Supplemental Digital Content is available in the text
This case-control study revealed associations of adverse events in spinal surgery with secondary complications, neurological recovery and dysphagia after traumatic injury to cervical spinal cord. In addition, length of stay and treatment costs were higher when spinal surgery adverse events occurred in patients with spinal fractures involving spinal cord injury.
Keywords: acute traumatic spinal cord injury, cervical spine fracture, dysphagia, length of stay, neurological outcome, spinal surgery adverse events, spinal surgery complications, surgical management, time of surgery, treatment costs, treatment-relevant complications
Study Design.
Monocenter case-control study.
Objective.
Effects of spinal surgical adverse events (SSAE) on clinical and functional outcome, length of stay, and treatment costs after traumatic cervical spinal cord injury (SCI).
Summary of Background Data.
Traumatic SCI is a challenge for primary care centers because of the emergency setting and complex injury patterns. SSAE rates of up to 15% are reported for spine fractures without SCI. Little is known about SSAE after traumatic SCI and their outcome relevance.
Methods.
Acute traumatic cervical SCI patients were enrolled from 2011 to 2017. Cases with and without SSAE were compared regarding neurological recovery, functional outcome, secondary complications, mortality, length of stay, and treatment costs. Adjusted logistic regression and generalized estimating equation models were calculated for the endpoints ASIA impairment scale (AIS)-conversion and dysphagia. All analyses were run in the total and in a propensity score matched sample.
Results.
At least one SSAE occurred in 37 of 165 patients (22.4%). Mechanical instability and insufficient spinal decompression were the most frequent SSAE with 13 (7.9%) or 11 (6.7%) cases, respectively. The regression models adjusted for demographic, injury, and surgery characteristics demonstrated a reduced probability for AIS-conversion related to SSAE (OR [95% CI] 0.14 [0.03–0.74]) and additionally to single-sided ventral or dorsal surgical approach (0.12 [0.02–0.69]) in the matched sample. Furthermore, SSAE were associated with higher risk for dysphagia in the matched (4.77 [1.31–17.38]) and the total sample (5.96 [2.07–17.18]). Primary care costs were higher in cases with SSAE (median (interquartile range) 97,300 [78,200–112,300]) EUR compared with cases without SSAE (52,300 [26,700–91,200]) EUR.
Conclusion.
SSAE are an important risk factor after acute traumatic cervical SCI with impact on neurological recovery, functional outcome, and healthcare costs. Reducing SSAE is a viable means to protect the limited intrinsic capacity for recovery from SCI.
Level of Evidence: 4
Primary surgical care of acute traumatic spinal cord injury (SCI) is a challenge for trauma centers because the complex injury patterns require timely surgical treatment.1 Although the incidence of traumatic SCI is relatively low,2 individuals with SCI are vulnerable as the possibilities of restoring their mostly severe neurofunctional deficits are limited and clinical trials on neurorestorative therapies require an optimal starting point.3 Thus, secondary insults to the spinal cord due to spinal surgery adverse events (SSAE) are likely to have considerable impact on the clinical outcome after SCI.
Studies on spinal surgery in general report SSAE rates of 10%4,5 andofupto15%forthetreatmentofspinefractures.6 After SCI, SSAE rates are probably even higher. Nevertheless, SCI-specific literature on SSAE is scarce. Moreover, differences in the etiology of spine fractures,6–9 anatomical regions with associated organ and vascular systems10–12; surgical approaches,5,12 the time of SSAE occurrence,13–15 and SSAE causality16 complexify the interpretation and generalizability of published evidence. Moreover, as vertebral fractures with SCI require a timely and sufficient decompression of the spinal canal in order to limit ischemic secondary damage to the swollen spinal cord, insufficient decompression is an SSAE of particular relevance after SCI.1
In the setting of a Level I trauma center with specialized SCI care, this study investigated the frequency and type of SSAE in acute traumatic cervical SCI and the impact of SSAE on the clinical, functional, and health economic outcome after primary surgical care.
MATERIALS AND METHODS
Study Overview
The retrospective monocenter case-control study enrolled n = 165 patients with acute traumatic cervical SCI between 2011 and 2017 (Figure 1). Patients 18 years of age or older who underwent initial spinal surgery at the study center or at referring external spine centers were included. Postoperative treatment and subsequent spinal surgery procedures, including revision surgery, were performed in the study center. All data were stored in the electronic hospital information systems “Medico Portal” (Cerner Health Services, Idstein, Germany), “ICM Portal” (Drägerwerk, Lübeck, Germany), or the radiograph archiving and communications system “IntelliSpace PACS Enterprise” (Philips Healthcare Informatics, Hamburg, Germany) and compiled for statistical evaluation using a versioned database. The treatment costs stored in the cost and activity accounting software “WICO” (Cerner Health Services) were calculated with the software “eisTIKAKUT” (KMS AG, Unterhaching, Germany) as provided for the Institute for the payment system in hospitals (InEK, Siegburg, Germany). The study was approved by the Ethics Committee of Charité - Universitätsmedizin Berlin, Germany (EA2/015/15).
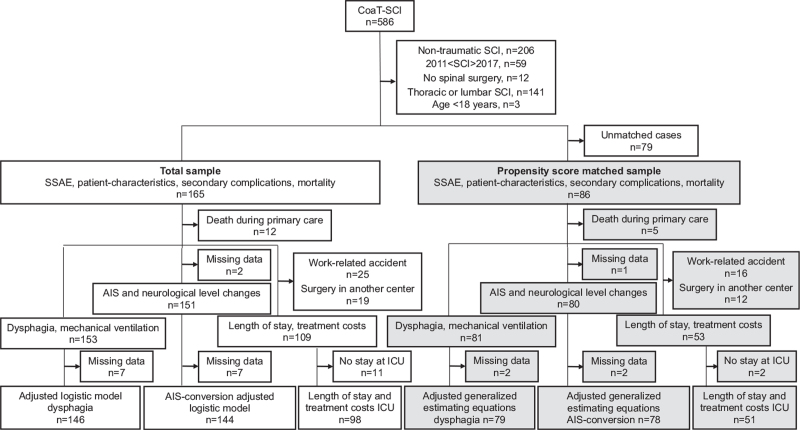
Figure 1.
Data analysis chart. The analysis of SSAE was performed in the total sample (white boxes) and in a propensity score matched sample (gray boxes). In the total sample the effects of SSAE were analyzed using logistic or linear regression. In the matched sample generalized estimating equations considering the match-ID as a cluster were used. AIS indicates ASIA impairment scale; ICU, intensive care unit; SCI, spinal cord injury; SSAE, spinal surgery adverse events.
Variable Definitions
SSAE were defined as any unexpected adverse event directly or indirectly related to the spinal surgery. SSAE were divided into (i) adverse events during surgery, that is, insufficient spinal decompression, malpositioned osteosynthesis, spinal cord compression, vessel injury, (ii) indirect adverse events of surgery, that is, positional damage, cerebellar hemorrhage, and (iii) postoperative adverse events, that is, mechanical instability, wound infection, wound hematoma, cerebrospinal fistula, and retropharyngeal scarring requiring revision surgery. The data on adverse events were collected from the medical records and post-surgery magnetic resonance imaging (MRI), computed tomography (CT)-scans or spine x-ray. A distinction between minor and major adverse events16,17 was not made in this study as the observation period was limited to the phase of primary care.
The baseline data comprised age, sex, body mass index (BMI), Charlson Comorbidity Index (CCI), AO Spine Upper Cervical Injury and Subaxial Injury Classification System (AO-classification), and ASIA impairment scale (AIS). Characteristics of primary surgical treatment analyzed were the surgical approach (dorsal, ventral, combined), the number and duration of spine surgery procedures, and nighttime surgery outside of regular working hours (5 pm to 7 am). Secondary complications of SCI were defined based on clinical diagnoses requiring specific treatment and the burden of SCI-associated secondary complications was calculated as their sum.
Neurological outcome was described by changes in the AIS and in the neurological level using the international standards for neurological classification of spinal cord injury definitions.18 Functional outcome parameters were temporary and permanent dysphagia diagnosed through otorhinolaryngologic or logopedic examination, mechanical ventilation status, and tracheostoma status at discharge. Patients who died during primary care were excluded from the analysis of neurological and functional outcome (Figure 1).
The discharge from primary care was defined based on the healthcare sponsor's eligibility criteria for rehabilitation (supplementary Table 1). Because of different health insurance regulations, only patients with work-related accidents are allowed to undergo inpatient rehabilitation at the study center subsequently to primary care. Therefore, in order to avoid bias in the health economic data, patients with work-related accidents were excluded from the analysis of length of stay and treatment costs. Furthermore, patients who underwent primary spine surgery in other centers, were excluded as data on surgery and ICU costs were missing in these cases (Figure 1).
Statistical Analysis
For propensity score matching of cases with and without SSAE, the matching variables, age (smoothed), CCI (smoothed), AIS at admission (exact), and sex (exact) were used. Nearest neighbor 1:3 matching with both discard and a caliper of 0.2 was used and combined with GAMlogit smoothing of continuous variables. Before and after matching, the groups with and without SSAE were compared using standardized mean differences (SMD). SMD less than or equal to 0.2 or more than or equal to 0.2 were considered as relevant group differences. All subsequent analyses were performed in the total sample and the matched sample (Figure 1). In the total sample, logistic or ordinal regression models were used to estimate SSAE effects on binary or ordinal variables, respectively. In the matched sample, generalized estimating equations (GEE) considering the match-ID as a cluster were used. The models with AIS conversion or with dysphagia were calculated also as multiple models in order to adjust for demographics at baseline, injury, and surgery characteristics. For estimation of SSAE effects on continuous variables, linear regression models were calculated in the total sample and GEE models were applied in the matched sample. The SSAE-related mortality was analyzed using the Kaplan–Meier method. A two-sided significance level of α = 0.05 was used. All P-values have to be interpreted cautiously, as no adjustment for multiple testing was applied in this exploratory study. The statistical software used was SPSS for Windows (Version 25, IBM Corp, Armonk, NY, USA) and RStudio (Version 1.2.5042; Survival package 3.1–12; Survimer 0.4.6).
RESULTS
Spinal Surgery Adverse Events (SSAE)
After traumatic spine injury with cervical SCI (n = 165), at least one SSAE occurred in 37 patients (22.4%) during primary care (Table 1). More than one SSAE occurred in nine patients (5.5%). The most frequent SSAE during surgery was insufficient decompression of the spinal canal followed by malpositioned osteosynthesis. There were three affections of spinal cord tissue due to compression and two injuries to the vertebral artery during spine surgery. Mechanical instability was the most frequent postoperative SSAE, followed by wound infections, hematoma, or retropharyngeal scarring. There were no indirect adverse events during surgery (Table 1).
TABLE 1.
Types of Spinal Surgery Adverse Events
| Type of SSAE | Total Sample n = 165 |
| Patients with SSAE, n (%) | 37 (22.4) |
| Patients with more than 1 SSAE, n (%) | 9 (5.5) |
| Direct adverse events during surgery | |
| Insufficient spinal decompression, n (%) | 11 (6.7) |
| Malpositioned osteosynthesis, n (%) | 7 (4.2) |
| Vessel injuries, n (%) | 2 (1.2) |
| Spinal cord compression, n (%) | 3 (1.8) |
| Indirect adverse events during surgery | |
| Perioperative visual loss, n (%) | – |
| Postoperative cerebellar hemorrhage, n (%) | – |
| Postoperative adverse events | |
| Mechanical instability, n (%) | 13 (7.9) |
| Wound infection, n (%) | 4 (2.4) |
| Wound hematoma, n (%) | 3 (1.8) |
| Retropharyngeal scarring, n (%) | 3 (1.8) |
| Cerebrospinal fistula, n (%) | – |
SSAE indicates spinal surgery adverse events.
Baseline Demographics and Injury Characteristics
Differences in baseline characteristics of patients with and without SSAE are expressed as effect sizes using SMDs (Table 2). In the total sample, older age and higher CCI were weakly associated with higher rates of SSAE indicated by SMDs of 0.28 or 0.27, respectively. Female sex was strongly associated with less frequent SSAE (SMD –0.78). The body mass index (BMI) had no effects on SSAE rates (SMD 0.02). Categories of underweight or high-grade obesity were rarely represented in the study population (supplemental Table 2). The effects of the distribution of spine fracture types according to the AO-classification (SMD 0.19) and of SCI severity classified by the AIS (SMD –0.20) were marginal (Table 2). After propensity score matching (n = 86), the effects regarding age, sex, CCI, and AIS and were appropriately reduced or remained appropriately small in terms of BMI and AO-classification (Table 2).
TABLE 2.
Demographic Baseline and Injury Characteristics of the Groups With and Without Spinal Surgery Adverse Events
| Total Sample | |||||
| Variable | Group Without SSAE | n | Group With SSAE | n | SMD (95% CI) |
| Age, median (IQR) | 61.4 (50.2–76.1) | 128 | 69.1 (54.7–78.0) | 37 | 0.28 (–0.09–0.65) |
| Gender, female (%) | 34 (26.6) | 128 | 3 (8.1) | 37 | –0.78 (–1.46–0.09) |
| CCI, median (IQR) | 0 (0–2) | 128 | 1 (0–3) | 37 | 0.27 (–0.1–0.64) |
| BMI, median (IQR) | 25.4 (23.6–27.6) | 124 | 26.0 (23.4–28.0) | 36 | 0.02 (–0.35–0.39) |
| AO-classification, A: B: C, n (%) | 28: 44: 54 (22.2: 34.9: 42.9) | 128 | 6: 12: 19 (16.2: 32.4: 51.4) | 37 | 0.19 (–0.18–0.55) |
| AIS at admission, A: B: C: D (%) | 52: 8: 19: 49 (40.6: 6.3: 14.8: 38.3) | 128 | 15: 8: 4: 10 (40.5: 21.6: 10.8: 27.0) | 37 | -0.20 (–0.57–0.17) |
| Matched Sample | |||||
| Variable | Group Without SSAE | n | Group With SSAE | n | SMD (95% CI) |
| Age, median (IQR) | 57.4 (45.5–75.3) | 60 | 64.0 (46.7–78.0) | 26 | 0.19 (–0.27–0.65) |
| Gender, female (%) | 6 (10.0) | 60 | 3 (11.5) | 26 | 0.09 (–0.72–0.9) |
| CCI, median (IQR) | 0 (0–2) | 60 | 0 (0–2.3) | 26 | –0.12 (–0.58–0.34) |
| BMI, median (IQR) | 25.3 (23.5–27.0) | 59 | 25.9 (23.1–28.2) | 25 | 0.11 (–0.36–0.58) |
| AO-classification, A: B: C, n (%) | 10: 23: 27 (16.7: 38.3: 45.0) | 60 | 5:8: 13 (29.2: 30.8: 50.0) | 26 | 0.03 (–0.43–0.49) |
| AIS at admission, A: B: C: D (%) | 32: 7: 4: 17 (53.3: 11.7: 6.7: 28.3) | 60 | 15: 4: 2: 8 (53.8: 11.5: 7.7: 26.9) | 26 | –0.02 (–0.48–0.44) |
SMD ≤–0.2 or ≥0.2 were considered as group differences relevant for propensity score matching. Matching variables: Age (smoothed), gender (exact), CCI (smoothed), AIS at admission (exact). Matching criteria: nearest neighbor matching combined with caliper matching, caliper = 0.2; method = gamlogit for smoothing, ratio = 1:3.
AIS indicates ASIA impairment scale; CCI, Charlson Comorbidity Index; IQR, interquartile range; SMD, standardized mean difference; SSAE, spinal surgery adverse events.
Primary surgical care characteristics, including type of surgical approach, surgery during nighttime, or number and duration of spine surgery had weak to moderate effects on SSAE (supplemental Table 3). The strongest effect was observed for a single sided (ventral or dorsal) surgical approach which was associated with more SSAE both in the total sample (SMD 0.28) and in the matched sample (SMD 0.35) compared with a combined approach.
Secondary Complications and Mortality
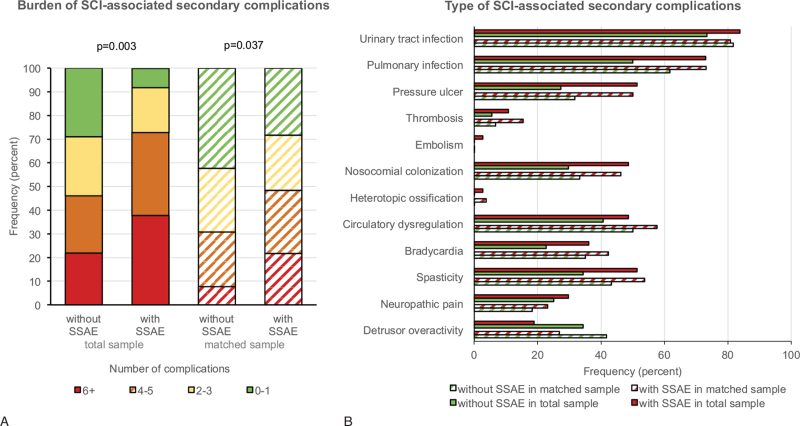
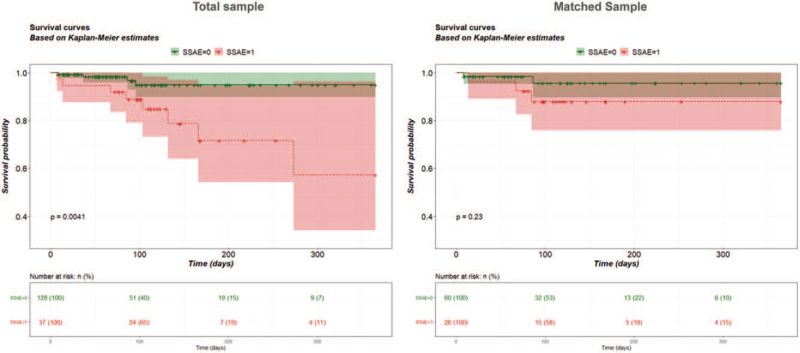
The analysis of SCI-related secondary complications revealed a clearly higher burden of complications in patients with SSAE in the total sample and in the propensity score matched sample (Figure 2A). The most frequent secondary complications in patients with SSAE compared with patients without SSAE were pressure ulcers, nosocomial colonization, and spasticity (Figure 2B). The Kaplan–Meier analysis of mortality during primary care resulted in a higher risk of death in the group with SSAE compared with the group without SSAE in the total sample. This effect was much smaller in the matched sample (Figure 3).
Figure 2.
SCI-associated secondary complications. The cumulative occurrence of different secondary complications during primary care is expressed as burden of complications (A) and the frequencies of complications (B) are described in the total sample (solid bars) and a propensity score matched sample (striped bars). Explorative P-values were calculated using unadjusted models of ordinal regression in the total sample and in the matched sample generalized estimating equation considering the match-id as a cluster. SSAE indicates spinal surgery adverse events.
Figure 3.
Cumulative survival. Kaplan–Meier analysis of mortality during primary care up to 1 year in the total sample (left) and a propensity score matched sample (right). Shaded areas indicate the 95% CI of the survival curves. Cases were censored at discharge (vertical bars). Groups with or without SSAE were compared using the log-rank test. SSAE indicates spinal surgery adverse events.
Neurological Outcome
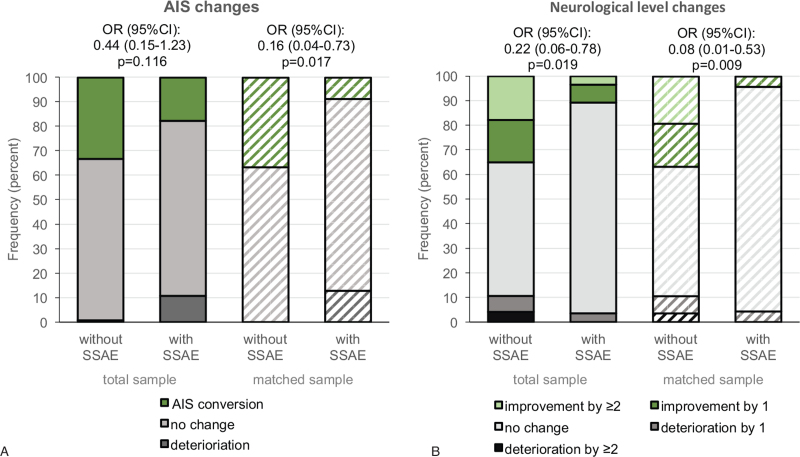
In the total and in the matched sample, the group with SSAE experienced more events of AIS-deterioration compared with the group without SSAE (total sample 10.7% vs. 0.8%; matched sample 12.8% vs. 0%) as well as a lower rate of AIS-conversion (total sample 17.7% vs. 33.1%; matched sample 8.7% vs. 36.6%) (Figure 4A). The recovery pattern of the neurological level was similar to the pattern of AIS-conversion (Figure 4B). In the adjusted logistic regression in the total sample, none of the explanatory variables, except the AO-classification, was clearly associated with AIS-conversion (Table 3). In the GEE in the matched sample, additionally to the AO-classification, incomplete SCI AIS B-D was positively associated with AIS-conversion (OR [95% CI] 7.03 [1.78–27.72]). Furthermore, SSAE (0.14 [0.03–0.74]), a surgical approach either from dorsal or ventral (0.12 [0.02–0.69]) and higher CCI scores (0.73 [0.53–0.99]) were associated with a reduced probability for AIS-conversion (Table 3). Subgroup analysis regarding effects of insufficient spinal decompression on AIS-conversion revealed that none of the patients with insufficient spinal decompression experienced an AIS-conversion, which was not the case in patients with other SSAE or no SSAE. The proportion of patients with deterioration in the AIS was similar across the SSAE subgroups (supplementary Table 4).
Figure 4.
Neurological outcome. Changes in the AIS (A) and the neurological level (B) in the total sample (solid bars) and a propensity score matched sample (striped bars) from admission to discharge. Effects of SSAE on AIS-conversion (green vs. grey area) or neurological level improvement (green vs. grey area) were analyzed using unadjusted models of binary logistic regression in the total sample and in the matched sample generalized estimating equations considering the match-id as a cluster. AIS indicates ASIA impairment scale; SSAE, spinal surgery adverse events.
TABLE 3.
Regression Analysis of AIS-Conversion
| Multiple Logistic Regression in the Total Sample | ||
| n = 144 | ||
| Dependent Variable: AIS-Conversion (No = 0, Yes = 1) | Nagelkerkes R2 = 0.19 –2 Log-likelihood: 153.0 | |
| Covariates | Odds Ratio (95% CI) | P-Value |
| Age (per 1 year increase) | 0.99 (0.97–1.02) | 0.56 |
| Gender (male) | 2.07 (0.73–5.89) | 0.17 |
| BMI (per one point increase) | 1.05 (0.94–1.16) | 0.40 |
| CCI (per one point increase) | 0.88 (0.67–1.15) | 0.36 |
| AIS at admission (AIS B-D) | 2.0 (0.87–4.62) | 0.10 |
| AO-classification, overall | – | 0.042 |
| C (reference) | – | |
| B | 3.24 (1.27–8.25) | 0.014 |
| A | 2.37 (0.83–6.81) | 0.11 |
| Surgical approach (ventral or dorsal) | 0.45 (0.19–1.06) | 0.069 |
| Place of spinal surgery (at trial center) | 2.45 (0.73–8.21) | 0.15 |
| SSAE (yes) | 0.62 (0.2–1.93) | 0.41 |
| Multiple Generalized Estimating Equation Model in the Matched Sample | ||
| n = 78 | ||
| Dependent Variable: AIS-Conversion (No = 0, Yes = 1) | Quasi-Likelihood: 86.8 | |
| Covariates | Odds Ratio (95% CI) | P-Value |
| Age (per 1 year increase) | 0.99 (0.96–1.02) | 0.50 |
| Gender (male) | 2.42 (0.1–76.01) | 0.62 |
| BMI (per one point increase) | 1.03 (0.87–1.22) | 0.73 |
| CCI (per one point increase) | 0.73 (0.53–0.99) | 0.043 |
| AIS at admission (AIS B-D) | 7.03 (1.78–27.72) | 0.005 |
| AO-classification, overall | – | – |
| C (reference) | – | – |
| B | 3.14 (1.03–9.67) | 0.045 |
| A | 5.70 (0.93–34.84) | 0.059 |
| Surgical approach (ventral or dorsal) | 0.12 (0.02–0.69) | 0.017 |
| Place of spinal surgery (at trial center) | 2.81 (0.87–9.25) | 0.086 |
| SSAE (yes) | 0.14 (0.03–0.74) | 0.021 |
Excluded: patients who died during primary care.
AIS indicates ASIA impairment scale; BMI, body mass index; CCI, Charlson Comorbidity Index; SSAE, spinal surgery adverse events.
Functional Outcome
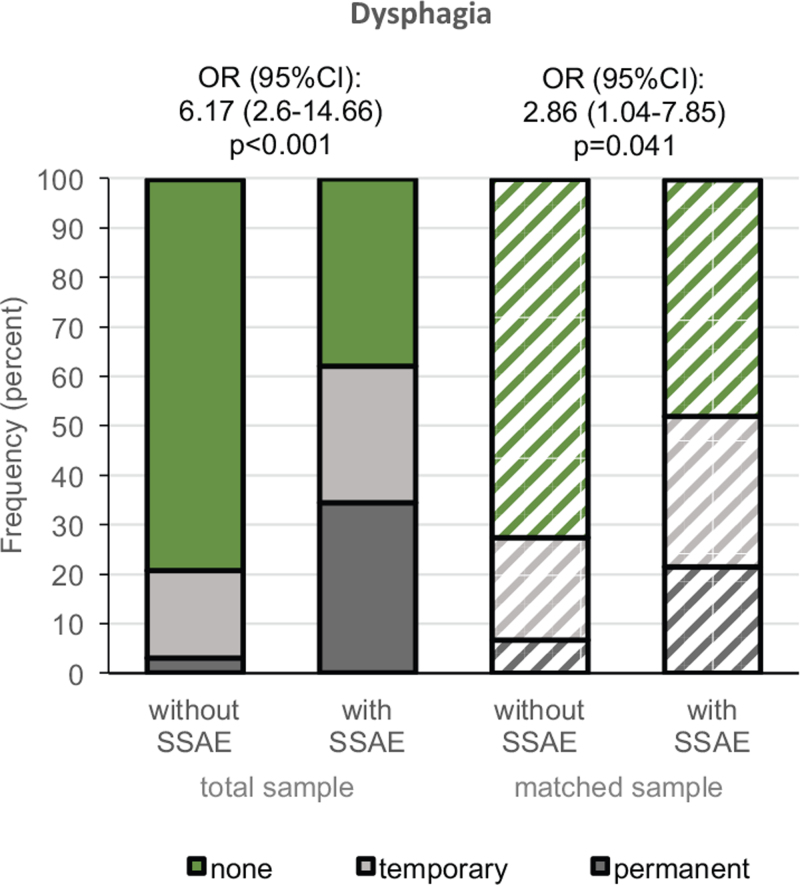
The rate of dysphagia was higher in the group with SSAE compared with the group without SSAE in both samples (Figure 5). This effect was observed for permanent (total sample 34.3% vs. 3.0%; matched sample 21.5% vs. 6.7%) and for temporary dysphagia (total sample 27.6% vs. 17.7%; matched sample 30.4% vs. 20.7%). The adjusted logistic regression model in the total sample revealed an increased risk for dysphagia in patients with SSAE (OR [95% CI] of 5.96 [2.07–17.18]) and the GEE in the matched sample yielded similar results (4.77 [1.31–17.38]). Clear additional effects were observed for the AIS with a reduced risk for dysphagia after incomplete SCI AIS B-D in the total (0.27 [0.11–0.66]) and in the matched sample (0.17 [0.05– 0.56]). The risk for dysphagia was not related to anterior surgical access in both models (Table 4). The explorative subgroup analysis has not revealed any association of insufficient spinal decompression with dysphagia (supplemental Table 4).
Figure 5.
Transient and permanent dysphagia. Rates of dysphagia at discharge in the total sample (solid bars) and a propensity score matched sample (striped bars). Effects of SSAE on dysphagia status (green vs. grey area) were analyzed using unadjusted models of binary logistic regression in the total sample and in the matched sample generalized estimating equation considering the match-id as a cluster. AIS indicates ASIA impairment scale; SSAE, spinal surgery adverse events.
TABLE 4.
Regression Analysis of Dysphagia
| Multiple Logistic Regression in the Total Sample | ||
| n = 146 | ||
| Dependent Variable: Dysphagia (No = 0, Yes = 1) | Nagelkerkes R2 = 0.41 –2 Log-likelihood: 125.42 | |
| Independent Variables | Odds Ratio (95% CI) | P-Value |
| Age (per 1 year increase) | 1.05 (1.02–1.08) | 0.004 |
| Gender (male) | 6.72 (1.64–27.44) | 0.008 |
| BMI (per one point increase) | 1.0 (0.89–1.13) | 0.98 |
| CCI (per one point increase) | 1.11 (0.86–1.44) | 0.41 |
| AIS at admission (AIS B-D) | 0.27 (0.11–0.66) | 0.005 |
| AO-classification | – | 0.79 |
| C (reference) | – | – |
| B | 0.75 (0.27–2.09) | 0.58 |
| A | 0.68 (0.2–2.4) | 0.55 |
| Surgical approach (ventral) | 2.29 (0.65–8.08) | 0.20 |
| Place of spinal surgery (at trial center) | 0.63 (0.21–1.91) | 0.41 |
| SSAE (yes) | 5.96 (2.07–17.18) | 0.001 |
| Multiple Generalized Estimating Equation Model in the Matched Sample | ||
| (n = 79) | ||
| Dependent Variable: Dysphagia (No = 0, Yes = 1) | Quasi-Likelihood: 94.9 | |
| Independent Variables | Odds Ratio (95% CI) | P-Value |
| Age (per 1 year increase) | 1.03 (0.99–1.06) | 0.11 |
| Gender (male)∗ | – | – |
| BMI (per one point increase) | 1.11 (0.85–1.45) | 0.43 |
| CCI (per one point increase) | 1.27 (0.88–1.82) | 0.20 |
| AIS at admission (AIS B-D) | 0.17 (0.05–0.56) | 0.003 |
| AO-classification | – | – |
| C (reference) | – | – |
| B | 0.73 (0.14–3.77) | 0.71 |
| A | 1.01 (0.30–3.92) | 0.91 |
| Surgical approach (ventral or combined) | 3.17 (0.44–22.62) | 0.25 |
| Place of spinal surgery (at trial center) | 0.38 (0.11–1.28) | 0.12 |
| SSAE (yes) | 4.77 (1.31–17.38) | 0.018 |
Excluded: patients who died during primary care.
Gender could not be included in the model as a complete separation applies in the matched sample (no cases of dysphagia in female patients).
AIS indicates ASIA impairment scale; BMI, body mass index; CCI, Charlson Comorbidity Index; SSAE, spinal surgery adverse events.
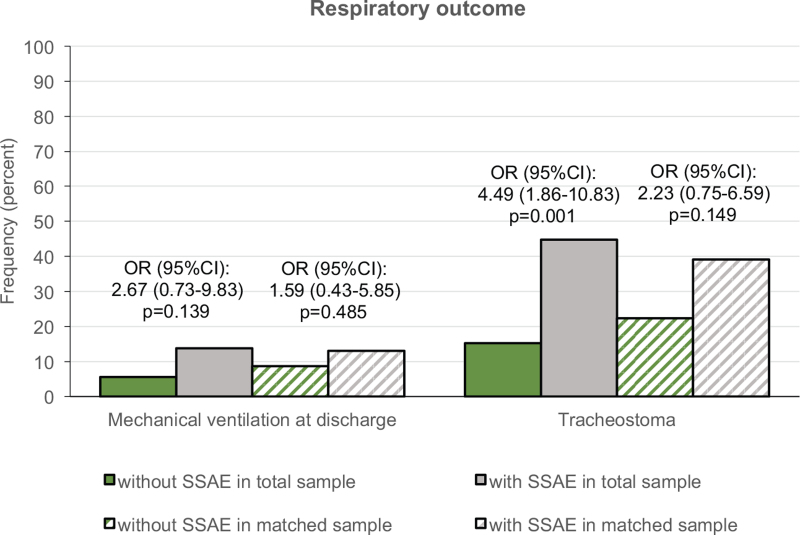
In the analysis of mechanical ventilation at discharge no considerable effects of SSAE on respiratory independence were detectable in both samples (Figure 6). In the group with SSAE more patients had a tracheostoma at discharge compared with the group without SSAE in the total sample, this effect was smaller in the matched sample (Figure 6).
Figure 6.
Respiratory independence. Frequency of mechanical ventilation and tracheostoma at discharge in the total sample (solid bars) and a propensity score matched sample (striped bars). Effects of SSAE on mechanical ventilation or tracheostoma status at discharge were analyzed using unadjusted models of binary logistic regression in the total sample and in the matched sample generalized estimating equations considering the match-id as a cluster. SSAE indicates spinal surgery adverse events.
Length of Stay and Treatment Costs
The length of stay was clearly higher in the group with SSAE (101.3 [86.2–120.6] days) (median interquartile range [IQR]) compared with the group without SSAE (72.6 [34.8–116.5] days) in the total sample. The stay in ICU was also longer in the SSAE group (24.8 [12.0–37.9] days) than in the group without SSAE (10.0 [2.7–28.5]). The association of SSAE with length of stay was less clear in the matched sample (Table 5).
TABLE 5.
Length of Stay and Treatment Costs
| Total Sample | |||||
| Length of Stay | Group Without SSAE | n | Group With SSAE | n | P-Valuea |
| Intensive care unit; days, median (IQR) | 10 (2.7–28.5) | 80 | 24.8 (12.0–37.9) | 18 | 0.030 |
| Total length of stay, days, median (IQR) | 72.6 (34.8–116.5) | 91 | 101.3 (86.2–120.6) | 18 | 0.012 |
| Treatment Costs | Group Without SSAE | n | Group with SSAE | n | P-Valuea |
| Surgery; €∗1000, median (IQR) | 4.7 (2.7–6.9) | 90 | 7.6 (5.0–9.9) | 17 | 0.003 |
| Intensive care unit; €∗1000, median (IQR) | 8.7 (2.1–31.1) | 76 | 29.1 (3.6–44.2) | 18 | 0.033 |
| Spinal cord unit; €∗1000, median (IQR) | 27.7 (11.7–40.7) | 90 | 42.6 (28.8–47.8) | 18 | 0.034 |
| Total costs; €∗1000, median (IQR) | 52.3 (26.7–91.2) | 91 | 97.3 (78.2–112.3) | 18 | 0.001 |
| Matched Sample | |||||
| Length of Stay | Group Without SSAE | n | Group With SSAE | n | P-Valuea |
| Intensive care unit; days, median (IQR) | 13.0 (2.3–36.5) | 37 | 28.5 (16.4–46.0) | 14 | 0.10 |
| Total length of stay, days, median (IQR) | 72.9 (47.1–144.5) | 39 | 104.4 (86.2–120.6) | 14 | 0.56 |
| Treatment Costs | Group Without SSAE | n | Group With SSAE | n | P-Valuea |
| Surgery; €∗1000, median (IQR) | 4.9 (2.9–6.9) | 38 | 7.7 (4.9–9.8) | 14 | 0.014 |
| Intensive care unit; €∗1000, median (IQR) | 13.2 (2.3–40.0) | 34 | 31.9 (16.6–57.2) | 14 | 0.095 |
| Spinal cord unit; €∗1000, median (IQR) | 30.3 (14.4–51.5) | 38 | 42.6 (28.8–47.8) | 14 | 0.85 |
| Total costs; €∗1000, median (IQR) | 52.9 (27.8–128.8) | 39 | 93.4 (78.2–115.9) | 14 | 0.14 |
Excluded: work-related accidents, patients who received the first surgery in another center, and patients who died during primary care.
Explorative P-values were calculated in the total sample using univariate linear regression and in the matched sample using generalized estimating equation considering the match-ID as a cluster.
IQR indicates interquartile range; SSAE, spinal surgery adverse events.
The cumulative treatment costs (median [IQR]) were higher in the group with SSAE compared with the group without SSAE in the total sample (97,300 [78,200– 112,300] € vs. 52,300 [26,700–91,200] €) and to some extent also in the matched sample (93,400 [78,200– 115,900] € vs. 52,900 [27,800–128,800] €). The analysis in the matched sample revealed that these effects were related primarily to spinal surgery costs and treatment costs in the ICU but not to the costs for treatment in the SCI-unit (Table 5).
DISCUSSION
The overall SSAE rate of 22.4% in this study was higher compared with studies on spinal surgery for any reason or for spine fractures without SCI reporting SSAE rates between 10% and 15%.4–6 The rather high SSAE rate in this study is most likely related to the relevance of particular types of SSAE after SCI and to more complex injury patterns.
In this study, SSAE were classified as direct or indirect SSAE occurring during surgery or as post-surgery SSAE. Knop et al6 distinguished direct SSAE as inappropriate fracture correction, access-related complications, or insufficient spinal decompression. Their study on SSAE in spine surgery performed for any underlying reason reported only singular cases of insufficient spinal decompression.6 In this study, however, insufficient spinal decompression was the most frequent SSAE during surgery observed in 6.7% of the SCI population. Noteworthy, none of the patients with insufficient decompression has improved neurologically. In addition, Knop et al6 report a rate of 1% pedicle misalignment of one screw, which is lower than the 4.2% rate in this study. This can possibly be explained by more complex injury patterns after SCI, although only weak associations of SSAE with higher AO-classifications of the spine fracture or a more severe AIS grades of SCI were observed.
The frequencies of post-surgery SSAE such as wound infections in this study were similar to studies that included any reasons for spinal surgery.4–6,15,19 This also holds true for rates of hematoma after surgery of spinal fractures.4,6 Indirect SSAE were not observed in this study, which may be related to its relatively small sample size. Indirect SSAE are rare but serious events. The permanent loss of visual acuity due to abdominal positioning was reported elsewhere to occur in 0.028% to 1.3%20 and life-threatening postoperative cerebellar hemorrhages occurred in 0.08%.21
Among the patient's baseline characteristics, sex had the strongest effect on SSAE with a reduced probability for SSAE in female patients. Comorbidities and older age were weakly associated with higher SSAE rates. Other studies consider similar pre-existing conditions as risk factors in spinal surgery.4,7,11,15 The fact that, unlike in other stud-ies,22 no associations of the BMI with SSAE or outcome were observed, may be due to very few cases with higher grades of obesity in our sample.23,24 Despite the challenge of emergency surgery for complex spinal injuries, this study did not reveal any association of surgery performed at nighttime with the occurrence of SSAE in the setting of a level I trauma center.
Frequent secondary complications in studies combining all spinal surgery procedures are 1.8% urinary tract infections,4,15 0.8% thrombosis,4,12,15 0.4% pulmonary embolism.4,12 The mortality rates of up to 0.4% in general5,12,15 are higher after procedures in the cervical spine (0.9%)11 or for fracture treatment (1%).6,25 This study revealed a much higher burden of secondary complications and a higher mortality rate after spinal surgery in cases with cervical SCI. Comparing this study to other SCI-specific studies7–9,26 reveals similar rates of secondary complications. Nevertheless, SSAE may contribute to a higher susceptibility for specific secondary complications as the association of SSAE with these complications was also observed in the matched sample. In contrast, higher mortality in the SSAE group was only detectable in the total sample but not in the matched sample. Thus, it can be assumed that the mortality was more related to the patient's injury and baseline conditions rather than to SSAE.
Regarding neurological outcome, the 12.8% rate of AIS-deterioration in the group with SSAE in the matched sample is similarly high compared with other studies on spinal surgery of any etiology with neurological deterioration rates of up to 17%.6,10,12,27 Moreover, in the matched sample in this study, also a lower probability of AIS-conversion was associated with SSAE. With regard to the relevance of SSAE subtypes, that is, insufficient spinal decompression, the observation that none of the patients with this type of SSAE experienced an AIS-conversion in contrast to patients with other SSAE should be interpreted cautiously, as the SSAE subgroups are of a small size. Also, the additional association of a single-sided surgical approach (ventral or dorsal) with poor neurological outcome has to be interpreted carefully and warrants further investigation in prospective multicenter studies. However, the results underline that SSAE may not only be associated with sudden neurological deterioration but are of particular relevance for SCI patients because SSAE can further restrict their already limited capacity for neurological recovery.
SSAE were most robustly associated with transient and especially with permanent dysphagia in the total and in the matched sample in this study. Depending on study design and definitions, swallowing disorders after surgery in the anterior cervical spine region occur in 1% to 71% of cases.28 If divided into temporary and permanent, the rate of permanent swallowing disorder reported elsewhere is 14% for fractures without SCI and 28% for fractures with tetraple-gia.28,29 In line with these data, the rate of permanent dysphagia is 21.5% to 34.5% in this study. Noteworthy, the increased risk for dysphagia in patients with SSAE was independent of additional effects of a ventral surgical approach, which had no clear effect in the multiple models. Thus, dysphagia after spinal surgery after cervical SCI is most likely related to SSAE rather than to surgery in the anterior cervical region per se.
The increased length of stay in the group with SSAE can be considered as being related to surgery revisions, neurological deterioration, or delayed recovery.4,11,15 Increased length of stay is mirrored by higher treatment costs, particularly for spine surgery and stay in ICU, both of which are accounting for the increase in total costs as also observed in other studies.11 The generally longer stay in primary care in this study compared to acute SCI studies in other countries,8,9 can be explained by differences in health care systems and definitions of primary care.
Limitations of this study are its retrospective monocenter design and the end of follow-up at discharge from primary SCI care. Thus, confirmative studies are required to gain insights on SSAE effects on long-term outcome. Nevertheless, the dataset of this study enables a disease-specific and comprehensive investigation of SSAE and their consequences in cervical SCI. In the light of the epidemiological change, the highly vulnerable group of cervical SCI patients is becoming increasingly relevant.3 In addition, risk factors on the individual practitioner level, such as the degree of spinal surgery training could not be evaluated due to the small number of spine surgeons in the monocenter setting. Hamilton et al27 and Smith et al25 showed that specialist training or certification is important to improve the outcome. This was also demonstrated for procedures performed in the presence of a senior physician or experienced spine surgeon in the operating room.4,11
CONCLUSION
Surgery of cervical spine fractures with SCI is associated with a higher frequency of SSAE compared with other reasons for spinal surgery. Risk markers for SSAE are male sex and to a lesser extent older age, comorbidities, motor complete SCI, or a single sided surgical approach. SSAE are associated with a higher burden of SCI-associated secondary complications, a poorer neurological outcome, higher rates of permanent dysphagia, and higher surgery and treatment costs after cervical SCI. For these reasons a consistent high-quality management in the emergency setting in specialized spine centers is required to protect outcome at risk in SCI patients with fractures to the cervical spine. Improving surgical management to reduce SSAE and subsequently poor neurological outcome and dysphagia is an achievable target that can not only influence the patients’ quality of life but also ensure the baseline for future therapeutic interventions.
Key Points
Spinal surgery adverse events in the primary treatment of traumatic spinal fractures occur in approximately one quarter of patients with acute cervical spinal cord injury.
Most prevalent spinal surgery adverse events after traumatic injury to the cervical spinal cord are mechanical instability and insufficient decompression of the spinal cord.
Spinal surgery adverse events after cervical spinal cord injury are associated with poor neurological outcome.
Swallowing disorders associated with spinal surgery adverse event occur independently of the surgical approach after cervical spinal cord injury.
Adverse events in spinal surgery for traumatic cervical spinal cord injury result in higher healthcare costs, especially for surgery and intensive care.
Supplementary Material
Acknowledgments
The authors would like to thank our doctoral candidates Johanna Wollschlaeger, Magdalena Hoppe, and Erik Prilipp for their work in source data verification. In recognition of Dr Andreas Niedeggen for his many years of work in the field of spinal cord injury treatment.
Footnotes
The manuscript submitted does not contain information about medical device(s)/drug(s).
No funds were received in support of this work.
Relevant financial activities outside the submitted work: grants.
Supplemental digital content is available for this article.
References
- 1.Wilson JR, Tetreault LA, Kwon BK, et al. Timing of decompression in patients with acute spinal cord injury: a systematic review. Global Spine J 2017; 7: (3 suppl): 95S–115S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jazayeri SB, Beygi S, Shokraneh F, et al. Incidence of traumatic spinal cord injury worldwide: a systematic review. Eur Spine J 2015; 24:905–918. [DOI] [PubMed] [Google Scholar]
- 3.Badhiwala JH, Ahuja CS, Fehlings MG. Time is spine: a review of translational advances in spinal cord injury. J Neurosurg Spine 2018; 30:1–18. [DOI] [PubMed] [Google Scholar]
- 4.Kimmell KT, Algattas H, Joynt P, et al. Risk modeling predicts complication rates for spinal surgery. Spine (Phila Pa 1976) 2015; 40:1836–1841. [DOI] [PubMed] [Google Scholar]
- 5.Schoenfeld AJ, Carey PA, Cleveland AW, et al. Patient factors, comorbidities, and surgical characteristics that increase mortality and complication risk after spinal arthrodesis: a prognostic study based on 5,887 patients. Spine J 2013; 13:1171–1179. [DOI] [PubMed] [Google Scholar]
- 6.Knop C, Bastian L, Lange U, et al. Complications in surgical treatment of thoracolumbar injuries. Eur Spine J 2002; 11:214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bourassa-Moreau É, Mac-Thiong J-M, Ehrmann Feldman D, et al. Complications in acute phase hospitalization of traumatic spinal cord injury: does surgical timing matter? J Trauma Acute Care Surg 2013; 74:849–854. [DOI] [PubMed] [Google Scholar]
- 8.Wilson JR, Arnold PM, Singh A, et al. Clinical prediction model for acute inpatient complications after traumatic cervical spinal cord injury: a subanalysis from the Surgical Timing in Acute Spinal Cord Injury Study. J Neurosurg Spine 2012; 17:46–51. [DOI] [PubMed] [Google Scholar]
- 9.van Weert KCM, Schouten EJ, Hofstede J, et al. Acute phase complications following traumatic spinal cord injury in Dutch level 1 trauma centres. J Rehabil Med 2014; 46:882–885. [DOI] [PubMed] [Google Scholar]
- 10.Ghobrial GM, Williams KA, Arnold P, et al. Iatrogenic neurologic deficit after lumbar spine surgery: a review. Clin Neurol Neurosurg 2015; 139:76–80. [DOI] [PubMed] [Google Scholar]
- 11.Marquez-Lara A, Nandyala SV, Hassanzadeh H, et al. Sentinel events in cervical spine surgery. Spine (Phila Pa 1976) 2014; 39:715–720. [DOI] [PubMed] [Google Scholar]
- 12.Smith JS, Fu K-MG, Polly DW, et al. Complication rates of three common spine procedures and rates of thromboembolism following spine surgery based on 108,419 procedures: a report from the Scoliosis Research Society Morbidity and Mortality Committee. Spine (Phila Pa 1976) 2010; 35:2140–2149. [DOI] [PubMed] [Google Scholar]
- 13.Hemmer S, Almansour H, Pepke W, et al. [A new classification of surgical complications in adult spinal deformity]. Orthopade 2018; 47:335–340. [DOI] [PubMed] [Google Scholar]
- 14.Whitmore RG, Stephen JH, Vernick C, et al. ASA grade and Charlson Comorbidity Index of spinal surgery patients: correlation with complications and societal costs. Spine J 2014; 14:31–38. [DOI] [PubMed] [Google Scholar]
- 15.Bekelis K, Desai A, Bakhoum SF, et al. A predictive model of complications after spine surgery: the National Surgical Quality Improvement Program (NSQIP) 2005–2010. Spine J 2014; 14:1247–1255. [DOI] [PubMed] [Google Scholar]
- 16.Lebude B, Yadla S, Albert T, et al. Defining “complications” in spine surgery: neurosurgery and orthopedic spine surgeons’ survey. J Spinal Disord Tech 2010; 23:493–500. [DOI] [PubMed] [Google Scholar]
- 17.Dekutoski MB, Norvell DC, Dettori JR, et al. Surgeon perceptions and reported complications in spine surgery. Spine (Phila Pa 1976) 2010; 35:S9–21. [DOI] [PubMed] [Google Scholar]
- 18.Kirshblum SC, Waring W, Biering-Sorensen F, et al. Reference for the 2011 revision of the International Standards for Neurological Classification of Spinal Cord Injury. J Spinal Cord Med 2011; 34:547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith JS, Shaffrey CI, Sansur CA, et al. Rates of infection after spine surgery based on 108,419 procedures: a report from the Scoliosis Research Society Morbidity and Mortality Committee. Spine (Phila Pa 1976) 2011; 36:556–563. [DOI] [PubMed] [Google Scholar]
- 20.Uribe A, Baig M, Peunte E, et al. Current intraoperative devices to reduce visiual loss after spine surgery. Neurosurg Focus 2012; 33:E14. [DOI] [PubMed] [Google Scholar]
- 21.Worm PV, Dalla-Corte A, Brasil AVB, et al. Cerebellar hemorrhage as a complication of spine surgery. Surg Neurol Int 2019; 10:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katsevman GA, Daffner SD, Sedney CL, et al. Complexities of spine surgery in obese patient populations: a narrative review. Spine J 2020; 20:501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wen H, DeVivo MJ, Chen Y, et al. The impact of body mass index on one-year mortality after spinal cord injury. J Spinal Cord Med 2019; 15:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hales CM, Carroll MD, Ogden CL, et al. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. NCHS Data Brief 2020; No. 360. Available at: https://www.cdc.gov/nchs/products/databriefs/db360.htm. Accessed December 04, 2020. [Google Scholar]
- 25.Smith JS, Saulle D, Chen C-J, et al. Rates and causes of mortality associated with spine surgery based on 108,419 procedures: a review of the scoliosis research society morbidity and mortality database. Spine (Phila Pa 1976) 2012; 37:1975–1982. [DOI] [PubMed] [Google Scholar]
- 26.Aito S. Gruppo Italiano Studio Epidemiologico Mielolesioni GISEM Group. Complications during the acute phase of traumatic spinal cord lesions. Spinal Cord 2003; 41:629–635. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton DK, Smith JS, Sansur CA, et al. Rates of new neurological deficit associated with spine surgery based on 108,419 procedures: a report of the scoliosis research society morbidity and mortality committee. Spine (Phila Pa 1976) 2011; 36:1218–1228. [DOI] [PubMed] [Google Scholar]
- 28.Joaquim AF, Murar J, Savage JW, et al. Dysphagia after anterior cervical spine surgery: a systematic review of potential preventative measures. Spine J 2014; 14:2246–2260. [DOI] [PubMed] [Google Scholar]
- 29.Liebscher T, Niedeggen A, Estel B, et al. Airway complications in traumatic lower cervical spinal cord injury: a retrospective study. J Spinal Cord Med 2015; 38:607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.