with classical Hodgkin lymphoma after brentuximab vedotin failure. J Clin Oncol 2016; 34:3733–3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen R, Zinzani PL, Fanale MA, et al. Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J Clin Oncol 2017; 35:2125–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sharma P, Allison JP. The future of immune checkpoint therapy. Science 2015; 348:56–61. [DOI] [PubMed] [Google Scholar]
- 67.Zhang Y, Chen L. Classification of advanced human cancers based on tumor immunity in the microenvironment (TIME) for cancer immunotherapy. JAMA Oncol 2016; 2:1403–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature 2017; 541:321–330. [DOI] [PubMed] [Google Scholar]
- 69.Thorsson V, Gibbs DL, Brown SD, et al. The immune landscape of cancer. Immunity 2018; 48:812–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70▪▪.Bagaev A, Kotlov N, Nomie K, et al. Conserved pan-cancer microenvironment subtypes predict response to immunotherapy. Cancer Cell 2021; 39:845–865. e7. [DOI] [PubMed] [Google Scholar]; By integrating transcriptomics and genomics data, comprehensive analysis and visualization are more helpful for the discovery of biomarkers and the personalization of treatment plans.
- 71.Wang Q, Li M, Yang M, et al. Analysis of immune-related signatures of lung adenocarcinoma identified two distinct subtypes: implications for immune checkpoint blockade therapy. Aging 2020; 12:3312–3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72▪▪.Derks S, de Klerk LK, Xu X, et al. Characterizing diversity in the tumor-immune microenvironment of distinct subclasses of gastroesophageal adenocarcinomas. Ann Oncol 2020; 31:1011–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article describes the significant heterogeneity of TME among different tumor subtypes, which may provide directions for the development of more precise antidrug resistance strategies.
- 73.Zhou YJ, Zhu GQ, Lu XF, et al. Identification and validation of tumour microenvironment-based immune molecular subgroups for gastric cancer: immunotherapeutic implications. Cancer Immunol Immunother 2020; 69:1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bedognetti D, Ceccarelli M, Galluzzi L, et al. Toward a comprehensive view of cancer immune responsiveness: a synopsis from the SITC workshop. J Immunother Cancer 2019; 7:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De With M, Hurkmans DP, Oomen-de Hoop E, et al. Germline variation in PDCD1 is associated with overall survival in patients with metastatic melanoma treated with anti-PD-1 monotherapy. Cancers 2021; 13:1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Parakh S, Musafer A, Paessler S, et al. PDCD1 polymorphisms may predict response to anti-PD-1 blockade in patients with metastatic melanoma. Front Immunol 2021; 12:672521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kula A, Dawidowicz M, Kiczmer P, et al. The role of genetic polymorphism within PD-L1 gene in cancer. Review. Exp Mol Pathol 2020; 116:104494. [DOI] [PubMed] [Google Scholar]
- 78.Nomizo T, Ozasa H, Tsuji T, et al. Clinical impact of single nucleotide polymorphism in PD-L1 on response to nivolumab for advanced nonsmall-cell lung cancer patients. Sci Rep 2017; 7:45124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Minari R, Bonatti F, Mazzaschi G, et al. PD-L1 SNPs as biomarkers to define benefit in patients with advanced NSCLC treated with immune checkpoint inhibitors. Tumori 2021; 3008916211014954.doi: 10.1177/03008916211014954. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 80.Matsumoto A, Nakashima C, Kimura S, et al. ALDH2 polymorphism rs671 is a predictor of PD-1/PD-L1 inhibitor efficacy against thoracic malignancies. BMC Cancer 2021; 21:584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81▪▪.Sayaman RW, Saad M, Thorsson V, et al. Germline genetic contribution to the immune landscape of cancer. Immunity 2021; 54:367–386. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article provides evidence for the common germline variant effects in tumor immune response and rare mutations in susceptibility genes that may affect tumor immune response.
- 82.Wang J, Zeng H, Zhang H, Han Y. The role of exosomal PD-L1 in tumor immunotherapy. Transl Oncol 2021; 14:101047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Poggio M, Hu T, Pai CC, et al. Suppression of exosomal PD-l1 induces systemic antitumor immunity and memory. Cell 2019; 177:414–427. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Diaz AA. Exosomal PD-L1 induces immunosuppressive nonclassical monocytes. Neuro Oncol 2020; 22:901–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen G, Huang AC, Zhang W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018; 560:382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86▪▪.Morrissey SM, Yan J. Exosomal PD-L1: roles in tumor progression and immunotherapy. Trends Cancer 2020; 6:550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article describes the mechanisms of exosome PD-L1 mediated immunosuppression, points out the good prospects and unanswered questions of using exosome PD-L1 as a marker of immunotherapy response.
- 87▪.Sun Y, Guo J, Yu L, et al. PD-L1+ exosomes from bone marrow-derived cells of tumor-bearing mice inhibit antitumor immunity. Cell Mol Immunol 2021; 18:2402–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reveals a new mechanism of tumor immune escape, in which PD-L1+exosomes from bone marrow-derived cells plays an important role.
- 88.Ayala-Mar S, Donoso-Quezada J, González-Valdez J. Clinical implications of exosomal PD-L1 in cancer immunotherapy. J Immunol Res 2021; 2021:8839978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Colombo M, Moita C, van Niel G, et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci 2013; 126:5553–5565. [DOI] [PubMed] [Google Scholar]
- 90.Monypenny J, Milewicz H, Flores-Borja F, et al. ALIX regulates tumor-mediated immunosuppression by controlling EGFR activity and PD-L1 presentation. Cell Rep 2018; 24:630–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci 2018; 75:193–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ostrowski M, Carmo NB, Krumeich S, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol 2010; 12:19–30. su1-13. [DOI] [PubMed] [Google Scholar]
- 93.Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018; 359:91–97. [DOI] [PubMed] [Google Scholar]
- 94.Wu Q, Liu J, Wu S, Xie X. The impact of antibiotics on efficacy of immune checkpoint inhibitors in malignancies: a study based on 44 cohorts. Int Immunopharmacol 2021; 92:107303. [DOI] [PubMed] [Google Scholar]
- 95.Hwang SR, Higgins A, Castillo Almeida NE, et al. Effect of antibiotic use on outcomes in patients with Hodgkin lymphoma treated with immune checkpoint inhibitors. Leuk Lymphoma 2021; 62:247–251. [DOI] [PubMed] [Google Scholar]
- 96▪▪.Chalabi M, Cardona A, Nagarkar DR, et al. Efficacy of chemotherapy and atezolizumab in patients with nonsmall-cell lung cancer receiving antibiotics and proton pump inhibitors: pooled post hoc analyses of the OAK and POPLAR trials. Ann Oncol 2020; 31:525–531. [DOI] [PubMed] [Google Scholar]; This comprehensive analysis evaluated the impact of ATB and PPI on the prognosis of patients randomized between ICI and chemotherapy, suggested that clinicians should be cautious when using ATB and PPI for patients treated with ICIs.
- 97.Iglesias-Santamaría A. Impact of antibiotic use and other concomitant medications on the efficacy of immune checkpoint inhibitors in patients with advanced cancer. Clin Transl Oncol 2020; 22:1481–1490. [DOI] [PubMed] [Google Scholar]
- 98▪.Gaucher L, Adda L, Séjourné A, Joachim C, et al. Associations between dysbiosis-inducing drugs, overall survival and tumor response in patients treated with immune checkpoint inhibitors. Ther Adv Med Oncol 2021; 13:17588359211000591. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study investigates whether a variety of drugs known to change the intestinal flora affect the clinical benefit of patients receiving ICIs treatment.
- 99▪▪.Derosa L, Routy B, Fidelle M, et al. Gut bacteria composition drives primary resistance to cancer immunotherapy in renal cell carcinoma patients. Eur Urol 2020; 78:195–206. [DOI] [PubMed] [Google Scholar]; This study suggests that TKIs and ATBs may affect the microbial composition and the success of immunotherapy in renal cell carcinoma patients.
- 100▪.Cortellini A, Tucci M, Adamo V, et al. Integrated analysis of concomitant medications and oncological outcomes from PD-1/PD-L1 checkpoint inhibitors in clinical practice. J Immunother Cancer 2020; 8:e001361. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article describes that baseline steroids, systemic antibiotics, and PPI have adverse effects on immunomodulation.
- 101▪.Hopkins AM, Kichenadasse G, Karapetis CS, et al. Concomitant antibiotic use and survival in urothelial carcinoma treated with atezolizumab. Eur Urol 2020; 78:540–543. [DOI] [PubMed] [Google Scholar]; This article shows that the use of antibiotics is associated with poorer survival outcomes in UC patients treated with atezolizumab.
- 102.Yang M, Wang Y, Yuan M, et al. Antibiotic administration shortly before or after immunotherapy initiation is correlated with poor prognosis in solid cancer patients: An up-to-date systematic review and meta-analysis. Int Immunopharmacol 2020; 88:106876. [DOI] [PubMed] [Google Scholar]
- 103.Guven DC, Acar R, Yekeduz E, et al. The association between antibiotic use and survival in renal cell carcinoma patients treated with immunotherapy: a multicenter study. Curr Probl Cancer 2021; 100760.doi: 10.1016/j.currproblcancer.2021.100760. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 104.Huang L, Chen X, Zhou L, et al. Antibiotic exposure windows and the efficacy of immune checkpoint blockers in patients with cancer: a meta-analysis. Ann Palliat Med 2021; 10:2709–2722. [DOI] [PubMed] [Google Scholar]
- 105.Chambers LM, Michener CM, Rose PG, et al. Impact of antibiotic treatment on immunotherapy response in women with recurrent gynecologic cancer. Gynecol Oncol 2021; 161:211–220. [DOI] [PubMed] [Google Scholar]
- 106.Tsikala-Vafea M, Belani N, Vieira K, et al. Use of antibiotics is associated with worse clinical outcomes in patients with cancer treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Int J Infect Dis 2021; 106:142–154. [DOI] [PubMed] [Google Scholar]
- 107.Yu Y, Zheng P, Gao L, et al. Effects of antibiotic use on outcomes in cancer patients treated using immune checkpoint inhibitors: a systematic review and meta-analysis. J Immunother 2021; 44:76–85. [DOI] [PubMed] [Google Scholar]
- 108.Pinato DJ, Howlett S, Ottaviani D, et al. Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer. JAMA Oncol 2019; 5:1774–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mohiuddin JJ, Chu B, Facciabene A, et al. Association of antibiotic exposure with survival and toxicity in patients with melanoma receiving immunotherapy. J Natl Cancer Inst 2021; 113:162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Derosa L, Hellmann MD, Spaziano M, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and nonsmall-cell lung cancer. Ann Oncol 2018; 29:1437–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Khan U, Ho K, Hwang EK, et al. Impact of use of antibiotics on response to immune checkpoint inhibitors and tumor microenvironment. Am J Clin Oncol 2021; 44:247–253. [DOI] [PubMed] [Google Scholar]
- 112.Tinsley N, Zhou C, Tan G, et al. Cumulative antibiotic use significantly decreases efficacy of checkpoint inhibitors in patients with advanced cancer. Oncologist 2020; 25:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Arbour KC, Mezquita L, Long N, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with nonsmall-cell lung cancer. J Clin Oncol 2018; 36:2872–2878. [DOI] [PubMed] [Google Scholar]
- 114.Scott SC, Pennell NA. Early use of systemic corticosteroids in patients with advanced NSCLC treated with nivolumab. J Thorac Oncol 2018; 13:1771–1775. [DOI] [PubMed] [Google Scholar]
- 115.Fucà G, Galli G, Poggi M, et al. Modulation of peripheral blood immune cells by early use of steroids and its association with clinical outcomes in patients with metastatic nonsmall cell lung cancer treated with immune checkpoint inhibitors. ESMO Open 2019; 4:e000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Svaton M, Zemanova M, Zemanova P, et al. Impact of concomitant medication administered at the time of initiation of nivolumab therapy on outcome in nonsmall cell lung cancer. Anticancer Res 2020; 40:2209–2217. [DOI] [PubMed] [Google Scholar]
- 117▪.Iorgulescu JB, Gokhale PC, Speranza MC, et al. Concurrent dexamethasone limits the clinical benefit of immune checkpoint blockade in glioblastoma. Clin Cancer Res 2021; 27:276–287. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article suggests that simultaneous dexamethasone treatment may be detrimental to immunotherapy for GBM patients.
- 118.Ricciuti B, Dahlberg SE, Adeni A, et al. Immune checkpoint inhibitor outcomes for patients with nonsmall-cell lung cancer receiving baseline corticosteroids for palliative versus nonpalliative indications. J Clin Oncol 2019; 37:1927–1934. [DOI] [PubMed] [Google Scholar]
- 119.De Giglio A, Mezquita L, Auclin E, et al. Impact of intercurrent introduction of steroids on clinical outcomes in advanced nonsmall-cell lung cancer (NSCLC) patients under immune-checkpoint inhibitors (ICI). Cancers 2020; 12:2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Petrelli F, Signorelli D, Ghidini M, et al. Association of steroids use with survival in patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Cancers 2020; 12:546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Marinelli D, Giusti R, Mazzotta M, et al. Palliative- and nonpalliative indications for glucocorticoids use in course of immune-checkpoint inhibition. Current evidence and future perspectives. Crit Rev Oncol Hematol 2021; 157:103176. [DOI] [PubMed] [Google Scholar]
- 122▪.Skribek M, Rounis K, Afshar S, et al. Effect of corticosteroids on the outcome of patients with advanced nonsmall cell lung cancer treated with immune-checkpoint inhibitors. Eur J Cancer 2021; 145:245–254. [DOI] [PubMed] [Google Scholar]; The results of this study show the complexity of the effects of steroids, and different reasons for medication may have distinct effects on clinical outcomes.
- 123.Kichenadasse G, Miners JO, Mangoni AA, et al. Effect of concomitant use of antihypertensives and immune check point inhibitors on cancer outcomes. J Hypertens 2021; 39:1274–1281. [DOI] [PubMed] [Google Scholar]
- 124.Tozuka T, Yanagitani N, Yoshida H, et al. Impact of renin-angiotensin system inhibitors on the efficacy of anti-PD-1/PD-L1 antibodies in NSCLC patients. Anticancer Res 2021; 41:2093–2100. [DOI] [PubMed] [Google Scholar]
- 125▪.Medjebar S, Truntzer C, Perrichet A, et al. Angiotensin-converting enzyme (ACE) inhibitor prescription affects nonsmall-cell lung cancer (NSCLC) patients response to PD-1/PD-L1 immune checkpoint blockers. Oncoimmunology 2020; 9:1836766. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article suggests that ACEI may be related to the impaired prognosis and tumor immunosuppressive status of advanced NSCLC patients treated with ICIs.
- 126.Wang DY, McQuade JL, Rai RR, et al. The impact of nonsteroidal anti-inflammatory drugs, beta blockers, and metformin on the efficacy of anti-PD-1 therapy in advanced melanoma. Oncologist 2020; 25:e602–e605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127▪.Cantini L, Pecci F, Hurkmans DP, et al. High-intensity statins are associated with improved clinical activity of PD-1 inhibitors in malignant pleural mesothelioma and advanced nonsmall cell lung cancer patients. Eur J Cancer 2021; 144:41–48. [DOI] [PubMed] [Google Scholar]; This study shows that statins may be related to the better clinical efficacy of ICIs.
- 128.Li M, Zeng C, Yao J, Ge Y, et al. The association between proton pump inhibitors use and clinical outcome of patients receiving immune checkpoint inhibitors therapy. Int Immunopharmacol 2020; 88:106972. [DOI] [PubMed] [Google Scholar]
- 129.Hussain N, Naeem M, Pinato DJ. Concomitant medications and immune checkpoint inhibitor therapy for cancer: causation or association? Hum Vaccin Immunother 2021; 17:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Banna GL, Cantale O, Bersanelli M, et al. Are anti-PD1 and anti-PD-L1 alike? The nonsmall-cell lung cancer paradigm. Oncol Rev 2020; 14:490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang N, Tu J, Wang X, Chu Q. Programmed cell death-1/programmed cell death ligand-1 checkpoint inhibitors: differences in mechanism of action. Immunotherapy 2019; 11:429–441. [DOI] [PubMed] [Google Scholar]
- 132.Zalba S, Contreras-Sandoval AM, Martisova E, et al. Quantification of pharmacokinetic profiles of PD-1/PD-L1 antibodies by validated ELISAs. Pharmaceutics 2020; 12:595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ponce LF, García-Martínez K, León K, Valiente PA. Exploring the conformational dynamics of PD1 in complex with different ligands: What we can learn for designing novel PD1 signaling blockers? Proteins 2021; 89:141–148. [DOI] [PubMed] [Google Scholar]
- 134.Córdova-Bahena L, Velasco-Velázquez MA. Anti-PD-1 And Anti-PD-L1 antibodies as immunotherapy against cancer: a structural perspective. Rev Invest Clin 2020; 73:008–016. [DOI] [PubMed] [Google Scholar]
- 135.Lee HT, Lee SH, Heo YS. Molecular interactions of antibody drugs targeting PD-1, PD-L1, and CTLA-4 in immuno-oncology. Molecules 2019; 24:1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Davda J, Declerck P, Hu-Lieskovan S, et al. Immunogenicity of immunomodulatory, antibody-based, oncology therapeutics. J Immunother Cancer 2019; 7:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hock BD, McKenzie JL, Strother M, et al. Functional effects of immune complexes formed between pembrolizumab and patient-generated antidrug antibodies. Cancer Immunol Immunother 2020; 69:2453–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.De Sousa Linhares A, Battin C, Jutz S, et al. Therapeutic PD-L1 antibodies are more effective than PD-1 antibodies in blocking PD-1/PD-L1 signaling. Sci Rep 2019; 9:11472. [DOI] [PMC free article] [PubMed] [Google Scholar]