Objective:
To assess efficacy and safety of dolutegravir (DTG) + lamivudine (3TC) vs. DTG + tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) in treatment-naive adults with HIV-1 in the prespecified 144-week secondary analyses of GEMINI-1 and GEMINI-2.
Design:
Identical, multicenter, phase III, randomized, non-inferiority studies (double-blind through 96 weeks).
Methods:
Participants with HIV-1 RNA ≤500 000 copies/ml and no major viral resistance mutations to nucleoside reverse transcriptase inhibitors, nonnucleoside reverse transcriptase inhibitors, or protease inhibitors were randomized 1:1 to once-daily DTG + 3TC or DTG + TDF/FTC.
Results:
At week 144, DTG + 3TC (N = 716) was noninferior to DTG + TDF/FTC (N = 717) in proportion of participants achieving HIV-1 RNA <50 copies/ml (Snapshot algorithm) in the pooled analysis (82% vs. 84%, respectively; adjusted treatment difference [95% confidence interval (CI)], −1.8% [−5.8, 2.1]), GEMINI-1 (−3.6% [−9.4, 2.1]), and GEMINI-2 (0.0% [−5.3, 5.3]). Twelve DTG + 3TC participants and nine DTG + TDF/FTC participants met protocol-defined confirmed virologic withdrawal (CVW) criteria; none developed treatment-emergent resistance. One DTG + 3TC participant who did not meet CVW criteria developed M184V at week 132 and R263R/K at week 144, conferring a 1.8-fold change in susceptibility to DTG; non-adherence to therapy was reported. Significantly fewer drug-related adverse events occurred with DTG + 3TC vs. DTG + TDF/FTC (20% vs. 27%; relative risk [95% CI], 0.76 [0.63–0.92]). Renal and bone biomarker changes favored DTG + 3TC.
Conclusions:
Three-year durable efficacy, long-term tolerability, and high barrier to resistance support first-line use of DTG + 3TC for HIV-1 treatment (see Supplemental Digital Content 1; video abstract).
Keywords: dolutegravir, integrase strand transfer inhibitor, nucleoside reverse transcriptase inhibitor, treatment-naive, two-drug regimen
Introduction
As people with HIV (PWH) require lifelong therapy, effective treatments with limited toxicity are needed [1]. Two-drug regimens (2DRs) reduce the number of antiretroviral drugs PWH are exposed to and can potentially decrease treatment-associated toxicity and costs [1]. To ensure comparable efficacy with three-drug regimens (3DRs), the optimal antiretroviral agents in a 2DR should have potent and durable antiviral activity and a high barrier to resistance [1].
Dolutegravir (DTG) is a potent integrase strand transfer inhibitor (INSTI) with a high barrier to resistance, supporting its inclusion in a 2DR [2], especially with the well tolerated nucleoside reverse transcriptase inhibitor (NRTI) lamivudine (3TC) [3–5]. In the week 48, primary analyses of the phase III GEMINI-1 and GEMINI-2 studies in treatment-naive adults, the 2DR DTG + 3TC was noninferior to the 3DR DTG + tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) in achieving HIV-1 RNA <50 copies/ml [6]. The subsequent week 96 analysis demonstrated the durable efficacy of DTG + 3TC, as evidenced by its sustained noninferiority vs. DTG + TDF/FTC [7]. Few participants met confirmed virologic withdrawal (CVW) criteria, and no treatment-emergent resistance mutations were detected. As a result, the DTG/3TC single-tablet, fixed-dose combination 2DR was approved by the European Medicines Agency and US Food and Drug Administration (FDA) in 2019 [8,9]. Additionally, the US Department of Health and Human Services, European AIDS Clinical Society, and International Antiviral Society–USA 2020 guidelines classify DTG + 3TC as a recommended initial antiretroviral regimen for most PWH, with exceptions for individuals with HIV-1 RNA >500 000 copies/ml, hepatitis B virus (HBV) co-infection, or in whom therapy is started before the results of HIV genotypic resistance testing for reverse transcriptase or HBV testing are available [10–12].
As 2DRs are an emerging and important treatment option for PWH, it is critical to assess their long-term durability and genetic barrier to resistance. Here, we present efficacy and safety data from the prespecified 144-week secondary analyses of the GEMINI studies.
Methods
GEMINI-1 (NCT02831673) and GEMINI-2 (NCT02831764) are identical, multicenter, phase III, randomized, non-inferiority studies that were double-blind through 96 weeks and conducted at 187 centers in 21 countries. Protocols for GEMINI-1 and GEMINI-2 are available at https://www.viiv-clinicalstudyregister.com/study/204861#ps and https://www.viiv-clinicalstudyregister.com/study/205543#ps, respectively. Methods, including ethical compliance information, have previously been published [6,7] and are described briefly below.
After screening, eligible participants aged ≥18 years with HIV-1 infection, ≤10 days of prior antiretroviral therapy (ART), and screening plasma HIV-1 RNA 1000–500 000 copies/ml were randomized 1:1 to receive a once-daily 2DR of DTG 50 mg + 3TC 300 mg or a once-daily 3DR of DTG 50 mg plus TDF 300 mg/FTC 200 mg. Participants were stratified by screening HIV-1 RNA (≤100 000 or >100 000 copies/ml) and screening CD4+ cell count (≤200 or >200 cells/cells/μl) and treated in a double-blind randomized phase from day 1 to week 96, followed by an open-label randomized phase from week 96 to week 148. Adverse events (AEs) and laboratory assessments were performed through week 148, whereas other assessments were generally conducted through week 144; HIV-1 RNA was assessed at week 144, with re-testing occurring at week 148 for participants with HIV-1 RNA ≥50 copies/ml at the week 144 visit.
Plasma for HIV-1 RNA analysis was collected at baseline and weeks 4, 8, 12, 16, 24, 36, 48, and every 12 weeks thereafter until week 144 and quantitated using the Abbott RealTime HIV-1 assay (Abbott Molecular, Des Plaines, Illinois, USA). Participants met CVW criteria if a second and consecutive HIV-1 RNA value met any of the following: decrease from baseline in plasma HIV-1 RNA <1 log10 copies/ml, unless HIV-1 RNA <200 copies/ml, by week 12; confirmed plasma HIV-1 RNA ≥200 copies/ml at or after week 24; or plasma HIV-1 RNA ≥200 copies/ml after previous confirmed suppression to HIV-1 RNA <200 copies/ml. Participants who met CVW criteria discontinued the study, and plasma samples from day 1 and the initial elevated viral load sample were used for genotypic and phenotypic resistance tests (Monogram Biosciences, San Francisco, California, USA).
Adverse events, concomitant medications, and physical examinations were assessed at all study visits. Participants who became pregnant were discontinued from the study. Testing for fasting lipids and glucose, urinalysis, and renal and bone biomarkers was conducted at baseline and up to week 144. Patient-reported changes in health-related quality of life were assessed at baseline and up to week 144 using the EuroQol-5 Dimensions-5 Levels (EQ-5D-5L) questionnaire [13].
The primary endpoint of GEMINI-1 and GEMINI-2 was the proportion of participants with plasma HIV-1 RNA <50 copies/ml at week 48 using the FDA Snapshot algorithm (missing, switch, or discontinuation = failure) [14] in the intention-to-treat-exposed (ITT-E) population. Endpoints for the week 144 secondary analysis included proportion of participants with HIV-1 RNA <50 copies/ml at week 144, change in CD4+ cell count from baseline, incidence of emergent mutations conferring genotypic and/or phenotypic resistance to study treatments in participants meeting CVW criteria, and proportion of participants with HIV-1 RNA <50 copies/ml in participant subgroups. Safety outcomes included incidence and severity of AEs and proportion of participants who discontinued treatment because of AEs. Changes from baseline in weight, renal and bone biomarkers, lipids, and inflammatory (interleukin-6 and high-sensitivity C-reactive protein) biomarkers were also assessed at week 144.
All participants who were randomized and received ≥1 dose of study medication were included in the ITT-E and safety populations. The per-protocol population consisted of the ITT-E population but excluded participants with significant protocol violations. The analyses reflect a cutoff date based on an individual participant's upper bound of the analysis window for week 144 (or week 148, depending on the assessment).
The proportion of participants with HIV-1 RNA <50 copies/ml at week 144 was analyzed using a Cochran–Mantel–Haenszel test stratified by baseline plasma HIV-1 RNA, baseline CD4+ cell count, and individual study. Sensitivity analyses were performed in the per-protocol population. Baseline characteristics, response rates by study visit or participant subgroup (using Snapshot algorithm), and AEs were summarized using descriptive statistics, with relative risk (95% CI) reported for drug-related AEs. The proportion of participants without treatment-related discontinuation equals failure (TRDF) was estimated using the Kaplan–Meier nonparametric method. Changes from baseline in CD4+ cell count and lipids were analyzed using a mixed-effect repeated-measures model.
Results
Study participants
Overall, 1433 participants in GEMINI-1 and GEMINI-2 were randomized and received ≥1 dose of study medication (2DR, N = 716; 3DR, N = 717; Supplemental Digital Content [SDC] 2, figure showing trial profile). At baseline (day 1), 20% (n = 293) of participants had HIV-1 RNA >100 000 copies/ml, and 8% (n = 118) had CD4+ cell count ≤200 cells/μl (Table 1). There were 1266 participants who entered the open-label phase at week 96 (2DR, n = 620; 3DR, n = 646). Through week 148, 19% (n = 134) of the DTG + 3TC group and 17% (n = 123) of the DTG + TDF/FTC group were discontinued from the study. Reasons for discontinuation are shown in SDC 2.
Table 1.
Demographics and baseline characteristics in the pooled ITT-E population from GEMINI-1 and GEMINI-2.
| Demographic or characteristic | DTG + 3TC (N = 716) | DTG + TDF/FTC (N = 717) |
| Age, median (range), years | 32 (18–72) | 33 (18–70) |
| Sex, n (%) | ||
| Female | 113 (16) | 98 (14) |
| Male | 603 (84) | 619 (86) |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 215 (30) | 232 (32) |
| Not Hispanic or Latino | 501 (70) | 485 (68) |
| Race, n (%) | ||
| White | 484 (68) | 499 (70) |
| African American/African heritage | 90 (13) | 71 (10) |
| Asian | 71 (10) | 72 (10) |
| Other | 71 (10) | 75 (10) |
| HIV-1 RNA, mean (SD), (log10 copies/ml) | 4.4 (0.7) | 4.4 (0.6) |
| ≤100 000 copies/ml, n (%) | 576 (80) | 564 (79) |
| >100 000 copies/ml, n (%)a | 140 (20) | 153 (21) |
| CD4+ cell count, mean (SD), (cells/μl) | 462.0 (219.2) | 461.3 (213.1) |
| ≤200, n (%) | 63 (9) | 55 (8) |
| >200, n (%) | 653 (91) | 662 (92) |
3TC, lamivudine; DTG, dolutegravir; FTC, emtricitabine; ITT-E, intention-to-treat–exposed; TDF, tenofovir disoproxil fumarate.
Includes 28 participants with HIV-1 RNA ≥500 000 copies/ml: 13 in the DTG + 3TC group and 15 in the DTG + TDF/FTC group.
Efficacy
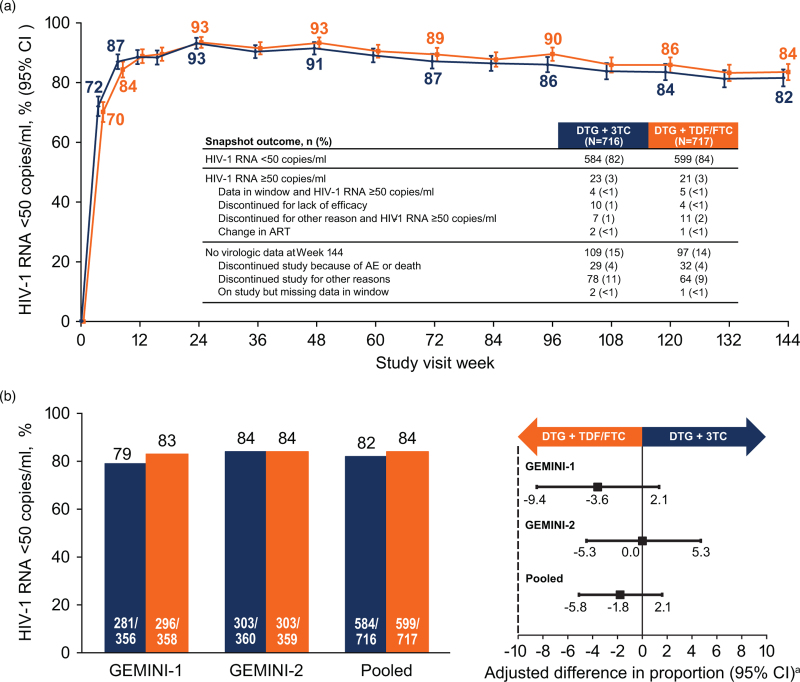
Analysis of virologic outcomes showed a similar proportion of participants with HIV-1 RNA <50 copies/ml (Snapshot) in either treatment group at each visit through week 144 (Fig. 1a). In the pooled ITT-E analysis, 82% of participants in the DTG + 3TC group and 84% in the DTG + TDF/FTC group achieved HIV-1 RNA <50 copies/ml (adjusted treatment difference [95% CI], −1.8% [−5.8, 2.1]; Fig. 1b). Using a 10% noninferiority margin, DTG + 3TC remained noninferior to DTG + TDF/FTC at week 144. Non-inferiority was also supported by the pooled per-protocol sensitivity analysis, as 83% (575/689) of participants in the DTG + 3TC group and 85% (584/685) in the DTG + TDF/FTC group achieved HIV-1 RNA <50 copies/ml (adjusted treatment difference [95% CI], −1.7% [−5.5, 2.1]. In GEMINI-1, 79% of participants in the DTG + 3TC group and 83% in the DTG + TDF/FTC group achieved HIV-1 RNA <50 copies/ml at week 144; the corresponding percentages in GEMINI-2 were 84% vs. 84%; Fig. 1b). In the pooled analysis, the proportion of participants in the Snapshot HIV-1 RNA ≥50 copies/ml category was 3% in both treatment groups (Fig. 1a). The majority of Snapshot failures that occurred in both treatment groups were due to non-treatment-related reasons (SDC 3, table showing Snapshot outcomes in individual and pooled studies). In the prespecified TRDF analysis at week 144, 5% of participants in each treatment group discontinued for treatment-related reasons (treatment difference [95% CI], 0.6% [−1.7, 2.9]). Adjusted mean change from baseline to week 144 in CD4+ cell count was 301.9 cells/μl in the DTG + 3TC group and 299.9 cells/μl in the DTG + TDF/FTC group.
Fig. 1.
Snapshot analysis of the proportion of participants with plasma HIV-1 RNA <50 copies/ml (a) by visit and (b) in the GEMINI-1, GEMINI-2, and pooled ITT-E populations at week 144, with adjusted treatment differences (95% CI).
CI, confidence interval; DTG, dolutegravir; FTC, emtricitabine; ITT-E, intention-to-treat-exposed; 3TC, lamivudine; TDF, tenofovir disoproxil fumarate. aBased on Cochran–Mantel–Haenszel stratified analysis adjusting for baseline plasma HIV-1 RNA (≤100 000 vs. >100 000 copies/ml), baseline CD4+ cell count (≤200 vs. >200 cells/μl), and study (GEMINI-1 vs. GEMINI-2).
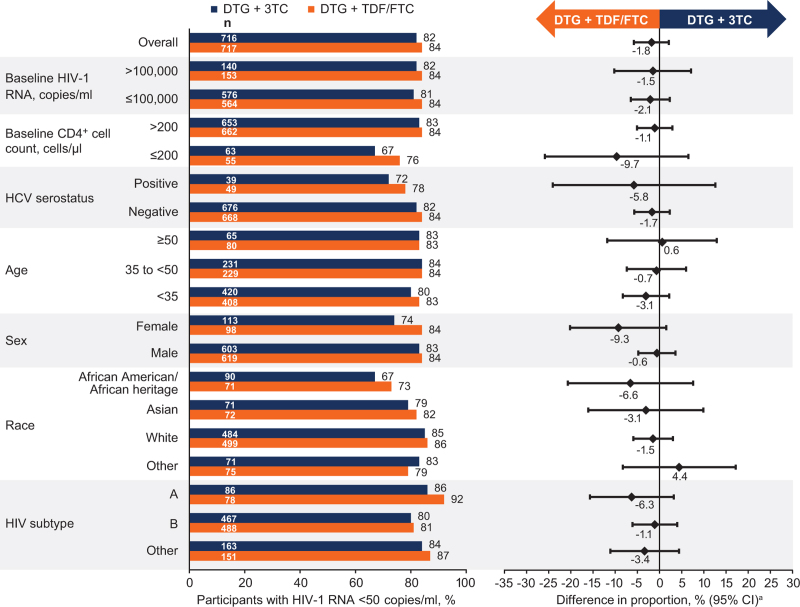
Proportion of participants with HIV-1 RNA <50 copies/ml in demographic and baseline characteristics subgroups (Fig. 2) was generally comparable across treatment groups and consistent with the overall week 144 results. Among participants with baseline HIV-1 RNA >100 000 copies/ml, 82% (115/140) and 84% (128/153) in the DTG + 3TC and DTG + TDF/FTC groups, respectively, achieved HIV-1 RNA <50 copies/ml (unadjusted treatment difference [95% CI], −1.5 [−10.2, 7.1]); the corresponding proportions were 81% (469/576) and 84% (471/564), respectively, for those with baseline HIV-1 RNA ≤100 000 copies/ml (unadjusted treatment difference [95% CI], −2.1 [−6.5, 2.3]; Fig. 2). Among 28 participants with HIV-1 RNA ≥500 000 copies/ml at baseline, the majority in both treatment groups achieved HIV-1 RNA <50 copies/ml (2DR, 77% [10/13]; 3DR, 80% [12/15]). In the subgroup of participants with baseline CD4+ cell count ≤200 cells/μl, 67% (42/63) in the DTG + 3TC group and 76% (42/55) in the DTG + TDF/FTC group achieved HIV-1 RNA <50 copies/ml (unadjusted treatment difference [95% CI], −9.7 [−25.9, 6.5]), with 83% (542/653) and 84% (557/662), respectively, achieving HIV-1 RNA <50 copies/ml for those with baseline CD4+ cell count >200 cells/μl (unadjusted treatment difference [95% CI], −1.1 [−5.1, 2.9]). In the CD4+ ≤200 cells/μl subgroup, most Snapshot failures were due to non-treatment-related reasons in both treatment groups (SDC 4, table showing reasons for virologic nonresponse in this subgroup). Proportions of participants without treatment-related discontinuations at week 144 (TRDF analysis) with baseline CD4+ cell count ≤200 cells/μl were 91% in the DTG + 3TC group and 92% in the DTG + TDF/FTC group (SDC 5, figure showing Snapshot and TRDF analyses).
Fig. 2.
Snapshot analysis of the proportion of participants with plasma HIV-1 RNA <50 copies/ml by subgroup in the ITT-E population at week 144.
aAdjusted difference for overall population (DTG + 3TC – DTG + TDF/FTC) and 95% CI are based on a stratified analysis (adjusting for plasma HIV-1 RNA, CD4+ cell count, and study) using Cochran–Mantel–Haenszel weights (meeting noninferiority based on 10% margin); unadjusted difference for subgroups calculated by proportion on DTG/3TC − proportion on DTG + TDF/FTC. CI, confidence interval; 3TC, lamivudine; DTG, dolutegravir; FTC, emtricitabine; HCV, hepatitis C virus; ITT-E, intention-to-treat-exposed; TDF, tenofovir disoproxil fumarate.
Across both studies, 12 participants in the DTG + 3TC group (1 since week 96) and 9 in the DTG + TDF/FTC group (2 since week 96) met protocol-defined CVW criteria through week 144 (SDC 6, table showing all CVWs through week 144); none had treatment-emergent INSTI or NRTI resistance mutations. M184V was detected at week 132 and R263R/K was detected at week 144 in one participant in the DTG + 3TC group. The participant achieved HIV-1 RNA <50 copies/ml at week 4, was suppressed through week 120, had elevated viral load at week 132 (considered due to nonadherence by investigator) followed by re-suppression 4 weeks later, therefore not meeting CVW criteria. Further low-level viral load elevations occurred at week 144 and at withdrawal from study for lack of efficacy at week 148 (SDC 7, figure showing viral load and resistance profile). After week 144, the sponsor was notified that a local laboratory had performed genotypic resistance testing at the investigator's discretion using samples collected ∼1 week after the week 132 visit, revealing the NRTI resistance mutation M184V. The central laboratory initiated resistance testing using samples from the week 132 and 144 visits: M184V at week 132 (SVW criteria time point) was confirmed; at week 144, both M184V and the DTG resistance-associated mutation mixture R263R/K were detected, the latter conferring a 1.8-fold change in susceptibility to DTG. After study withdrawal, the participant started once-daily DTG + cobicistat-boosted darunavir and re-suppressed.
Changes from baseline to week 144 in EQ-5D-5L Health State Utility score were similar between groups (adjusted treatment difference [95% CI], 0.004 [−0.003, 0.012]; P = 0.273; SDC 8, table showing health outcomes results). Adjusted mean change from baseline EQ-5D visual analog scale was greater with DTG + 3TC compared with DTG + TDF/FTC (adjusted treatment difference [95% CI], 1.2 [0.3–2.2]; P = 0.012). However, this difference is unlikely to be clinically relevant.
Safety
Through week 144, overall AE profiles were similar between treatment groups. Consistent with previous results [6,7], diarrhea, nasopharyngitis, and headache were among the most common AEs in the pooled safety population (Table 2). In the DTG + 3TC group, the proportion of participants with drug-related AEs (146/716; 20%) was significantly lower compared with the DTG + TDF/FTC group (192/717; 27%; relative risk [95% CI], 0.76 [0.63–0.92]). These differences were due to more participants with grade 1 events such as nausea in the DTG + TDF/FTC group vs. the DTG + 3TC group. Proportions of participants with serious AEs were similar between groups: DTG + 3TC, 11%; DTG + TDF/FTC, 12%. Overall, 10 participants experienced drug-related serious AEs (2DR: suicidal ideation, n = 2; psychotic disorder, n = 1; substance-induced psychotic disorder, n = 1; hepatotoxicity, n = 1; 3DR: suicidal ideation, n = 2; suicide attempt, n = 1; cholelithiasis, n = 1; rhabdomyolysis, n = 1). Three fatal AEs occurred in the DTG + 3TC group (acute myocardial infarction, n = 1; Burkitt's lymphoma, n = 1; coronary artery disease, n = 1); one fatal AE of unknown cause occurred in the DTG + TDF/FTC group. None of these events were considered drug related by the investigator. Adverse events leading to withdrawal were reported in 4% of participants in the DTG + 3TC group (31/716) and 5% in the DTG + TDF/FTC group (33/717; Table 2). During the open-label phase (week 96 to week 144), proportions of participants with any AEs (2DR, 59%; 3DR, 58%), drug-related AEs (2DR, 2%; 3DR, 3%), serious AEs (2DR, 2%; 3DR, 4%), and AEs leading to discontinuation (2DR, 1%; 3DR, 2%) were similar between groups. Overall, 11 pregnancies were reported, with three resulting in live births of healthy infants, three resulting in spontaneous abortions within the first trimester (no congenital anomalies reported), and four resulting in elective abortions. The pregnancy outcome for one participant was unknown.
Table 2.
Summary of AEs in the pooled safety population from GEMINI-1 and GEMINI-2 through week 144.
| n (%) | DTG + 3TC (N = 716) | DTG + TDF/FTC (N = 717) |
| Any AE | 613 (86) | 625 (87) |
| AEs occurring in ≥7% of participants in either group through week 144 | ||
| Diarrhea | 99 (14) | 106 (15) |
| Nasopharyngitis | 93 (13) | 127 (18) |
| Headache | 84 (12) | 91 (13) |
| Upper respiratory tract infection | 84 (12) | 61 (9) |
| Syphilis | 64 (9) | 70 (10) |
| Pharyngitis | 62 (9) | 64 (9) |
| Vitamin D deficiency | 53 (7) | 42 (6) |
| Back pain | 49 (7) | 48 (7) |
| Insomnia | 46 (6) | 59 (8) |
| Nausea | 29 (4) | 63 (9) |
| Arthralgia | 24 (3) | 47 (7) |
| Drug-related AEs | 146 (20) | 192 (27) |
| Grade 2–5 drug-related AEs | 58 (8) | 69 (10) |
| Grade 2–5 drug-related AEs occurring in ≥1% of participants | ||
| Headache | 8 (1) | 8 (1) |
| Serious AEs | 76 (11) | 85 (12) |
| AEs leading to withdrawal from study | 31 (4) | 33 (5) |
| AEs of interest leading to withdrawal from study | ||
| Psychiatric | 11 (2) | 8 (1) |
| Renal-relateda | 2 (<1) | 12 (2) |
| Osteoporosis | 0 | 2 (<1) |
3TC, lamivudine; AE, adverse event; DTG, dolutegravir; FTC, emtricitabine; TDF, tenofovir disoproxil fumarate.
Includes events of renal impairment, renal failure, chronic kidney disease, renal tubular disorder, glomerular filtration rate decreased, blood creatinine increased, and creatinine renal clearance decreased.
Increased weight was reported as an AE in 2% of participants in both treatment groups (2DR, n = 16 [grade 1, n = 10; grade 2, n = 5; grade 3, n = 1]; 3DR, n = 12 [grade 1, n = 8; grade 2, n = 4]) and led to discontinuation in one participant in the DTG + 3TC group. Obesity was reported as an AE in four participants in the DTG + 3TC group (grade 1, n = 2; grade 2, n = 2) and five in the DTG + TDF/FTC (grade 1, n = 2; grade 2, n = 2; grade 3, n = 1). Overall mean (SD) change in weight from baseline to week 144 was 3.7 (6.8) kg in the DTG + 3TC group and 2.4 (7.6) kg in the DTG + TDF/FTC group, and mean (SD) change in body mass index was 1.2 (2.3) kg/m2 and 0.8 (2.8) kg/m2, respectively (SDC 9, figure showing weeks 96 and 144 change from baseline in weight and BMI overall and week 144 by sex). Within each treatment group, mean (SD) change in weight from baseline was higher in men (2DR, 3.8 [6.8] kg; 3DR, 2.5 [7.8] kg) than women (2DR, 2.7 [6.7] kg; 3DR, 1.8 [6.6]; SDC 9).
Biomarkers and adverse events of special interest
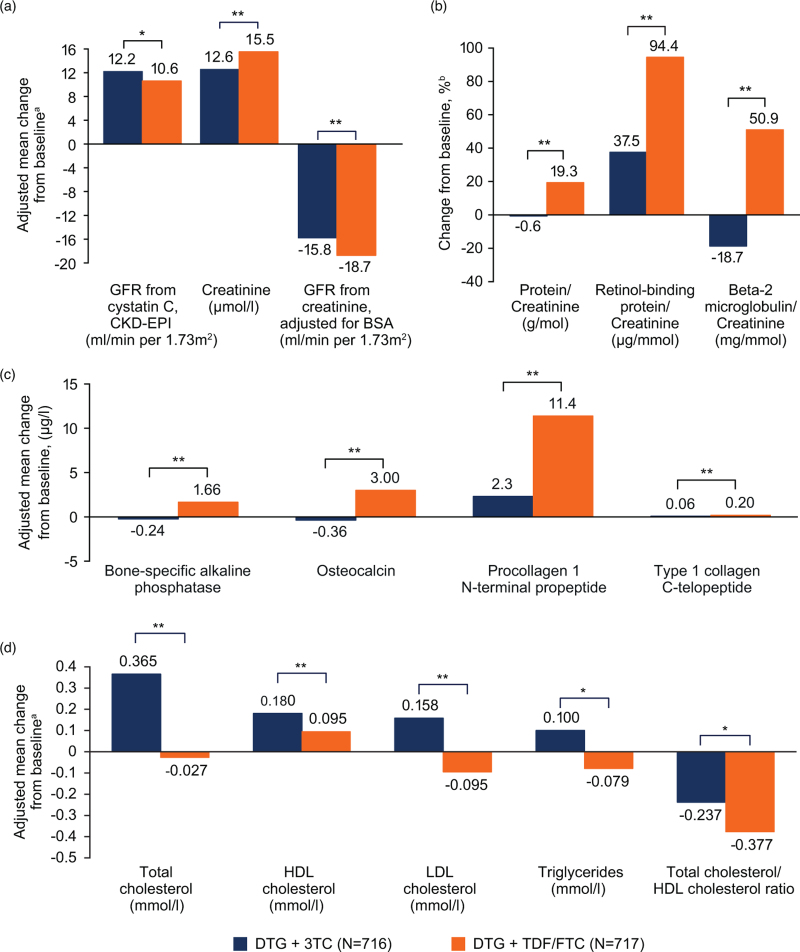
At week 144, changes in renal biomarkers significantly favored DTG + 3TC vs. DTG + TDF/FTC (Fig. 3a and b), with fewer renal function–related AEs leading to discontinuation (2DR, n = 2; 3DR, n = 12; Table 2). Significant changes in bone turnover biomarkers favoring DTG + 3TC vs. DTG + TDF/FTC were observed at week 144 (Fig. 3c). There were no osteoporosis AEs with DTG + 3TC and 5 grade 2 osteoporosis AEs with DTG + TDF/FTC, 2 of which were considered drug related and led to treatment discontinuation. Significant differences between treatment groups in adjusted mean change from baseline were observed for all lipid parameters. Changes in lipid parameters at week 144 generally favored DTG + TDF/FTC (Fig. 3d). However, increases in high-density lipoprotein (HDL) cholesterol with resultant decreases in total cholesterol/HDL cholesterol ratio were observed in both groups. There were minimal or no changes from baseline at week 144 in inflammatory biomarkers in both treatment groups (interleukin-6 [median (interquartile range) for both groups: 0.0 (0.0, 0.0) ng/l) and high-sensitivity C-reactive protein [median (interquartile range): DTG + 3TC, 0.0 (−0.9, 0.8) mg/l; DTG + TDF/FTC, −0.2 (−0.9, 0.5) mg/l).
Fig. 3.
Adjusted mean change from baseline in (a) serum or plasma renal biomarkers, (b) ratios of urine renal biomarkers, (c) serum bone turnover biomarkers, and (d) serum or plasma lipids at week 144.
CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration equation; DTG, dolutegravir; FTC, emtricitabine; GFR, glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; 3TC, lamivudine; TDF, tenofovir disoproxil fumarate. aWeek 144 analysis used a mixed-effect repeated-measures model. Mean change from baseline adjusted for study, treatment, visit, baseline HIV-1 RNA, baseline CD4+ cell count, age, sex, race, baseline biomarker value, treatment-by-visit interaction, and baseline biomarker value-by-visit interaction. For renal biomarkers, mean change from baseline was also adjusted for presence of diabetes and presence of hypertension. For bone biomarkers, mean change was also adjusted for body mass index, smoking status, and current vitamin D use. bEstimated from geometric mean ratios for baseline and week 144. Based on the same model as plasma/serum markers except adjusting for loge-transformed baseline biomarker. Normal ranges per National Cholesterol Education Program criteria for lipid parameters: total cholesterol (desirable), <5.18 mmol/l; HDL cholesterol, 1.04 to <1.56 mmol/l; LDL cholesterol (optimal), <2.59 mmol/l; triglycerides, <1.7 mmol/l; TC/HDL ratio, <3.5. ∗P < 0.01. ∗∗P < 0.001.
Discussion
Among contemporary HIV treatment options, DTG-based 2DRs are potentially beneficial for PWH as they offer noninferior efficacy and exposure to fewer antiretroviral drugs. The continued noninferiority of DTG + 3TC vs. DTG + TDF/FTC observed through 144 weeks in the present studies further supports the durable virologic efficacy of the 2DR DTG + 3TC as initial therapy for PWH. High proportions of participants (>80%) achieved HIV-1 RNA <50 copies/ml in both treatment groups, and response rates were similar between groups regardless of baseline viral load or CD4+ cell count. Data through week 96 from the TANGO study, in which participants switched to the 2DR of DTG/3TC, provide further evidence of long-term efficacy of this 2DR [15]. Additionally, through week 144 of the GEMINI studies, DTG + 3TC was well tolerated, with a significantly lower rate of drug-related AEs vs. DTG + TDF/FTC.
Importantly, consistent with previous studies of DTG-based 3DRs (e.g., SINGLE) [16], DTG + 3TC sustained a high barrier to resistance, with low numbers of participants meeting protocol-defined CVW criteria in this treatment group (n = 12; only one additional since week 96), none of whom had treatment-emergent resistance to INSTIs or NRTIs. Notably, in the single-group, phase II pilot study ACTG A5353 assessing DTG + 3TC in treatment-naive participants with HIV-1, three participants met virologic failure criteria, one of whom developed M184V and R263R/K mutations. All three had no detectable DTG levels at multiple time points while on treatment (3TC-triphosphate levels not measured); viral rebound was temporally linked to lapses in adherence [5]. In these larger, fully powered, randomized GEMINI-1 and GEMINI-2 studies, 1 participant with transiently increased HIV-1 RNA levels late in the study period (week 132; did not meet CVW criteria) developed the M184V and R263R/K mutations. While not possible to fully determine the cause of emergent resistance in this case, especially in the absence of drug concentrations, the transient HIV-1 RNA elevation to >60 000 copies/ml followed by re-suppression is consistent with adherence difficulties, and the investigator agreed this was a probable scenario. In addition, selective nonadherence to the individual components of the treatment regimen cannot be ruled out since DTG and 3TC were administered as separate tablets in the GEMINI studies; this would not be a risk with the fixed-dose combination tablet of DTG/3TC. Overall, these results demonstrate the high barrier to resistance with the 2DR of DTG + 3TC, which is critical to preserving treatment options for PWH who currently need to maintain lifelong therapy.
Safety outcomes at week 144 were consistent with week 48 and week 96 results, and no new signals emerged [6,7]; proportions of participants with any AEs, SAEs, drug-related AEs, and AEs leading to discontinuation during the open-label phase were comparable between treatment groups. There were few treatment-related discontinuations and a lower proportion of participants with drug-related AEs with DTG + 3TC vs. DTG + TDF/FTC. Mean weight increased from baseline in both groups, as would be expected in treatment-naive individuals. An AE of increased weight led to discontinuation in one DTG + 3TC participant. In both groups, mean changes in weight from week 96 to week 144 (2DR, 0.5 kg; 3DR, 0.3 kg) were consistent with expected values in the background population [7,17]. The favorable effects on renal and bone health observed for DTG + 3TC vs. DTG + TDF/FTC were sustained through week 144, as evidenced by fewer renal and osteoporosis clinical events. Of note, changes in renal and bone biomarkers from baseline to week 144 were generally consistent with patterns observed from baseline to week 48 and week 96 [6,7]. Within treatment groups, changes from baseline to week 96 were generally similar to changes from baseline to week 144, favoring the DTG + 3TC group [7]. In the GEMINI studies, the use of TDF/FTC as a backbone in the 3DR group was in line with standard of care and availability in participating countries at the time of study initiation; tenofovir alafenamide may have fewer renal and bone toxicities than TDF, which can be a consideration for some regions [18]. Changes in lipid parameters at week 144 relative to baseline generally favored the 3DR group, as observed at week 96, while favorable decreases in total cholesterol/HDL cholesterol ratio were observed in both treatment groups [7].
Numerically lower rates of Snapshot virologic response (HIV-1 RNA <50 copies/ml) were observed in DTG + 3TC participants in the female subgroup (2DR, n = 113; 3DR, n = 98), African American/African heritage subgroup (2DR, n = 90; 3DR, n = 71), and baseline CD4+ cell count ≤200 cells/μl subgroup (2DR, n = 63; 3DR, n = 55). The small number of participants in these subgroups limits the generalizability of the results in these populations. Of note, the majority of Snapshot failures were due to non-virologic reasons, which for the CD4+ cell count ≤200 cells/μl subgroup is also reflected in the TRDF analysis. Likewise, only a small number of participants (n = 28) had HIV-1 RNA ≥500 000 copies/ml at treatment initiation since screening HIV-1 RNA >500 000 copies/ml was exclusionary, though most participants (22/28; 79%) in this subgroup achieved HIV-1 RNA <50 copies/ml, and response rates were similar between treatment groups. Additionally, findings from the STAT study demonstrating efficacy of first-line treatment with DTG/3TC in treatment-naive participants in a test-and-treat setting, including those with low CD4+ cell counts and high viral loads, are consistent with those from the GEMINI studies [19].
Conclusion
GEMINI-1 and GEMINI-2 are the largest phase III trials of a 2DR in treatment-naive adults with HIV-1 infection. Results through 144 weeks reinforce the durable efficacy, high barrier to treatment-emergent resistance, and good tolerability of DTG + 3TC compared with a traditional 3DR of DTG + TDF/FTC. These results continue to support the recommended first-line use of this 2DR as a treatment option for ART-naive PWH.
Acknowledgements
The present study was funded by ViiV Healthcare. The authors thank the study participants; their families and caregivers; investigators (see SDC 10, which shows list of investigators) and site staff who participated in the study; and the ViiV Healthcare, GlaxoSmithKline, Pharmaceutical Product Development, and Parexel study team members. Editorial assistance was provided under the direction of the authors by Rebecca E. Slager, PhD, CMPP, and Jennifer Rossi, MA, ELS, MedThink SciCom, and was funded by ViiV Healthcare.
Funding: This study was funded by ViiV Healthcare.
Conflicts of interest
P.C. has served on advisory boards for GlaxoSmithKline (GSK), ViiV Healthcare, and Merck; served as an investigator for Abbott, Gilead, ViiV Healthcare, GSK, Merck, and Richmond; and has received honoraria for his speaking or chairing engagements from Abbott, GSK, Gilead, Merck, and ViiV Healthcare. J.S.M. has received lecture fees, sponsorship, and honoraria from Gilead, Stendhal, AbbVie, ViiV Healthcare, Janssen, and Merck Sharp & Dohme (MSD; all before 2019). J.R.A. has received advisory fees, speaker fees, and grant support from ViiV Healthcare, Janssen, Gilead, MSD, Alexa, and Teva. A.A. has served as a paid consultant to Gilead, Janssen, Merck, and ViiV Healthcare and received research funding from Gilead, Janssen, and ViiV Healthcare. R.O. has nothing to disclose. A.E.C. has received advisory fees from GSK, ViiV Healthcare, and Gilead; conference sponsorship from Gilead and Janssen; and speaker travel fees from GSK. C.-C.H. has received honoraria for speaking at educational events or consulting from AbbVie, Bristol-Myers Squibb (BMS), Gilead, Janssen, and ViiV Healthcare and has received research funding from BMS, Janssen, Gilead, Merck, and ViiV Healthcare. J.K.R. has received grant/research support from Gilead; served as a consultant/advisor to Abbott, AbbVie, Bionor, Gilead, Hexal, Janssen, Merck, and ViiV Healthcare; and was a speaker at educational events for AbbVie, Gilead, Janssen, and Merck. P.-M.G. has received grants from BMS and Janssen and has received honoraria and consulting fees from Gilead, ViiV Healthcare, and AbbVie. J.S., C.Y.M., M.U., K.A.P., K.Y.S., M.G., M.A., J.vW., and B.W. are employees of ViiV Healthcare and own stock in GSK. R.U., D.J.B., and L.C. are employees of GSK and own stock in GSK.
Author contributions: P.C., J.S., K.A.P., K.Y.S., M.G., M.A., and B.W. contributed to the conception of the study. P.C., J.S., R.U., M.U., K.A.P., K.Y.S., M.G., M.A., and B.W. contributed to the design of the study. P.C., J.S.M., J.R.A., A.A., R.O., A.E.C., C.-C.H., J.K.R., and P.-M.G. contributed to the acquisition of data. J.S., C.Y.M., R.U., D.J.B., M.U., L.C., and B.W. contributed to the analysis of data. P.C., J.S., C.Y.M., R.U., D.J.B., M.U., L.C., K.Y.S., J.vW., and B.W. contributed to the interpretation of data. J.S., C.Y.M., R.U., M.U., J.vW., and B.W. contributed to drafting the manuscript. All authors contributed to critically revising the manuscript for important intellectual content and approve the manuscript for publication.
Data sharing: Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
These data have previously been presented in part at HIV Drug Therapy Glasgow 2020; October 5–8, 2020; Virtual; Poster P018, and Conference on Retroviruses and Opportunistic Infections; March 6–10, 2021; Virtual; Science spotlight 1991.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
References
- 1.Back D. 2-Drug regimens in HIV treatment: pharmacological considerations. Germs 2017; 7:113–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brenner BG, Wainberg MA. Clinical benefit of dolutegravir in HIV-1 management related to the high genetic barrier to drug resistance. Virus Res 2017; 239:1–9. [DOI] [PubMed] [Google Scholar]
- 3.Quercia R, Perno C-F, Koteff J, Moore K, McCoig C, St Clair M, et al. Twenty-five years of lamivudine: current and future use for the treatment of HIV-1 infection. J Acquir Immune Defic Syndr 2018; 78:125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cahn P, Rolón MJ, Figueroa MI, Gun A, Patterson P, Sued O. Dolutegravir-lamivudine as initial therapy in HIV-1 infected, ARV-naive patients, 48-week results of the PADDLE (Pilot Antiretroviral Design with Dolutegravir LamivudinE) study. J Int AIDS Soc 2017; 20:21678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taiwo BO, Zheng L, Stefanescu A, Nyaku A, Bezins B, Wallis CL, et al. ACTG A5353: a pilot study of dolutegravir plus lamivudine for initial treatment of human immunodeficiency virus-1 (HIV-1)-infected participants with HIV-1 RNA <500000 copies/mL. Clin Infect Dis 2018; 66:1689–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cahn P, Sierra Madero J, Arribas JR, Antinori A, Ortiz R, Clarke AE, et al. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, noninferiority, phase 3 trials. Lancet 2019; 393:143–155. [DOI] [PubMed] [Google Scholar]
- 7.Cahn P, Sierra Madero J, Arribas JR, Antinori A, Ortiz R, Clarke AE, et al. Durable efficacy of dolutegravir plus lamivudine in antiretroviral treatment-naive adults with HIV-1 infection: 96-week results from the GEMINI-1 and GEMINI-2 randomized clinical trials. J Acquir Immune Defic Syndr 2020; 83:310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dovato. Summary of product characteristics. ViiV Healthcare; 2020. Available at: https://www.ema.europa.eu/en/documents/product-information/dovato-epar-product-information_en.pdf. [Accessed 18 January 2021] [Google Scholar]
- 9. US Food and Drug Administration. FDA approves first two-drug complete regimen for HIV-infected patients who have never received antiretroviral treatment. US Food and Drug Administration, April 8, 2019. Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-first-two-drug-complete-regimen-hiv-infected-patients-who-have-never-received. [Accessed 18 January 2021] [Google Scholar]
- 10. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Department of Health and Human Services, August 16, 2021. Available at: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/AdultandAdolescentGL.pdf. [Accessed 16 August 2021] [Google Scholar]
- 11. European AIDS Clinical Society. Guidelines version 10.1. European AIDS Clinical Society, October 2020. Available at: https://www.eacsociety.org/files/guidelines-10.1_30032021_1.pdf. [Accessed 18 January 2021] [Google Scholar]
- 12.Saag MS, Gandhi RT, Hoy JF, Landovitz RJ, Thompson MA, Sax PE, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2020 recommendations of the International Antiviral Society-USA Panel. JAMA 2020; 324:1651–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herdman M, Gudex C, Lloyd A, Janssen MF, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011; 20:1727–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. US Department of Health and Human Services. Human immunodeficiency virus-1 infection: developing antiretroviral drugs for treatment: guidance for industry. US Food and Drug Administration. Center for Drug Evaluation and Research (CDER), November 2015. Available at: https://www.fda.gov/media/86284/download. [Accessed 18 January 2021] [Google Scholar]
- 15.van Wyk J, Ajana F, Bisshop F, De Wit S, Osiyemi O, Portilla J, et al.Switching to DTG/3TC fixed-dose combination (FDC) is noninferior to continuing a TAF-based regimen (TBR) in maintaining virologic suppression through 96 weeks (TANGO Study). [Abstract O441]HIV Drug Therapy Glasgow 2020 5–8 October 2020. [Google Scholar]
- 16.Walmsley S, Baumgarten A, Berenguer J, Felizarta F, Florence E, Khuong-Josses MA, et al. Brief report: dolutegravir plus abacavir/lamivudine for the treatment of HIV-1 infection in antiretroviral therapy-naive patients: week 96 and week 144 results from the SINGLE randomized clinical trial. J Acquir Immune Defic Syndr 2015; 70:515–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hutfless S, Maruthur NM, Wilson RF, Gudzune KA, Brown R, Lau B, et al. Strategies to prevent weight gain among adults. Comparative effectiveness reviews, no. 97. Agency for Healthcare Research and Quality (US), March 2013. Available at: https://www.ncbi.nlm.nih.gov/books/NBK133218/. [Accessed 19 January 2021] [Google Scholar]
- 18.DeJesus E, Haas B, Segal-Maurer S, Ramgopal MN, Mills A, Margot N, et al. Superior efficacy and improved renal and bone safety after switching from a tenofovir disoproxil fumarate- to a tenofovir alafenamide-based regimen through 96 weeks of treatment. AIDS Res Hum Retroviruses 2018; 34:337–342. [DOI] [PubMed] [Google Scholar]
- 19.Rolle C-P, Berhe M, Singh T, Ortiz R, Wurapa A, Ramgopal M, et al. Dolutegravir/lamivudine as a first-line regimen in a test-and-treat setting for newly diagnosed people with HIV. AIDS 2021; 35:1957–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.